Summary
In mammalian cells, nonsense-mediated mRNA decay (NMD) generally requires that translation terminates sufficiently upstream of a post-splicing exon junction complex (EJC) during a pioneer round of translation. The subsequent binding of Upf1 to the EJC triggers Upf1 phosphorylation. We provide evidence that phospho-Upf1 functions after nonsense codon recognition during steps that involve the translation initiation factor eIF3 and mRNA decay factors. Phospho-Upf1 interacts directly with eIF3 and inhibits the eIF3-dependent conversion of 40S/Met-tRNAiMet/mRNA to translationally competent 80S/Met-tRNAiMet/mRNA initiation complexes to repress continued translation initiation. Consistent with phospho-Upf1 impairing eIF3 function, NMD fails to detectably target nonsense-containing transcripts that initiate translation independently of eIF3 from the CrPV IRES. There is growing evidence that translational repression is a key transition that precedes mRNA delivery to the degradation machinery. Our results uncover a critical step during NMD that converts a pioneer translation initiation complex to a translationally compromised mRNP.
Introduction
NMD plays an important role in the posttranscriptional control of human gene expression. Approximately 75% of human genes generate pre-mRNAs that undergo alternative splicing, and more than one-third of alternatively spliced transcripts are targeted for elimination by the NMD pathway (Lewis et al., 2003). Most alternatively spliced NMD targets appear to be generated in error (Pan et al., 2006). However, NMD also downregulates the level of other transcripts that are productive, including those that harbor upstream open reading frames, contain a UGA selenocysteine codon, or are autoregulated by the encoded protein (see, e.g., Mendell et al., 2004; Wittmann et al., 2006; Ni et al., 2007).
As a rule, NMD in mammals degrades newly synthesized mRNAs during a pioneer round of translation, which utilizes mRNA that is associated with the cap-binding protein (CBP) heterodimer CBP80/20 and may involve the loading of more than one ribosome (Ishigaki et al., 2001; Lejeune et al., 2002; Chiu et al., 2004; Lejeune et al., 2004; Hosoda et al., 2005; Kashima et al., 2006). The pioneer round precedes subsequent rounds of translation that utilize mRNA that is bound at its cap by eukaryotic translation initiation factor (eIF)4E. eIF4E-bound mRNA, which is the remodeled product of CBP80/20-bound mRNA, supports the bulk of cellular protein synthesis (Pestova et al., 2007; Lejeune et al., 2002). NMD generally occurs when translation during a pioneer round terminates more than ~50–55 nucleotides upstream of a splicing-generated exon-exon junction (Isken and Maquat, 2007). The role of exon-exon junctions in NMD reflects the splicing-dependent deposition of an exon junction complex (EJC) of proteins ~20–25 nucleotides upstream of junctions (Le Hir et al., 2001). The EJC consists of NMD factors Upf3 (also called Upf3a) or Upf3X (also called Upf3b), Upf2, and, presumably after translation terminates sufficiently upstream of the EJC, Upf1 (Kashima et al., 2006; Kim et al., 2001), which also associates with the CBP80/20 cap-binding complex (Hosoda et al., 2005). According to a current model, translation termination depends on the joining of the PIK-related protein kinase SMG-1 and Upf1 with the two translation termination factors eRF1 and eRF3 to form SURF (Kashima et al., 2006). If an EJC exists sufficiently downstream of the termination event, then SMG-1 and Upf1 binding to that EJC triggers Upf1 phosphorylation (Kashima et al., 2006; Wittmann et al., 2006). Upf1 phosphorylation leads to formation of the so-called decay-inducing complex (Kashima et al., 2006), and decay is initiated from either or both mRNA ends (Lehner and Sanderson, 2004; Lejeune et al., 2003; Lykke-Andersen, 2002; Unterholzner and Izaurralde, 2004; Yamashita et al., 2005). In comparison to CBP80/20-bound mRNA, eIF4E-bound mRNA lacks detectable EJCs and appears to be immune to NMD (Chiu et al., 2004; Hosoda et al., 2005; Ishigaki et al., 2001; Lejeune et al., 2002; Matsuda et al., 2007).
There is growing evidence that mRNA decay in eukaryotes requires an exit from translation so that the mRNA is accessible to degradative activities. For example, in Saccharomyces cerevisiae, eIF4E and the poly(A) binding protein inhibit decapping at least in part through their roles as general translational activators (Coller and Parker, 2004). Also in S. cerevisiae, Dhh1p and Pat1p, which target cytoplasmic mRNAs for decapping and facilitate their assembly into processing (P) bodies in response to glucose deprivation or amino acid starvation, have been shown to be general activators of translational repression (Coller and Parker, 2005). In mammalian cells, binding of the translational repressor TIAR to the 3′ untranslated region (UTR) of GADD45α mRNA, which is induced upon growth arrest or DNA damage, results in GADD45α mRNA translational repression and decay that is mediated by AUF-1 association with the 3′ UTR (Lal et al., 2006). Also in mammalian cells, 4E-transporter binding to eIF4E downregulates cap-dependent translation in a way that primes mRNA for localization to P bodies and decay (Ferraiuolo et al., 2005). More directly pertinent to the studies reported here, translational repression occurs in S. cerevisiae, although by an unknown mechanism, after nonsense codon recognition during the process of NMD (Muhlrad and Parker, 1999; Sheth and Parker, 2006). It follows that translational repression would likewise accompany NMD in mammalian cells.
In this report, we provide evidence that translational repression does indeed occur during NMD in mammalian cells. Since the trigger for decay appears to be the phosphorylation of Upf1, we first identified NMD factors that preferentially coimmunoprecipitate with wild-type (WT) Upf1, which is primarily hypophosphorylated (Pal et al., 2001; Yamashita et al., 2001), or either a mutated variant of Upf1 or WT Upf1 from okadaic acid (OA)-treated cells, each of which manifests a higher degree of phosphorylation than WT Upf1 from untreated cells. We found that WT Upf1 from untreated cells has a significantly higher affinity than hyperphosphorylated (phospho-) Upf1 for SURF constituents SMG-1 and eRF3. These interactions are thought to take place prior to or during nonsense codon recognition (Kashima et al., 2006). We also discovered that phospho-Upf1 has a significantly higher affinity for the NMD degradative factors Dcp1a, Xrn1, and Rrp4. According to current models, these interactions occur after nonsense codon recognition. Remarkably, phospho-Upf1 also preferentially interacts with the eIF2-GTP-Met-tRNAiMet ternary translation initiation complex via eIF3. We present a model in which the SMG-1-mediated Upf1 phosphorylation that occurs upon SMG-1 and Upf1 binding to an EJC triggers eIF3-dependent translational repression and mRNA decay. Consistent with this model, we find that phospho-Upf1 targets the translation initiation process by inhibiting the eIF3-dependent conversion of 40S/Met-tRNAiMet/mRNA to translationally competent 80S/Met-tRNAiMet/mRNA. Furthermore, CrPV IRES-mediated translation initiation, which is eIF3 independent, does not detectably support NMD, whereas EMCV IRES-mediated translation initiation, which is eIF3 dependent, does.
Results
WT Upf1 and Phospho-Upf1 Manifest Different Affinities for mRNP Proteins
Upf1 is a phosphoprotein (Pal et al., 2001), and Upf1 phosphorylation has been shown to influence Upf1 binding to SMG-5, SMG-7, protein phosphatase 2A, Upf2, and distinct isoforms of Upf3 (Kashima et al., 2006; Ohnishi et al., 2003). Upf1 phosphorylation by SMG-1 is essential for NMD (Brumbaugh et al., 2004; Yamashita et al., 2001), takes place upon Upf1 and SMG-1 binding to an EJC after translation terminates sufficiently upstream of the EJC (Kashima et al., 2006), and is promoted by EJC components (Kashima et al., 2006; Wittmann et al., 2006). We aimed to define which steps of NMD involve phosphorylated Upf1.
Initially, HeLa cells were transiently transfected with one of two pCMV-myc-UPF1R plasmids, each of which produces Upf1 that is resistant to an siRNA (the particular use of which is important in subsequent experiments). One plasmid, WT, encodes unmutated Upf1, which is primarily hypophosphorylated (Pal et al., 2001; Yamashita et al., 2001). The other plasmid, G495R/G497E, encodes Upf1 harboring amino acid substitutions at positions 495 and 497 within the ATPase/RNA helicase domain. These substitutions are predicted from studies of Upf1 in Caenorhabiditis elegans to result in inefficient Upf1 dephosphorylation and, therefore, hyperphosphorylated (phospho)-Upf1 (Page et al., 1999). Two days after transfection, cells were lysed. Protein was analyzed before and after immunoprecipitation (IP) using anti-myc or, to control for nonspecific IP, mouse (m)IgG.
Each myc-Upf1R derivative was produced in HeLa cells at a comparable level (Figure 1A, lanes 1 and 2, WB: α-myc), and this level was less than 2-fold above the level of endogenous HeLa cell Upf1 (Figure 1A, lanes 1 and 2, WB: Furthermore, each myc-Upf1R derivative was immunoprecipitated with comparable efficiency using anti-myc (Figure 1A, lanes 4 and 6, WB: α-Upf1 or α-myc), and neither was immunoprecipiated using mIgG (Figure 1A, lanes 3 and 5, WB: α-Upf1 or α-myc).
Figure 1. WT Upf1 Preferentially Interacts with SMG-1 and eRF3, whereas Phospho-Upf1 Preferentially Interacts with mRNA Decay Factors.
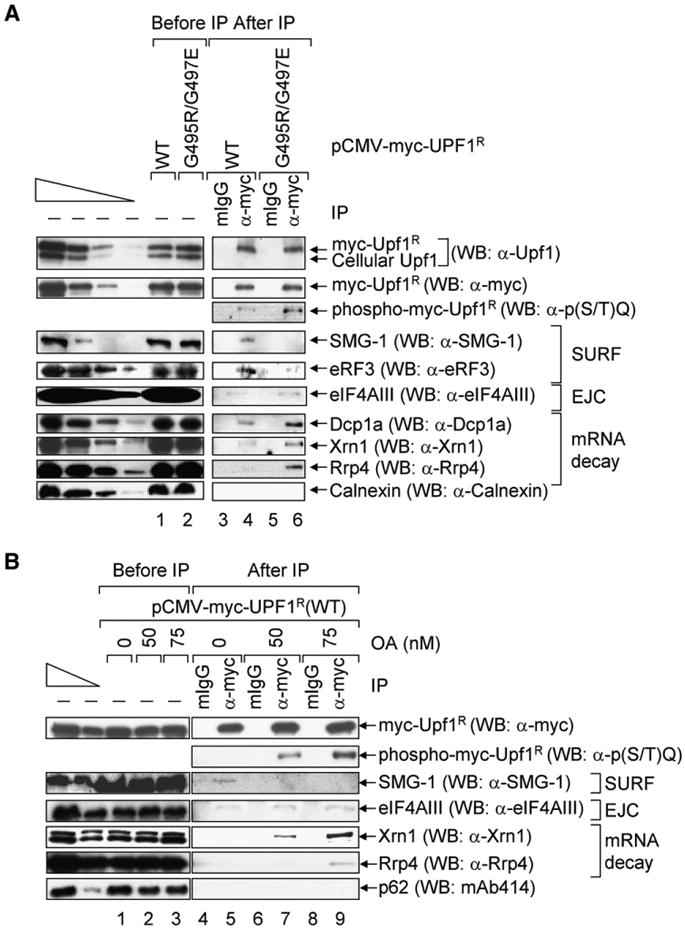
(A) HeLa cells (3–4 × 107) were transiently transfected with pCMV-myc-UPF1R(WT) or pCMV-myc-UPF1R(G495R/G497E) (10 µg). Two days later, total-cell lysate was immunoprecipitated using anti(α)-myc or mouse (m)IgG. Western blotting (WB) was performed using the specified antibodies. The four leftmost lanes analyzed decreasing amounts of protein before (−) IP.
(B) Essentially as in (A), except cells were transiently transfected with pCMV-myc-UPF1R(WT) and treated with the specified amount of OA for 3 hr prior to harvesting. All results are representative of three independently performed experiments.
Using samples after IP, myc-Upf1R(G495R/G497E) was confirmed to be hyperphosphorylated relative to myc-Upf1R(WT) as indicated by the 4- to 5-fold higher reactivity of anti-phospho(S/T)Q with myc-Upf1R(G495R/G497E) relative to myc-Upf1R(WT) (Figures 1A, lanes 4 and 6, WB: α-p(S/T)Q; Figure S1A available online) that was not detectable upon phosphatase treatment (Figure S1A). SMG-1 and eRF3 preferentially immunoprecipitated with myc-Upf1R(WT) compared to myc-Upf1R(G495R/G497E) (Figure 1A, lanes 4 and 6, WBs: α-SMG-1 and α-eRF3). By contrast, the EJC core constituent eIF4AIII manifested a very slight preference for myc-Upf1R(G495R/G497E) compared to myc-Upf1R(WT) (Figure 1A, lanes 4 and 6, WB: α-eIF4AIII), and degradative activities known to function in NMD, including the decapping activator Dcp1a, the 5′-to-3′ exonuclease Xrn1, and the exosomal component Rrp4, exhibited appreciable preference for myc-Upf1R(G495R/G497E) (Figure 1A, lanes 4 and 6, WBs: α-Dcp1a, α-Xrn1, and α-Rrp4). The weak interaction of Upf1 with eIF4AIII probably reflects its very transient nature. As a negative control, Calnexin was not detectably immunoprecipitated under any condition (Figure 1A, lanes 3–6, WB: α-Calnexin). The preferential affinities of SMG-1 for hypophosphorylated Upf1 and of mRNA degradative activities and, to a very slight extent, eIF4AIII for phospho-Upf1 were corroborated by treating HeLa cells transiently expressing myc-Upf1(WT) with 0, 50, or 75 nM OA prior to IP (Figure 1B, lanes 5, 7, and 9). OA is a potent inhibitor of protein phosphatase 2A (Haystead et al., 1989) that results in the accumulation of phospho-Upf1 (Figure 1B, lanes 7 and 9; Figure S1B).
Our findings indicate that hypophosphorylated Upf1 preferentially interacts with mRNP constituents at steps that are thought to precede or occur during translation termination. In contrast, phospho-Upf1 preferentially interacts with mRNP constituents, presumably largely during the pioneer round of translation, at steps that are envisioned to take place after translation termination, including the recruitment of mRNA decay factors.
Upf1 Decreases Cap-Dependent and HCV IRES-Dependent Translation Initiation but Not CrPV IRES-Dependent Translation Initiation
mRNAs that are targeted for decapping generally undergo translational repression (Coller and Parker, 2005). Since NMD in mammalian cells involves decapping (Lejeune et al., 2003; Unterholzner and Izaurralde, 2004), mRNAs that are slated for NMD should also undergo translational repression. We hypothesized that translational repression during NMD is likely to involve phospho-Upf1 for a number of reasons. First, the trigger for NMD is Upf1 binding to an EJC after translation terminates sufficiently upstream of the EJC, which results in Upf1 phosphorylation (Kashima et al., 2006; Wittmann et al., 2006; Figure 1). Second, Upf1 remains hyperphosphorylated during subsequent steps that include recruiting mRNA decay factors (Figure 1). Therefore, phospho-Upf1 would be expected to exist during what we propose would be an intermediate step of translational repression.
As noted above, NMD in mammalian cells is largely if not exclusively restricted to the translation of CBP80/20-bound mRNA during a pioneer round of translation. At present, analyzing NMD provides the only functional assay for this round of translation, which constitutes only a very small fraction of cellular translation (Ishigaki et al., 2001; Lejeune et al., 2002). However, the pioneer translation initiation complex shares many constituents with the translation initiation complex that supports the bulk of cellular protein synthesis, including eIF2, eIF3, eIF4G, PABPC1, and ribosomes (Chiu et al., 2004; Hosoda et al., 2006; Ishigaki et al., 2001; Lejeune et al., 2002, 2004). Moreover, NMD, like the bulk of cellular translation, is inhibited by suppressor tRNA, anisomysin, cycloheximide, emetine, pactamycin, and puromycin (Isken and Maquat, 2007). Furthermore, the Upf factors have been implicated as effectors of bulk cellular translation (see Discussion in Supplemental Data). Additionally, Upf1 binds eRF1 and eRF3 (Czaplinski et al., 1998; Kashima et al., 2006; Figure 1A), which function during both pioneer and subsequent rounds of translation termination. Therefore, it is reasonable to propose that phospho-Upf1 would affect the bulk of cellular translation as it affects the pioneer round of translation.
Initially, we assayed for an effect of Upf1 on the bulk of cellular translation by downregulating the cellular abundance of Upf1 using in vitro-synthesized siRNA. HeLa cells were transfected with Upf1 siRNA or a nonspecific control siRNA (Kim et al., 2005). Two days later, cells were retransfected with three plasmids: a pmCMV-Gl test plasmid (Ishigaki et al., 2001) that was either nonsense free (Norm) or contained a premature termination codon (39Ter), a phCMV-MUP reference plasmid (Ishigaki et al., 2001), and a pR/HCV/F or pR/CrPV/F dual Renilla (R) and Firefly (F) luciferase reporter plasmid (Figure 2A; Kim et al., 2005; Wilson et al., 2000). The test plasmids provided an assay for NMD. The reference plasmid controlled for variations in the efficiencies of cell transfection and RNA recovery. The reporter plasmids monitored the efficiencies of cap-dependent translation initiation and, respectively, Hepatitis C Virus (HCV) internal ribosome entry site (IRES)-dependent or Cricket Paralysis Virus (CrPV) IRES-dependent translation initiation. One day after the second transfection, total-cell protein was analyzed to assess the efficiency of Upf1 downregulation. Additionally, total-cell RNA was purified for the analysis of individual mRNAs by RT-PCR to determine the efficiency of NMD and the abundance of R/HCV/F or R/CrPV/F mRNA. Cell lysates were also subjected to dual Luciferase (Luc) activity assays.
Figure 2. Downregulating the Cellular Abundance of Upf1 Increases Cap-Dependent and HCV IRES-Dependent Translation but Not CrPV IRES-Dependent Translation.

(A) Schematic representations of the bicistronic constructs that encode Renilla (R) and Firefly (F) Luciferase (Luc). HeLa cells (2 × 106) were transiently transfected with Upf1 or control siRNA. Two days later, cells were retransfected with a pmCMV-Gl test plasmid, either Norm or 39Ter, the reference phCMV-MUP plasmid, and a reporter plasmid that was either pR/HCV/F or pR/CrPV/F.
(B) Western blotting (WB) was used to quantitate the level of Upf1 relative to Vimentin.
(C) RT-PCR was used to quantitate the levels of Gl and MUP mRNAs in transfections with either pR/HCV/F or pR/CrPV/F. Numbers below the figure represent the level of Gl mRNA after normalization to the level of MUP mRNA, where the normalized level of Gl Norm mRNA in the presence of each siRNA or no (−) siRNA was defined as 100%.
(D) Luminometry was used to quantitate FLucand RLuc activities, which were normalized to levels of R/HCV/F or R/CrPV/F mRNA to control for variations in transfection efficiencies. The normalized levels of Luc activity in transfections with control siRNA were defined as 1. Results are the average of two independently performed experiments.
Results of western blotting indicated that, relative to control siRNA, Upf1 siRNA reduced the level of cellular Upf1 ~20-fold (Figure 2B). Results of RT-PCR revealed that downregulating Upf1 also inhibited the NMD of Gl 39Ter mRNA 3- to 4-fold (Figure 2C), consistent with reports that Upf1 is essential for NMD (Isken and Maquat, 2007).
Additionally, downregulating Upf1 increased the level of cap-dependent or HCV IRES-dependent translation 4- to 6-fold (Figure 2D). Downregulating Upf1 was of little consequence to CrPV IRES-dependent translation but, again, increased the level of cap-dependent translation (Figure 2D). Importantly, downregulating Upf1 using Upf1(A) siRNA (Kim et al., 2005), which targets a different region of UPF1 mRNA than does Upf1 siRNA, also increased the level of cap-dependent and HCV IRES-dependent translation but not CrPV IRES-dependent translation (Figure S2; data not shown for HCV IRES-dependent translation). Therefore, the changes observed with Upf1 siRNA are not the result of off-target effects. In contrast to Upf1, neither Upf2 nor Upf3X were found to affect any type of translation initiation (Figures S3 and S4).
Cap-dependent and IRES-dependent translation differ in their modes of initiation but not elongation or termination (Jackson, 2005). Therefore, our results indicate that downregulating Upf1 upregulates translation initiation but not detectably later steps of protein synthesis. Cap-dependent translation initiation requires all eIFs, HCV IRES-dependent translation initiation requires eIF2 and eIF3, and CrPV IRES-dependent translation initiation requires no eIFs (Doudna and Sarnow, 2007). Therefore, our results suggest that Upf1 inhibits translation initiation by targeting eIF2, eIF3, or both.
CrPV IRES-Dependent Translation Initiation Fails to Support NMD
If Upf1 inhibits translation initiation by targeting eIF2 and/or eIF3, then translation initiation from the CrPV IRES should not support NMD. To test this hypothesis, plasmids were generated (Figure 3A) that produce RLuc-Gl (R-Gl) mRNA, either Norm or 39Ter, containing the CrPV IRES or, as a positive control, the EMCV IRES, which depends on all canonical eIFs (Doudna and Sarnow, 2007) and supports NMD (Holbrook et al., 2006). The EMCV IRES was chosen for analysis since its activity in vivo can be selectively reduced by mutating the functionally critical GCGA tetraloop (Robertson et al., 1999). This is important since protein production from the CrPV IRES is less than from the EMCV IRES in cycling cells (Fernandez et al., 2002; Humphreys et al., 2005). To generate an EMCV IRES that initiates translation with an efficiency comparable to the CrPV IRES, the EMCV IRES(WT) GCGA tetraloop was mutated to GTTA. Furthermore, a hairpin structure that blocks ribosomes initiating at the cap was inserted upstream of each IRES to ensure that translation initiates only at the IRES (Holbrook et al., 2006; Woeller et al., 2008). Cos cells were transiently transfected with Control or Upf1 siRNA. Cells were subsequently transfected with each IRES-containing construct and, to control for variations among transfections, pcDNA FLuc and phCMV-MUP. Cos cells were used since the activities of the EMCV IRES(GTTA) and the CrPV IRES were more similar in Cos cells than in HeLa cells (data not shown), and equivalent activities are critical if we aim to compare the dependence of IRES-initiated NMD on eIF2 or eIF3 function.
Figure 3. Translation Initiation from the CrPV IRES, Unlike Translation Initiation from the EMCV IRES, Fails to Support NMD.
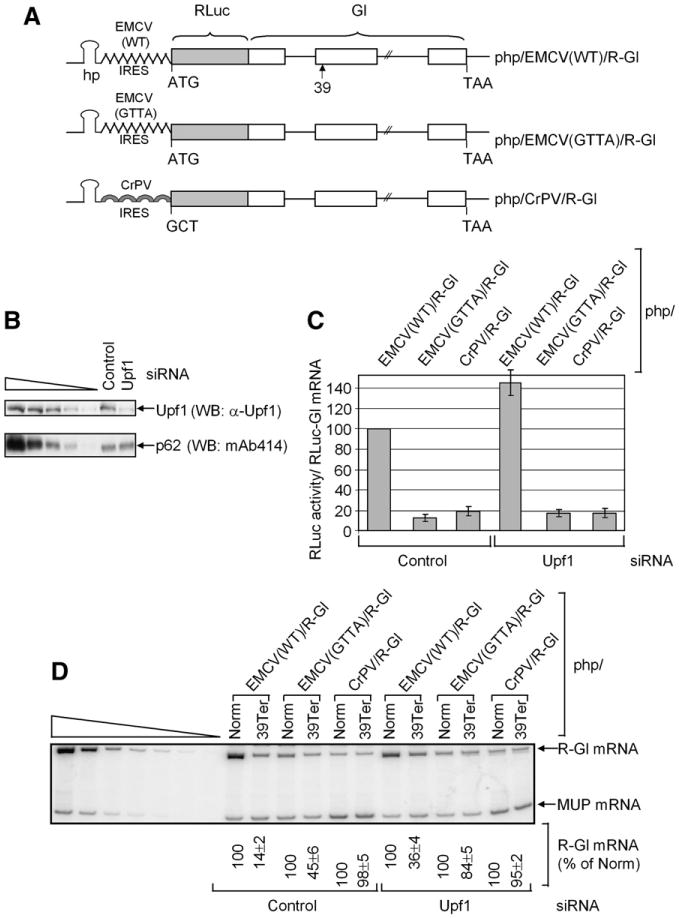
Cos cells (3 × 106) were transiently transfected with control or Upf1 siRNA and, 2 days later, with php/EMCV(WT)/R-Gl, php/EMCV(GTTA)/R-Gl or php/CrPV/R-Gl, either Norm or 39Ter (10 μg), pFLuc (0.5 μg), and phCMV-MUP (0.3 μg).
(A) Diagrams of each R-Gl construct. In the case of 39Ter, 39 specifies the position of the nonsense codon.
(B) Western blotting (WB) was used to quantitate the level of Upf1 relative to p62.
(C) Luminometry was used to quantitate RLuc and FLuc activities. RLuc activities were normalized to FLuc activities, to control for variations among protein preparations, and R-Gl mRNA levels, to control for variations in cell transfection efficiencies. The normalized level of RLuc activity from php/EMCV(WT)/R-Gl in the presence of control siRNA was defined as 100%. Error bars represent the mean ± standard deviation (SD) of three independently performed experiments.
(D) RT-PCR of R-Gl and MUP mRNAs. Numbers below the figure represent the levels of R-Gl mRNA after normalization to the level of MUP mRNA, where the normalized level of R-Gl Norm mRNA in the presence of each php plasmid was defined as 100%. Results are the average of three independently performed experiments.
Western blotting confirmed that, relative to control siRNA, Upf1 siRNA reduced the cellular abundance of Upf1 ~10-fold (Figure 3B). In the presence of control siRNA, mutating the GCGA tetraloop to GTTA reduced the level of EMCV IRES-mediated translation from 100% to 15% ± 3% (Figure 3C). CrPV IRES-mediated translation was 20% ± 4% the level of EMCV IRES(WT)-mediated translation (Figure 3C). The comparable strengths of the EMCV IRES(GTTA) and CrPV IRES allowed us to compare the efficiency of NMD elicited by each IRES in the absence of significant differences due to translation initiation efficiencies and, thus, nonsense codon recognition. In the presence of Upf1 siRNA, the detectable increase in EMCV IRES(WT)-initiated and EMCV IRES(GTTA)-initiated RLuc activities but not CrPV IRES-initiated RLuc activity provided the first indication that Upf1 may differently affect the EMCV and CrPV IRESes. RT-PCR revealed that both EMCV IRES(WT)-initiated translation and EMCV IRES(GTTA)-initiated translation supported NMD whereas CrPV IRES-initiated translation did not (Figure 3D). Additionally, EMCV IRES(WT)-supported NMD and EMCV IRES(GTTA)-supported NMD were inhibited by Upf1 siRNA (Figure 3D). Notably, the IRES-supported NMD of R-Gl mRNA in Cos cells was less efficient than the cap-dependent NMD of Gl mRNA in HeLa cells (compare Figure 3D to Figure 2C). The difference in efficiency may be partly explained by the lower efficiency of IRES-mediated translation initiation compared to cap-dependent translation initiation (data not shown). However, the use of different cell types and reporter mRNAs probably also contributed to the observed differences. We conclude that NMD requires eIF2 and/or eIF3, which is consistent with the hypothesis that Upf1 mediates translational repression by targeting one or both of these initiation factors.
Upf1 Phosphorylation Represses Translation Initiation
Our results predict that phospho-Upf1 inhibits cap-dependent translation more efficiently than WT Upf1 for two reasons: (1) Upf1 becomes hyperphosphorylated just prior to when translational repression during NMD would be expected to occur, and (2) Upf1 remains hyperphosphorylated while associated with mRNA decay factors (Figure 1). To test this prediction, we measured the effect of phospho-Upf1 expression on translation.
HeLa cells were transiently transfected with Upf1 siRNA or control siRNA, the latter of which was used solely to determine the efficacy of Upf1 siRNA. Two days later, cells were retransfected with (1) pmCMV-Gl, either Norm or 39Ter, (2)phCMV-MUP, (3) pR/CrPV/F, and (4) pCMV-myc, pCMV-myc-UPF1R(WT), or pCMV-myc-UPF1R(G495R/G497E), the latter two of which provide an siRNA-resistant source of Upf1.
Western blotting using anti-Upf1 demonstrated that the level of cellular Upf1 was downregulated to ~14% of normal (Figure 4A). Furthermore, each myc-Upf1R was indeed resistant to siRNA-mediated downregulation and restored the cellular level of Upf1 to near normal (Figure 4A). RT-PCR analysis of Gl and MUP mRNAs revealed that in the presence of Upf1 siRNA myc-Upf1R(WT) expression resulted in more efficient NMD than myc-Upf1R(G495R/G497E) expression (Figure 4B). These results are expected considering that cycles of Upf1 phosphorylation and dephosphorylation are essential to NMD (Page et al., 1999; Sun et al., 1998; Yamashita et al., 2001). Additionally, the increased production of cap-dependent RLuc activity relative to CrPV IRES-dependent FLuc activity brought about by downregulating Upf1 was inhibited more effectively by myc-Upf1R(G495R/G497E) than by myc-Upf1R(WT) (Figure 4C). Consistent with these findings, treating cells with OA, which produces phospho-Upf1 (Figures 1B and S1B), also inhibited NMD (Figure 4D). OA, which has been shown to inhibit the dephosphorylation of eIF4E and increase bulk cellular translation (Bu et al., 1993), increased RLuc activity (Figure 4E) as expected. These data suggest that phospho-Upf1 is largely responsible for the Upf1-mediated inhibition of translation initiation of CBP80/20-bound mRNA.
Figure 4. Phosphorylated Upf1 Represses Cap-Dependent but Not CrPV IRES-Dependent Translation Initiation.

(A–C) HeLa cells (1 × 106) were transiently transfected with control or Upf1 siRNA. Two days later, cells were transfected with pCMV-myc-UPF1R(WT) or pCMV-myc-UPF1R(G495R/G497E). (A) Western blotting (WB) was used to quantitate the level of myc-Upf1 relative to endogenous Upf1 and SMG-1. (B) RT-PCR analysis of Gl and MUP mRNAs. As in Figure 2C, except the normalized levels of Gl Norm mRNA in transfections that included each pCMV-myc expression vector were defined as 100%. (C) Relative levels of Luc activity.
(D and E) HeLa cells(3 × 106) were treated with the specified amount of OA for 3hr prior to harvesting. (D) RT-PCR of Gl and MUP mRNAs. (E) Relative RLuc activity normalized to the level of RLuc mRNA, where the normalized level in the absence of OA is defined as 1. Results are the average of three independently performed experiments. Error bars in (C) and (E) represent the mean ± SD of three independent experiments.
Upf1 Phosphorylation Promotes Upf1 Binding to eIF3
Since Upf1 may inhibit translation initiation by targeting eIF2 and/or eIF3, we investigated the interaction of Upf1 with each of these eIFs. Results demonstrated that Upf1 coimmunoprecipitates with eIF3 and eIF2 (Figure S5A). However, downregulating Upf1 was of no consequence to the phosphorylation status of eIF2α (Figure S5B), which is important considering that phosphorylated eIF2α inhibits translation initiation (Proud, 2005). Far-western analyses using eIF2 or eIF3 purified from HeLa cells (Brown-Luedi et al., 1982) and baculovirus-produced and purified FLAG-Upf1(WT) (Hosoda et al., 2005) indicated that the interaction of FLAG-Upf1 with eIF2 is likely to be indirect but the interaction of FLAG-Upf1 with the eIF3a subunit is apt to be direct (Figure S5C). Notably, FLAG-Upf1 coimmunoprecipitated with HA-eIF2α(WT) but not detectably with HA-eIF2α(S51D) (Figure S5D), the latter of which is an inactive phosphomimetic variant (Kaufman et al., 1989) that cannot recycle to form the eIF2-GTP-Met-tRNAiMet ternary complex (Ernst et al., 1987). Therefore, the co-IP of FLAG-Upf1 with eIF2 is probably because FLAG-Upf1 coimmunoprecipitates with the ternary complex via its direct interaction with eIF3.
Our finding that Upf1 interacts with eIF3, together with data suggesting that Upf1 phosphorylation inhibits translation initiation, predict that phospho-Upf1 would have a higher affinity for eIF3 than does WT Upf1. This prediction was tested in three ways, each of which used our two sources of phospho-Upf1. In the first test, anti-myc was used to immunoprecipitate myc-Upf1R(WT) or myc-Upf1R(G495R/G497E) from HeLa cells as described earlier (Figure 1A). Western blotting using anti-Upf1 revealed that myc-Upf1R(WT) and myc-Upf1R(G495R/G497E) were immunoprecipitated with comparable efficiencies, and mIgG failed to immunoprecipitate either protein (Figure 5A, lanes 3–6). Western blotting using anti-eIF3a demonstrated that myc-Upf1R(G495R/G497E), relative to myc-Upf1R(WT), immunoprecipitated the eIF3a subunit ~3.5-fold more efficiently (Figure 5A, lanes 4 and 6). Similarly, western blotting using anti-eIF3 detected both eIF3a and eIF3b/c subunits, suggesting that the entire eIF3 complex was associated with Upf1. Consistent with this interpretation, anti-eIF3f, which reacts more efficiently than anti-eIF3 with the integral f subunit of eIF3, detected eIF3f in the IP of myc-Upf1R(G495R/G497E) (Figure 5A, lanes 4 and 6). As expected if the increased reactivity of myc-Upf1R(G495R/G497E) relative to myc-Upf1R(WT) with eIF3 was due to Upf1 phosphorylation, myc-Upf1R(WT) from OA-treated cells reacted ~3-fold more strongly with eIF3 than did myc-Upf1R(WT) from untreated cells (Figure 5B, lanes 4 and 6).
Figure 5. Upf1 Phosphorylation Promotes Upf1 Binding to eIF3 Subunits.

(A) HeLa cells were transiently transfected with pCMV-myc-UPF1R(WT) or pmCMV-UPF1R(G495R/G497E). Western blotting (WB) was performed using the designated antibody (α) before (−) and after immunoprecipitation (IP) using anti-myc or mouse (m) IgG. The dot specifies a nonspecific band that does not interfere with the analysis.
(B) HeLa cells were transiently transfected with pCMV-myc-UPF1R(WT) and treated with 0 or 75 nM OA for 3 hr prior to harvesting. Cell lysates were subjected to western blot analysis before and after IP.
(C) HeLa cells were transiently transfected with control or Upf1 siRNA and 2 days later with pmCMV-UPF1R(G495R/G497E). Cell lysates were immunoprecipitated using anti-eIF3a or mIgG, and western blotting was performed as noted.
(D) As in (B) except IP was performed using anti-eIF3a or mIgG.
(E) Coomassie blue staining of baculovirus-produced FLAG-Upf1(WT) and FLAG-Upf1(G495R/G497E).
(F) Far-western analysis (FW) of the specified purified proteins using baculovirus-produced FLAG-Upf1(WT) (left) or FLAG-Upf1(G495R/G497E) (right) followed by western blotting using anti-FLAG. eIF3 subunits and BSA were also stained using Coomassie blue (middle).
(G) Coomassie blue staining of baculovirus-produced FLAG-Upf1(WT) from insect cells that were (+) or were not (−) treated with 75 nM OA for 3 hr prior to harvesting.
(H) As in (F), except proteins shown in (G) were used as probes in the far-western analysis.
In the second test, HeLa cells were transfected with either control or Upf1 siRNA. Two days later, cells were retransfected with pmCMV-myc-Upf1R(G495R/G497E). Lysates were immunoprecipitated using anti-eIF3a or, to control for nonspecific IP, mIgG. Western blotting using anti-eIF3a revealed that comparable amounts of eIF3a were immunoprecipitated regardless of the siRNA treatment (Figure 5C, lanes 4 and 6). Western blotting of samples before IP using anti-Upf1 showed that the abundance of myc-Upf1R(G495R/G497E) was ~3-fold above the level of cellular Upf1 in the presence of control siRNA (Figure 5C, lane 1), and Upf1 siRNA reduced the level of cellular Upf1 to 10% of normal (Figure 5C, lane 2). However, western blotting of samples after IP demonstrated that only myc-Upf1R(G495R/G497E) and not endogenous Upf1 was immunoprecipiated (Figure 5C, lanes 4 and 6). As expected if the increased reactivity of eIF3a with myc-Upf1R(G495R/G497E) relative to myc-Upf1R(WT) was due to Upf1 phosphorylation, eIF3a reacted more strongly with myc-Upf1R(WT) and cellular Upf1 when they derived from OA-treated cells than when they derived from untreated cells (Figure 5D, lanes 4 and 6).
In the third test, equal amounts of baculovirus-produced and purified FLAG-Upf1(WT) and FLAG-Upf1(G495R/G497E) (Figure 5E) were used in far-western analyses to probe blots of purified HeLa cell eIF3, bovine serum albumin (BSA) as a negative indicator, and two concentrations of FLAG-BAP to control for variations in blotting and reactivity with anti-FLAG (Figure 5F, left and right panels). In parallel, all three proteins were subjected to Coomassie blue staining prior to blotting so that they could be visualized and compared to far-western results (Figure 5F, middle panel). We found that FLAG-Upf1(G495R/G497E) had a ~3-fold stronger affinity for eIF3a than FLAG-Upf1(WT) under conditions where the reactivity of FLAG-BAP with anti-FLAG was comparable (Figure 5F, compare left and right panels). Furthermore, analyses of equal amounts of purified baculovirus-produced FLAG-Upf1(WT) purified from virus-infected cells that were or were not treated with OA (Figure 5G) revealed that OA treatment enhanced the reactivity of FLAG-Upf1(WT) with eIF3a (Figure 5H, compare left and right panels). Taken together, all of our findings suggest that phospho-Upf1 promotes translational repression by binding to eIF3.
Phospho-Upf1 Inhibits the Conversion of 40S-Bound mRNA to 80S-Bound mRNA
If Upf1 phosphorylation promotes translational repression, then baculovirus-produced FLAG-Upf1(WT) purified from OA-treated insect cells and baculovirus-produced FLAG-Upf1(G495R/G497E) purified from untreated insect cells would be expected to inhibit mRNA translation in rabbit reticulocyte lysates (RRLs) when compared to either baculovirus-produced FLAG-Upf1(WT) from untreated insect cells or BSA. To test this prediction, capped and polyadenylated RLuc mRNA was synthesized in vitro (Figure S6A) and added to RRLs that had been supplemented with equal amounts of one of the three FLAG-Upf1 proteins (Figure S6B, left panel), each of which manifested the expected phosphorylation status (Figure S6B, right panel). Results revealed that FLAG-Upf1(WT) from OA-treated cells and FLAG-Upf1(G495R/G497E) from untreated cells preferentially inhibited the production of RLuc activity relative to either FLAG-Upf1(WT) from untreated cells or BSA (Figure S6C). Furthermore, the extent of inhibition roughly correlated with the degree of Upf1 phosphorylation, although it is possible that not all Upf1 phosphorylation sites function equally in translational repression. These data indicate that phospho-Upf1 inhibits RLuc mRNA translation initiation in vitro.
If Upf1 phosphorylation promotes translational repression by inhibiting eIF3 function, then phospho-Upf1 could preclude (1) binding of the ternary complex to the 40S ribosomal subunit to form the 40S/Met-tRNAiMet preinitiation complex, (2) recruitment of the preinitiation complex to mRNA, or (3) joining of the 60S ribosomal subunit to the mRNA-bound preinitiation complex to form the 80S/Met-tRNAiMet/mRNA complex (Hinnebusch, 2006). To gain insight into how phospho-Upf1 inhibits translation initiation, we used a so-called shift-assay (Darnbrough et al., 1972). Briefly, in vitro-aminoacylated [35S]Met-tRNAiMet was added to elongation-inhibited RRLs to generate [35S]Met-labeled ternary complexes. The progressive association of ternary complexes with 40S ribosomal subunits, mRNA, and, finally, 60S ribosomal subunits was monitored using polysome profiling (Figure S7; Darnbrough et al., 1972). Shift assays were performed in the presence of BSA, FLAG-Upf1(WT), or FLAG-Upf1(G495R/G497E), which were added to RRLs prior to the addition of charged [35S]Met-tRNAiMet and in vitro-synthesized RLuc mRNA (Figure 6A). Results revealed that, relative to either BSA or FLAG-Upf1(WT), FLAG-Upf1(G495R/G497E) inhibited the formation of 80S/[35S]Met-tRNAiMet/mRNA initiation complexes (Figures 6A and 6B).
Figure 6. Phospho-Upf1 Inhibits the Conversion of 40S/[35S]Met-tRNAiMet/mRNA to Translationally Competent 80S/[35S]Met-tRNAiMet/mRNA in RRLs.

BSA, baculovirus-produced FLAG-Upf1(WT), or baculovirus-produced FLAG-Upf1(G495R/G497E) were added to RRLs prior to the addition of charged [35S]Met-tRNAiMet and RLuc mRNA.
(A) [35S]Met was quantitated (colored plot) and superimposed on absorbance profiles of subpolysomes and polysomes that were separated by sucrose gradient centrifugation. The positions of 40S, 60S, and 80S complexes are specified.
(B) Superimposition of the [35S]Met distribution in sucrose gradients of FLAG-Upf1(WT) or FLAG-Upf1(G495R/G497E) as shown in (A).
(C) RT-PCR of RLuc mRNA in sucrose gradients.
In theory, the observed inhibition could be due to a block in either the recruitment of preinitiation complexes to mRNA or the joining of 60S ribosomal subunits to mRNA-bound preinitiation complexes to form 80S/Met-tRNAiMet/mRNA complexes. To distinguish between these two possibilities, the distribution of RLuc mRNA in profiles was measured directly using RT-PCR. Results revealed that while FLAG-Upf1(G495R/G497E) inhibited the formation of 80S/Met-tRNAiMet/mRNA complexes, there was no significant effect on the recruitment of 40S/Met-tRNAiMet to mRNA (Figure 6C). Notably, using 0.1 M aurintricarboxylic acid to prevent the binding of mRNA to 40S ribosomal subunits demonstrated that ribosome-free RLuc mRNA sedimented in poly-some gradients to a lighter region than 40S ribosomal subunits (Figure S8). Thus, RLuc mRNA that sediments at 40S indeed represents true translation initiation complexes. We conclude that phospho-Upf1 inhibits the joining of 60S ribosomal subunits to mRNA-bound 40S initiation complexes.
Discussion
Our studies have uncovered a previously unappreciated step of translational repression during the process of mammalian cell NMD. This step is triggered by Upf1 phosphorylation (Figure 7). Data indicate that hypophosphorylated Upf1, which constitutes the majority of cellular Upf1, is involved in early steps of the pioneer round of translation. These steps include translation termination since hypophosphorylated Upf1 has a stronger affiliation than phospho-Upf1 for SMG-1 and eRF3 (Figure 1; Kashima et al., 2006), all of which constitute the SURF complex that has been proposed to function in nonsense codon recognition (Kashima et al., 2006). Provided a post-splicing EJC exists more than ~25–30 nucleotides downstream of a termination event, then Upf1 and SMG-1 of the SURF complex are thought to join the EJC and Upf1 undergoes phosphorylation (Kashima et al., 2006; Wittmann et al., 2006). Phospho-Upf1 has higher affinity than hypophosphorylated Upf1 for eIF3 (Figure 5), so as to elicit translational repression (Figures 2, 3, 4, 6, and S6). Phospho-Upf1 also has a higher affinity for mRNA degradative activities (Figure 1). We propose that the steps of translational repression precede mRNA decay and, by precluding additional ribosome loading and otherwise promoting mRNP remodeling, make the NMD target physically accessible to degradative activities. While we find that the rate of translation in RRLs in the presence of phospho-Upf1 eventually returns to the rate in the presence of hypophosphorylated Upf1 (Figure S6C), it should be noted that RRLs fail to recapitulate important aspects of cellular metabolism, including mRNA decay after translational repression.
Figure 7. Model for Phospho-Upf1-Mediated Repression of Translation Initiation.
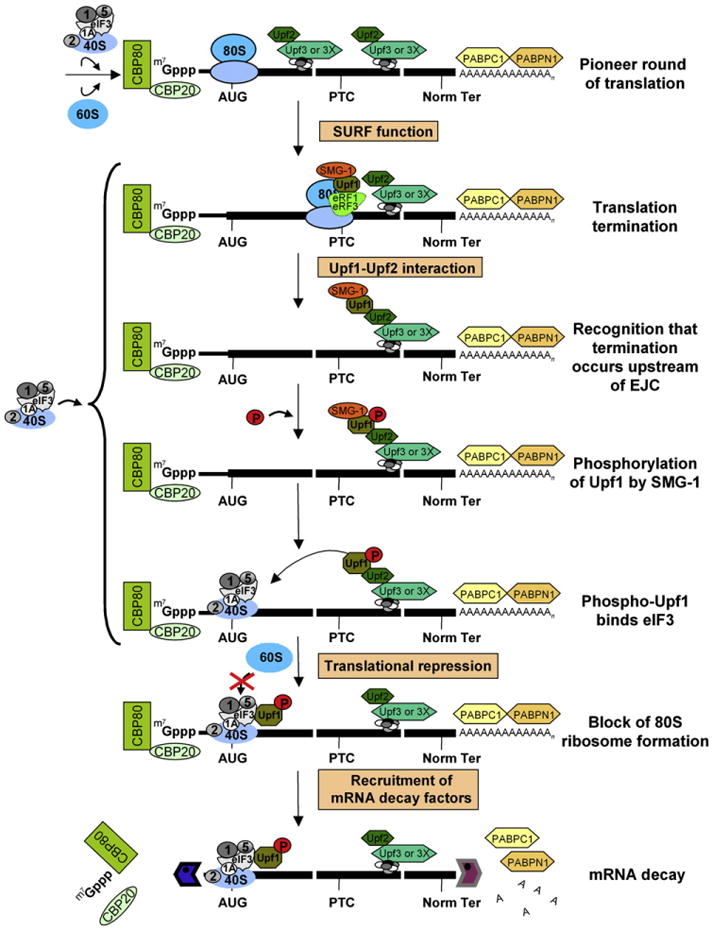
Upf1, which is primarily hypophosphorylated, forms the SURF complex together with SMG-1, eRF1, and eRF3 when translation terminates during a pioneer round, which involves CBP80/20-bound mRNA. If termination occurs sufficiently upstream of a post-splicing EJC, e.g., at the specified premature termination codon (PTC), then Upf1 and SMG-1 associate with the EJC. Upon association, SMG-1 phosphorylates Upf1. Phospho-Upf1 then represses translation by binding to eIF3 subunits and inhibiting the eIF3-mediated joining of 60S ribosomal subunits to 40S/Met-tRNAiMet/mRNA. Phospho-Upf1 also recruits mRNA decay factors, including Dcp1a, Xrn1, and Rrp4.
Evidence that phospho-Upf1 mediates translational repression by inhibiting eIF3 function derives from the finding that phospho-Upf1 has a higher affinity for eIF3 than hypophosphorylated Upf1 (Figures 5 and S5). Furthermore, phospho-Upf1 inhibits cap-dependent and HCV IRES-dependent translation (Figures 4 and S6; data not shown), which rely on eIF3, but not CrPV-dependent translation, which occurs independently of eIF3 (Figure 4). Additionally, phospho-Upf1 precludes the eIF3-mediated conversion of 40S/Met-tRNAiMet/mRNA to translationally competent 80S/Met-tRNAiMet/mRNA (Figure 6). Consistent with phospho-Upf1 mediating translational repression during NMD by targeting eIF3, nonsense-containing mRNA that initiates translation in an eIF3-independent mechanism using the CrPV IRES was found to be immune to NMD (Figure 3). In contrast, a nonsense-containing mRNA that initiates translation in an eIF3-dependent mechanism using the EMCV IRES(GTTA) is subject to NMD even though this IRES supports nearly the same level of translation as the CrPV IRES (Figure 3). While we do not know how phospho-Upf1 binding to eIF3 inhibits eIF3 function, additional support for eIF3 function during a pioneer round of translation derives from the recent report that eIF3e, which is a nonessential eIF3 subunit, is required for NMD (Morris et al., 2007).
The mechanism of translational repression during mammalian cell NMD may overlap the more generalized type of translational repression that is mediated in yeast by the decapping activator Dhh1p (Coller and Parker, 2005). Rather than targeting an essential translation factor, data indicate that Dhh1p works to promote the assembly of a translationally repressed mRNP that is inaccessible to translation initiation factors. However, it is possible that the phospho-Upf1-mediated inhibition of eIF3 function could be accompanied by the assembly of a translationally repressed complex that, like the yeast complex, involves the assembly of Lsm1-7 and other constituents. Consistent with this possibility, downregulating Lsm1 inhibits the NMD of Gl and GPx1 mRNAs (F. Lejeune and L.E.M., unpublished data). The translational repression that typifies mammalian cell NMD undoubtedly results in mRNA sequestration away from ribosomes and other components of the translational machinery, possibly in P bodies (Parker and Sheth, 2007). This would allow physical access of the mRNA to the decapping and 5′-to-3′ exonucleolytic machinery, which functions during NMD in mammalian cells (Lehner and Sanderson, 2004; Lejeune et al., 2003; Lykke-Andersen, 2002; Unterholzner and Izaurralde, 2004) and is enriched in P bodies (Parker and Sheth, 2007). Consistent with the view that phospho-Upf1 may deliver translationally repressed NMD targets to sites of decapping and exonucleolytic decay, it was recently reported that Upf1 dephosphorylation occurs in P bodies, and a block in Upf1 dephosphorylation results in the accumulation of Upf1, other NMD factors, and NMD substrates in P bodies (Durand et al., 2007).
Experimental Procedures
Plasmid Constructions
See Supplemental Data.
Cell Transfections, siRNA-Mediated Downregulation, and Immunoprecipitations
HeLaCCl2 orCos-7 cells were cultured in DMEM that was supplemented with 10% FBS (Invitrogen). When noted, cells were treated with siRNA as previously reported (Kim et al., 2005). After 2 days, cells were transfected using Lipofectamine 2000 (Invitrogen) (Hosoda et al., 2006) with the specified plasmids and harvested 1 day later. Where indicated, cells were incubated with 0, 50, or 75 nM OA (Sigma) 3 hr prior to harvesting. Protein was prepared using passive lysis buffer (Promega), and RNA was purified using Trizol reagent (GIBCO-BRL) before or after immunoprecipitation (Kim et al., 2005).
Western Blotting
Protein was electrophoresed in SDS-polyacrylamide, transferred to Hybond ECL nitrocellulose (GE Healthcare), and probed (see Supplemental Data for antibody details).
RT-PCR
FLuc, RLuc, Gl, and MUP mRNAs were amplified and analyzed as described (Kim et al., 2005). R-Gl mRNA was amplified using 5′-CCTGAGGAGAAGTCTGCCG-3′ (sense) and 5′-GGGTTTAGTGGTACTTGTGAGC-3′ (anti-sense).
Luciferase Activity Assays
Assays were performed using the Dual-Luciferase Reporter Assay System (Promega) and a FB12 Luminometer (Berthold).
Far-Western Analyses
Blots of BSA (New England Biolabs), FLAG-BAP (Sigma), and purified eIF3 (Brown-Luedi et al., 1982) were probed with baculovirus-produced FLAG-Upf1(G495R/G497E) or FLAG-Upf1(WT) purified from SF21 cells that either were or were not treated with 75 nM of OA for 3 hr, and interactions were detected using anti-FLAG (Hosoda et al., 2005).
In Vitro Synthesis of RLuc mRNA
To generate RLuc mRNA, phRL-CMV(Promega) was linearized using XbaI (NE Biolabs) and transcribed using T7 RNA polymerase (Roche) in the presence of 5′Gppp Cap (Promega).
Aminoacylation of tRNAiMet
Calf liver tRNA(Clontech; 24 μg) was aminoacylated in vitro using 10 μg of purified His-tagged methionyl-tRNA synthetase (Stanley, 1974). Reactions (200 μl) containing 500 μCi of [35S]Met (GE Healthcare, 37TBq/mmol), 10 μM of unlabeled methionine, 40 mM Tris (pH 7.5), 15 mM MgCl2, 10 mM ATP, and 1 U/ml of RNasin (Promega) were incubated for at 37°C for 30 min (Pestova and Hellen, 2001). They were subsequently extracted with acidic phenol (pH 4.7), filtered using a Nuc-trap column (Invitrogen), and precipitated using ethanol.
In Vitro Shift Assays
Shift assays were performed essentially as described (Darnbrough et al., 1972) and detailed in Supplemental Data.
Supplementary Material
Acknowledgments
We thank Peter Sarnow for pR/CrPV/F, Sung Key Jang for pR/HCV/F, Bill Merrick for anti-eIF2, Nahum Sonenberg for anti-eIF3b and anti-eIF4AIII, Deanna Janzen and Adam Geballe for anti-eRF3, Jens Lykke-Andersen for anti-Upf1 and anti-Dcp1a, Ger Pruijn for anti-Rrp4, Tom Parsons for anti-eIF3a, Mark Nelson for anti-eIF3f, and Paul Anderson for pMT2-HA-eIF2α plasmids. We also are grateful to Richard Jackson and Graham Belsham for helpful information. This work was supported by NIH grants GM074593 to L.E.M. and GM073732 to J.W.B.H. N.H. was supported in part by a Fellowship from the Japan Society for the Promotion of Science.
Footnotes
Supplemental Data: Supplemental Data include Results, Discussion, Experimental Procedures, References, and eight figures and can be found with this article online at http://www.cell.com/cgi/content/full/133/2/314/DC1/.
References
- Brown-Luedi ML, Meyer LJ, Milburn SC, Yau PM, Corbett S, Hershey JW. Protein synthesis initiation factors from human HeLa cells and rabbit reticulocytes are similar: comparison of protein structure, activities, and immunochemical properties. Biochemistry. 1982;21:4202–4206. doi: 10.1021/bi00261a002. [DOI] [PubMed] [Google Scholar]
- Brumbaugh KM, Otterness DM, Geisen C, Oliveira V, Brognard J, Li X, Lejeune F, Tibbetts RS, Maquat LE, Abraham RT. The mRNA surveillance protein hSMG-1 functions in genotoxic stress response pathways in mammalian cells. Mol Cell. 2004;14:585–598. doi: 10.1016/j.molcel.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bu X, Haas DW, Hagedorn CH. Novel phosphorylation sites of eukaryotic initiation factor-4F and evidence that phosphorylation stabilizes interactions of the p25 and p220 subunits. J Biol Chem. 1993;268:4975–4978. [PubMed] [Google Scholar]
- Chiu SY, Lejeune F, Ranganathan AC, Maquat LE. The pioneer translation initiation complex is functionally distinct from but structurally overlaps with the steady-state translation initiation complex. Genes Dev. 2004;18:745–754. doi: 10.1101/gad.1170204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J, Parker R. Eukaryotic mRNA decapping. Annu Rev Biochem. 2004;73:861–890. doi: 10.1146/annurev.biochem.73.011303.074032. [DOI] [PubMed] [Google Scholar]
- Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–886. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaplinski K, Ruiz-Echevarria MJ, Paushkin SV, Han X, Weng Y, Perlick HA, Dietz HC, Ter-Avanesyan MD, Peltz SW. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 1998;12:1665–1677. doi: 10.1101/gad.12.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C, Hunt T, Jackson RJ. A complex between met-tRNA F and native 40S subunits in reticulocyte lysates and its disappearance during incubation with double-stranded RNA. Biochem Biophys Res Commun. 1972;48:1556–1564. doi: 10.1016/0006-291x(72)90891-1. [DOI] [PubMed] [Google Scholar]
- Doudna JA, Sarnow P. Translation initiation by viral internal ribosome entry sites. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 129–154. [Google Scholar]
- Durand S, Cougot N, Mahuteau-Betzer F, Nugyen CH, Grieson DL, Bertrand E, Tazi J, Lejeune F. Inhibition of nonsense-mediated mRNA decay (NMD) by a new chemical molecule reveals the dynamic of NMD factors in P bodies. J Cell Biol. 2007;178:1145–1160. doi: 10.1083/jcb.200611086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst H, Duncan RF, Hershey JW. Cloning and sequencing of complementary DNAs encoding the alpha-subunit of translational initiation factor eIF-2. Characterization of the protein and its messenger RNA. J Biol Chem. 1987;262:1206–1212. [PubMed] [Google Scholar]
- Fernandez J, Yaman I, Sarnow P, Snider MD, Hatzoglou M. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2alpha. J Biol Chem. 2002;277:19198–19205. doi: 10.1074/jbc.M201052200. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo MA, Basak S, Dostie J, Murray EL, Schoenberg DR, Sonenberg N. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J Cell Biol. 2005;170:913–924. doi: 10.1083/jcb.200504039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead TA, Sim AT, Carling D, Honnor RC, Tsukitani Y, Cohen P, Hardie DG. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337:78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci. 2006;31:553–562. doi: 10.1016/j.tibs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Holbrook JA, Neu-Yilik G, Gehring NH, Kulozik AE, Hentze MW. Internal ribosome entry sequence-mediated translation initiation triggers nonsense-mediated decay. EMBO Rep. 2006;7:722–726. doi: 10.1038/sj.embor.7400721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda N, Kim YK, Lejeune F, Maquat LE. CBP80 promotes interaction of Upf1 with Upf2 during nonsense-mediated mRNA decay in mammalian cells. Nat Struct Mol Biol. 2005;12:893–901. doi: 10.1038/nsmb995. [DOI] [PubMed] [Google Scholar]
- Hosoda N, Lejeune F, Maquat LE. Evidence that poly(A) binding protein C1 binds nuclear pre-mRNA poly(A) tails. Mol Cell Biol. 2006;26:3085–3097. doi: 10.1128/MCB.26.8.3085-3097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys DT, Westman BJ, Martin DI, Preiss T. Micro-RNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc Natl Acad Sci USA. 2005;102:16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106:607–617. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Isken O, Maquat LE. Quality control of eukaryotic mRNA: safe-guarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–1856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- Jackson RJ. Alternative mechanisms of initiating translation of mammalian mRNAs. Biochem Soc Trans. 2005;33:1231–1241. doi: 10.1042/BST0331231. [DOI] [PubMed] [Google Scholar]
- Kashima I, Yamashita A, Izumi N, Kataoka N, Morishita R, Hoshino S, Ohno M, Dreyfuss G, Ohno S. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006;20:355–367. doi: 10.1101/gad.1389006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ, Davies MV, Pathak VK, Hershey JW. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989;9:946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN, Kataoka N, Dreyfuss G. Role of the nonsense-mediated decay factor hUpf3 in the splicing-dependent exon-exon junction complex. Science. 2001;293:1832–1836. doi: 10.1126/science.1062829. [DOI] [PubMed] [Google Scholar]
- Kim YK, Furic L, Desgroseillers L, Maquat LE. Mammalian Staufen1 recruits Upf1 to specific mRNA 3′ UTRs so as to elicit mRNA decay. Cell. 2005;120:195–208. doi: 10.1016/j.cell.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Lal A, Abdelmohsen K, Pullmann R, Kawai T, Galban S, Yang X, Brewer G, Gorospe M. Posttranscriptional derepression of GAD-D45alpha by genotoxic stress. Mol Cell. 2006;22:117–128. doi: 10.1016/j.molcel.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Le Hir H, Gatfield D, Izaurralde E, Moore MJ. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001;20:4987–4997. doi: 10.1093/emboj/20.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner B, Sanderson CM. A protein interaction framework for human mRNA degradation. Genome Res. 2004;14:1315–1323. doi: 10.1101/gr.2122004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune F, Ishigaki Y, Li X, Maquat LE. The exon junction complex is detected on CBP80-bound but not eIF4E-bound mRNA in mammalian cells: dynamics of mRNP remodeling. EMBO J. 2002;21:3536–3545. doi: 10.1093/emboj/cdf345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune F, Li X, Maquat LE. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating, and exonucleolytic activities. Mol Cell. 2003;12:675–687. doi: 10.1016/s1097-2765(03)00349-6. [DOI] [PubMed] [Google Scholar]
- Lejeune F, Ranganathan AC, Maquat LE. eIF4G is required for the pioneer round of translation in mammalian cells. Nat Struct Mol Biol. 2004;11:992–1000. doi: 10.1038/nsmb824. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Green RE, Brenner SE. Evidence for the wide-spread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc Natl Acad Sci USA. 2003;100:189–192. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J. Identification of a human decapping complex associated with hUpf proteins in nonsense-mediated decay. Mol Cell Biol. 2002;22:8114–8121. doi: 10.1128/MCB.22.23.8114-8121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda D, Hosoda N, Kim YK, Maquat LE. Failsafe nonsense-mediated mRNA decay does not detectably target eIF4E-bound mRNA. Nat Struct Mol Biol. 2007;14:974–979. doi: 10.1038/nsmb1297. [DOI] [PubMed] [Google Scholar]
- Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- Morris C, Wittmann J, Jack HM, Jalinot P. Human INT6/eIF3e is required for nonsense-mediated mRNA decay. EMBO Rep. 2007;8:596–602. doi: 10.1038/sj.embor.7400955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D, Parker R. Recognition of yeast mRNAs as “nonsense containing” leads to both inhibition of mRNA translation and mRNA degradation: implications for the control of mRNA decapping. Mol Biol Cell. 1999;10:3971–3978. doi: 10.1091/mbc.10.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JZ, Grate L, Donohue JP, Preston C, Nobida N, O'Brien G, Shiue L, Clark TA, Blume JE, Ares M., Jr Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes Dev. 2007;21:708–718. doi: 10.1101/gad.1525507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Yamashita A, Kashima I, Schell T, Anders KR, Grimson A, Hachiya T, Hentze MW, Anderson P, Ohno S. Phosphorylation of hUPF1 induces formation of mRNA surveillance complexes containing hSMG-5 and hSMG-7. Mol Cell. 2003;12:1187–1200. doi: 10.1016/s1097-2765(03)00443-x. [DOI] [PubMed] [Google Scholar]
- Page MF, Carr B, Anders KR, Grimson A, Anderson P. SMG-2 is a phosphorylated protein required for mRNA surveillance in Caenorhabditis elegans and related to Upf1p of yeast. Mol Cell Biol. 1999;19:5943–5951. doi: 10.1128/mcb.19.9.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M, Ishigaki Y, Nagy E, Maquat LE. Evidence that phosphorylation of human Upfl protein varies with intracellular location and is mediated by a wortmannin-sensitive and rapamycin-sensitive PI 3-kinase-related kinase signaling pathway. RNA. 2001;7:5–15. doi: 10.1017/s1355838201000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Saltzman AL, Kim YK, Misquitta C, Shai O, Maquat LE, Frey BJ, Blencowe BJ. Quantitative microarray profiling provides evidence against widespread coupling of alternative splicing with nonsense-mediated mRNA decay to control gene expression. Genes Dev. 2006;20:153–158. doi: 10.1101/gad.1382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Pestova TV, Hellen CU. Preparation and activity of synthetic unmodified mammalian tRNAi(Met) in initiation of translation in vitro. RNA. 2001;7:1496–1505. doi: 10.1017/s135583820101038x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Lorsch JR, Hellen CUT. The mechanism of translation initiation in eukaryotes. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 87–128. [Google Scholar]
- Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16:3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Robertson ME, Seamons RA, Belsham GJ. A selection system for functional internal ribosome entry site (IRES) elements: analysis of the requirement for a conserved GNRA tetraloop in the encephalomyocarditis virus IRES. RNA. 1999;5:1167–1179. doi: 10.1017/s1355838299990301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U, Parker R. Targeting of aberrant mRNAs to cytoplasmic processing bodies. Cell. 2006;125:1095–1109. doi: 10.1016/j.cell.2006.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WM., Jr Specific aminoacylation of the methionine-specific tRNA's of eukaryotes. Methods Enzymol. 1974;29:530–547. doi: 10.1016/0076-6879(74)29049-9. [DOI] [PubMed] [Google Scholar]
- Sun X, Perlick HA, Dietz HC, Maquat LE. A mutated human homologue to yeast Upf1 protein has a dominant-negative effect on the decay of nonsense-containing mRNAs in mammalian cells. Proc Natl Acad Sci USA. 1998;95:10009–10014. doi: 10.1073/pnas.95.17.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L, Izaurralde E. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol Cell. 2004;16:587–596. doi: 10.1016/j.molcel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Powell MJ, Hoover SE, Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol Cell Biol. 2000;20:4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann J, Hol EM, Jack HM. hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol Cell Biol. 2006;26:1272–1287. doi: 10.1128/MCB.26.4.1272-1287.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeller C, Gaspari M, Isken O, Maquat LE. NMD resulting from EMCV IRES-directed translation initiation appears restricted to CBP80/20-bound mRNA. EMBO Rep. 2008 doi: 10.1038/embor.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Ohnishi T, Kashima I, Taya Y, Ohno S. Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein kinase, associates with components of the mRNA surveillance complex and is involved in the regulation of nonsense-mediated mRNA decay. Genes Dev. 2001;15:2215–2228. doi: 10.1101/gad.913001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Chang TC, Yamashita Y, Zhu W, Zhong Z, Chen CY, Shyu AB. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nat Struct Mol Biol. 2005;12:1054–1063. doi: 10.1038/nsmb1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


