Abstract
Tinea pedis is a chronic fungal infection of the feet, very often observed in patients who are immuno-suppressed or have diabetes mellitus. The practicing allergist may be called upon to treat this disease for various reasons. Sometimes tinea infection may be mistaken for atopic dermatitis or allergic eczema. In other patients, tinea pedis may complicate allergy and asthma and may contribute to refractory atopic disease. Patients with recurrent cellulitis may be referred to the allergist/immunologist for an immune evaluation and discovered to have tinea pedis as a predisposing factor. From a molecular standpoint, superficial fungal infections may induce a type2 T helper cell response (Th2) that can aggravate atopy. Th2 cytokines may induce eosinophil recruitment and immunoglobulin E (IgE) class switching by B cells, thereby leading to exacerbation of atopic conditions. Three groups of fungal pathogens, referred to as dermatophytes, have been shown to cause tinea pedis: Trychophyton sp, Epidermophyton sp, and Microsporum sp. The disease manifests as a pruritic, erythematous, scaly eruption on the foot and depending on its location, three variants have been described: interdigital type, moccasin type, and vesiculobullous type. Tinea pedis may be associated with recurrent cellulitis, as the fungal pathogens provide a portal for bacterial invasion of subcutaneous tissues. In some cases of refractory asthma, treatment of the associated tinea pedis infection may induce remission in airway disease. Very often, protracted topical and/or oral antifungal agents are required to treat this often frustrating and morbid disease. An evaluation for underlying immuno-suppression or diabetes may be indicated in patients with refractory disease.
Keywords: Tinea Pedis, cellulitis, dermatitis, immunity, antifungal agents, trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, Asthma, Allergy, Type 2 T helper cytokines, IgE
Introduction
Dermatophytic infection of the skin can manifest in different anatomical regions of the body and have been accordingly named. Thus, tinea capitis affects the scalp, tinea barbae- the face, tinea unguum- the nails, tinea manuum- the hands, and tinea cruris- the groin area. Tinea pedis, also known as athlete's foot, is a chronic fungal infection of the feet and is the focus of this review. Tinea pedis is estimated to be the second most common skin disease in the United States, behind acne [1], and up to 15% of the population may manifest the disease [2,3]. Tinea pedis presents as pruritic, erythematous, inflamed regions on the feet that may be located on the sole (vesicular type) or lateral aspects (moccasin type) of the foot and sometimes between the toes (interdigital type). Three main genera of fungi may cause tinea pedis, Trichophyton, Epidermophyton, and Microsporum. Other, nondermatophtye, fungi like Malassezia furfur, corynebacterium minutissimum, and Candida species may also cause tinea pedis but fall outside the scope of this review [4,5]. These fungi may be spread from soil (geophilic), animals (zoophilic), or humans (anthropophilic) as well as through contact with fomites.
Tinea pedis may present to the practicing allergist/immunologist in several ways. It may be mistaken for plantar eczema or dermatitis. It may complicate the management of the atopic patient and fungal pathogens themselves may aggravate asthma or atopic dermatitis. Occasionally, a patient may be referred for evaluation of recurrent cellulitis resulting from a tinea pedis infection rather than from immune deficiency. For these reasons, the allergist/immunologist must be prepared to evaluate, diagnose, and treat tinea pedis. This review will discuss the clinical features of tinea pedis infection, the pathogens incriminated, and the current treatment options for patients with this disease.
Pathogens
Three species of fungi, Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum are together responsible for the vast majority of cases of tinea pedis throughout the world. Of these keratinophilic organisms, Trichophyton rubrum is the most common pathogen associated with chronic tinea pedis, while other fungal pathogens have also been associated with the disorder and are listed in Table 1. The factors affecting the transmission of these dermatophytic pathogens are dependent on the source of infection, which is usually either human (anthropophilic), animal (zoophilic) or soil (geophilic).
Table 1.
Pathogens That Cause Tinea Pedis
| 1. Trichophyton |
| a. T. rubrum |
| b. T. mentagrophytes |
| c. T. tonsurans |
| 2. Epidermophyton |
| a. E. floccosum |
| 3. Microsporum |
| a. M. canis |
The most common anthropophilic dermatophyte infection seen is T. rubrum. A recent study showed that T. rubrum accounted for over 76% of all dermatophyte infections, including tinea pedis [1] and may account for over 2/3 of all tinea pedis infections. The spread of infections with this pathogen have been attributed to large population movements during World War II. Outbreaks of infection of glabrous skin have been associated with contact with infected, desquamated skin scales. This may occur in military camps and in factories.
T. rubrum appears in two forms. The first is typically white and fluffy in appearance with several aerial hyphae and is called the "downy form". The granular form of T. rubrum, however, is flat and has no aerial hyphae [6]. It is easily confused with T. mentagrophytes which is similar in appearance and causes a more inflammatory form of tinea pedis. T. rubrum has several club shaped microconidia that form along the length of the hyphae [7]. Conidia are asexual spores that form at the tip of conidiophores; in the same species, these are either large (macroconidia) or small (microconidia). T. rubrum is not just common in tinea pedis but in other tinea infections as well.
Trichophyton mentagrophytes is morphologically and characteristically similar to T. rubrum. Both have a downy or granular appearance and are sometimes indistinguishable under the microscope. T. mentagrophytes species can be pale yellow on the underside while T. rubrum is mostly, but not always, wine colored on the bottom [6]. T. mentagrophytes is zoophilic and affects many animal species including rodents, cats, dogs, and horses. Microsporum canis, however, is probably the most prevalent of the zoophilic dermatophytes.
Trichophyton tonsurans is another anthropophilic fungus that causes tinea pedis. T. tonsurans is not a common cause of tinea pedis but its prevalence is increasing in North America [6]. Cultures of this fungus have short, septate hyphae with several microconidia that vary from tear drop to club shape. Many chlamydoconidia, asexual conidia produced from the hyphae, may be seen but macroconidia are usually rare. T. tonsurans colonies can range in color from white to brown with an underside ranging from yellow to red [7].
Another pathogen known to cause tinea pedis and is responsible for 5% of tinea pedis infections is Epidermophyton floccosum [6]. E. floccosum is an anthropophilic fungus found worldwide and has been incriminated in several types of tinea infections. Colonies of this fungus are flat and grainy and range in color from yellow to brown. E. floccosum has septate hyphae with club shaped macroconidia. No microconidia are observed in this species, but chlamydoconidia can sometimes be seen in older colonies [7].
Microsporum canis is a zoophilic fungus contracted from dogs that is a rarer cause of tinea pedis infections [6]. Colonies of M. canis are white with a yellow underside and fluffy appearance while some remain colorless [7]. Their hyphae are septate, their microconidia are club shaped, they have many macroconidia, and their cell wall is usually thick [7]. Lesions caused by this fungus are usually more severe and are often characterized by erythema [6].
Clinical syndromes
Depending on the pathogen and anatomical distribution, tinea pedis may present in a given patient as one of several syndromes. Typically, three variants are seen and include the interdigital (Figure 1), moccasin (Figures 2A and 2B), and vesicobullous forms (Figure 3) of the disease. Some patients may present with recurrent cellulitis and aggressive attempts have to be made to identify the presence of tinea pedis as therapy for this condition can alleviate recurrences of cellulitis. In other patients, asthma exacerbations have been linked to the presence of tinea pedis and immune/allergic responses to these pathogens. These clinical syndromes are discussed in greater detail in the following sections.
Figure 1.
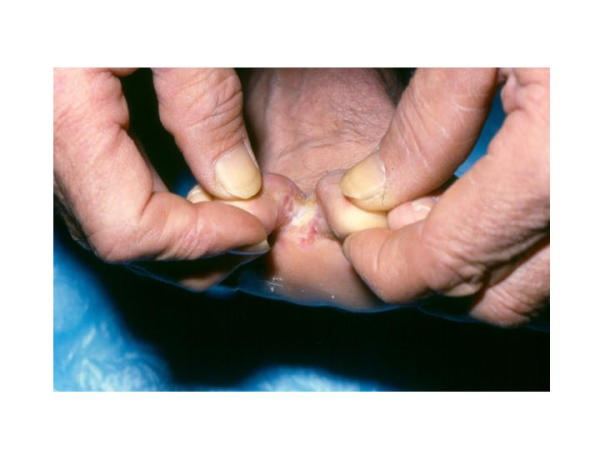
Interdigital tinea pedis affecting the space between the third and fourth digits. Tinea infections between the toes are common due to high moisture content and occlusion and often present with itching, burning, and/or malodor. This figure shows a man with dry-type tinea pedis in the third interspace. (Photograph kindly provided by Dr. Stuart Leicht, Division of Dermatology, East Tennessee State University).
Figure 2.
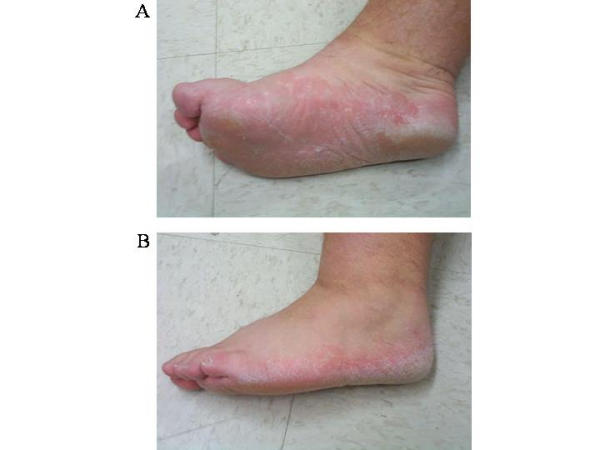
Bilateral moccasin type tinea pedis lesions. Moccasin type tinea pedis is a more prolonged form of tinea infection that surrounds the sole and lateral aspects of the foot in a slipper or moccasin distribution. 2A shows hyperkeratotic skin on the medial and bottom portions of the foot. 2B shows moccasin type tinea pedis on the lateral portion of the foot.
Figure 3.
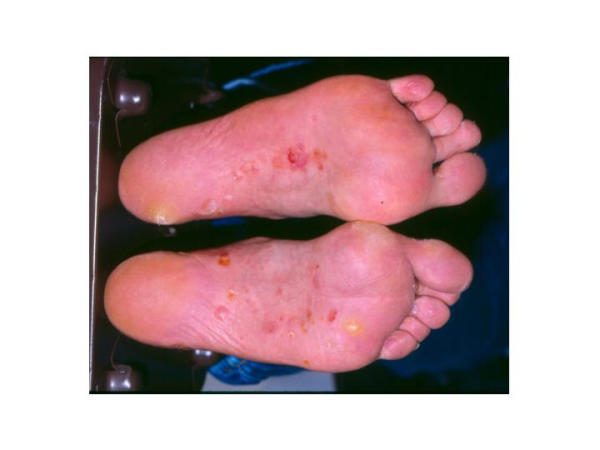
Vesiculobullous type tinea pedis on the plantar surface of the feet. This type of tinea pedis usually causes pustules or vesicles on the instep and plantar surfaces of the feet. Bacterial infection should be ruled out by microscopy or culture. (Photograph kindly provided by Dr. Stuart Leicht, Division of Dermatology, East Tennessee State University.)
Interdigital-type tinea pedis
Interdigital tinea pedis is the most common form and usually manifests in the interspace of the fourth and fifth digits and may spread to the underside of the toes (Figure 1) [4,8]. Patients often complain of itching and burning sensations on the feet accompanied by malodor. There are generally two types of interdigital tinea pedis. The first is a scaly, dry type called dermatophytosis simplex. The skin of the interdigital space is dry with low-grade peeling. This form is usually asymptomatic except for occasional pruritus. The second type is symptomatic and presents with wet, macerated interdigital spaces. Dermatophytosis complex, as it is called, may have fissuring of the interspace along with hyperkeratosis, leukokeratosis, or erosions [9]. Wet conditions along with fungal invasion increases the incidence of bacterial infection in these patients by breaching cutaneous integrity. In the case of T. metagrophytes, bullae may form. In patients with Human Immunodeficiency Virus (HIV) infection and/or others with impaired T cell function, extensive spread of tinea lesions to the dorsum of the foot is seen [10]. The infection may also become resistant to therapy in some of these patients.
In some cases as described recently, tinea pedis may mimic bacterial cellulitis due to the inflammatory component [11]. This may further lead to complications and skin maceration and damage. As summarized by Semel and Golden, tinea pedis was discovered in 20 of 24 episodes (84%) of cellulitis studied [12]. Common bacterial coinfection in descending order of frequency in their study included beta hemolytic streptococci (85%), Staphylococcus aureus (45%) and enterobacteriaceae (35%). These authors suggested that cultures of interdigital areas for fungal pathogens combined with antistreptolysin-O titers (ASO titers) may provide more useful information in patients with recurrent cellulitis than other routine studies [12]. A similar syndrome of recurrent cellulitis with tinea pedis in patients who have undergone saphenous vein grafting for coronary artery disease has been described almost two decades ago [13]. Besides tinea pedis, other interdigital inflammatory conditions need to be included in the differential diagnosis and include erythrasma, impetigo, pitted keratolysis, Candida intertrigo, and Pseudomonus aeruginosa interdigital infection [14]. These can often be differentiated by clinical features, gram stains, and cultures of aspirated material.
Moccasin-type tinea pedis
The moccasin type is a more severe, prolonged form of tinea pedis that covers the bottom and lateral aspects of the foot. Its appearance is that of a slipper or moccasin covering the foot, hence the name (Figures 2A and 2B). T. rubrum is most commonly associated with moccasin type tinea pedis. The skin of the inflamed area in this type of infection is often scaly and hyperkeratotic with erythema around the soles and sides of the foot [4,8]. Papules may also be noted around the demarcation line of erythema that surrounds the foot. Either foot may be affected, however bilateral involvement is most often seen [14]. Subungual onychomycosis coexisting with moccasin type dermatophytosis is most often caused by T. rubrum as well. The differential diagnosis to be considered here are psoriasis; dyshidrotic, atopic, or allergic eczematous dermatitis; pitted keratolysis; and various keratodermas [14]. This form responds to itraconazole and other antifungal agents.
Vesiculobullous tinea pedis
Vesiculobullous tinea pedis is the third type of dermatophyte infection of the feet. Occasionally a pustular variant may be seen. This type comprises pustules or vesicles on the instep and adjacent plantar surfaces of the feet and is less common (Figure 3) [8]. Bacterial infection needs to be considered in the differential diagnosis and ruled out by microscopy and/or culture. Fluid filled vesicles are usually clear but pus usually indicates secondary bacterial infection, most often with Staphylococcus aureus or group A Streptococcus. This form of tinea pedis may be associated with dermatophytid or "ID" reaction [14]. KOH preparations of the aspirate should be examined for presence of hyphae. Bullous impetigo, allergic contact dermatitis, dyshidrotic eczema, and bullous disease all need to be considered in the differential diagnosis [14].
Predisposing factors
Over the past decade, many fungi that were once thought not to infect humans have suddenly emerged as human pathogens. A number of host factors have contributed to the growing incidence of these fungi in tinea pedis infections (Table 2). Due to an increase in immune deficiency diseases (AIDS) and increased numbers of patients receiving chemotherapy, steroids, organ transplants, and parenteral nutrition, the incidence of tinea pedis has increased [6]. The overall health of the patient is another important risk factor in developing tinea pedis. Patients who are obese, elderly, or have systemic problems (diabetes mellitus) are at increased risk [6]. Other local factors that may contribute infection are trauma, excessive moisture, occlusive clothing, and frequent usage of public showers and pools. A recent study in Israel showed that repeated foot washing among school children might lead to delipidation and pH changes in the stratum corneum which would favor fungal growth [15].
Table 2.
Predisposing Risk Factors for Tinea Pedis
| A. Host Factors |
| 1. Immunosuppression |
| a.) Chemotherapy |
| b.) Immunosuppressive Drugs |
| c.) Steroids |
| d.) Organ Transplant |
| e.) Acquired Immunodeficiency Syndrome (AIDS) |
| 2. Poorly controlled diabetes mellitus |
| 3. Obesity |
| 4. Age |
| B. Local Factors |
| 1. Trauma |
| 2. Occlusive Clothing |
| 3. Public Showering |
| 4. Moist Conditions |
Complications
Lower extremity cellulitis
Cellulitis is a bacterial infection of the subcutaneous layers of the skin, which usually stems from a skin lesion or wound. Common predisposing factors for cellulitis include trauma, ulceration, venous and lymphatic insufficiency, and peripheral vascular disease. Tinea pedis infections, most often interdigital type, may be complicated by cellulitis and has been discussed in the article earlier. Wet, occlusive conditions developing in the infected interspaces lead to maceration and fissuring of the skin. This weakens the natural barrier of the skin and may serve as an entry point for several kinds of pathogenic bacteria. β-hemolytic streptococci (group A, B C, F, and G), Staphylcoccus aureus, Streptococcus pneumoniae, and gram negative bacilli have all been associated with cellulitis, with β-hemolytic streptococci being the most prevalent [16].
Semel and Goldin describe 24 episodes of lower extremity cellulitis in 22 patients, 20 of which had tinea pedis [12]. Of the 20 patients with athlete's foot, all had gram-positive bacteria isolated from their ipsilateral interdigital web space, and 85% (17 of the 20 cases) of these had β-hemolytic streptococci present. β-hemolytic streptococci were found significantly more often in patients with cellulitis and tinea pedis than in tinea pedis patients alone (p < 0.01). No β-hemolytic streptococci were isolated from control patients without tinea pedis or cellulitis. They conclude, but do not prove, that athlete's foot may be a common entry point for bacteria that cause cellulitis.
Saphenous venectomy has also been associated with increased incidence of cellulitis and tinea pedis infection may be a complicating factor [16,17]. However, the infection may not arise until many years after surgery. A patient with recurrent cellulitis may be suspected to have an underlying immune deficiency and may be referred to the allergist/immunologist. A thorough examination of the patient's feet and inter-digital spaces will often reveal evidence of recent or active tinea pedis. Treatment of this condition could result in amelioration of the cellulitis episodes. Evaluation for and treatment of any underlying disorders that led to a predisposition to tinea pedis (such as diabetes mellitus, obesity, hygiene) will also be necessary.
In some patients, gram-negative infection may complicate tinea pedis. Gram-positive bacteria usually dominate the interdigital spaces of the feet and upon infection with dermatophytes, bactericidal products similar to penicillin may inhibit their growth. This may result in a predominant growth of gram-negative bacteria like pseudomonas, proteus, and klebsiella, leading to gram-negative cellulitis [18]. In such situations, demonstration of fungal elements may become difficult as excessive gram-negative bacterial presence may inhibit fungal growth and make it harder to detect fungal hyphae on KOH preparations. Antifungal agents released from the gram-negative bacteria may also contribute to decreased presence of fungus. Hence, cultures of skin scraping may demonstrate gram-negative bacteria but no fungal pathogens. Aggressive treatment of the underlying fungal as well as the gram-negative bacterial infection can, however, lead to amelioration of cellulitis in these patients.
Tinea unguum (onychomycosis)
Tinea unguium is a fungal infection, usually with a dermatophyte, on the matrix, plate, or nail bed commonly associated with tinea pedis. Like tinea pedis infections, T. rubrum is the major cause of subungual onychomycosis. Not all thick, brittle, and discolored nails are due to dermatophyte infections. Onychomycosis accounts for about one third of fungal skin infections but only about half of onychomycosis infections are caused by a dermatophyte [19]. This means a KOH prep or fungal culture should be done before instating treatment.
Dermatophytid and Majocchi's granuloma
Dermatophytid, also referred to as the "ID" reaction, is an immunological reaction secondary to tinea pedis along with other tinea infections. It most often causes vesicular or pustular eruptions near the site of infection or on the palms and fingers of the hands [14]. Dermatophytid reactions may be the only sign in asymptomatic tinea pedis infections. The reaction usually subsides with antifungal therapy. In some patients with follicular invasion, a residual granuloma develops that is sterile. This is referred to as Majocchi's granuloma and it resolves with time.
Asthma and atopic disease
Case reports of asthma associated with dermatophytic infections have been described. Ward and coworkers described "Trichophyton asthma" in 12 adult patients with chronic rhinitis and asthma who also demonstrated immediate hypersensitivity to Trichophyton spp [20]. 10 out of the 12 patients also demonstrated positive immediate broncho-constrictive reactions to extracts of T. tonsurans. These patients had eosinophilia and chronic rhinosinusitis in addition to features compatible with "intrinsic or late onset asthma". Mungan et al. described coinfection with T. rubrum in patients with chronic asthma who also had evidence of tinea pedis [21]. The dominant pathogens were T. rubrum (63.1%) and T. mentagrophytes (35.5%). These investigators suggested that patients with intrinsic asthma needed to be tested for sensitivity to Trichophyton spp. Other investigators have also discussed a possible relationship between dermatophytic infections and asthma [22,23]. Hurliman and Fah described 2 patients who presented with tinea unguum caused by T. rubrum but also had underlying asthma, rhinitis, and eczema [23]. The allergic disease improved rapidly when the patients were treated with terbinafine and relapsed rapidly after this therapy was discontinued. Klein and coworkers described a patient with atopic dermatitis and concurrent tinea pedis and onychomycosis [24]. Skin cultures were positive for T. rubrum and atopic eczema flares improved with the use of antifungal agents [24]. As reviewed by Leyden, mocassin-type tinea pedis occurs more often in patients with an atopic background [25]. Byrld and coworkers found an association between tinea pedis and risk for vesicular eruption on the hands of patients [26]. The use of systemic antifungal therapies and ketoconazole shampoos led to improvement in 5 patients with atopic dermatitis complicated by dermatophytosis [27]. Mungan et al. demonstrated that trichophyton hypersensitivity may also be seen in patients with intrinsic asthma (nonallergic asthma) suggesting that both allergic and nonallergic asthma may have components of fungal sensitivity [21]. The role of Mallasezia (pityrosporium species) in atopic eczema has been extensively reviewed by several investigators [28-31]. Ward and coworkers demonstrated that treatment of 11 patients with late onset asthma complicated by dermatophytosis with fluconazole led to improved lung function and symptoms, and decreased steroid usage [32]. This was also echoed in a report by Elewski and Schwartz [22]. Mandez and colleagues also reported on one patient with urticaria complicating dermatophytosis [33]. Treatment of the fungal dermatitis led to clearing of the urticaria. This, being an anecdotal report, requires confirmation in larger studies.
Molecular immunopathogenesis
The relationship between fungal dermatitis and atopic disease is interesting. In one study, peripheral blood mononuclear cells from some patients with atopic dermatitis released interleukin-5 (IL-5) in response to stimulation by fungal antigens [34]. This response appeared to correlate with severity of facial dermatitis in these patients. Kanda and colleagues also demonstrated expression of the Th2 cytokine IL-4 from peripheral blood mononuclear cells in response to stimulation by Trichophyton rubrum [35]. Johansson and coworkers showed that a positive response to patch testing with Malassezia furfur in atopic dermatitis patients correlated with a Th2 cytokine response (IL-4, IL-5, and IL-13) in peripheral blood mononuclear cells [36]. The possible interactions of dermatophytes with T-cells and triggering of atopic disease is shown in Figure 4. Activation of cutaneous and/or circulating T cells by fungal antigens could induce a Th2-dominant response leading to elaboration of IL-4, IL-5, and IL-13. The first two cytokines can lead to two pivotal effects: synthesis of IgE by B cells and endothelial activation leading to eosinophil recruitment by a vascular cell adhesion molecule (VCAM)-very late activating antigen 4 (VLA-4) adhesion molecule pathway. Both IgE and eosinophils play a prominent role in atopic disease. IgE in the presence of antigen can cross-link mast cells leading to further cytokine, chemokine, and mediator production. The other cytokine, IL-5, enhances eosinophil production from the bone marrow, eosinophil activation, survival, recruitment, and degranulation. These processes can result in expression of atopic disease, from rhinitis and asthma to atopic dermatitis.
Figure 4.
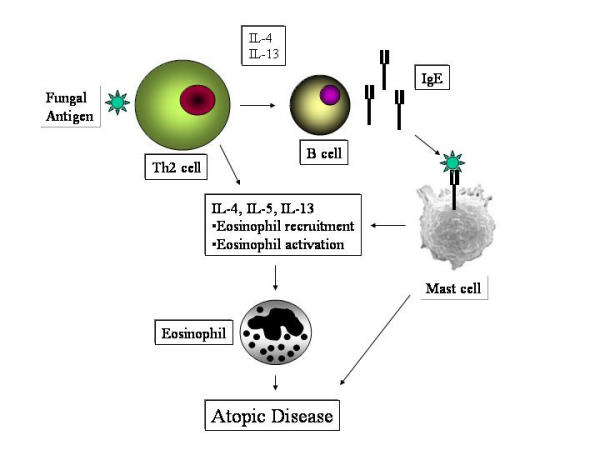
Activation of cutaneous and/or circulating T cells by fungal antigens could induce a Th2-dominant response leading to elaboration of IL-4, IL-13 and IL-5. The first 2 cytokines can lead to two pivotal effects: Synthesis of IgE by B cells and endothelial activation leading to eosinophil recruitment by a vascular cell adhesion molecule (VCAM)-very late activating antigen 4 (VLA-4) adhesion molecule pathway. Both IgE and eosinophils play a prominent role in atopic disease. IgE in the presence of antigen can cross-link mast cells leading to further cytokine, chemokine and mediator production. The other cytokine, IL-5, enhances eosinophil production from the bone marrow, eosinophil activation, survival, recruitment and degranulation. These processes can result in expression of atopic disease, from rhinitis and asthma to atopic dermatitis.
Diagnosis
Tinea pedis infections are typically easy to distinguish and diagnose. However, complete identification of the causative fungi should be established to confirm diagnosis and ensure proper treatment. Diagnosis of tinea pedis is based on history and clinical appearance of the feet in addition to direct microscopy of a potassium hydroxide (KOH) preparation. Cultures or histological examinations are rarely required. A Wood's lamp is not usually helpful in diagnosing tinea pedis but can be used to rule out other diagnoses like infection with Malassezia furfur [1] or erythrasma [14]. Malassezia furfur and Corynebacterium minutissimum both fluoresce under ultraviolet light while other common dermatophytes do not. KOH preparations are simple, inexpensive, efficient, and widely used. The KOH preparation also has an excellent positive predictive value. Occasionally, false negative results may be obtained, especially if treatment has already begun.
To prepare a KOH slide of the suspected fungi, the procedure has to be followed carefully. First of all, skin cells from the periphery of the affected area are scrapped off onto a glass microscope slide with a sterile scalpel blade. One to two drops of 10% KOH solution is dropped onto the slide and heated gently to dissolve skin cells while keeping the fungal hyphae intact (Figure 5). Slide preparations should then be examined under low or medium power of a microscope. If a vesicle is being examined, it may be unroofed so the inside material may be examined.
Figure 5.
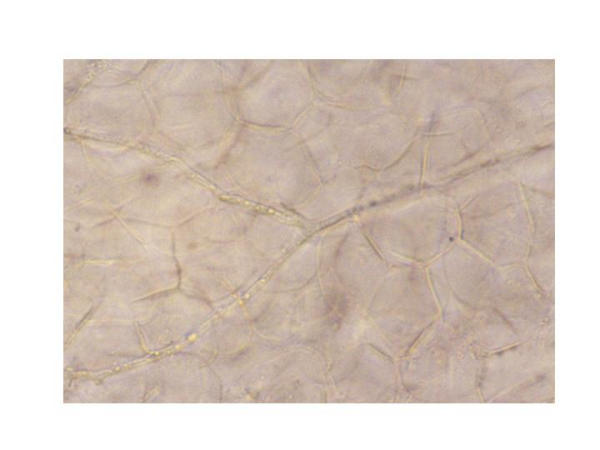
KOH preparation of fungal hyphae. This slide shows the branched hyphae of T. rubrum. Scrapings from the outer edge of a tinea lesion should be taken with a sterile scalpel, placed on a slide and KOH applied. KOH dissolves the epithelial cells seen in the background and leaves the fungus to be viewed by microscopy.
Treatment
Tinea Pedis usually responds to topical antifungal agents such as ketoconazole, terbinafine, econazole, or cicloprox creams. In the case of interdigital infection, antifungal powder preparations may be more effective. Topical treatment may need to be continued for at least 4 weeks and recurrences are common. For refractory or resilient disease, oral antifungals may be required. Treatment options and dosing are explained in greater detail below and are reviewed in Table 3. Oral treatment with griseofulvin, itraconazole, fluconazole, and terbinafine may all be effective for this disease. Drug resistance, adverse effects of medications and drug interactions are all major hurdles to successful completion of therapy. An evaluation for etiologies for immunosupression such as new onset diabetes or malignancy may be indicated in selected patients. Possible drug-drug interactions are listed in Table 4, adapted from "Oral antifungal drug interactions" by Katz and Gupta [37].
Table 3.
List of Oral Antifungal Therapies, Their Dosages, and Their Possible Side Effects
| DRUG | DOSE | SIDE EFFECTS |
| 1. Griseofulvin* | 375–500 mg qd 3–6 months | Nausea, Hepatotoxic, Photosensitivity, Headache, Leukopenia+, Neutropenia+ |
| 2. Azoles | ||
| A. Ketoconazole** | 200–400 mg qd 6–8 weeks | Hepatotoxic, Hepatitis Idiosyncratic rxn, Nausea, Vomiting, Diarrhea |
| B. Itraconazole | 100 mg qd 2–4 weeks | Abdominal pain, Increased transaminases |
| 400 mg qd 1 week | ||
| C. Fluconazole | 50 mg qd 6 weeks | Hepatotoxic+, Anaphylaxis |
| 100 mg qd 8 weeks | ||
| 150 mg q week 6 weeks | ||
| 3. Allylamines | ||
| A. Terbinafine | 125 mg qd 8 weeks | Headache, Rash, Cholestatic hepatitis, Blood dyscrasia Steven-Johnson Syndrome |
| 250 mg qd 2–6 weeks |
* Contraindicated in pregnancy ** Contraindicated in nursing women, + Rare side effect
Table 4.
List of Possible Drug-Drug Interactions with Oral Antifungal Therapy
| 1. Griseofulvin: |
| Aspirin, Oral contraceptives, Phenobarbital, Porfimer, Theophylline, Warfarin |
| 2. Ketoconazole: |
| Alcohol, Oral hypoglycemics, Phenytoin |
| 3. Itraconazole: |
| Alfentanil, Alprazolam, Amphotericin B, Agenerase, Antacids, Atorvastatin, Bexarotene, Buspirone, Bussulfan, Carbamazepine, Cilostazol, Cimetidine, Cisapride, Citalopram, Clarithromycin, Cyclosporine, Diazepam, Didanosine, Digotoxin, Docetaxel, Dofetilide, Erythromycin, Famatidine, Felodipine, Grapefruit juice, Haloperidol, Indinavir, Isoniazid, Lansoprazole, Lovastatin, Methylprednisone, Midazolam, Nevirapine, Nifedipine, Omeprazole, Oral hypoglycemics, Phenobarbital, Phenytoin, Pimazide, Quinidine, Ranitidine, Rifabutin, Rifampin, Retonavir, Saquinavir, Sildenafil, Simvastatin, Sirolimus, Sodium bicarbonate, Sucralfate, Tacrolimus, Triazolam, Trimetrexate, Verapamil, Vincristine, Warfarin |
| 4. Fluconazole: |
| Amphotericin B, Celecoxib, Cimetidine, Cisapride, Citalopram, Cyclosporine, Defetilide, Felodipine, Glipiside, Glyburide, Hydrochlorothiazide, Lovastatin, Midazolam, Oral hypoglycemics, Phenytoin, Pimozide, Prednisone, Quinidine, Rifabutin, Rifampin, Sildenafil, Simvastatin, Sirolimus, Tacrolimus, Theophylline, Trizolam, Warfarin, Zidovudine |
| 5. Terbinafine: |
| Cimetidine, Cyclosporine, Nortriptyline, Rifampin, Terfenadine, Theophylline, Warfarin |
Non-pharmacological
Non-pharmacological treatment focuses on educating patients about the predisposing factors, and the chronic nature of disease. Also, measures that are aimed at eliminating the moisture that provides the environment for infection and its recurrence should be discussed fully with the patients. Instructions about wearing open-toed shoes and avoiding skin maceration are essential.
Pharmacological
Topical treatment
Data has shown that topical antifungal treatment fails to cure about one-third of patients with tinea pedis [38]. However, most of the relapses were due to poor compliance. So it must be emphasized that the patient should continue topical treatment at least one week after symptoms have resolved. The application area should include normal skin about 2 cm beyond the affected area. Many studies have compared the efficacy and rate of relapse upon applying different topical antifungal agents. None of them showed 100% superiority of one treatment or regimen over the other. A comparison study by Suschka et al. between clotrimazole once daily 1% cream and ketoconazole twice/day 2% cream showed that clotrimazole once per day is as effective as twice per day ketoconazole [39]. Another randomized, double-blind parallel group study compared efficacy and tolerability of topical terbinafine 1% cream, twice a day for one week, with clotrimazole 1% cream, twice a day for 3 weeks, and showed that terbinafine achieves a cure rate more rapidly than clotrimazole and in a shorter amount of time [3]. Topical corticosteroids combined with topical antifungal agents (for example, in the form of clotrimazole-betamethasone) provide rapid symptomatic relief in addition to eradication of the causative organism. With long-term use of topical glucocorticosteroids atrophy and steroid-induced rosacea and striae may occur. These combination agents are relatively contraindicated in children under12 years of age [1].
Systemic antifungal therapy
Systemic treatment may be required for patients who have failed with topical antifungal therapy. It can also be used as a first line therapy in patients with severe disease like hyperkeratotic lesions or in patients who are immunosuppressed [40]. A list of common oral therapies and their dosages are listed in Table 3.
Griseofulvin concentrates in the stratum corneum of the skin and causes mitotic arrest during microtubule spindle formation in actively growing fungi [41]. Cure is difficult to achieve and the recurrence rate is high. Blood counts and serum chemistry including renal and hepatic function should be checked regularly throughout treatment, as Griseofulvin may have toxic effects on the liver.
The azoles, as a group, include ketoconazole, itraconazole, and fluconazole. Doses, indications for usage, and adverse effects are all readily available in textbooks. Table 4 provides a list of important drug-drug interactions for this group of medications that may influence treatment decisions and/or influence patient outcomes. Ketoconazole treatment is indicated when griseofulvin therapy has failed or when the patient cannot tolerate it. Blood counts and serum chemistry should be monitored more frequently during this therapy. Dosage adjustment may not be necessary in patients with renal failure. Absorption is improved by food, so food intake should be encouraged with the ketoconazole treatment. Due to its structural difference, itraconazole has greater efficacy and less toxicity as compared with other azoles. Also its absorption is enhanced by coadministration with food. No dose adjustment is needed in renal failure. Fluconazole has more potency, less toxicity, and wider spectrum of action than earlier azoles. A comparison study by Nozickova et al in 1998 between fluconazole at 50 mg/day for 6 weeks and fluconazole at 150 mg/week for 6 weeks showed no significant difference in cure rates between the two [42]. Fluconazole and itroconazole should not be given together with terfenadine, astemizole, and cisapride in view of the risk of cardiac arrhythmia.
Another group of drugs classified as allylamines include terbinafine as a member. These drugs act by destroying the fungal cell wall at a much earlier stage in its development than the azoles. Terbinafine may be the most effective treatment for tinea pedis. However, as a cost-effective option it is not the first line of therapy. Blood counts, platelet count, and liver enzymes should be repeated every 4–6 weeks with this therapy. Allylamines must be stopped if liver enzyme measurement exceeds twice the normal level.
Conclusions
Tinea pedis is a common dermatophyte infection of the feet. Classifying the type and causative organism of tinea pedis is important for proper treatment of the patient. The easiest way to test for fungal presence is by microscopy of a KOH preparation, but complete identification of the organism requires culturing. Tinea pedis infection can contribute not only to fungal dermatitis but also to flares of eczema and asthma. Tinea pedis may lead to severe bacterial cellulitis. Aggressive treatment of tinea pedis can be associated with improvements in atopic dermatitis, asthma and cellulitis in affected individuals.
Competing interests
None declared.
Authors' contributions
MA did the research and writing of the paper. SF did the research and writing of the paper. MS helped review the paper. GK did the writing and the major review of the paper.
Acknowledgments
Acknowledgments
This report was supported by NIH grants AI-43310 and HL-63070, and the Department of Internal Medicine at East Tennessee State University.
Contributor Information
Muhannad Al Hasan, Email: alhasan@etsu.edu.
S Matthew Fitzgerald, Email: fitzgers@etsu.edu.
Mahnaz Saoudian, Email: Saoudian@etsu.edu.
Guha Krishnaswamy, Email: krishnas@etsu.edu.
References
- Weinstein A, Berman B. Topical treatment of common superficial tinea infections. Am Fam Physician. 2002;65:2095–2102. [PubMed] [Google Scholar]
- Bell-Syer SE, Hart R, Crawford F, Torgerson DJ, Tyrrell W, Russell I. Oral treatments for fungal infections of the skin of the foot. Cochrane Database Syst Rev. 2002:CD003584. doi: 10.1002/14651858.CD003584. [DOI] [PubMed] [Google Scholar]
- Patel A, Brookman SD, Bullen MU, Marley J, Ellis DH, Williams T, Barnetson RS. Topical treatment of interdigital tinea pedis: terbinafine compared with clotrimazole. Australas J Dermatol. 1999;40:197–200. doi: 10.1046/j.1440-0960.1999.00360.x. [DOI] [PubMed] [Google Scholar]
- Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;67:101–108. [PubMed] [Google Scholar]
- Vander Straten MR, Hossain MA, Ghannoum MA. Cutaneous infections dermatophytosis, onychomycosis, and tinea versicolor. Infect Dis Clin North Am. 2003;17:87–112. doi: 10.1016/s0891-5520(02)00065-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi MG. Dermatophytosis: epidemiological and microbiological update. J Am Acad Dermatol. 2000;43:S120–S124. doi: 10.1067/mjd.2000.110378. [DOI] [PubMed] [Google Scholar]
- Sutton DA, Fothergill AW, Rinaldi MG. Guide to Clinically Significant Fungi. 1998.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132–133. doi: 10.1016/S0190-9622(00)90022-7. [DOI] [PubMed] [Google Scholar]
- Leyden JJ, Kligman AM. Interdigital athlete's foot: new concepts in pathogenesis. Postgrad Med. 1977;61:113–116. doi: 10.1080/00325481.1977.11712222. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Skelton HG, Yeager J, Ledsky R, McCarthy W, Baxter D, Turiansky GW, Wagner KF, Turianski G. Cutaneous findings in HIV-1-positive patients: a 42-month prospective study. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR) J Am Acad Dermatol. 1994;31:746–754. doi: 10.1016/s0190-9622(94)70236-5. [DOI] [PubMed] [Google Scholar]
- Sweeney SM, Wiss K, Mallory SB. Inflammatory tinea pedis/manuum masquerading as bacterial cellulitis. Arch Pediatr Adolesc Med. 2002;156:1149–1152. doi: 10.1001/archpedi.156.11.1149. [DOI] [PubMed] [Google Scholar]
- Semel JD, Goldin H. Association of athlete's foot with cellulitis of the lower extremities: diagnostic value of bacterial cultures of ipsilateral interdigital space samples. Clin Infect Dis. 1996;23:1162–1164. doi: 10.1093/clinids/23.5.1162. [DOI] [PubMed] [Google Scholar]
- Baddour LM, Bisno AL. Recurrent cellulitis after coronary bypass surgery. Association with superficial fungal infection in saphenous venectomy limbs. JAMA. 1984;251:1049–1052. doi: 10.1001/jama.251.8.1049. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB, Johnson RA, Wolff K, Suurmond D. Fungal Infections of the Skin and Hair. In: Cooke Darlene, Englis Mariapaz Ramos, Morriss John M, editor. Color Atlas and Synopsis of Clinical Dermatology Common and Serious Diseases. 4. McGraw Hill Medical Publishing Division; 2001. pp. 684–707. [Google Scholar]
- Leibovici V, Evron R, Dunchin M, Strauss-Leviatan N, Westerman M, Ingber A. Population-based epidemiologic study of tinea pedis in Israeli children. Pediatr Infect Dis J. 2002;21:851–854. doi: 10.1097/00006454-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Baddour LM. Cellulitis syndromes: an update. Int J Antimicrob Agents. 2000;14:113–116. doi: 10.1016/S0924-8579(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Baddour LM. Recent Considerations in Recurrent Cellulitis. Curr Infect Dis Rep. 2001;3:461–465. [PubMed] [Google Scholar]
- Day MR, Day RD, Harkless LB. Cellulitis secondary to web space dermatophytosis. Clin Podiatr Med Surg. 1996;13:759–766. [PubMed] [Google Scholar]
- Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663–668. [PubMed] [Google Scholar]
- Ward GW, Jr, Karlsson G, Rose G, Platts-Mills TA. Trichophyton asthma: sensitisation of bronchi and upper airways to dermatophyte antigen. Lancet. 1989;1:859–862. doi: 10.1016/S0140-6736(89)92863-8. [DOI] [PubMed] [Google Scholar]
- Mungan D, Bavbek S, Peksari V, Celik G, Gugey E, Misirligil Z. Trichophyton sensitivity in allergic and nonallergic asthma. Allergy. 2001;56:558–562. doi: 10.1034/j.1398-9995.2001.056006558.x. [DOI] [PubMed] [Google Scholar]
- Elewski BE, Schwartz HJ. Asthma induced by allergy to Trichophyton rubrum. J Eur Acad Dermatol Venereol. 1999;12:250–253. doi: 10.1016/S0926-9959(99)00024-0. [DOI] [PubMed] [Google Scholar]
- Hurlimann A, Fah J. Asthma, rhinitis and dermatitis triggered by fungal infection: therapeutic effects of terbinafine. Dermatology. 2001;202:330–332. doi: 10.1159/000051668. [DOI] [PubMed] [Google Scholar]
- Klein PA, Clark RA, Nicol NH. Acute infection with Trichophyton rubrum associated with flares of atopic dermatitis. Cutis. 1999;63:171–172. [PubMed] [Google Scholar]
- Leyden JL. Tinea pedis pathophysiology and treatment. J Am Acad Dermatol. 1994;31:S31–S33. doi: 10.1016/s0190-9622(08)81264-9. [DOI] [PubMed] [Google Scholar]
- Bryld LE, Agner T, Menne T. Relation between vesicular eruptions on the hands and tinea pedis, atopic dermatitis and nickel allergy. Acta Derm Venereol. 2003;83:186–188. doi: 10.1080/00015550310007184. [DOI] [PubMed] [Google Scholar]
- Forte WC, Santos de Menezes MC, Cipolli Guerra de Oliveira SM, Bruno S. Atopic dermatitis with mononuclear phagocytic activity deficiency. Allergol Immunopathol (Madr) 2002;30:263–266. doi: 10.1016/s0301-0546(02)79135-0. [DOI] [PubMed] [Google Scholar]
- Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, Ogawa H, Nishikawa A. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002;40:1363–1367. doi: 10.1128/JCM.40.4.1363-1367.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita T, Suto H, Unno T, Tsuboi R, Ogawa H, Shinoda T, Nishikawa A. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J Clin Microbiol. 2001;39:3486–3490. doi: 10.1128/JCM.39.10.3486-3490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayser P, Gross A. IgE antibodies to Malassezia furfur, M. sympodialis and Pityrosporum orbiculare in patients with atopic dermatitis, seborrheic eczema or pityriasis versicolor, and identification of respective allergens. Acta Derm Venereol. 2000;80:357–361. doi: 10.1080/000155500459303. [DOI] [PubMed] [Google Scholar]
- Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000;38:337–341. doi: 10.1080/mmy.38.5.337.341. [DOI] [PubMed] [Google Scholar]
- Ward GW, Jr, Woodfolk JA, Hayden ML, Jackson S, Platts-Mills TA. Treatment of late-onset asthma with fluconazole. J Allergy Clin Immunol. 1999;104:541–546. doi: 10.1016/s0091-6749(99)70321-0. [DOI] [PubMed] [Google Scholar]
- Mendez J, Sanchez A, Martinez JC. Urticaria associated with dermatophytosis. Allergol Immunopathol (Madr) 2002;30:344–345. doi: 10.1016/s0301-0546(02)79151-9. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Akiyama K. [Malassezia and atopic dermatitis] Nippon Ishinkin Gakkai Zasshi. 2003;44:65–69. doi: 10.3314/jjmm.44.65. [DOI] [PubMed] [Google Scholar]
- Kanda N, Tani K, Enomoto U, Nakai K, Watanabe S. The skin fungus-induced Th1- and Th2-related cytokine, chemokine and prostaglandin E2 production in peripheral blood mononuclear cells from patients with atopic dermatitis and psoriasis vulgaris. Clin Exp Allergy. 2002;32:1243–1250. doi: 10.1046/j.1365-2745.2002.01459.x. [DOI] [PubMed] [Google Scholar]
- Johansson C, Eshaghi H, Linder MT, Jakobson E, Scheynius A. Positive atopy patch test reaction to Malassezia furfur in atopic dermatitis correlates with a T helper 2-like peripheral blood mononuclear cells response. J Invest Dermatol. 2002;118:1044–1051. doi: 10.1046/j.1523-1747.2002.01758.x. [DOI] [PubMed] [Google Scholar]
- Katz HI, Gupta AK. Oral antifungal drug interactions: a mechanistic approach to understanding their cause. Dermatol Clin. 2003;21:543–563. doi: 10.1016/s0733-8635(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Bell-Syer SE, Hart R, Crawford F, Torgerson DJ, Young P, Tyrrell W, Williams H, Russell I. A systematic review of oral treatments for fungal infections of the skin of the feet. J Dermatolog Treat. 2001;12:69–74. doi: 10.1080/095466301317085336. [DOI] [PubMed] [Google Scholar]
- Suschka S, Fladung B, Merk HF. Clinical comparison of the efficacy and tolerability of once daily Canesten with twice daily Nizoral (clotrimazole 1% cream vs. ketoconazole 2% cream) during a 28-day topical treatment of interdigital tinea pedis. Mycoses. 2002;45:91–96. doi: 10.1046/j.1439-0507.2002.00724.x. [DOI] [PubMed] [Google Scholar]
- Markova T. What is the Most Effective Treatment for Tinea Pedis (Athlete's Foot)? The Journal of Family Practice. 2002;51:21. [PubMed] [Google Scholar]
- Moossavi M, Bagheri B, Scher RK. Systemic antifungal therapy. Dermatol Clin. 2001;19:35–52. doi: 10.1016/S0738-081X(01)00203-6. [DOI] [PubMed] [Google Scholar]
- Nozickova M, Koudelkova V, Kulikova Z, Malina L, Urbanowski S, Silny W. A comparison of the efficacy of oral fluconazole, 150 mg/week versus 50 mg/day, in the treatment of tinea corporis, tinea cruris, tinea pedis, and cutaneous candidosis. Int J Dermatol. 1998;37:703–705. doi: 10.1046/j.1365-4362.1998.00541.x. [DOI] [PubMed] [Google Scholar]


