Abstract
Skeletal muscle tissue engineering (SMTE) aims to repair or regenerate defective skeletal muscle tissue lost by traumatic injury, tumor ablation, or muscular disease. However, two decades after the introduction of SMTE, the engineering of functional skeletal muscle in the laboratory still remains a great challenge, and numerous techniques for growing functional muscle tissues are constantly being developed. This article reviews the recent findings regarding the methodology and various technical aspects of SMTE, including cell alignment and differentiation. We describe the structure and organization of muscle and discuss the methods for myoblast alignment cultured in vitro. To better understand muscle formation and to enhance the engineering of skeletal muscle, we also address the molecular basics of myogenesis and discuss different methods to induce myoblast differentiation into myotubes. We then provide an overview of different coculture systems involving skeletal muscle cells, and highlight major applications of engineered skeletal muscle tissues. Finally, potential challenges and future research directions for SMTE are outlined.
Introduction
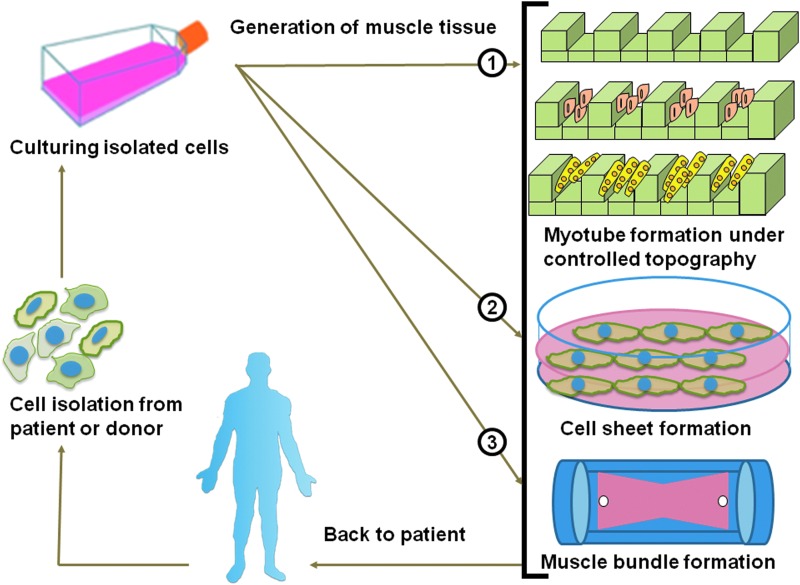
Approximately 45% of the mass of the human adult body is muscle tissue. Muscles play an important role in locomotion, prehension, mastication, ocular movement, and other dynamic events, including body metabolism regulation. Myopathy, traumatic injury, aggressive malignant tumor extraction, and muscle denervation are the most common clinical reasons for therapeutic or cosmetic reconstructive muscle surgery. Therefore, the engineering of muscles as clinical substitutes for various medical applications is beneficial. In this context, skeletal muscle tissue engineering (SMTE) focuses on the development of engineered tissues capable of repairing or replacing normal function in defective muscles. The concept of SMTE (Fig. 1) involves the culture of muscle cells that are harvested either from the patient or a donor, with or without the use of tissue scaffolds to generate functional muscle that can be implanted in the patient's body.1 Further, SMTE also has great potential for drug screening,2,3 construction of hybrid mechanical muscle actuators,4,5 robotic devices,6–8 and as a potential food source containing engineered meat.9
FIG. 1.
Schematic illustration of the concept of skeletal muscle tissue engineering showing three different types of culture techniques for generating muscle tissue. Color images available online at www.liebertpub.com/teb
Muscle tissue can be classified as smooth muscle, cardiac muscle, and skeletal muscle, which have been extensively reviewed previously.10–13 However, as the properties of engineered muscles are still far from their natural counterparts, we aim to review and address the methodology for building skeletal muscle with recent insights and to overcome the barriers between different fields of research to provide a better understanding of the nature of muscle and practical ways to engineer muscle tissue. Specifically, we review and update recent findings on the methodology and various technical aspects of SMTE, including cell alignment and differentiation. We start by addressing the structure and organization of muscle tissue and then describe useful methods to align myoblasts cultured in vitro, since cell alignment is a prerequisite for the formation of myotubes. We also address the molecular basics of myogenesis and describe different methods to induce myoblast differentiation into myotubes. We then give an overview of different coculture systems involving skeletal muscle cells, and highlight major applications of engineered skeletal muscle tissues. Finally, we conclude with a discussion of potential challenges and future research directions for SMTE.
Muscle Tissue Organization
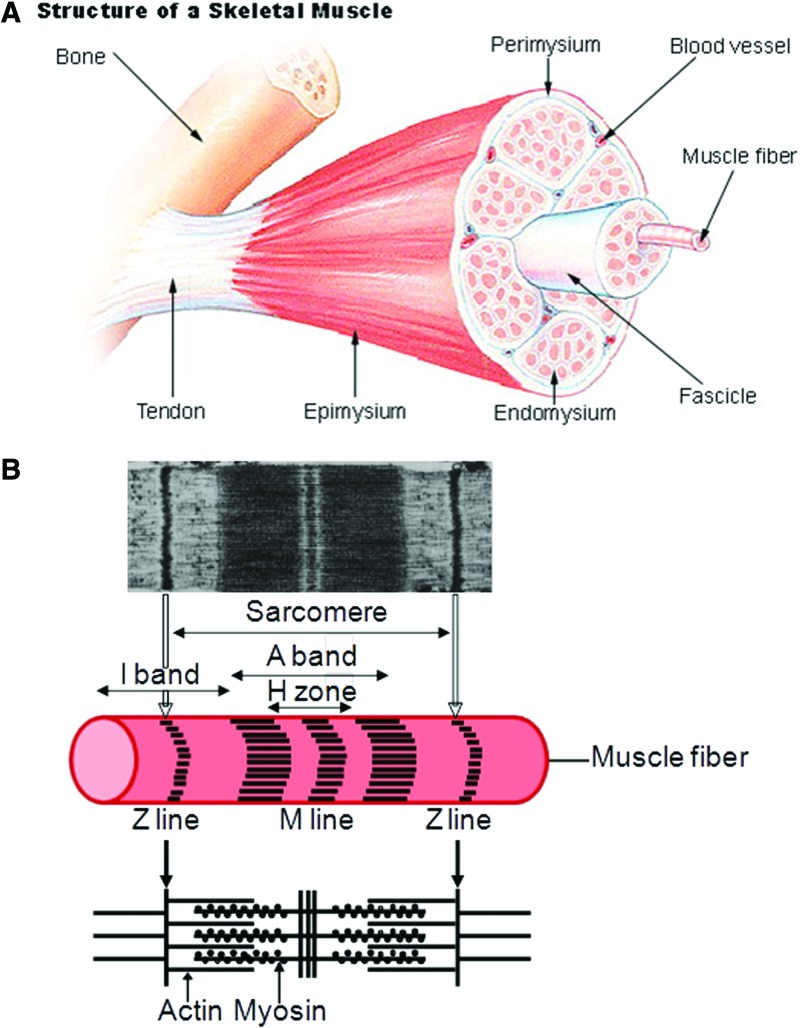
The human body has over 600 skeletal muscles that are linked to bones and are involved in anatomical position, locomotion, preemption, mastication, and other dynamic events. These muscles are comprised of multiple bundles of muscle fibers that are formed by the fusion of undifferentiated myoblasts into long cylindrical, multinucleated structures called myotubes (Fig. 2).14 Major components of the myotubes include the plasma membrane or sarcolemma, the cytoplasm or sarcoplasm, and the peripheral flattened multinuclei. The sarcoplasm is notably filled by myofibrils, which are composed of the cytoplasmic proteins myosin (thick filament) and actin (thin filament) in repeated units called sarcomeres that are aligned along the cell axis. Under a microscope, each sarcomere appears delimited by two dark lines (Z lines) of dense proteins. Between these two Z lines are two light bands (I bands) containing actin filaments, separated by a dark band (A band) containing myosin filaments that overlap each other. The A band has also a lighter central zone (H zone) that does not overlap with the I bands when the muscle is in relaxed state; the H zone is separated in two parts by a middle dark line (M line). As sarcomeres of different myofibrils are also aligned with each other in skeletal and cardiac muscle cells (but not in smooth muscle cells), the myofibers appear striated. When the muscle contracts, the actin filaments are pulled along the myosin filament toward the M line, and the overlapping area between the myosin and actin filaments increases, whereas the H zone decreases and the muscle becomes shorter.
FIG. 2.
Anatomy of a skeletal muscle and a sarcomere. (A) From SEER training on structure of skeletal muscle, U.S. National Institute of Health, National Cancer Institute (12 July 2012). http://training.seer.cancer.gov/anatomy/muscular/structure.html (B) Micrograph of a sarcomere adapted with permission from Sosa et al.14 Copyright © 1994, Elsevier, and schematic. Color images available online at www.liebertpub.com/teb
Other important components of this contractile machinery found in the sarcoplasm are the sarcoplasmic reticulum, where calcium ions are stored and used for the muscle activation; the T tubules, which are used as the pathway for the action potential; and proteins, such as troponin and tropomyosin, which are linked to the actin filaments to prevent their interaction with myosin filaments when the muscle is in a relaxed state. Skeletal muscles differ in their phenotypes, and muscle fibers in humans are classified into three categories (I, IIa, and IIx [or IIb]) according to their myosin heavy chain (MyHC) isoforms.15 Type I fibers are red because of the presence of myoglobin. They have a high mitochondrial content and rely on oxidative metabolism to generate ATP. These fibers express slow-twitch MyHCs and are suited for endurance. Type II fibers are white because of the absence of myoglobin and rely on glycolytic metabolism to generate ATP. They express fast-twitch MyHCs and are suited for fast bursts of power. This difference in twitch speed between muscle fibers results not only from differences in MyHC protein isoforms, which induce a difference in sliding velocity between the actin and the myosin filaments in the sarcomeres, but also from Ca2+ sequestering components, such as sarcoplasmic reticulum Ca2+ ATPases that are expressed as different isoforms in type I and type II fibers.16 Thus, the in vitro development of muscle tissue with high functionality and good contractibility requires mimicking of this muscular structure, particularly to generate aligned muscle fibers; therefore, we describe in the “Engineering of Skeletal Muscle Tissues In Vitro” section some useful methods to align myoblasts cultured in vitro, as this cell alignment is necessary to the formation of myotubes.
Engineering of Skeletal Muscle Tissues In Vitro
Although the first contractile skeletal muscle tissue from a chicken embryo leg was cultured in vitro by Lewis about hundred years ago,17 the challenge of building large-scale muscle tissue with functional properties has persisted. Since the late 1970s, many approaches and techniques have emerged to study the development of muscle tissues. Notably, Vandenburgh and Kaufman developed an in vitro model for stretch-induced hypertrophy of a skeletal muscle tissue construct embedded in a collagen gel.18 Later, in the early 1990s, the first three-dimensional (3D) muscle construct was grown in vitro by Strohman et al.,19 who grew a monolayer of myoblasts on a membrane that detached after differentiation and formed 3D contractile muscle tissue, which was later termed “myooids” by Dennis and colleagues.20 More recently, Lam et al. showed that aligned myotubes formed by the prealignment of myoblasts on a micropatterned polydimethylsiloxane (PDMS) layer can be transferred from the PDMS substrate into a fibrin gel, thus allowing for the formation of a 3D free-standing construct with higher muscle fiber content and force production.21 The size of the construct did not exceed 1 mm in diameter because of the limited diffusion capacity in the tissue. Thus, the use of synthetic polymers and advanced patterning techniques has allowed SMTE to progress. Currently, micro- and nanofabrication techniques enhance the possibility to create tissues.22 When engineering a skeletal muscle tissue, one of the key points is to prealign the cells to obtain increased muscle fiber formation, as shown previously by Lam and colleagues.21 To this end, many techniques (for reviews on micro/nanofabrication see Ramalingam and Khademhosseini,23 Khademhosseini and Peppas,24 Zorlutuna et al.,25 and Ostrovidov et al.26), such as soft lithography,27 hot embossing,28 electrospinning,29 photolithography and solvent casting,30 passive or active stretching,31 and the use of electrical fields,32,33 have been applied to create an environment that induces cell alignment. In the following subsections, we review useful methods to align myoblasts in vitro.
Cell alignment by topography
It has long been known that cell behavior is influenced by surface features.34 Thus, numerous studies have focused on the effects of different topographical features, such as size and geometry, on the cellular response.35–38 Among these topographical features, parallel grooves are among the most-studied patterns to elongate muscle cells in one direction. The first studies aimed to determine how the cells sense their environment and what causes the cells to undergo alignment. Thus, grooved patterns with different widths and depths were tested. For example, Evans et al. generated micropatterned grooves with depths ranging from 40 nm to 6 μm and widths ranging from 5 to 100 μm on silicon substrates by etching with conventional photolithographic methods and studied myoblast direction and alignment along the grooves.39 They showed that shallow grooves with a depth of 40–140 nm did not significantly affect myoblast alignment, whereas significant cell alignment was achieved with deep grooves that had a width of 5–12 μm and a depth of 2–6 μm. Additionally, Clark et al. showed that nanosized grooves with a width of 130 nm and a depth of 210 nm also induced myoblast alignment.40 In addition, because they observed that myotubes with identical diameters formed in grooves with different widths, Clark et al. hypothesized that lateral fusion of myoblasts was not a possible mechanism in myotube formation. Therefore, they cultured myoblasts on ultrafine grating (grooves with a width of 130 nm and a depth of 210 nm and ridges with a width of 130 nm) that strongly aligned the myoblasts, and showed that myoblasts fused in end-to-end configurations.41
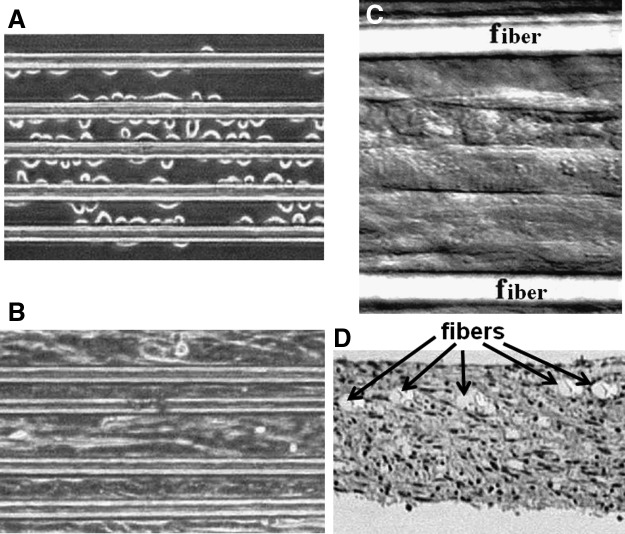
To easily fabricate groove/ridge micro- and nanopatterns without requiring a clean room, alternative methods to photolithography have also been used. Thus, since they contain nano/microgrooves, commercially CD-R and DVD-R in polycarbonate have been used for directing cell alignment or for patterning polymers.42,43 Abrasive paper has also been proposed to easily produce parallel grooves on a surface at low cost to direct the alignment of myoblasts.44 Similarly, Jiang et al. fabricated sinusoidal-wavy-grooved (size ranging between 0.1 and 10 μm) micropatterns on a PDMS surface by stretching a PDMS slab and then subjecting it to extended oxidation under low pressure before relaxing it. For this continuous topography without sharp edges, they showed that sharp-edge features were not necessary to induce contact guidance.45 Another study by Lam et al. focused on the effects of wave periodicity on C2C12 cells and showed that a wavelength of 6 μm was optimal to induce myoblast and myotube alignment.46 These topography–cell interaction studies opposed the theory proposed by Curtis and Clark, who suggested that cell guidance on groove-ridge patterns is mostly governed by groove depth.37,47 Although numerous studies have suggested that cells sense and grow on predefined topography, the mechanism by which the cells sense the topography is not well understood. However, filopodia are involved in this detection because they extend in front of the cells and probe the topographic features.48 This topographical surface guidance is the foundation of several approaches used for designing scaffolds in 2D and 3D. For instance, Neumann et al. used arrays of parallel polymer fibers with thicknesses of 10 to 50 μm and spacings of 30 to 95 μm to generate a scaffold for engineering a C2C12 myoblast sheet. They showed that by using this method, it was possible to generate a continuous contractile aligned muscle sheet with fiber spacing of up to 55 μm49 (Fig. 3).
FIG. 3.
C2C12 cells cultured on an array of large fibers. (A) Thirty minutes after seeding. (B) Gaps between fibers were closed after 5 weeks of culture and a cell sheet was formed. (C) After 10 weeks in culture, myotubes were striated (DIC image). (D) Cross-section of the muscular tissue fixed and stained by hematoxylin and eosin. The fiber plane is in the upper section (scale bars for [A–D] 50 μm). Reprinted with permission from Neumann et al.49 Copyright © 2003, Mary Ann Liebert, Inc.
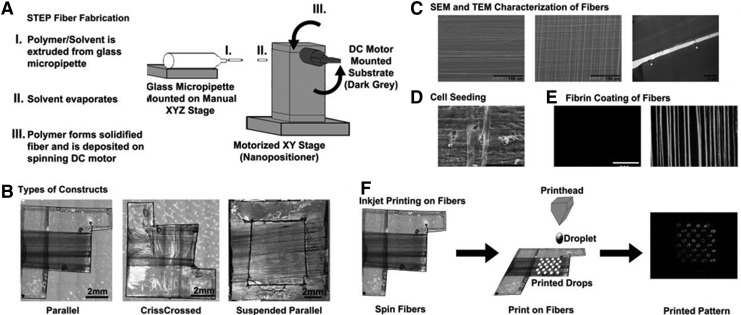
In another example, to build a muscle-tendon-bone tissue in one step, Ker et al. used a spinneret-based tunable engineered parameter technique to fabricate polystyrene fiber (655-nm diameter) arrays that they coated with extracellular matrix (ECM) proteins, such as fibronectin, and bioprinted with patterns of two different growth factors. By combining topographical and chemical cues to mimic the in vivo environment, they showed that although the fibers induced C2C12 cell alignment by topography, the localized presence of growth factors induced myoblast differentiation in tenocytes and osteoblasts, and the absence of growth factors enabled differentiation in myotubes50 (Fig. 4).
FIG. 4.
Fiber-fabrication procedure by Polystyrene Spinneret-based Tunable Engineered Parameter (STEP). (A) Schematic showing the building of fibers by STEP. (B) STEP fiber types that can be fabricated by one set of fibers running in a parallel manner (left), two sets of fibers running perpendicular to each other (middle), and one set of fibers running in a parallel manner with a hollowed-out support base (right). (C) SEM and TEM pictures used to quantify the STEP fiber diameter and length. (D) SEM image showing the cell attachment to the polystyrene STEP fibers. (E) Images in fluorescence of polystyrene STEP fibers coated with Alexa649-conjugated fibrin (right) and uncoated (left). (F) Polystyrene STEP fibers can be printed on by inkjet printing. (Scale bars: B, 2 mm; C, 100 μm and right photo 2 μm; D, 100 μm; E, 200 μm). Reprinted with permission from Ker et al.50 Copyright © 2011, Elsevier.
Nanotopography also greatly influences cell contact guidance.51 In groove/ridge patterns, the contact guidance cues are efficient in aligning cells for groove sizes up to 100 μm. With the development of methods to fabricate materials from micro- to nanoscale, new advancements of SMTE became available and materials with nanofeatures are of great interest. Indeed, cells in vivo evolve in an ECM environment, which comprises a material with nanoscale features that is composed of different proteins. For example, collagen fibrils found in the ECM usually have a length of ∼10 μm and a width of 260–410 nm.52,53 The fabrication of materials with nano-cues and nano-signals that are able to interact with cells and mimic the natural environment of the ECM can have tremendous applications in tissue engineering. Several studies have used columns, protrusions, pits, nodes, or nano-islands as substrates and have shown that small features, such as 11-nm columns, 35-nm pits, nanoposts (pointed columns), or 20-nm islands, promote cell adhesion, whereas increases in the size of these features decreases cell adhesion.54 Moreover, it has been shown that the symmetry of these features as well as the surface roughness also affects the adhesion of cells.55–58 Surface topography affects not only cell orientation and elongation but also the cell differentiation. For example, adult rat hippocampal progenitor cells cultured on a patterned polystyrene dish with 16-μm-wide grooves overexpressed neuronal marker (class III β-tubulin) when compared with smooth substrates.59 Electrospinning has also been used to fabricate aligned nanofiber scaffolds to induce the alignment of myoblasts.29,60–62 The 3D structure of the electrospun fibers resembles the physical structure of native collagen fibrils or ECM.63 However, although electrospun scaffolds are 3D structures, in many studies, the dense packing of fibers inhibits cell infiltration into the fiber network, and cells proliferate mostly on the top side of the electrospun fiber to generate a tissue similar to that formed using other 2D topographic substrates.64 Recently, direct electrospinning of a 3D aligned nanofibrous tube has been realized, promoting cell alignment and myotube formation.65 In another attempt to scale down the topography features, Dugan and coworkers employed oriented cellulose nanowhiskers with a diameter of 10–15 nm on a glass surface. They showed (Fig. 5) that myoblasts effectively sense the topography of such a surface and that myotubes resulting from myoblast fusion were nearly oriented in line with the direction of the cellulose nanowhiskers.66 This study clearly shows that cells are sensitive to topographical features, which affect cell orientation, shape, and differentiation.
FIG. 5.
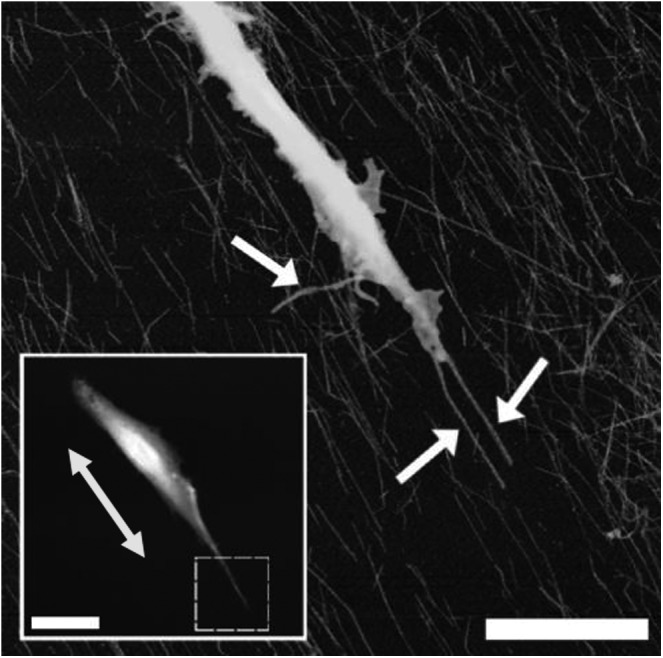
Oriented cellulose nanowhiskers of 10–15-nm diameter were produced by spin-coating. The response of myoblasts to the surfaces was assessed by atomic force microscopy 12 h after seeding. The inset picture shows a large-scale image of the whole cell (inset scale bar=20 μm), whereas the yellow arrow indicates the direction of the nanowiskers. The main image shows a closeup scan of the area bounded by the dashed box in the inset picture (scale bar=5 μm), whereas the white arrows indicate filopodia. Reprinted with permission from Dugan et al.66 Copyright © 2010, American Chemical Society.
To mimic in vivo muscle tissue, engineering of a 3D structure from aligned myotubes is needed. Zhao et al. have shown that a multilayered construct of aligned myotubes can be obtained by seeding additional myoblasts on a first layer of aligned myotubes formed in a groove (2-μm width and depth)/ridge micropattern.38 In addition, Hume et al. recently showed that if C2C12 cells aligned well in small grooves (≤100 μm) and did not align in large grooves in 2D culture, then their behavior changed in 3D culture.67 Thus, C2C12 cells were able to align in larger grooves (width of 200 μm and depth of 200 μm) when layered into the grooves. This study highlights the importance of 3D cultures in tissue engineering applications.
In an attempt to generate 3D tissue cultures in an environment that allows cells to assume a shape and exhibit matrix adhesion similar to that of native tissues, hydrogels have been extensively studied.68 Hydrogels can be generated from synthetic (e.g., poly(ethylene glycol) [PEG]) or natural polymers (e.g., collagen, chitosan, and hyaluronic acid). To generate skeletal muscle tissue, myoblasts must proliferate, migrate, align, and fuse to form a functional construct. ECM-derived hydrogel-like collagen or fibrin gel contains fibrils. These tiny protein nanofibers play the role of natural 3D topographical cues to guide the cells.69,70 Thus, Lanfer et al. used a microfluidic device to create highly aligned type I collagen matrices and showed that myotube assembly and alignment were influenced by the topographical feature of collagen fibrils.71 Hydrogel molding was also considered for guiding myoblasts in a method developed by Bian and coworkers. In their work, a PDMS mold was used to cast cell-laden hydrogels resulting in myotube alignment depending on the geometry and size72 (Fig. 6).
FIG. 6.
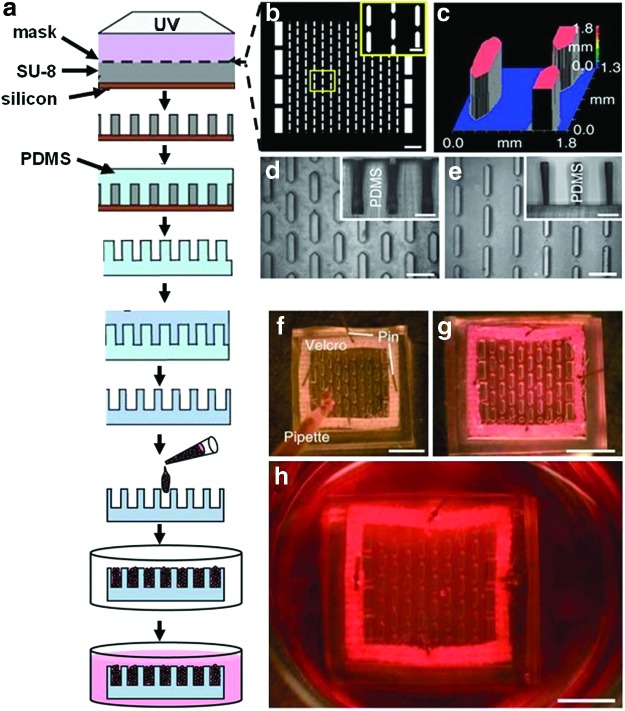
(a) A Silicon wafer coated with SU-8 was patterned by UV through (b) a photomask (scale bars=2 mm; inset=500 μm). (c) Optical profile of the master mold. (d) Polydimethylsiloxane (PDMS)-negative replica (scale bars=1 mm; inset vertical cross-section 500 μm). (e) PDMS-positive replica (scale bars=1 mm; inset vertical cross-section 500 μm). (f) Cells in hydrogel prepolymer solution were poured in the PDMS mold and incubated at 37°C to allow (g) hydrogel polymerization. (h) The culture medium was then added and the cells were cultured for 2 weeks. The hydrogel was fixed on a Velcro frame (scale bars in f–h 5 mm). Reprinted with permission from Bian et al.72 Copyright © 2009, Nature Publishing Group. Color images available online at www.liebertpub.com/teb
A composite 3D scaffold made of parallel glass fibers within a collagen gel has been used to direct the growth and differentiation of primary human masseter muscle derived cells.73,74 The effect of the cell density on the maturation and contractile ability of an engineered muscle in a collagen gel was also studied by the same group.75 In another study, gelatin, which is a hydrolyzed derivative of collagen, has been methacrylated to become photocrosslinkable. This acrylated gelatin showed promising aspects for supporting cell proliferation, and cell-laden photopatterned methacrylated gelatin was successfully used to direct, elongate, and align myoblasts in a 3D hydrogel environment76,77 (Fig. 7). These techniques rely on the limitation of cell migration by molding them in groove-like structures, which induced their alignment in a 3D environment improving their functionalities.
FIG. 7.
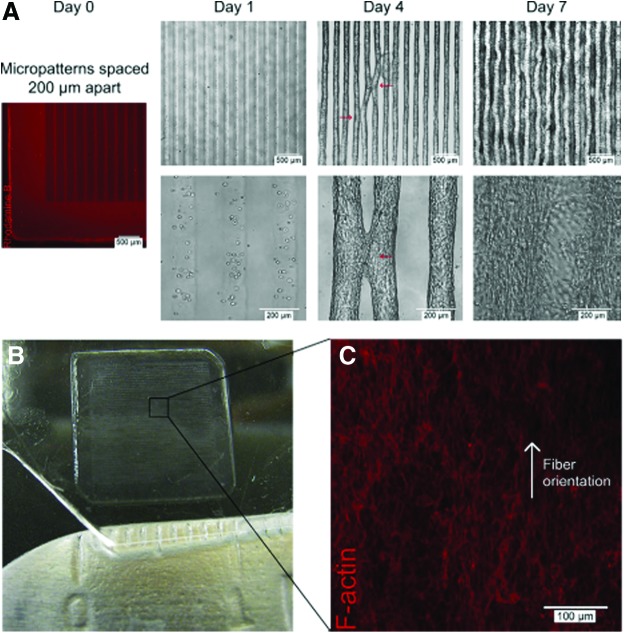
3T3 fibroblasts encapsulated in 5% GelMA hydrogels patterned into rectangular microconstructs [50 μm (w)×800 μm (l)×150 μm (h) with 200-μm interlines]. (A) Hydrogel stained with Rhodamine B showing initial microconstruct at day 0 and phase-contrast images of cell-laden microconstructs at days 1, 4, and 7 of culture. The red arrows indicate points of contact between neighboring lines at day 4 of culture, with tissue convergence at day 7 of culture. (B) Three-dimensional (3D) tissue construct (1×1 cm2) at day 7 of culture. (C) F-Actin staining showing the middle of the 3D tissue construct with aligned actin fibers. Reprinted with permission from Aubin et al.77 Copyright © 2010, Elsevier. Color images available online at www.liebertpub.com/teb
Cell alignment by surface patterning
Surface patterning is a general term used to describe the modification of a biomaterial's surface by patterning techniques. Soft lithography, which was introduced by the Whitesides group in the late 1990s to facilitate microfluidic device fabrication and fast prototyping, has also been used to pattern surfaces.78 This technique is based on the use of an elastomeric master that is easy to mold or emboss and can be used directly as substrate for biological applications or as mold. Among the elastomers used, PDMS is the most popular elastomer for biological applications, and the construction of a PDMS master is related to another mold prepared by conventional photolithography approaches.79–81 Soft lithography is widely used for the patterning of cells and proteins through using patterning techniques such as microcontact printing, microfluidic patterning, and stencil micropatterning.23,82,83 To guide cells on a surface, patterning of ECM proteins, such as collagen, fibronectin, or laminin, is widely used, as is the printing of self-assembled monolayers (SAMs) with cell-repellant molecules, such as PEG derivatives, poly(ethylene oxide)-b-poly(propylene oxide) (PEO-PPO) block copolymer, or copolymer surfactant with primary hydroxyl groups, thereby limiting the area where cells can settle. A combination of printed cell repellant and cell-adhesive molecules could also be used84–86 (Fig. 8). Another technique is showed by Shimizu et al., who used a stencil membrane to micropattern myoblasts and form a pattern of single myotubes87 (Fig. 9). Direct patterning of myoblasts by inkjet printing techniques has been also done to improve the cell alignment and tissue formation.88
FIG. 8.
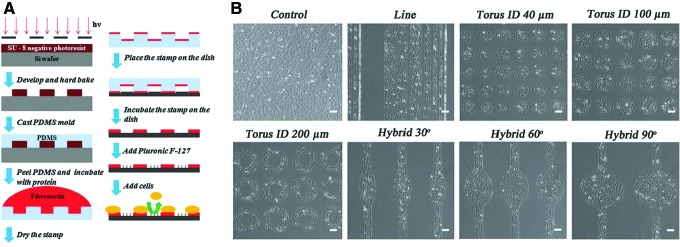
Micropatterned substrate building process and cell culture. (A) Schematic showing the preparation of the micropatterned substrate. (B) Phase-contrast images of the different micropatterns with cells: lines of different widths (300 μm, 150 μm, 80 μm, 40 μm, 20 μm, and 10 μm), tori of different inner diameters (40 μm, 100 μm, and 200 μm), and hybrid patterns of different arc degrees (30°, 60°, and 90°), (scale bar=100 μm). Reprinted with permission from Bajaj et al.84 Copyright © 2011, Royal Society of Chemistry. Color images available online at www.liebertpub.com/teb
FIG. 9.
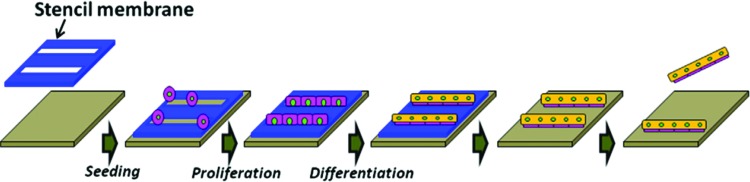
Schematic showing the micropatterning of myotubes by the use of a thin PDMS stencil membrane. A BSA-coated membrane was attached on the surface of a Petri dish and C2C12 were seeded on the membrane. After culturing in differentiation medium, the membrane was peeled off under a microscope to free the micropatterned myotubes, which can be removed. Reprinted with permission from Shimizu et al.87 Copyright © 2010, Elsevier. Color images available online at www.liebertpub.com/teb
Cell patterning has been mostly used to study cell behavior, such as cell migration, proliferation, cell–cell interactions, and drug screening, in a 2D environment. However, this approach is also appealing for the creation of 3D tissue-like constructs via cell-sheet-based tissue engineering. Indeed, various methods exist for the harvesting of prepatterned cell sheets. For example, Nagamine et al. used a fibrin gel to embed aligned myotubes into a 3D hydrogel system.89 Similarly, Huang et al. transferred aligned myotubes from a parallel micropattern of poly(2-hydroxyethyl methacrylate) (pHEMA) to a type I collagen gel overlaid on the micropattern. After 3 days of culture, the collagen sheet was rolled around a biodegradable polymeric mandrel to fabricate a tubular muscle-like construct with aligned myotubes.90 Polymeric nanomembranes, with exceptional flexibility were also micropatterned by microcontact printing with lines of fibronectin plus multiwalled carbon nanotubes (MWNTs) to enhance the cell alignment and myotube formation and then rolled up to fabricate a tubular structure.91 In an interesting study, prevascularized 3D muscle tissue was constructed by stacking multiple layers of endothelial cells sandwiched by myoblast sheets.92 Petri dishes covalently grafted with the temperature-responsive polymer poly(N-isopropylacrylamide) were used to harvest the different cell sheets, and the handling of the cell sheets was secured by a plunger coated with a hydrogel matrix. By patterning hydrophilic polymer on thermoresponsive surface and by using a plunger coated with gelatin to harvest the different cell sheets of human skeletal myoblasts, Takahashi et al. showed that an anisotropic cell sheet placed on the top of four random cell sheets stacked together induced the myoblasts and the ECM alignment in the whole construct.93 Recently, Guillaume-Gentil et al. described a method to fabricate and harvest micropatterned heterotypic cell sheets by local electrochemical dissolution of a polyelectrolyte coating94 (Fig. 10). Such methods introduce the possible creation of cocultured harvestable cell sheets and the growth of more complex tissue constructs via cell-sheet-based tissue engineering.
FIG. 10.
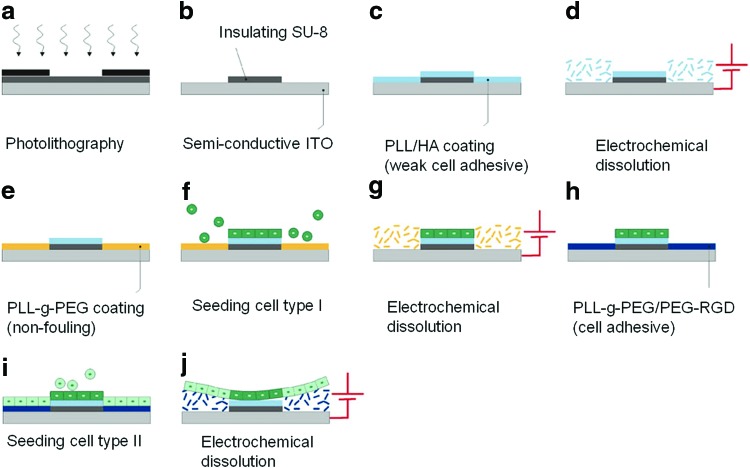
Micropatterning of heterotypic cell sheets. (a) Patterning of an SU-8 layer spin coated on an indium tin oxide (ITO) electrode by UV through a photomask. (b) After development, SU-8 micropatterns are formed on the ITO electrode. (c) The substrate is then coated with a weak cell-adhesive polyelectrolyte and (d) subjected to electrochemical polarization, which induced the dissolution of the polyelectrolyte only from the ITO regions. (e) The ITO regions are backfilled with a cell-repellent polymer PLL-g-PEG and (f) a first cell type is seeded whereas the nonadherent cells are washed away. (g) The PLL-g-PEG monolayer is then removed by a second electrochemical step. (h) The ITO regions are backfilled with PLL-g-PEG/PEG-RGD, which is a cell-adhesive monolayer. (i) The second cell type is seeded and the nonadherent cells are washed away. (j) After 1 day of culture, the PLL-g-PEG/PEG-RGD monolayer is dissolved by a final electrochemical step, which allows the whole cell sheet to detach. Indeed, due to their weak interaction with the substrate, the cells on weak cell-adhesive regions also detach easily. PEG, poly(ethylene glycol). Reprinted with permission from Guillaume-Gentil et al.94 Copyright © 2010, Springer Science+Business Media, LLC. Color images available online at www.liebertpub.com/teb
Cell alignment by mechanical stimulation
Lack of stimulation and mechanical force causes muscle degeneration, as occurs in disabled individuals or during skeletal muscle atrophy in the microgravity of spaceflight. Although the role of mechanical stimulation has been widely studied in gene regulation, endogenous protein regulation, accumulation, and metabolic products,95,96 it has been less studied as a tool in SMTE. However, it has been reported that under continuous uniaxial strain, avian myoblasts and L6 rat skeletal muscle cells cultured on an elastic substratum differentiated into myotubes oriented parallel to the direction of strain, whereas under stretching/relaxation cycles, the myotubes were aligned perpendicular to the stretch direction.31,97,98 Other studies of myoblasts encapsulated in a collagen hydrogel and treated by continuous uniaxial strain also showed the formation of myotubes parallel to the direction of the strain.5,99,100 One hypothesis to explain the difference in the angle of cell and myotube orientation between cells cultured under continuous strain or under stretching/relaxing cycles is the appearance of micro-ripples or micro-cracks in the matrix or in the elastomer surface perpendicular to the stretch direction, as observed for PDMS surfaces treated with stretching/relaxing cycles.101 The passive tension observed during the coalescence of a collagen gel has also been used to align myoblasts between two posts to form aligned myotubes.3
Cell alignment by magnetic or electrical fields
Electrical fields are often used in single-cell manipulation and cell sorting techniques but less in the engineering of a whole tissue. Indeed, a cell placed in an alternating electrical field polarizes into a dipole and is subjected to a dieletrophoretic force F102 given by the formula:
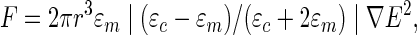 |
Where r is the radius of the cell, ɛm is the medium permittivity, ɛc is the cell permittivity, ∇E is the magnitude of the electrical field, and |(ɛc−ɛm)/(ɛc+2ɛm)| is the real part of the Clausius-Mossotti factor.103 If |(ɛc−ɛm)/(ɛc+2ɛm)|>0, then a positive dielectrophoresis (DEP) force F exists that induces the cell to move toward regions with a high electrical field. If |(ɛc−ɛm)/(ɛc+2ɛm)|<0, a negative DEP force F exists that repels the cell toward regions of low electrical field. As the magnitude of the DEP force F is inversely proportional to the electrode gap, the electrodes are usually designed to be close to each other to allow the induction of an electrical field of several hundred V/cm that is suitable for cell manipulation.104 Thus, DEP has been used to pattern several cell types for coculture and tissue engineering applications105–107 (Fig. 11).
FIG. 11.
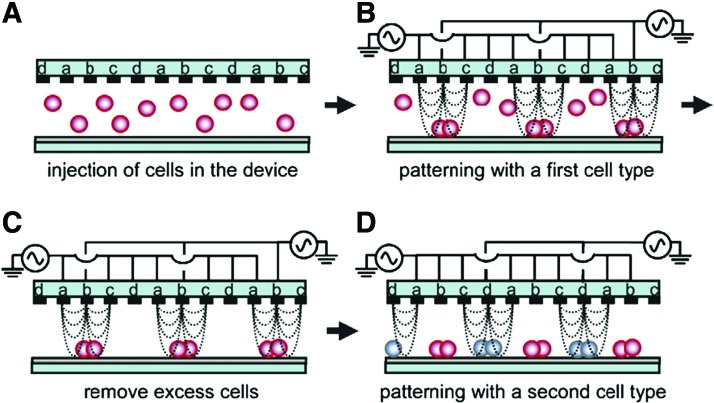
Patterning of two different cell populations by dielectrophoresis (DEP). (A) An interdigitated array of four-electrode subunit was used to pattern cells. (B) By applying an AC voltage, the n-DEP force was induced and cells were directed toward weaker region of electric field strength. (C) Cells in excess were removed. (D) A second cell type was loaded into the device and guided to other areas by changing the AC voltage mode. Reprinted with permission from Suzuki et al.107 Copyright © 2008, Elsevier. Color images available online at www.liebertpub.com/teb
Ramon-Azcon and colleagues used DEP to pattern C2C12 myoblasts in a hydrogel matrix, which resulted in a highly aligned muscle tissue construct.32,33 MWNTs have also been included into hydrogel and aligned by DEP improving the hydrogel electrical conductivity and favoring the cell alignment and the myotube formation.108 Some notable characteristics of the DEP method include accuracy, high cell manipulation speed, and the ability to scale-up.109 It has also been shown that a static magnetic field alone can induce the alignment of L6 myoblasts.110 However, the mechanism underlying this phenomenon is not well understood. Yamamoto et al. reported that C2C12 cells were elongated along the axis of a magnetic field after endocytosis of magnetic nanoparticles.111 By using this method of magnetic-force-based tissue engineering,112 which promotes tissue organization under a magnetic field, Akiyama et al. fabricated 3D tissue architecture,113 whereas Yamamoto et al. fabricated 200-μm-thick skeletal muscle tissue.111,114 To fabricate a 1.9-mm-thick skeletal muscle tissue, Yamamoto et al. combined the application of a magnetic field to C2C12 cells loaded with magnetic nanoparticles to induce tissue organization with the use of cell culture in a perfused hollow fiber reactor that allowed the maintenance of high cell density by supplying oxygen and nutrients.115
Induction of Cell Differentiation from Myoblasts to Myotubes
Cell differentiation
Myogenesis is the differentiation process that drives the formation of multinucleated myotubes. However, cell proliferation and phenotypic differentiation are mutually exclusive events. Therefore, myoblasts have to exit the cell cycle to enter into myogenesis. During this process, mononucleated myoblasts, which have a spindle or a polygonal shape, migrate toward each other and aggregate. Following this cell adhesion, the myoblasts align in an end-to-end configuration41 by the parallel apposition of their membranes.116 Membrane fusion then occurs, and the cells fuse together to generate a multinucleated structure.117 Initially, myoblasts fuse with each other to form small nascent myotubes, which subsequently fuse with additional myoblasts to form large and mature myotubes.118,119 All of these successive events are regulated by numerous factors, such as transcription factors, and involve several protein regulatory mechanisms, which are discussed in the following subsections.
Transcription factors
Nuclear factor of activated T cell (NFAT) is regulated by calcium and is involved in the transcription of numerous cytokines, such as IL-2, IL-3, IL-4, IL-5, and tumor necrosis factor α (TNFα).120 Upon calcium activation, calcineurin dephosphorylates the NFAT protein, which translocates to the nucleus, binds to DNA, and actives gene transcription. Three isoforms of this protein (NFATc1, c2, and c3) are present in myoblasts, and their translocation to the nucleus occurs at different stages of myogenesis. Thus, NFATc3 is activated only in myoblasts, and NFATc1 and NFATc2 are activated in myotubes.121 NFATc2 is notably involved in nascent myotube formation and growth.122 Myogenic regulatory factors (MRFs) include MyoD, myogenin, Myf-5, and MRF4 (also called Herculin or Myf-6),123 which are expressed exclusively in skeletal muscle. Each of these factors activates myogenesis and forcing their expression in a variety of nonmuscle cell types converts these cells into muscle cells.124 These factors are characterized by a 70-amino-acid sequence containing a basic helix-loop-helix structure (bHLH) that is a DNA-binding domain.125 Mutagenesis studies have shown that the HLH motif is required for dimerization, whereas the adjacent basic motif is involved in DNA binding and targets a CANNTG sequence known as the E-box.126 MyoD is a 318-amino-acid phosphorylated nuclear protein. MyoD and Myf-5 can functionally substitute for one another to activate the muscle differentiation program127 and play a crucial role in the determination and maintenance of myogenic identity. Both of them are able to activate their own transcription and to cross-activate the other MRFs. MyoD mRNA and Myf-5 mRNA are expressed before and after myoblast differentiation. When activated, MyoD induces cells to permanently exit the cell cycle by increasing the expression of p21, which is an inhibitor of the cyclin-dependent kinases (Cdks).128,129 If MyoD and Myf5 have been shown to specify the myogenic lineage in a redundant manner and therefore are involved in the generation of myoblasts, then Myogenin plays a major role in the differentiation of myoblasts into multinucleated myotubes and its expression marks the entry of myoblasts into the differentiation pathway.130 However, the trigger that switches cells from proliferation to differentiation remains unknown. Andres and Walsh have shown that myogenin is expressed early before cell cycle exit and that the next step into myogenesis involves an increase in the number of cells expressing myogenin and the cell-cycle inhibitor p21.131 Indeed, Cdks are enzymes that are involved in cell-cycle progression and their inhibition by cyclin-dependent kinase inhibitors, such as p21 and p57, which act in a redundant way, induces G1/S transition arrest and cell cycle exit concomitantly with increased activity of retinoblastoma protein (Rb), which together with myogenin activates the differentiation.132 It has been shown that the differentiation of myotubes can be reversed in cells with low expression of myogenin, thereby inducing myotube dislocation into mononucleated cells capable of DNA replication.133 MRF4 is also able to induce myogenic differentiation.134 However, MRF4 is usually expressed during the later phase of myogenesis during myotube maturation.135 Gap junctions are present on myoblasts prior to myotube formation and disappear after cell fusion. Studies have shown that blocking gap junctions with a compound such as octanol not only impairs myoblast fusion136 but also inhibits the activation of myogenin and MRF4.137 The myocyte-specific enhancer factor-2 (MEF-2) is a nuclear factor that activates muscle-specific transcription and belongs to the MCM1, Agamous, Deficiens and Serum response factor (MADS) box family of transcription factors.138 The MADS-box is a motif of 57 amino acids that is localized at the N-terminus of MEF-2 members and is a DNA-binding domain. It is located adjacent to a sequence of 29 amino acids referred to as the MEF2 domain that reinforces DNA binding and dimerization.139 In vertebrates, MEF-2 members include MEF-2 A, MEF-2 B, MEF-2 C, and MEF-2 D, all of which bind to an A+T-rich DNA sequence [CTA(A/T)4TAG].140 The MEF-2 family cooperates with the MRF family in the activation of muscle gene expression via a direct interaction between the respective DNA-binding domains, which results in a protein–protein association that synergistically increases the transcription and myogenic activity of MRF members.141–144
Other proteins that regulate myogenesis
Because myogenesis is regulated by a complex signaling pathway with multiple entry points and steps, many extra- and intra-cellular molecules and proteins positively or negatively affect this pathway. For example, growth factors, such as fibroblast growth factor (FGF) and transforming growth factor β (TGF-β), are potent inhibitors of myoblast differentiation.145,146 FGF impairs the binding of myogenic helix-loop-helix proteins to DNA by inducing the phosphorylation of their basic region by protein kinase C (PKC).147 TGF-β inhibits the activity of myogenin and MyoD without affecting their ability to bind DNA.148 It has been shown that TGF-β can induce the translocation of MEF-2 from the nucleus to the cytoplasm, thereby preventing it from participating in an active transcriptional complex.149 Table 1 depicts some of the molecules involved in the regulation of myogenesis,122 and we suggest that readers refer to other articles for detailed examples of various signaling pathways involved in myogenesis.150–153
Table 1.
Molecules Acting on Mammalian Myogenesis
| Molecule name | Effect on | Reference |
|---|---|---|
| Membrane proteins | ||
| Integrins (VLA-4, β1), integrin receptor VCAM-1 | Myoblast fusion | 384 |
| Nephrin | Myotube accretion | 385 |
| K+ ion channel, T-type Ca2+ channel | Intracellular Ca2+ | 386,387 |
| Epidermal growth factor receptor | Myoblast differentiation | 388 |
| Protein GRP94 | Myoblast fusion | 389 |
| ADAM 12, Calveolin-3 | Myoblast fusion | 384,390 |
| Notch receptor | Satellite cell regulation | 327,391 |
| Mannose receptor | Myotube accretion | 119 |
| Intracellular proteins | ||
| Calpain, Calmodulin | Myoblast fusion | 392 |
| Calcineurin | Myoblast recruitment | 393 |
| AMPKinases | Protein catabolism | 187,394 |
| NFATC(1,2,3) | Gene activation | 394 |
| Yap | Hippo signaling | 395 |
| MAP Kinases | MEF2, stress signaling, activator mTORC1 | 150,185 |
| TSC1-TSC2 | mTORC1 inhibitor | 167 |
| mTOR | Regulation protein anabolism | 166 |
| FoxO/Smad | Protein catabolism | 176 |
| Extracellular factors | ||
| PGE 1, PGF2α, arachidonic acid | Myoblast fusion | 159,396 |
| IL-4, IL-6, LIF | Myoblast fusion, satellite cells | 397 |
| Ca2+ | Signaling pathway | 387 |
| Cathepsin B | Autophagy | 398 |
| IGF-1, Insulin, Androgen, GH | Protein anabolism | 399 |
| Myostatin, glucocorticoids | Protein catabolism | 227,400 |
| NO | Regulation satellite cell, myoblast fusion | 401 |
Reprinted and adapted with permission from Horsley and Pavlath.122 Copyright © 2004, S. Karger AG Basel.
Molecular induction of myoblast differentiation
Serum deprivation and other biochemical inductions
Myoblast differentiation can be molecularly induced by the absence of inhibitory molecules or by the presence of stimulatory molecules, and the surface chemistry of the substrate on which cells are cultured also plays a key role. Thus, serum deprivation is often used to induce myoblast differentiation into myotubes. Indeed, for myoblasts, the decision to proliferate or to differentiate is determined by the presence or the absence of serum. Among the different factors that downregulate the MRF members, Id (for inhibitor of DNA binding) is an HLH-protein that has the HLH motif but lacks the adjacent basic motif involved in DNA binding.154 Id is expressed at a high level in proliferating cells and is downregulated by serum starvation.155 By forming nonfunctional heterodimers with MRF members, Id impairs their ability to bind DNA.156 The expression of several genes, including c-Fos and c-Jun, is also rapidly induced by serum and represses the transcriptional activation induced by myogenin and MyoD.147 Another effect of serum deprivation is cell-cycle arrest via Cdk inactivation.132 In contrast, because myogenesis is regulated by a complex signaling pathway as described just now, the addition of certain molecules or proteins into the culture medium may induce or favor myoblast differentiation. For example, by using the nitric oxide (NO)–generator DETA-NO, Pisconti et al. showed in vitro in C2C12 cells and in vivo in mice that NO via cGMP induced the generation of follistatin (FST), which induces myoblast fusion.157 Similarly, it has been shown that myoblasts express netrin-3 and its cell-surface receptor neogenin. Treatment of C2C12 cells with recombinant netrin induces myotube promotion and NFAT activation, which results in the formation of larger myotubes when compared with controls.158 Arachidonic acid supplementation also enhances myotube growth.159 Cytokines and growth factors may also activate the JAK-STAT pathway regulating positively (or negatively) the myogenic differentiation.160 Material surface chemistry and ECM may also favor myoblast differentiation. Indeed, in vivo, muscle fibers are wrapped by basal lamina, which is an ECM that contains mainly laminin, collagen IV, collagen I, and proteoglycans. As ECM is a key component of the cellular environment, some research groups have used directly the ECM extracted from muscles to improve cell culture and differentiation in 2D systems,161,162 whereas others have used a 3D hydrogel environment mixed with different ECM components.163 ECM acts on cell-cycle progression and cell differentiation through the binding of transmembrane integrin receptors and the activation of signaling cascades. Chemical patterning of the material surface by SAMs is also a versatile technique for surface modification23,164 that can be used to induce cell differentiation. Indeed, SAMs and notably alkanethiols, which are molecules composed of a head group with a sulfhydryl (-SH) and a long alkyl tail that can be functionalized, form spontaneously ordered monolayers through the adsorption of their head group to the surface of the substrate. By using fibronectin-coated SAM surfaces to present defined functional chemicals to C2C12 cells, Lan et al. showed higher myoblast differentiation on surfaces with functional groups following the order OH>CH3>NH2=COOH, which was correlated with higher α5β1 integrin binding.165
Cellular regulation of protein anabolism
In cells, the protein homeostasis is balanced by protein synthesis and degradation to sustain anabolic processes and energy production. This important regulation is mainly controlled by the mammalian target of rapamycin (mTOR) signaling pathway.166 mTOR is a 289-kDa serine/threonine kinase that forms two different complexes 1 (mTORC1) and 2 (mTORC2), respectively, sensitive and insensitive to rapamycin.167 The mTORC1 complex is formed by mTOR, the regulatory-associated protein of mTOR (Raptor), the mammalian lethal with SEC13 protein 8 (mLST8, also known as G protein β-subunit-like protein [GβL]), and the proline-rich Akt substrate-40 kDa (PRAS40), whereas mTORC2 complex is formed by mTOR, the rapamycin-insensitive companion of mTOR (Rictor), and mLST8.168,169 When activated, mTORC1 promotes the cell growth by enhancing the protein synthesis through the phosphorylation and activation of the ribosomal S6 kinase (S6K), forms 1 (S6K1) and 2 (S6K2), activating downstream the RNA translation.170 Moreover, phosphorylated mTORC1 induces also the phosphorylation and inhibition of the translational repressor named eukaryotic initiation factor binding protein 1 (4EBP1) by inducing its dissociation from the eukaryotic translation initiation factors 4E (eIF4E) that assembles with eIF4G and eIF4A to form the trimeric complex eIF4F initiating the RNA translation.171 Upstream, mTORC1 is regulated by the tuberous sclerosis complex also named hamartin-tuberin complex (TSC1-TSC2), which inhibits mTORC1 by stimulating the GTPase activity of the protein Ras homologue enriched in brain (Rheb) via the GTPase activating protein (GAP) domain of TSC2, increasing the conversion of Rheb-GTP into Rheb-GDP.167 The TSC1-TSC2 complex is a hub of signal transduction modulating mTORC1 activity in function of the signals received from a large number of different signaling pathways. This allows mTORC1 to sense among different signals the level in amino acid, in energy (ATP), in oxygen, and in growth signaling for regulating the cellular growth.
Upstream to TSC1-TSC2, one of the important signalization pathways transduced the signal by Akt also named protein kinase B (PKB). Akt is a serine/threonine kinase that has three isoforms, Akt1, Akt2, and Akt3,172 and is a central node of signalization, notably by growth factors.173 Akt acts positively on the protein synthesis regulation by directly phosphorylating TSC2 inhibiting the GAP activity of TSC1-TSC2 complex toward Rheb, which allows the accumulation of Rheb-GTP and the activation of mTORC1.174 Moreover, Akt can also inhibit the protein degradation by phosphorylating members of the forkhead box O (FoxO) family of transcription factors impairing them to translocate into the nucleus. The FoxO proteins control the ubiquitin-proteasome and autophagy-lysosome systems, which are two main proteolytic pathways in cell.175,176 Therefore in muscle, the activation of FoxO1 and FoxO3 induces their translocation from the cytosol to the nucleus to promote the transcription of genes, such as the muscle atrophy F-box also named atrogin1 (MAFbx),177 the muscle ring finger-1 (MuRF1),178 and the regulated in development and DNA damage response-1 (REDD1),179 inducing muscular atrophy. Upstream, Akt is regulated by the phosphatidylinositol-3-kinase (PI3K), which upon activation recruits its substrate phosphatidylinositol-4-5- biphosphate (PIP2) to generate the second messenger phosphatidylinositol-3-4-5- triphosphate (PIP3), which in association with the 3′-phosphoinositide-dependent kinase-1 (PDK1) activate Akt.180
However, other signaling pathways, such as the extracellular signal regulated kinase (ERK) pathway, the p38 mitogen activated protein kinase (MAPK) pathway, or the 5′ adenosine monophosphate-activated protein kinase (AMPK) pathway, can act positively or negatively on the TSC1-TSC2 complex.181 The ERK pathway activates mTORC1 by phosphorylating and inhibiting TSC2 via p90 ribosomal S6 kinase.182 ERK also upregulates the protein anabolism by phosphorylating and inhibiting FoXO3a protein, which is then degraded via the ubiquitin proteasome pathway.183 The P38/MAPK is involved in proinflammatory cytokines and other stress signals.184 The p38/MAPK pathway activates mTORC1 by inhibiting the TSC1-TSC2 complex via the phosphorylation of TSC2.185 The AMPK pathway acts as a cellular energy sensor.186 In low-energy (intracellular ATP) status, 5′ adenosine monophosphate (AMP) level increases and activates AMPK. To increase the energy production via catabolic processes, AMPK directly phosphorylates TSC2 and Raptor impairing mTORC1 to phosphorylate its substrate and to activate the protein synthesis pathway.187,188 The level of oxygen can also be sensed through the AMPK pathway since in hypoxia condition the ATP level will be reduced. Another mechanism involves the activation of TSC2 due to its dissociation from the inhibitor proteins 14-3-3 after the binding of REDD1, which is upregulated under hypoxia condition.189 Other signaling mechanisms have also been observed. Thus, the TNFα phosphorylates TSC1 via the inhibitory κB kinase β (IKKβ) enhancing the dissociation and inactivation of the TSC1-TSC2 complex, which activates the mTORC1 pathway and the protein synthesis.190 The glycogen synthase kinase 3 (GSK3) inhibits mTORC1 by phosphorylating and activating TSC2 via AMPK. However, Wnt signaling inhibits the phosphorylation and activation of TSC2 by the GSK3 that upregulates mTORC1.181 Thus, important cell signaling networks are complex, containing several points of regulation, signal divergence, and crosstalk with other signaling cascades.
Muscle anabolism induced by growth factors
Muscle growth is regulated by hormones, such as growth hormone (GH), insulin like growth factor-1 (IGF-I) and 2 (IGF-II), testosterone, and 5-α-dihydrotestosterone (DHT).191,192 It is well known that testosterone injection favors protein synthesis, whereas androgen deficiency induced muscle atrophy, reduction of IGF-1 level, reduction of muscle androgen receptor (AR) expression, and increase of fat store.193 Classically, androgens like testosterone mediated their effects via the AR.194 AR is a 110-kDa ligand-inducible transduction factor localized in the cytoplasm complexed by heat shock proteins (e.g., HSP 90 and HSP 70) and other chaperones.195 Upon ligand binding, AR is released from its chaperones, translocates to the nucleus, binds to the androgen-responsive elements, and actives directly, or indirectly via the recruitment of coactivators or corepressors, the gene transcription.196 This mechanism of signalization defined the genomic pathway. However, testosterone may also act faster trough nongenomic pathways involving surface membrane receptors.197,198 Thus, in the AR-negative rat L6 cell line, testosterone signaling involves a G-protein-coupled receptor with increase of intracellular calcium (Ca2+) acting as second messenger, and inducement of the cell proliferation via the PKC and ERK, while the cell differentiation was induced through the protein kinase A (PKA).199 Testosterone signal can also activate the MAPK pathway via the tyrosine kinase Src interacting with AR or the epidermal growth factor receptor.200–202 AR has also been shown to interact directly with the p85α subunit of PI3K to activate the PI3K/Akt pathway.203 Since AR can activate numerous signaling pathways, the development of AR ligands dissociating the anabolic effects from the androgenic effects of androgens, named selective androgen receptor modulators, is an important direction of research for therapeutic applications.194 Recent studies have shown that the anabolic effect of testosterone on muscle is transduced via the Akt/TSC2/mTORC1 pathway previously described.204,205
Insulin, IGF-I, and IGF-II are produced by the liver under the stimulation of GH and have also an anabolic effect on muscle.206 After binding its receptor (IR) insulin activates the insulin receptor substrate (IRS-1) by tyrosine phosphorylation, which acts as docking site for proteins with Src homology 2 (SH2) domains, such as the P85 subunit of PI3K. This leads to the generation of the second messenger PIP3 and the activation of Akt/TSC2/mTORC1 pathway resulting in anabolic effect on muscle.167,175 However, a feedback mechanism exists to regulate the insulin signaling since the activation of S6K1 by mTORC1 induces the serine/threonine phosphorylation of IRS-1 reducing its stability.207,208 It has also been shown that insulin activated the phosphorylation of PRAS40 by Akt impairing its inhibitor effects on mTORC1.209
IGF-I and IGF-II are also important regulators of muscle mass.210 As insulin, they can in addition be produced by muscles and act in an autocrine/paracrine fashion. Similarly, they signal through the PI3K/Akt/TSC2/mTORC1 pathway but via their respective receptors IGF-IR and IGF-IIR.197,211,212 A negative autoregulatory loop of myogenesis has also been observed, since IGF-I can also activate the phospholipase C gamma through the PI3K pathway promoting the release of calcium from the intracellular stores, which induces the transcription of myostatin via calcineurin/NFAT pathway.213,214 During myogenesis, it has been shown that IGF-II auto-upregulates its gene expression via PI3K/Akt and P38 MAPK pathways, while downregulates IGF-I gene expression through mTOR.215 Interestingly in a point of view of biomaterial engineering, the focal adhesion kinase is required for the anabolic signal of IGF-I mediated through TSC2/mTOR/S6K1 pathway.216,217 Under exercise (mechanical or electrical stimulation), human muscles express spliced variants of the IFG-I gene that produce three isoforms of IGF-I.218 One isoform is IGF-IEa similar in action to the IGF-I produced by the liver, the second is IGF-IEb, whereas the third one is IGF-IEc, also named mechanogrowth factor (MGF), containing an E domain of 49 bases that modulates the signalization.219–221 IGF-IEa has been involved in the promotion of myoblast differentiation222 whereas MGF has been shown to recruit and to stimulate the proliferation of satellite cells (SCs) in correlation with the activation of the ERK pathway.223,224 The IGF-I signaling pathway crosstalks with the androgen signaling pathway since androgens increase the IGF-I level in serum and the IGF-I mRNA expression in muscle.193,225
Muscle catabolism induced by myostatin
In cells, the protein catabolism is secured via both the ubiquitin proteasome system and the autophagy/lysosome pathway with the activation of FoXO, NF-κB, and Smads transcription factors, whereas the protein anabolism is secured by the PI3/Akt/mTORC1 pathway.226,227 Myostatin or growth differentiation factor 8 (GDF-8) belongs to the TGF-β family, which are known inhibitors of myogenic differentiation and muscle growth.228 Myostatin is secreted by muscle and acts as an autocrine/paracrine fashion inhibiting myoblast differentiation, and maintaining SCs quiescents.229 After cleavage of the promyostatin complex, the mature myostatin binds to the transmembrane receptor activin receptor type IIB (ActRIIB), which recruits the type 1 transmembrane activin receptor-like kinase 4 or 5 (ALK4 or ALK5).230 This induces the phosphorylation of Smad proteins (Smad name comes from mothers against decapentaplegic) 2 and 3, and the recruitment of Smad 4 to form a complex Smad 2,3,4, which translocates from the cytosol to the nucleus and actives the gene transcription of atrophy-related genes or “atrogenes,” such as Murf-1 an Atrogin-1.227 As there is a balance between protein catabolism and protein anabolism, if the protein catabolism is enhanced then the protein anabolism is decreased and vice versa. Thus, myostatin inhibits the Akt/mTORC1/S6K and the p38/MAPK pathways, whereas it enhances the FoXO protein activity and the IκBα/NF-κB pathway.213,231 Inhibition of myostatin signaling allows the rescue of muscle loss. FST is a secreted glycoprotein that antagonizes members of the TGF-β family like myostatin232 and rescues impaired myoblast differentiation by myostatin.233 The transcriptional coactivator PGC-1α is induced in muscle by exercise and favors mitochondrial genesis, resistance to muscle atrophy, and endurance.226 A spliced variant, PGC-1α4, also induced by exercise represses myostatin expression and favors hypertrophy by inducing IGF-I.234 It has also been shown that a combination of micro-distrophin gene replacement and FST restored muscle function in dystrophic mice.235 Indeed, myostatin upregulation has been observed in many diseases involving muscle loss, such as muscular dystrophy, sarcopenia, and cancer cachexia. Therefore, targeting myostatin with myostatin inhibitors for pharmacological applications is interesting.236,237
Electric and magnetic induction of myoblast differentiation
Mechanical and electrical stimulation are linked to muscle tissue formation. In the laboratory, an electrical stimulator can be used to easily mimic neuronal muscle stimulation with controlled parameters.238 In one example, Flaibani et al. applied 3-ms pulses with an amplitude of 70 mV/cm for 30 s to muscle precursor cells cultured on a micropatterned poly(L-lactic acid) membrane.239 They observed an increase in myotube density with a 30% increase in the release of nitrite (NO2−), which is a signaling molecule that is involved in myoblast fusion and myotube growth.157 Kawahara et al. applied 2-ms pulses of 50 V for 5 min/day to L6 rat myoblast cultures and observed accelerated myotube differentiation with the formation of thick myotubes followed by contracting striated muscle cells.240 The electrical stimulation was achieved by plunging electrodes into the culture medium. The drawback of this method is that most of the current lines cross under the culture medium rather than under the cells. In addition, electrolysis and the release of toxic products in the medium may result from this type of setup and should be avoided. Thus, to focus the current toward the myotubes, Kaji et al. cultured C2C12 cells on a conductive porous membrane and adopted a vertical setup for the electrodes.241 To protect the cells, Nagamine et al. transferred cultured myotubes to a fibrin gel, which they placed on microelectrode arrays for electrical stimulation (amplitude 2 V, duration 3 ms, frequency 10 Hz, train 1 s, and interval 10 s). The fibrin gel had been previously coated with a conductive polymer to improve interfacial electrical capacity.89 Other groups have also opted to use electrically conductive polymers to provide a matrix environment with safer electrical stimulation for the cells. In such cases, the cells can be encapsulated in the conductive polymer, seeded on the conductive polymer, or encapsulated in a polymer placed on electrodes. Thus, Sirivisoot and Harisson studied the formation of myotubes on electrically inductive composite scaffolds generated from electrospun polyurethane (PU) and carbon nanotubes.242 They showed that increased myotube formation was correlated with increased electrical conductivity because of the presence of carbon nanotubes. Also, Sekine et al. printed poly(3,4-ethylenedioxythiophene) (PEDOT) electrodes on an agarose hydrogel and electrically stimulated contractile myotubes embedded in a fibrin gel deposited on the PEDOT/agarose sheet,243 whereas Ido et al. embedded PEDOT electrodes in a hydrogel.244 In another strategy, Mawad et al. combined the advantages of hydrogels (e.g., mechanical properties, hydrated environment, and biocompatibility) and conducting polymers to build a conducting polymer hydrogel on which C2C12 cells proliferated.245 Ku et al. electrospun a blend of polycaprolactone with polyaniline to combine the topographical constraint of aligned fibers with electrical conductivity and observed a synergistic effect on myotube formation.246
Electrical stimulation not only increases the myotube density by increasing the speed of formation but also changes the nature of the muscle fibers and acts at the molecular level of muscle fiber formation. For example, after 8 days of conventional differentiation, C2C12 myotubes lacked spontaneous contractibility because of negligible sarcomere architecture. Fujita et al. used electrical pulses to study the effects of Ca2+ oscillation on the assembly of functionally active sarcomeres.247 They observed an increase in striated myotubes that peaked at 2 h and then decreased during stimulation with a 24-ms electrical pulse of 40 V/mm at 1 Hz. When they applied a lower-frequency signal (0.1 Hz), the striation was delayed and peaked at 12 h of stimulation. When they applied a high-frequency signal (10 Hz) for 2 h, they did not induce sarcomere assembly and did not observe contractile activity. This electrically induced contractile activity appeared to be mediated through the protease activity of calpain and also involved ECM-integrin engagement. Another study248 (Fig. 12) by the same group showed that electrical stimulation induced a switch from fast MyHC chain (type II) to slow MyHC chain (type I); consequently, the muscle fiber phenotype changed under stimulation.249
FIG. 12.
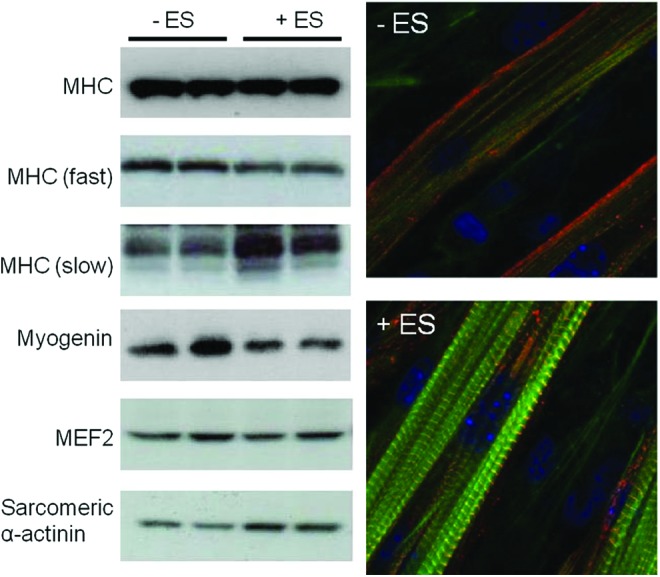
Effects of electrical stimulation on muscular protein and sarcomere development. C2C12 myotubes were stimulated 24 h with electrical pulses (40 V, 1 Hz, 2 ms duration). The cell lysates were analyzed by western blot (left) and cells were fixed (right) and stained with DAPI for nucleus (blue), anti-sarcomeric α-actinin antibody for sarcomeric α-actinin (red), and phalloidin for MHC (green). Reprinted with permission from Nedachi et al.248 Copyright © 2008, The American Physiological Society. Color images available online at www.liebertpub.com/teb
Some studies have also focused on the effects of combinations of stimulation types applied to cells. For example, Liao et al. combined mechanical and electrical stimulation by culturing C2C12 cells on PU mats with different diameters and fiber orientations under stretching and 10-ms electrical pulses of 20 V.250 They showed a net improvement of myotube striation and contractile protein (myosin, MyHC, and α-actinin) secretion under electromechanical stimulation but did not observe a significant benefit of bistimulation over monostimulation (mechanical or electrical only). Magnetic induction has also been used to induce myoblast differentiation. For example, Yuge and Kataoka introduced magnetic microparticles (0.05–0.1 μm Ø) into the cytoplasm of rat myoblast L6 cells by electroporation and then cultured the cells in magnetic fields of 0.01, 0.03, or 0.05 T.251 They observed that cells aligned and elongated along the N-S direction of the magnetic lines and that the formation of myotubes accelerated with the intensity of the magnetic field. Complete differentiation was obtained with striated myotube formation, and the myotube size also increased. Another study showed that a magnetic field of 80 mT orthogonal to the cell plane promoted myogenic differentiation and myotube hypertrophy without requiring other treatments, such as the introduction of magnetic particles into cells.110 Interestingly, when cells were treated with 5 μg/mL of TNFα, which is an inhibitor of myoblast differentiation,252 the exposure of cells to the magnetic field restored the myogenic differentiation. Clearly, electrical stimulation with or without other types of stimulation has important effects on myoblast differentiation and allows faster myotube formation, higher myotube density, increased myotube size, and a higher degree of myotube maturation.
Mechanical induction of myoblast differentiation
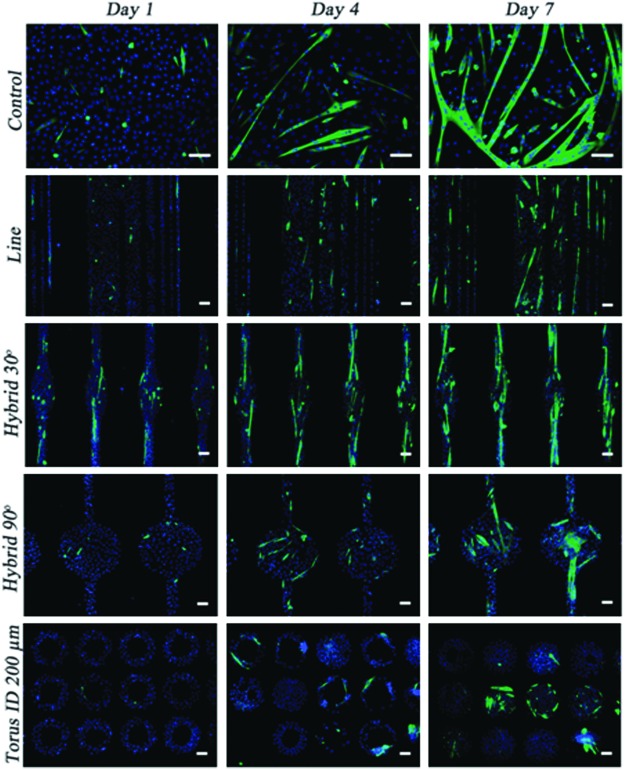
It is known that in body-building, muscular work through resistance training results in muscles with increased diameter. Therefore, it is interesting to analyze the effects of mechanical stimulation on myogenesis. Cells can be mechanically stimulated by topography, the stiffness of the material, and stretching. We previously described (“Cell alignment by topography” section) the degree to which topography can influence cellular alignment. Because myoblasts fuse mainly in an end-to-end configuration, cellular alignment seems to be required to obtain myotubes. Curiously, when Charest et al. analyzed the differentiation of C2C12 cells cultured on topographic patterns consisting of embossed ridges and grooves or arrays of holes with sizes ranging from 5 to 75 μm on polycarbonate coated with SAM and fibronectin, they concluded that the topography strongly influenced myoblast alignment but had no effect on the differentiation of the myoblasts.253 Their analysis, however, was mainly based on the measurement of sarcomeric myosin expression, and no images of myotubes were presented. In contrast, Bajaj et al. showed that the geometrical cues of a substrate significantly affect myoblast differentiation.84 They cultured C2C12 cells on dishes with different geometrical fibronectin coatings for 1 week and observed that myotubes with a hybrid line-torus shape had a two- and threefold increase in their fusion index when compared with myotubes on line and torus patterns, respectively (Fig. 13).
FIG. 13.
Topographical effects on C2C12 differentiation and myotube formation at different days in differentiation culture medium. Cells were cultured on unpatterned (control) and patterned substrates with different shapes (scale bar=100 μm) and stained with DAPI for nucleus (blue) and anti-MHC (green). Reprinted with permission from Bajaj et al.84 Copyright © 2011, Royal Society of Chemistry. Color images available online at www.liebertpub.com/teb
Similarly, when Aviss et al. prepared a scaffold of aligned poly(lactic-co-glycolic) acid (PLGA) fibers by electrospinning and cultured C2C12 cells for 14 days, they initially observed cell alignment with the fiber axis after 30 min of culture, followed by cell differentiation into long multinucleated myotubes aligned along the fibers.29 Our group also showed that topographical features can favor myoblast differentiation into myotubes.254
The stiffness of the material is also an important parameter in biomaterial and tissue engineering. Indeed, many studies have shown that on a material with different degrees of stiffness, cells migrate toward locations with the preferred stiffness.255 Engler et al. studied the effect of material stiffness on myoblast differentiation by culturing C2C12 cells on collagen-micropatterned polyacrylamide (PA) gels with different degrees of stiffness for 2 and 4 weeks.256 Although all cultures formed myotubes, myotubes only fully differentiated and reached myosin striation on gels of intermediate stiffness (8–11 kPa) (Fig. 14). The plot of material stiffness versus myotube striation fitted a Gaussian curve with an optimal modulus of 12 kPa that maximized myosin striation, whereas myotubes on gels with low (<5 kPa) or high stiffness (>17 kPa) had only poor or no striation. This optimal modulus value closely matched the elasticity of C2C12 myotubes, which is 12–15 kPa,257 and the native skeletal muscle tissue stiffness value, which is 12±4 kPa,258 as measured by atomic force microscopy on extensor digitorum longus muscles harvested from C57 mice.
FIG. 14.
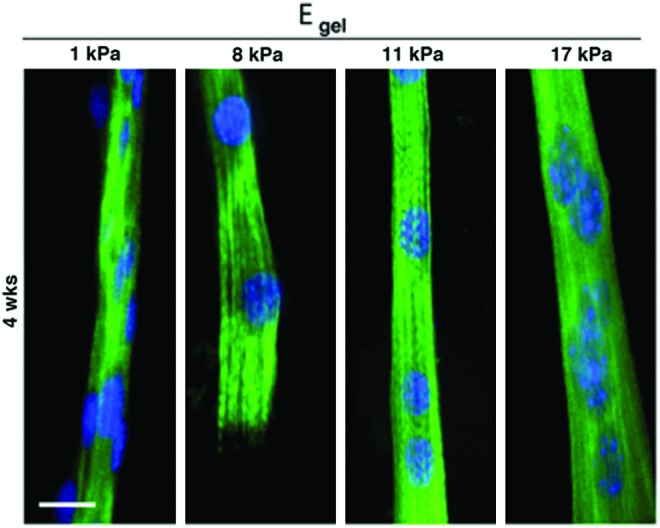
Stiffness effects on myotube striation at 4 weeks in differentiation culture medium. C2C12 were cultured on collagen-patterned substrates with different stiffness (scale bar=20 μm) and stained with DAPI for nucleus (blue) and anti-MHC (green). Myosin striation occurred only when cells were cultured on intermediated stiffness substrate. Reprinted with permission from Engler et al.256 Copyright © 2004, Rockefeller University Press. Color images available online at www.liebertpub.com/teb
Other studies also showed that the degree of myoblast differentiation depends on material stiffness. For example, Ren et al. generated biopolymeric films of poly(L-lysine)/hyaluronan with a controlled stiffness ranging from 3 kPa (native film) to 100 kPa (low-cross-linked films) and 400 kPa (high-cross-linked films).118 After culturing C2C12 cells on these films for 1 week in differentiation medium, they observed that myotubes were short and thick on soft films, whereas they were elongated and thin on stiff films and on a polystyrene dish, which was used as a control. Myotube striation increased with the stiffness of the film, from 14% (with a ∼350-kPa film) to 43% (with a ∼400-kPa film) and 69% (with the polystyrene plastic dish; 1 MPa). By using surfaces with different concentrations of silk and tropoelastin, Hu et al. studied the impact of surface roughness and stiffness on the differentiation of myogenic and osteogenic lineages.259 They showed that C2C12 cells preferred low surface roughness with high stiffness. Regarding myoblast differentiation under stretching, the recent literature is controversial. Globally, the consensus is that stretching stimulation increases myoblast proliferation but decreases myoblast differentiation.260–264 In SCs, it also seems that stretching induces NO production and hepatocyte growth factor secretion, both of which are involved in SC activation.265–267 However, the literature diverges concerning MRF expression by myoblasts under stretching. Kook et al. found that mechanical stretching stimulated C2C12 cell proliferation but inhibited differentiation into myotubes through the continuous phosphorylation of p38 MAP kinase, which decreases the level of MyoD expression.263 Akimoto et al. cultured C2C12 cells on a silicon membrane (BioFlex) that was stretched with a 20% elongation for 24 h at a frequency of 10 cycles/min (2-s on time, 4-s off time) and observed a decrease in the expression of MyoD and myocyte nuclear factor-α when compared with the nonstretched culture.268 Similarly, Kuang et al. observed a reduction of myogenin expression in C2C12 cells cultured under stretching stimulation.261 In contrast, another study by Abe et al. used a similar system (Flexercell) to culture C2C12 cells stretched to 15% elongation with cycles of 1-s on stretch and 1-s off stretch and observed an increase of MyoD and other myogenic factors after stretching for 12 and 24 h.269 Similarly, Gomes et al. reported increased MyoD expression 24 h after a passive stretching session on in vivo rat muscles.270 Another study using adipose-derived stem cells demonstrated an upregulation of MyoD when the cells were stimulated by stretching.271 Similarly, by using myoblasts loaded in an anchored mixture of collagen-Matrigel and submitted to frequent strain, Powell et al. demonstrated a 12% and 40% increase in myotube diameter and density, respectively.272 Although several studies have established the role of mechanotransduction in myogenesis activation, notably through the p38 MAPK pathway,273,274 the effects of stretching on myoblast differentiation are not yet well understood. A standardized stretching process may be beneficial to better compare results from different research groups.
Coculture with Skeletal Muscle Cells
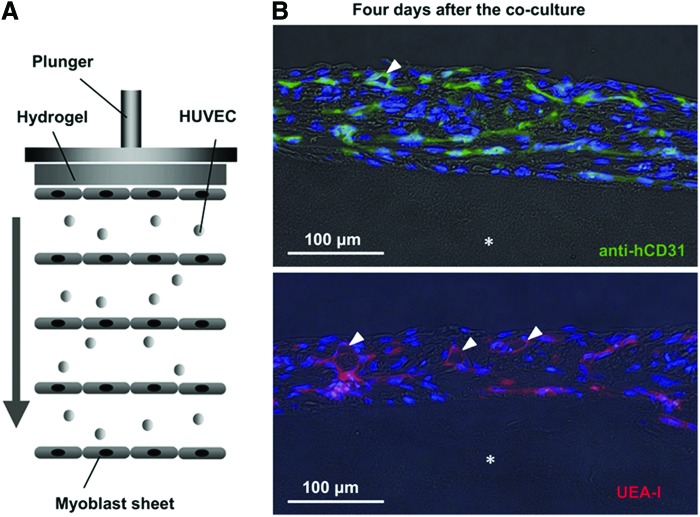
Skeletal muscle with in vivo functionality requires the cooperation of major types of tissues, including muscle tissue, nervous tissue, vascular tissue, and connective tissue. Thus, a skeletal muscle is infused by capillaries and connected to nerve branches, whereas several connective tissues cover the whole muscle (epimysium), the different muscle bundles (perimysium), and the muscle fibers (endomysium). The muscle is also linked to the bone by a tendon, which is a highly ordered connective tissue275,276 (see Fig. 2). These connective tissues are formed from collagen fibers with diameters of 600 to 1800 nm. They are connected to each other when interfacing and have several functions, including giving the muscle its final shape, resisting passive stretch, protecting different tissues from damage, distributing the force generated by muscle fibers, and serving as an ECM for muscle fibers. In skeletal muscle tissue, collagen and other ECM proteins are mainly secreted by interstitial fibroblasts.277,278 Therefore, interactions between muscle and fibroblasts are very important.279 It has been shown that C2C12 myoblasts cocultured on a fibroblast layer formed mature and highly contractile myotubes, with a higher differentiation rate than myoblasts cultured on a collagen-coated substrate.280 Mathew et al. also showed that fibroblastic connective tissues regulate the development and maturation of muscle fibers.281 Ricotti et al. observed higher myoblast differentiation when murine myoblasts cultured on dermal human fibroblasts seeded on micropatterned PA gel were stimulated through piezoelectrical effects by ultrasound, after cell internalization of boron nitride nanotubes supplemented in the culture medium.282 In another study, engineered vascularized muscle tissue with fibroblast participation was achieved in vitro on a highly porous biodegradable copolymer (PLGA-PLLA) sponge scaffold in a triple-culture condition, and it was shown that fibroblasts strongly promoted the formation and stabilization of endothelial vessels in the construct because of increased expression of vascular endothelial growth factor.283 When these muscle tissue constructs were implanted in mice, tissue prevascularization was shown to improve the vascularization, blood perfusion, and survival of the implanted muscle tissue. These results are encouraging because the construction of thick tissues has been limited by the lack of vascularization. Other studies have also attempted to build vascularized muscle tissue. For example, Sasagawa et al. described a method based on cell sheet tissue engineering to fabricate vascularized tissue.92 By using thermoresponsive polymer-coated dishes, they generated several layers of endothelial and muscle cells, which they harvested and then stacked together to form a sandwich-like construct of alternated HUVECs and myoblast sheets (Fig. 15). When the constructs were cultured in vitro, capillary-like structures formed in the five layers of the construct. When the constructs were implanted subcutaneously in nude mice, anastomosis with the host vascularization system and survival of the construct were observed.
FIG. 15.
Prevascularization of five-layer myoblast sheet constructs in vitro. (A) Schematic showing the construct made of human umbilical vein endothelial cells (HUVECs) sandwiched between myoblast cell layers. (B) HUVECs in the construct were stained with anti-human CD31 antibody (green, upper photo) or UEA-I (red, lower photo) whereas nuclei were stained with Hoechst 33342 (blue). HUVECs networked through the cell layers and formed capillary-like structures (white arrowheads) at day 4 of coculture. Asterisk shows the position of the fibrin gel used as substrate. Reprinted with permission from Sasagawa et al.92 Copyright © 2010, Elsevier. Color images available online at www.liebertpub.com/teb
Another study by Koffler et al. also used a triple-culture system of myoblasts, endothelial cells, and fibroblasts on a cellular bioscaffold composed of ECM proteins derived from pigs.284 These cell-loaded scaffolds were cultured in vitro for different durations (i.e., 1 day, 1, 2, and 3 weeks) before being transplanted into the abdominal wall of nude mice and were retrieved and analyzed 2 weeks after transplantation. One day of in vitro culture was sufficient to allow transplantation. Tissue (myofibers and vascular network) formation, organization, and integration with the host were poor at 2 weeks post-transplantation, whereas scaffolds cultured in vitro with preorganized tissues at 3 weeks demonstrated high tissue organization with dense-aligned muscle fibers and blood vessels, as well as anastomosis and full integration with the host environment. However, the construction of thick and highly vascularized tissues is still a challenge. It has been shown that muscle cells and endothelial cells affect each other through angiotensin II (ANG II). Indeed, in in vitro angiogenesis assays with HUVECs treated by ANG II, increases of 71% and 124% in tube length and branch point number were observed, respectively. Moreover, when HUVECs were cultured in conditioned media from differentiated muscle cells, the tube length and branch point number increased by 84% and 203%, respectively, when compared with controls.285 Other experiments showed that in growth medium, SCs and C2C12 cells expressed vein endothelial growth factor and its receptors to promote angiogenesis.286 Therefore, it can be concluded from these experiments that bi- and triculture systems are essential for future studies in this field.
Muscle tissue functionality is defined by muscle contractility, which is induced by neurons. Denervated muscles have shown a rapid loss of mass and contractility. Although it is possible to culture myoblasts and differentiate them to myotubes in the absence of innervation, it is not clear whether it is possible to develop muscle tissue in an entirely aneural culture environment. Because electrical stimulation is easy to set up in a laboratory and allows easy control of the stimulation parameters, many studies have used electrical stimulation of muscle rather than neural stimulation to induce muscle contraction. However, the hazardous side effects observed with electrical stimulation suggest that this method of inducing muscle contractility is not applicable for long-term tissue culture. Therefore, neural stimulation of muscle tissue may be more appropriate. Thus, coculture of nerve and muscle cells in vitro is widely used to study neuromuscular junction (NMJ) formation, function, and maintenance in nerve-muscle disorders. Modeling of signals exchanged at the NMJ may be clinically appropriate for spinal cord injury as well as muscle- and motoneuron-related diseases, such as amyotrophic lateral sclerosis,287 spinal muscular atrophy,288 and muscular dystrophy.289 For these reasons, nerve-muscle synaptogenesis is an active research area in tissue engineering and needs to be understood well because neurons transfer the action potential to muscle cells via the NMJ.
The NMJ is a synaptic structure between an axon terminal of a motor neuron and the motor endplate on a muscle fiber (Fig. 16).290 In vertebrates, it ensures the fast transmission of an action potential from the neuron to the muscle fiber through the release of the neurotransmitter acetylcholine (ACh) into the synaptic cleft. The binding of ACh to its receptor AChR on the muscle fiber ultimately cause muscle contraction. AChRs are evenly distributed along muscle fibers at a density of ∼1000/μm2. When motor neurons are added to muscle cultures, their axon terminals randomly contact myotubes, which induces the aggregation of AChRs291 at a density of >10,000/μm2 at the NMJ292 and the formation of shallow beds on the muscle fibers.293 The synaptic cleft is a space of ∼50 nm that separates the nerve from the muscle fiber sarcolemma. Basal lamina materials, including collagen IV, fibronectin, laminin, entactin, perlecan,294,295 and proteins, such as agrin, acetylcholinesterase, and neuregulin, invade the synaptic cleft. Then, postsynaptic invaginations called secondary synaptic folds, at the crests of which are localized AChRs,296 form on the muscle fibers in front of the axon terminal.297 The molecular mechanism underlying this process is governed by agrin, which is secreted by the nerve and induces AChR aggregation on the muscle fibers.298 Agrin clusters AChRs via the activation of the transmembrane muscle specific kinase (MuSK), which is selectively expressed in skeletal muscles, by binding its coreceptor, the transmembrane low-density lipoprotein receptor-related protein 4 (Lrp4).299,300 Another effector for AChR clustering is rapsyn (for receptor-associated protein at synapse), which anchors AChR at the synapse.301 Downstream, the signal transduction pathway that links MuSK activation to AChR aggregation is complex, and several pathways have been identified.290
FIG. 16.
SEM image of a rodent neuromuscular junction. Components are as follows: nerve terminal (N), muscle fiber (M), Schawnn cell (SC#), round synaptic vesicles containing ACh docked in the active zone of the nerve terminal (*), and synaptic cleft with basal lamina (SBL). Reprinted with permission from Wu et al.290 Copyright © 2010, The Company of Biologists Ltd.
To date, neuron-muscle cocultures have been studied using mouse, rat, chick, and human embryonic stem cell–derived C2C12 myotubes.302–305 It has been shown that the functional maturation of AChRs in the NMJ and differentiation of muscle cells is improved in nerve-muscle coculture systems.306 Muscle fiber maturation was also observed in vivo by Dhawan et al., who used implanted constructs of rat skeletal myoblasts in fibrin gels to investigate the effect of the host neural network on the engineered muscle. Their results indicate that neurotization of engineered skeletal muscle significantly increases force generation and NMJ development.307 In a recent study, Guo et al. established NMJ formation between human motoneurons and rat skeletal muscle in a serum-free culture system. Such studies bridge the findings from animal studies and applications in humans, and this human-cell-based tissue may be useful for drug screening and preclinical studies.308
The different coculture studies presented here with myoblasts, endothelial cells, fibroblasts, and neuronal cells demonstrate the importance of cell–cell interaction and communication. Such cell–cell contacts play a key role in cell maturation and differentiation, which will result in a higher-quality engineered organ through tissue engineering. Therefore, coculture is essential to engineer mature and functional muscle tissues.
Applications of Engineered Muscle Tissues
Regenerative medicine and stem cells
One of the main goals of muscle engineering is to develop muscle tissue for medical applications. Thus, the replacement of damaged, wounded, or nonfunctional muscle tissue is an important goal of most research in this field and has been extensively reviewed.1,309 Traditionally, regenerative strategies are based on ex-vivo-engineered constructs with autologous cells that can be reimplanted into the patient.310 SCs are stem cells that directly involve in muscle regeneration.311 They are located between the sarcolema of the muscle fibers and the basal lamina under a quiescent status (G0 phase) and are characterized by the expression of the transcription factors Pax7 and Myf5 but not MyoD or Myogenin.312 When the muscle fibers are damaged, SCs are activated and start to proliferate and to express MyoD. A symmetric/asymmetric mechanism of cell division induces the generation of SCs expressing Pax7+/Myf5− (basal cell in contact with the muscle fiber) that will contribute to the maintenance of the pool of SCs, and other SCs expressing Pax7+/Myf5+ (apical cell in contact with the other cell) that will differentiate and will fuse together into myotubes to regenerate the muscle fibers.313,314 Freshly harvested SCs have been used in injured or disease mice model that shows efficient myofiber regeneration.315 However, SCs cultured in vitro and then transplanted have shown decrease in cell proliferation and in their myogenic potential.316 In addition to their potential of generating different tissues, a large diversity and heterogeneity exist among stem cells; therefore, other cell types may also be used for muscle regeneration.317 Bone marrow stem cells, which contain hematopoietic stem cells and mesenchymal stem cells, have been shown to restore the SC pool and to generate myofibers in injured mice.318,319 However, bone marrow transplantation has not been reported efficient in therapy for muscle and further studies are required to define the subpopulation of bone marrow cells with myogenic potential in an animal model of muscle disease.312,320 Muscle side population cells (SPs) are localized in the interstitium between the muscle fibers, near the blood vessels, and are characterized by the expression of Sca-1+, ABCG2+, CD45−, CD43−, c-kit−, and Pax7−. SPs are able to differentiate into SCs and to form myotubes in monoculture or in coculture with myogenic cells.321–323 A subgroup of SPs characterized by Sca-1+, ABCG2+, CD45−, Pax7+, and Syndecan-4+ has been shown to regenerate skeletal muscle efficiently in an injured mouse model.324 Moreover, SPs injected intravenously were able to migrate toward the injured muscle for restoring it.325 Another group of cells from the interstitium are characterized by PW1+/Pax7− and are myogenic in vitro.326 When injected in vivo into injured muscle, these cells both proliferated to increase their own pool and differentiated into SCs (Pax7+).327 Muscle-derived stem cells (MDSCs) are characterized by Sca-1+, CD45−, CD34−, Flk1+, Desmin+, and M-cadherin− and are multipotent.328 Used in a model of dystrophic mice (mdx mice), MDSCs injected in the blood stream were able to migrate into the host muscle tissues and to regenerate myofibers and dystrophin expression.329 Platelet-rich plasma has been used in culture medium to improve in vitro the expansion of MDCs while maintaining their stemness.330 Mesoangioblast cells are characterized by CD34+, c-kit−, Flk1+, NKX2.5−, Myf5−, and Oct4− and are multipotent.312 They proliferate well in vitro maintaining their multipotency and have shown a particular efficiency in the treatment of different animal models of muscular dystrophy.331,332 Pericytes are derived from blood vessel and are characterized in human by CD45−, CD34−, CD56−, CD144−, CD146+, PDGFR-β1+, and NG2 proteoglycans+.333 Transplanted in dystrophic animal through blood stream, pericytes repopulated the SC niche and regenerated myofiber.333 AC133 cells or CD133+ cells are a subpopulation of hematopoietic cells with myogenic activity when cocultured with myogenic cells or with cells expressing Wnt.334 When injected into a muscle of dystrophic mice, cocultured AC133 cells replenished the SC niche and contributed in myofiber regeneration.335 An autologous transplantation of AC133 cells in a dystrophic boy demonstrated the safety of the therapeutic strategy used but did not offer substantial functional benefit.336 Embryonic stem cells (ESCs) are pluripotent and have great potential in therapy applications. However, immunogenic response, teratoma formation, and ethical concerns are the major hindrances of their use. The derivation of human ESCs into multipotent mesenchymal precursors followed by their differentiation into myoblasts allowed stable engraftment after transplantation into muscle-injured mice without teratoma formation.337 The use of ESC-derived embryoid bodies induced to myogenic differentiation has also been used and transplanted myoblasts in muscle-injured mice showed stable engraftment, myofiber regeneration, replenishment of the SC pool, and absence of teratoma.338 Induced pluripotent stem cells (iPS cells) are reprogrammed somatic cells into ESC-like state.339 The pluripotency is reprogrammed by nuclear transfer of four transcription factors (Oct3/4, Sox2, c-Myc, and Klf4).340 iPS cells can be generated from many cell types and different methods have been developed to avoid the use of viral vectors for the delivery of the four transcription factors.341,342 Moreover, iPS cells can be generated from genetic-disease tissues and can be used for specific disease study and drug screening.343 Myogenic progenitors can be derived from iPS cells and when transplanted in dystrophic mice have been shown to efficiently engraft, to replenish the SC pool, to regenerate dystrophin-positive myofibers, and to improve contractibility.344 Human iPS cells have also been generated from disease patient, genetically corrected, and transplanted in dystrophic mice showing specific functional tissue and gene restorations.345 In summary, stem cell therapy is a developing field and encouraging results have already been obtained in muscular regeneration. Some stem cells, such as mesangioblasts or AC133, are already in clinical-trial phase. Depending on the stem cell type, the main problems encountered are the difficulties of stem cell expansion in vitro and the loss of stemness, the difficulties of systemic delivery via blood stream due to the inability to cross the endothelial wall, the migration of the stem cells to the damaged muscle, and the survival of stem cells after injection. However, the techniques of cell derivation and of genetic modification open the field of stem cell therapy and make it full of potentialities.346 Recently, a new therapeutic strategy appeared with a recent shift toward an in-vivo-regenerative strategy, also called in situ tissue regeneration or endogenous regeneration, based on the recruitment and stimulation of progenitor cells in situ.347–350
Drug screening
Engineered skeletal muscle tissue is useful not only for reconstructive surgery but also for applications in drug screening. Indeed, engineered muscle tissue offers a physiological environment that should provide valuable information in multiple signaling pathways about drug efficiency, pharmacology, and toxicity.3 Such skeletal tissues used as a substrate can be either healthy or a model of human diseases and must be built in a reproducible way to allow standardization and comparison of results. As skeletal muscular tissues are notably involved in glucose homeostasis, any impairment of glucose signal transduction will decrease the glucose uptake by muscles under insulin stimulation and may contribute to type 2 diabetes development.351 Understanding this insulin activation pathway is therefore important. It has been shown that the glucose transporter GLUT4 is translocated from intracellular storage compartments to the plasma membrane under insulin stimulation.352,353 During this translocation, accelerated GLUT4-containing vesicle exocytosis was observed, as well as the involvement of the PI3K and Akt pathways.354,355 Thus, several investigators reported glucose uptake by differentiated C2C12 cells.356–358 However, to provide a model with higher insulin sensitivity, Hayata et al. established a new cell line named C2C12-IS and screened a chemical library in a high-throughput screening (HTS) study to search for compounds that promote glucose uptake.359 Although HTS is a powerful method that uses 2D-cell-based assays to screen a large amount of potential drugs, the use of cell cultures as a substrate does not necessarily match the in vivo complexity, which results in a great number of hits that fail in clinical trials.360,361 Indeed, many studies have now shown that cellular behavior in the 2D and 3D environment is different.67,362 In addition, the use of animal models for testing drug efficacy creates ethical problems, and the results derived from such models are difficult to translate to humans. Therefore, there is a great need for new substrates that mimic the complex biological architecture363 and support its physiological and metabolic function. To this end, some investigators have chosen to use simple organisms, such as zebrafish or nematode worms, for drug and genetic screening,364–366 whereas other groups have chosen to develop engineered muscle tissue3 (Fig. 17).
FIG. 17.
Engineering of miniature bioartificial muscles (mBAMs) on PDMS microposts in a 96-well plate for drug screening. PDMS microposts are 7-mm high, 7 mm Ø, and 4-mm apart. The mBAM shown in the well is 5 days postcasting whereas at day 7 the mBAM showed aligned myofibers after staining for sarcomeric tropomyosin. Reprinted with permission from Vandenburgh.3 Copyright © 2010, Mary Ann Liebert, Inc.
An important aspect of such muscular models that mimic the in vivo structure is to establish control of the contractibility of the engineered muscular tissue. This control can be achieved through neuronal or electrical stimulation. To this end, Nagamine et al. used microelectrode arrays to stimulate myotubes, and Kaji et al. showed that myotube contractibility was positively correlated with the glucose uptake.241,367 The current transition from 2D to 3D in vitro muscular models is also of great importance for drug testing.362 Finally, in the future, new directions for tissue drug screening, such as the individual screening of patient tissue through outpatient biopsy procedures, may also be possible.368,369 Moreover, iPS cells370 and ESCs371,372 represent potential sources of cells from which to engineer tissues for drug-screening applications.
Other applications
In addition to the aforementioned medical applications, engineered muscle tissue also has great potential in many other applications. Indeed, muscle is the best natural motility motor; therefore, an actuator made of living muscle would have several advantages regarding efficiency when compared with a synthetic actuator.373 Muscle tissue is also built by a hexagonal lattice of tightly packed filaments composed of actin and myosin chains, which has the best-known packing order and the lowest volume when compared with synthetic counterparts. These units of actin chains sliding on myosin chains are among the smallest, most well-organized motors and could be modified to generate miniaturized moving parts for micromechanical devices with several applications in biological microelectromechanical systems.374 The mechanical force developed during muscle contraction can also be converted into other forms of energy, such as electricity.375 Currently, researchers have attempted to harvest electricity from muscle contraction mostly for use as an energy source for implanted medical devices and other applications.376,377 Recently, Wang et al. were issued a patent in which the energy from the contractions of muscle cells cultured on an array of piezoelectric nanowires could be turned into electricity.378 Ishisaka et al. used pulsating heart cells as a micromechanical actuator to produce electricity by culturing heart muscle cells on a PDMS membrane, which transfers the vibration energy to piezoelectric fibers.379 These efforts show the use of muscles as electrical generators. In the context of the world energy crisis, there is a strong demand for new energy sources. Therefore, harvesting energy from muscle contractions may lead to important developments and applications in the future.
The food industry is another field in which engineered muscles could have applications. Indeed, meat is the most important source of protein in the human food cycle. Recent studies showed that engineered muscle tissues (cultured meat) as a new source of protein have several advantages over conventionally produced meat. For example, in comparison to conventionally produced meat, cultured meat reduces the use of energy by ∼7% to 45% (only poultry has a lower use of energy), greenhouse gas emissions by 78–96%, land use by 99%, and water use by 82–96% (Fig. 18). Finally, it was concluded that the overall impact on the environment from cultured meat production was substantially lower than those from conventionally produced meat.9 A tissue-engineered meat product and a method for producing such meat were also disclosed in a patent that gives a commercial prospective for such meat sources.380 Recently, Mark Post made the prototype burger of cultured meat from stem cells.381,382
FIG. 18.
Comparison of energy, greenhouse gas (GHG) emissions, and land and water used between different sources of meat production. Reprinted with permission from Tuomisto and Teixeira de Mattos.9 Copyright © 2011, American Chemical Society. Color images available online at www.liebertpub.com/teb
In addition, a patent has also been issued on artificially produced 3D muscle tissue, which shows the value of engineered muscle tissue for applications that may interest investors.383 All these examples, and others, clearly show the progress in SMTE toward clinically feasible, functional, engineered muscles, which could improve the life of patients.
Concluding Remarks
This article reviewed different methods and techniques for engineering muscle tissues. Although we provided some basics on the structure and organization of muscle for a better understanding of muscle function, we notably focused on cell alignment and cell differentiation through topographical constraints, mechanical stimuli, or electrical and magnetic fields. We also covered the effects of coculture systems for improving muscle tissue quality and highlighted some major applications of these engineered muscle tissues, such as drug screening or regenerative medicine. The research and development of engineered skeletal muscle tissues is in the beginning stages. Although numerous goals and applications have been defined, similar to the development of other tissue types, muscle tissue development is still limited by the lack of a vascularized network that is prerequisite for a large-scale tissue fabrication. Therefore, there is a great demand to develop new methods and techniques for the creation of prevascularized tissues. The alignment and differentiation of myoblasts in serum-free conditions is another challenge that needs to be considered, especially in clinical studies. Moreover, although some engineered skeletal muscles have applications in practical drug screening and miniaturized bioactuators, more research is necessary to introduce a practical muscle tissue for regenerative medicine. Until now, the properties of engineered muscles are far from those of native muscle tissue; notably, the thickness and strength of engineered tissues must be improved to achieve natural volumetric efficiency. This improvement in tissue efficiency will necessarily match with other possible developments in food resource and energy-harvesting systems.
Acknowledgment
This work was supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Disclosure Statement
No competing financial interests exist.
References
- 1.Klumpp D., Horch R.E., Kneser U., and Beier J.P.Engineering skeletal muscle tissue–new perspectives in vitro and in vivo. J Cell Mol Med 14,2622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandenburgh H., Shansky J., Benesch-Lee F., Barbata V., Reid J., Thorrez L., Valentini R., and Crawford G.Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37,438, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Vandenburgh H.High-content drug screening with engineered musculoskeletal tissues. Tissue Eng Part B Rev 16,55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal D., and Asada H.Co-fabrication of live skeletal muscles as actuators in A millimeter scale mechanical system. Robotics and Automation (ICRA), 2011 IEEE International Conference on, 2011, pp. 3251–3256 [Google Scholar]
- 5.Akiyama Y., Terada R., Hashimoto M., Hoshino T., Furukawa Y., and Morishima K.Rod-shaped tissue engineered skeletal muscle with artificial anchors to utilize as a bio-actuator. J Biomech Sci Eng 5,236, 2010 [Google Scholar]
- 6.Ho D.Engineering intelligent materials for the interrogation of bio-robotic architectures and regulatory networks. IEEE/RSJ International Conference on Intelligent Robots and Systems, Beijing, China, 2006, pp. 1849–1854 [Google Scholar]
- 7.Xi J., Schmidt J.J., and Montemagno C.D.Self-assembled microdevices driven by muscle. Nat Mater 4,180, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Kim J., Park J., Yang S., Baek J., Kim B., Lee S.H., Yoon E.-S., Chun K., and Park S.Establishment of a fabrication method for a long-term actuated hybrid cell robot. Lab Chip 7,1504, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Tuomisto H.L., and Teixeira de Mattos J.M.Environmental impacts of cultured meat production. Environ Sci Technol 45,6117, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi Y., Shimizu T., Yamato M., and Okano T.Concise review: cell therapy and tissue engineering for cardiovascular disease. Stem Cells Trans Med 1,136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Li Q., Qin J., and Gu Y.Musculature tissue engineering to repair abdominal wall hernia. Artif Organs 36,348, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Brack A.S., and Rando T.A.Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell 10,504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seene T., Kaasik P., and Riso E.-M.Review on aging, unloading and reloading: changes in skeletal muscle quantity and quality. Arch Gerontol Geriatr 54,374, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Sosa H., Popp D., Ouyang G., and Huxley H.E.Ultrastructure of skeletal muscle fibers studied by a plunge quick freezing method: myofilament lengths. Biophys J 67,283, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bottinelli R., and Reggiani C.Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73,195, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Periasamy M., and Kalyanasundaram A.SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35,430, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Lewis M.R.Rhytmical contraction of the skeletal muscle tissue observed in tissue cultures. Am J Physiol 38,153, 1915 [Google Scholar]
- 18.Vandenburgh H., and Kaufman S.In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203,265, 1979 [DOI] [PubMed] [Google Scholar]
- 19.Strohman R., Bayne E., Spector D., Obinata T., Micou-Eastwood J., and Maniotis A.Myogenesis and histogenesis of skeletal muscle on flexible membranes in vitro. In Vitro Cell Dev Biol 26,201, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Dennis R., and Kosnik P.Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim 36,327, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lam M.T., Huang Y.-C., Birla R.K., and Takayama S.Microfeature guided skeletal muscle tissue engineering for highly organized 3-dimensional free-standing constructs. Biomaterials 30,1150, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Khademhosseini A., Langer R., Borenstein J., and Vacanti J.P.Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A 103,2480, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramalingam M., and Khademhosseini A.Micropatterned biomaterials for cell and tissue engineering. In: Fisher J., and Mikos A.G., eds. Biomedical Engineering Handbook, 4th Edition. Boca Raton, USA: CRC Press, 2012, pp. 1–17 [Google Scholar]
- 24.Khademhosseini A., and Peppas N.A.Micro- and nanoengineering of biomaterials for healthcare applications. Adv Healthc Mater 2,10, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Zorlutuna P., Annabi N., Camci-Unal G., Nikkhah M., Cha J.M., Nichol J.W., Manbachi A., Bae H., Chen S., and Khademhosseini A.Microfabricated biomaterials for engineering 3D tissues. Adv Mater 24,1782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrovidov S., Seidi A., Ahadian S., Ramalingam M., and Khademhosseini A.Micro- and nanoengineering approaches to developing gradient biomaterials suitable for interface tissue engineering. In: Murugan Ramalingam E.J., Ramakrishna S., Khademhosseini A., eds. Micro and Nanotechnologies in Engineering Stem Cells and Tissues. Hoboken, New Jersey: John Wiley & Sons, Inc., 2013, pp. 52–79 [Google Scholar]
- 27.Wang P.-Y., Yu H.-T., and Tsai W.-B.Modulation of alignment and differentiation of skeletal myoblasts by submicron ridges/grooves surface structure. Biotechnol Bioeng 106,285, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Shen J.-Y., Chan-Park M.B.-E., Feng Z.-Q., Chan V., and Feng Z.-W.UV-embossed microchannel in biocompatible polymeric film: application to control of cell shape and orientation of muscle cells. J Biomed Mater Res B 77B,423, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Aviss K.J., Gough J.E., and Downes S.Aligned electrospun polymer fibers for skeletal muscle regeneration. Eur Cells Mater 19,193, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Altomare L., Gadegaard N., Visai L., Tanzi M.C., and Fare S.Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater 6,1948, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Pennisi C.P., Olesen C.G., de Zee M., Rasmussen J., and Zachar V.Uniaxial cyclic strain drives assembly and differentiation of skeletal myocytes. Tissue Eng Part A 17,2543, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Ahadian S., Ramon-Azcon J., Ostrovidov S., Camci-Unal G., Hosseini V., Kaji H., Ino K., Shiku H., Khademhosseini A., and Matsue T.Interdigitated array of Pt electrodes for electrical stimulation and engineering of aligned muscle tissue. Lab Chip 12,3491, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Ramon-Azcon J., Ahadian S., Obregon R., Camci-Unal G., Ostrovidov S., Hosseini V., Kaji H., Ino K., Shiku H., Khademhosseini A., and Matsue T.Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip 12,2959, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Curtis A.S.G.The mechanism of cells adhesion to glass. J Cell Biol 20,199, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikkhah M., Edalat F., Manoucheri S., and Khademhosseini A.Engineering microscale topographies to control the cell-substrate interface. Biomaterials 33,5230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh K.Y., Khademhosseini A., Yang J.M., Eng G., and Langer R.Soft lithographic patterning of hyaluronic acid on hydrophilic substrates using molding and printing. Adv Mater 16,584, 2004 [Google Scholar]
- 37.Curtis A., and Wilkinson C.Topographical control of cells. Biomaterials 18,1573, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y., Zeng H., Nam J., and Agarwal S.Fabrication of skeletal muscle constructs by topographic activation of cell alignment. Biotechnol Bioeng 102,624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans D.J.R., Britland S., and Wigmore P.M.Differential response of fetal and neonatal myoblasts to topographical guidance cues in vitro. Dev Genes Evol 209,438, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Clark P., Coles D., and Peckham M.Preferential adhesion to and survival on patterned laminin organizes myogenesis in vitro. Exp Cell Res 230,275, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Clark P., Dunn G.A., Knibbs A., and Peckham M.Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int J Biochem Cell Biol 34,816, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Anene-Nzelu C.G., Choudhury D., Li H., Fraiszudeen A., Peh K.-Y., Toh Y.-C., Ng S.H., Leo H.L., and Yu H.Scalable cell alignment on optical media substrates. Biomaterials 34,5078, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Anene-Nzelu C.G., Peh K.Y., Fraizsudeen A., Kuan Y.H., Ng S.H.G., Toh Y.C., Leo H.L., and Yu H.Scalable alignment of three-dimensional cellular constructs in a microfluidic chip. Lab Chip 13,4124, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Shimizu K., Fujita H., and Nagamori E.Alignment of skeletal muscle myoblasts and myotubes using linear micropatterned surfaces ground with abrasives. Biotechnol Bioeng 103,631, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Jiang X., Takayama S., Qian X., Ostuni E., Wu H., Bowden N., LeDuc P., Ingber D.E., and Whitesides G.M.Controlling mammalian cell spreading and cytoskeletal arrangement with conveniently fabricated continuous wavy features on poly(dimethylsiloxane). Langmuir 18,3273, 2002 [Google Scholar]
- 46.Lam M.T., Sim S., Zhu X., and Takayama S.The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 27,4340, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Clark P., Connolly P., Curtis A.S., Dow J.A., and Wilkinson C.D.Topographical control of cell behaviour: II. Multiple grooved substrata. Development 108,635, 1990 [DOI] [PubMed] [Google Scholar]
- 48.Dalby M.J., Riehle M.O., Johnstone H., Affrossman S., and Curtis A.S.G.Investigating the limits of filopodial sensing: a brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol Int 28,229, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Neumann T., Hauschka S.D., and Sanders J.E.Tissue engineering of skeletal muscle using polymer fiber arrays. Tissue Eng 9,995, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Ker E.D.F., Nain A.S., Weiss L.E., Wang J., Suhan J., Amon C.H., and Campbell P.G.Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 32,8097, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Ostrovidov S., Seidi A., and Ramalingam M.Introduction to nanobioscience: a tissue engineering point of view. In: Encyclopedia of Live Support Systems (EOLSS). Paris, France: UNESCO, Ch. 6.152.34, 2012 [Google Scholar]
- 52.Murugan R., and Ramakrishna S.Development of nanocomposites for bone grafting. Compos Sci Technol 65,2385, 2005 [Google Scholar]
- 53.Bozec L., van der Heijden G., and Horton M.Collagen fibrils: nanoscale ropes. Biophys Journal 92,70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalby M.Cellular response to low adhesion nanotopographies. Int J Nanomed 2,373, 2007 [PMC free article] [PubMed] [Google Scholar]
- 55.Curtis A.S.G., Gadegaard N., Dalby M.J., Riehle M.O., Wilkinson C.D.W., and Aitchison G.Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans Nanobiosci 3,61, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Gadegaard N., Martines E., Riehle M.O., Seunarine K., and Wilkinson C.D.W.Applications of nano-patterning to tissue engineering. Microelectron Eng 83,1577, 2006 [Google Scholar]
- 57.Webster T.J., Ergun C., Doremus R.H., Siegel R.W., and Bizios R.Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 21,1803, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Webster T.J., Schadler L.S., Siegel R.W., and Bizios R.Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng 7,291, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Recknor J.B., Sakaguchi D.S., and Mallapragada S.K.Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials 27,4098, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Huber A., Pickett A., and Shakesheff K.Reconstruction of spatially oriented myotubes in vitro using electrospun, paralelle microfibre arrays. Eur Cells Mater 14,56, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Huang C., Niu H., Wu J., Ke Q., Mo X., and Lin T.Needleless electrospinning of polystyrene fibers with an oriented surface line texture. J Nanomater 2012,1, 2012 [Google Scholar]
- 62.Ku S.H., and Park C.B.Combined effect of mussel-inspired surface modification and topographical cues on the behavior of skeletal myoblasts. Adv Healthc Mater 2,1445, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Murugan R., and Ramakrishna S.Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng 13,1845, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Pham Q.P., Sharma U., and Mikos A.G.Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng 12,1197, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Jana S., and Zhang M.Fabrication of 3D aligned nanofibrous tubes by direct electrospinning. J Mater Chem B 1,2575, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Dugan J.M., Gough J.E., and Eichhorn S.J.Directing the morphology and differentiation of skeletal muscle cells using oriented cellulose nanowhiskers. Biomacromolecules 11,2498, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Hume S.L., Hoyt S.M., Walker J.S., Sridhar B.V., Ashley J.F., Bowman C.N., and Bryant S.J.Alignment of multi-layered muscle cells within three-dimensional hydrogel macrochannels. Acta Biomater 8,2193, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Khademhosseini A., and Langer R.Microengineered hydrogels for tissue engineering. Biomaterials 28,5087, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Arnesen S., Mosler S., Larsen N.B., Gadegaard N., Purslow P.P., and Lawson M.A.The effects of collagen type I topography on myoblasts in vitro. Connect Tissue Res 45,238, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Wolberg A.S.Thrombin generation and fibrin clot structure. Blood Rev 21,131, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Lanfer B., Seib F.P., Freudenberg U., Stamov D., Bley T., Bornhäuser M., and Werner C.The growth and differentiation of mesenchymal stem and progenitor cells cultured on aligned collagen matrices. Biomaterials 30,5950, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Bian W., Liau B., Badie N., and Bursac N.Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc 4,1522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brady M.A., Lewis M.P., and Mudera V.Synergy between myogenic and non-myogenic cells in a 3D tissue-engineered craniofacial skeletal muscle construct. J Tissue Eng Regen Med 2,408, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Shah R., Knowles J.C., Hunt N.P., and Lewis M.P.Development of a novel smart scaffold for human skeletal muscle regeneration. J Tissue Eng Regen Med 2013. [Epub ahead of print]; DOI: 10.1002/term.1780 [DOI] [PubMed] [Google Scholar]
- 75.Mudera V., Smith A.S.T., Brady M.A., and Lewis M.P.The effect of cell density on the maturation and contractile ability of muscle derived cells in a 3D tissue-engineered skeletal muscle model and determination of the cellular and mechanical stimuli required for the synthesis of a postural phenotype. J Cell Physiol 225,646, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., and Khademhosseini A.Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31,5536, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aubin H., Nichol J.W., Hutson C.B., Bae H., Sieminski A.L., Cropek D.M., Akhyari P., and Khademhosseini A.Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 31,6941, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDonald J.C., Duffy D.C., Anderson J.R., Chiu D.T., Wu H., Schueller O.J.A., and Whitesides G.M.Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21,27, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Ostrovidov S., Jiang J., Sakai Y., and Fujii T.Membrane-based PDMS microbioreactor for perfused 3D primary rat hepatocyte cultures. Biomed Microdevices 6,279, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Ostrovidov S., Sakai Y., and Fujii T.Integration of a pump and an electrical sensor into a membrane-based PDMS microbioreactor for cell culture and drug testing. Biomed Microdevices 13,847, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Ostrovidov S., Mizuno J., Nakamura H., Inui H., Sakai Y., and Fujii T.Culturing embryos on endometrium tissue preformed in a microfluidic device: a new tool for ART (Assisted Reproductive Technology). 9th International Conference on Miniaturized Chemical and Biochemical Analysis Systems (MicroTAS), Boston, 2005, pp. 361–363 [Google Scholar]
- 82.Kane R.S., Takayama S., Ostuni E., Ingber D.E., and Whitesides G.M.Patterning proteins and cells using soft lithography. Biomaterials 20,2363, 1999 [DOI] [PubMed] [Google Scholar]
- 83.Khademhosseini A., Suh K.Y., Jon S., Eng G., Yeh J., Chen G.-J., and Langer R.A soft lithographic approach to fabricate patterned microfluidic channels. Anal Chem 76,3675, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Bajaj P., Reddy B., Millet L., Wei C., Zorlutuna P., Bao G., and Bashir R.Patterning the differentiation of C2C12 skeletal myoblasts. Integr Biol 3,897, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Kaji H., Kawashima T., and Nishizawa M.Patterning cellular motility using an electrochemical technique and a geometrically confined environment. Langmuir 22,10784, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Ahmed W.W., Wolfram T., Goldyn A.M., Bruellhoff K., Rioja B.A., Möller M., Spatz J.P., Saif T.A., Groll J., and Kemkemer R.Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials 31,250, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Shimizu K., Fujita H., and Nagamori E.Micropatterning of single myotubes on a thermoresponsive culture surface using elastic stencil membranes for single-cell analysis. J Biosci Bioeng 109,174, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Cui X., Gao G., and Qiu Y.Accelerated myotube formation using bioprinting technology for biosensor applications. Biotechnol Lett 35,315, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Nagamine K., Kawashima T., Ishibashi T., Kaji H., Kanzaki M., and Nishizawa M.Micropatterning contractile C2C12 myotubes embedded in a fibrin gel. Biotechnol Bioeng 105,1161, 2010 [DOI] [PubMed] [Google Scholar]
- 90.Huang N.F., Lee R.J., and Li S.Engineering of aligned skeletal muscle by micropatterning. Am J Transl Res 2,43, 2010 [PMC free article] [PubMed] [Google Scholar]
- 91.Fujie T., Ahadian S., Liu H., Chang H., Ostrovidov S., Wu H., Bae H., Nakajima K., Kaji H., and Khademhosseini A.Engineered nanomembranes for directing cellular organization toward flexible biodevices. Nano Lett [Epub ahead of print]; DOI: 10.1021/nl401237s, 2013 [DOI] [PubMed]
- 92.Sasagawa T., Shimizu T., Sekiya S., Haraguchi Y., Yamato M., Sawa Y., and Okano T.Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 31,1646, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Takahashi H., Shimizu T., Nakayama M., Yamato M., and Okano T.The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials 34,7372, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Guillaume-Gentil O., Gabi M., Zenobi-Wong M., and Vörös J.Electrochemically switchable platform for the micro-patterning and release of heterotypic cell sheets. Biomed Microdevices 13,221, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Zanchi N., and Lancha A.Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol 102,253, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Goodman C.A., Mayhew D.L., and Hornberger T.A.Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal 23,1896, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vandenburgh H., and Karlisch P.Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator. In Vitro Cell Dev Biol 25,607, 1989 [DOI] [PubMed] [Google Scholar]
- 98.Segurola R., Sumpio B., and Mills I.Strain-induced dual alignment of L6 rat skeletal muscle cells. In Vitro Cell Dev Biol Anim 34,609, 1998 [DOI] [PubMed] [Google Scholar]
- 99.Rhim C., Lowell D.A., Reedy M.C., Slentz D.H., Zhang S.J., Kraus W.E., and Truskey G.A.Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve 36,71, 2007 [DOI] [PubMed] [Google Scholar]
- 100.van der Schaft D.W.J., van Spreeuwel A.C.C., van Assen H.C., and Baaijens F.P.T.Mechanoregulation of vascularization in aligned tissue-engineered muscle: a role for vascular endothelial growth factor. Tissue Eng Part A 17,2857, 2011 [DOI] [PubMed] [Google Scholar]
- 101.Lin P.Spontaneous formation of one-dimensional ripples in transit to highly ordered two-dimensional herringbone structures through sequential and unequal biaxial mechanical stretching. Appl Phys Lett 90,2419031, 2007 [Google Scholar]
- 102.Matsue T., Matsumoto N., and Uchida I.Rapid micropatterning of living cells by repulsive dielectrophoretic force. Electrochim Acta 42,3251, 1997 [Google Scholar]
- 103.Martinez-Duarte R.Microfabrication technologies in dielectrophoresis applications–a review. Electrophoresis 33,3110, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Pethig R., Menachery A., Pells S., and De Sousa P.Dielectrophoresis: a review of applications for stem cell research. J Biomed Biotechnol 2010,1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaji H., Camci-Unal G., Langer R., and Khademhosseini A.Engineering systems for the generation of patterned co-cultures for controlling cell–cell interactions. Biochim Biophys Acta 1810,239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vermesh U., Vermesh O., Wang J., Kwong G.A., Ma C., Hwang K., and Heath J.R.High-density, multiplexed patterning of cells at single-cell resolution for tissue engineering and other applications. Angew Chem Int Ed 123,7516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki M., Yasukawa T., Shiku H., and Matsue T.Negative dielectrophoretic patterning with different cell types. Biosens Bioelectron 24,1043, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Ramón-Azcón J., Ahadian S., Estili M., Liang X., Ostrovidov S., Kaji H., Shiku H., Ramalingam M., Nakajima K., Sakka Y., Khademhosseini A., and Matsue T.Dielectrophoretically aligned carbon nanotubes to control electrical and mechanical properties of hydrogels to fabricate contractile muscle myofibers. Adv Mater 25,4028, 2013 [DOI] [PubMed] [Google Scholar]
- 109.Lennon E., Ostrovidov S., Senez V., and Fujii T.Dielectrophoresis, cell culture, and electrical impedance spectroscopy applied to adherent cells in a single biochip. International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 2006, pp. 165–168 [Google Scholar]
- 110.Coletti D., Teodori L., Albertini M.C., Rocchi M., Pristerà A., Fini M., Molinaro M., and Adamo S.Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytometry A 71A,846, 2007 [DOI] [PubMed] [Google Scholar]
- 111.Yamamoto Y., Ito A., Fujita H., Nagamori E., Kawabe Y., and Kamihira M.Functional evaluation of artificial skeletal muscle tissue constructs fabricated by a magnetic force-based tissue engineering technique. Tissue Eng Part A 17,107, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Ito A., Ino K., Hayashida M., Kobayashi T., Matsunuma H., Kagami H., Ueda M., and Honda H.Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng 11,1553, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Akiyama H., Ito A., Kawabe Y., and Kamihira M.Fabrication of complex three-dimensional tissue architectures using a magnetic force-based cell patterning technique. Biomed Microdevices 11,713, 2009 [DOI] [PubMed] [Google Scholar]
- 114.Yamamoto Y., Ito A., Kato M., Kawabe Y., Shimizu K., Fujita H., Nagamori E., and Kamihira M.Preparation of artificial skeletal muscle tissues by a magnetic force-based tissue engineering technique. J Biosci Bioeng 108,538, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Yamamoto Y., Ito A., Jitsunobu H., Yamaguchi K., Kawabe Y., Mizumoto H., and Kamihira M.Hollow fiber bioreactor perfusion culture system for magnetic force-based skeletal muscle tissue engineering. J Chem Eng Jpn 45,348, 2012 [Google Scholar]
- 116.Wakelam M.J.The fusion of myoblasts. Biochem J 228,1, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knudsen K.A., and Horwitz A.F.Tandem events in myoblast fusion. Dev Biol 58,328, 1977 [DOI] [PubMed] [Google Scholar]
- 118.Ren K., Crouzier T., Roy C., and Picart C.Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cell differentiation. Adv Funct Mater 18,1378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jansen K.M., and Pavlath G.K.Mannose receptor regulates myoblast motility and muscle growth. J Cell Biol 174,403, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rao A., Luo C., and Hogan P.G.Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15,707, 1997 [DOI] [PubMed] [Google Scholar]
- 121.Abbott K.L., Friday B.B., Thaloor D., Murphy T.J., and Pavlath G.K.Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell 9,2905, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Horsley V., and Pavlath G.K.Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs 176,67, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Yoshida N., Yoshida S., Koishi K., Masuda K., and Nabeshima Y.Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells'. J Cell Sci 111,769, 1998 [DOI] [PubMed] [Google Scholar]
- 124.Weintraub H., Tapscott S.J., Davis R.L., Thayer M.J., Adam M.A., Lassar A.B., and Miller A.D.Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A 86,5434, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T.K., Turner D., Rupp R., Hollenberg S., et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251,761, 1991 [DOI] [PubMed] [Google Scholar]
- 126.Edmondson D.G., Cheng T.C., Cserjesi P., Chakraborty T., and Olson E.N.Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol 12,3665, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rudnicki M.A., Schnegelsberg P.N.J., Stead R.H., Braun T., Arnold H.-H., and Jaenisch R.MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75,1351, 1993 [DOI] [PubMed] [Google Scholar]
- 128.Schneider J.W., Gu W., Zhu L., Mahdavi V., and Nadal-Ginard B.Reversal of terminal differentiation mediated by p107 in Rb−/− muscle cells. Science 264,1467, 1994 [DOI] [PubMed] [Google Scholar]
- 129.Halevy O., Novitch B.G., Spicer D.B., Skapek S.X., Rhee J., Hannon G.J., Beach D., and Lassar A.B.Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267,1018, 1995 [DOI] [PubMed] [Google Scholar]
- 130.Wright W.E., Sassoon D.A., and Lin V.K.Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56,607, 1989 [DOI] [PubMed] [Google Scholar]
- 131.Andres V., and Walsh K.Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol 132,657, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang P., Wong C., Liu D., Finegold M., Harper J.W., and Elledge S.J.p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev 13,213, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mastroyiannopoulos N.P., Nicolaou P., Anayasa M., Uney J.B., and Phylactou L.A.Down-regulation of myogenin can reverse terminal muscle cell differentiation. PLoS One 7,e29896, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kassar-Duchossoy L., Gayraud-Morel B., Gomes D., Rocancourt D., Buckingham M., Shinin V., and Tajbakhsh S.Mrf4 determines skeletal muscle identity in Myf5: Myod double-mutant mice. Nature 431,466, 2004 [DOI] [PubMed] [Google Scholar]
- 135.Rhodes S.J., and Konieczny S.F.Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev 3,2050, 1989 [DOI] [PubMed] [Google Scholar]
- 136.Mege R., Marc, Goudou D., Giaume C., Nicolet M., and Rieger F.Is intercellular communication via gap junctions required for myoblast fusion? Cell Commun Adhes 2,329, 1994 [DOI] [PubMed] [Google Scholar]
- 137.Proulx A., Merrifield P.A., and Naus C.C.G.Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. Dev Genet 20,133, 1997 [DOI] [PubMed] [Google Scholar]
- 138.Black B.L., and Olson E.N.Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol 14,167, 1998 [DOI] [PubMed] [Google Scholar]
- 139.Molkentin J.D., Black B.L., Martin J.F., and Olson E.N.Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Mol Cell Biol 16,2627, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin J.F., Schwarz J.J., and Olson E.N.Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci U S A 90,5282, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dodou E., Xu S.-M., and Black B.L.mef2c is activated directly by myogenic basic helix-loop-helix proteins during skeletal muscle development in vivo. Mech Dev 120,1021, 2003 [DOI] [PubMed] [Google Scholar]
- 142.Black B.L., Molkentin J.D., and Olson E.N.Multiple roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with MEF2. Mol Cell Biol 18,69, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Molkentin J.D., and Olson E.N.Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc Natl Acad Sci U S A 93,9366, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Molkentin J.D., Black B.L., Martin J.F., and Olson E.N.Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83,1125, 1995 [DOI] [PubMed] [Google Scholar]
- 145.Rao S.S., and Kohtz D.S.Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor β. J Biol Chem 270,4093, 1995 [DOI] [PubMed] [Google Scholar]
- 146.Vaidya T.B., Rhodes S.J., Taparowsky E.J., and Konieczny S.F.Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol 9,3576, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li L., Chambard J.C., Karin M., and Olson E.N.Fos and Jun repress transcriptional activation by myogenin and MyoD: the amino terminus of Jun can mediate repression. Genes Dev 6,676, 1992 [DOI] [PubMed] [Google Scholar]
- 148.Brennan T.J., Edmondson D.G., Li L., and Olson E.N.Transforming growth factor beta represses the actions of myogenin through a mechanism independent of DNA binding. Proc Natl Acad Sci U S A 88,3822, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Angelis L., Borghi S., Melchionna R., Berghella L., Baccarani-Contri M., Parise F., Ferrari S., and Cossu G.Inhibition of myogenesis by transforming growth factor β is density-dependent and related to the translocation of transcription factor MEF2 to the cytoplasm. Proc Natl Acad Sci U S A 95,12358, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lluis F., Perdiguero E., Nebreda A.R., and Munoz-Canoves P.Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol 16,36, 2006 [DOI] [PubMed] [Google Scholar]
- 151.Pietrangelo T., Guarnieri S., Fulle S., Fanò G., and Mariggiò M.Signal transduction events induced by extracellular guanosine 5′triphosphate in excitable cells. Purinergic Signal 2,633, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gundersen K.Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev 86,564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Naya F.J., and Olson E.MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11,683, 1999 [DOI] [PubMed] [Google Scholar]
- 154.Benezra R., Davis R.L., Lockshon D., Turner D.L., and Weintraub H.The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61,49, 1990 [DOI] [PubMed] [Google Scholar]
- 155.Olson E.N.Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol 154,261, 1992 [DOI] [PubMed] [Google Scholar]
- 156.Kreider B.L., Benezra R., Rovera G., and Kadesch T.Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science 255,1700, 1992 [DOI] [PubMed] [Google Scholar]
- 157.Pisconti A., Brunelli S., Di Padova M., De Palma C., Deponti D., Baesso S., Sartorelli V., Cossu G., and Clementi E.Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol 172,233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Kang J.-S., Yi M.-J., Zhang W., Feinleib J.L., Cole F., and Krauss R.S.Netrins and neogenin promote myotube formation. J Cell Biol 167,493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Markworth J.F., and Cameron-Smith D.Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX-2-dependent pathway. Am J Physiol Cell Physiol 304,C56, 2013 [DOI] [PubMed] [Google Scholar]
- 160.Jang Y.-N., and Baik E.J.JAK-STAT pathway and myogenic differentiation. JAKSTAT 2,e23282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.DeQuach J.A., Mezzano V., Miglani A., Lange S., Keller G.M., Sheikh F., and Christman K.L.Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One 5,e13039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stern M.M., Myers R.L., Hammam N., Stern K.A., Eberli D., Kritchevsky S.B., Soker S., and Van Dyke M.The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials 30,2393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hinds S., Bian W., Dennis R.G., and Bursac N.The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32,3575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mrksich M., and Whitesides G.M.Patterning self-assembled monolayers using microcontact printing: a new technology for biosensors? Trends Biotechnol 13,228, 1995 [Google Scholar]
- 165.Lan M.A., Gersbach C.A., Michael K.E., Keselowsky B.G., and García A.J.Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries. Biomaterials 26,4523, 2005 [DOI] [PubMed] [Google Scholar]
- 166.Laplante M., and Sabatini D.M.mTOR signaling at a glance. J Cell Sci 122,3589, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang J., and Manning B.D.The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 412,179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Frost R.A., and Lang C.H.mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology 26,83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guertin D.A., and Sabatini D.M.Defining the role of mTOR in cancer. Cancer Cell 12,9, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Fingar D.C., Salama S., Tsou C., Harlow E., and Blenis J.Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16,1472, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gingras A.-C., Raught B., and Sonenberg N.Regulation of translation initiation by FRAP/mTOR. Genes Dev 15,807, 2001 [DOI] [PubMed] [Google Scholar]
- 172.Manning B.D., and Cantley L.C.AKT/PKB signaling: navigating downstream. Cell 129,1261, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Frost R.A., and Lang C.H.Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 103,378, 2007 [DOI] [PubMed] [Google Scholar]
- 174.Huang J., and Manning B.A complex interplay between Akt, TSC2, and the two mTOR complexes. Biochem Soc Trans 37,217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sandri M., Barberi L., Bijlsma A.Y., Blaauw B., Dyar K.A., Milan G., Mammucari C., Meskers C.G.M., Pallafacchina G., Paoli A., Pion D., Roceri M., Romanello V., Serrano A.L., Toniolo L., Larsson L., Maier A.B., Munoz-Canoves P., Musaro A., Pende M., Reggiani C., Rizzuto R., and Schiaffino S.Signalling pathways regulating muscle mass in ageing skeletal muscle. The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 14,303, 2013 [DOI] [PubMed] [Google Scholar]
- 176.Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P., Burden S.J., Di Lisi, R., Sandri C., Zhao J., Goldberg A.L., Schiaffino S., and Sandri M.FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6,458, 2007 [DOI] [PubMed] [Google Scholar]
- 177.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., and Goldberg A.L.Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117,399, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., and Yancopoulos G.D.Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3,1014, 2001 [DOI] [PubMed] [Google Scholar]
- 179.Harvey K.F., Mattila J., Sofer A., Bennett F.C., Ramsey M.R., Ellisen L.W., Puig O., and Hariharan I.K.FOXO-regulated transcription restricts overgrowth of Tsc mutant organs. J Cell Biol 180,691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vanhaesebroeck B., and Alessi D.R.The PI3K-PDK1 connection: more than just a road to PKB. Biochem J 346,561, 2000 [PMC free article] [PubMed] [Google Scholar]
- 181.Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., Wang C.-y., He X., MacDougald O.A., You M., Williams B.O., and Guan K.-L.TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126,955, 2006 [DOI] [PubMed] [Google Scholar]
- 182.Roux P.P., Ballif B.A., Anjum R., Gygi S.P., and Blenis J.Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A 101,13489, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang J.-Y., Zong C.S., Xia W., Yamaguchi H., Ding Q., Xie X., Lang J.-Y., Lai C.-C., Chang C.-J., Huang W.-C., Huang H., Kuo H.-P., Lee D.-F., Li L.-Y., Lien H.-C., Cheng X., Chang K.-J., Hsiao C.-D., Tsai F.-J., Tsai C.-H., Sahin A.A., Muller W.J., Mills G.B., Yu D., Hortobagyi G.N., and Hung M.-C.ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 10,138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Keren A., Tamir Y., and Bengal E.The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol 252,224, 2006 [DOI] [PubMed] [Google Scholar]
- 185.Katholnig K., Kaltenecker C.C., Hayakawa H., Rosner M., Lassnig C., Zlabinger G.J., Gaestel M., Muller M., Hengstschlager M., Horl W.H., Park J.M., Saemann M.D., and Weichhart T.p38 alpha senses environmental stress to control innate immune responses via mechanistic target of rapamycin. J Immunol 190,1519, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kahn B.B., Alquier T., Carling D., and Hardie D.G.AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1,15, 2005 [DOI] [PubMed] [Google Scholar]
- 187.Mihaylova M.M., and Shaw R.J.The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13,1016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shaw R.J.LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol 196,65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.DeYoung M.P., Horak P., Sofer A., Sgroi D., and Ellisen L.W.Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev 22,239, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee D.-F., Kuo H.-P., Chen C.-T., Hsu J.-M., Chou C.-K., Wei Y., Sun H.-L., Li L.-Y., Ping B., Huang W.-C., He X., Hung J.-Y., Lai C.-C., Ding Q., Su J.-L., Yang J.-Y., Sahin A.A., Hortobagyi G.N., Tsai F.-J., Tsai C.-H., and Hung M.-C.IKKβ suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130,440, 2007 [DOI] [PubMed] [Google Scholar]
- 191.Deane C.S., Hughes D.C., Sculthorpe N., Lewis M.P., Stewart C.E., and Sharples A.P.Impaired hypertrophy in myoblasts is improved with testosterone administration. J Steroid Biochem Mol Biol 138,152, 2013 [DOI] [PubMed] [Google Scholar]
- 192.Urban R.J.Growth hormone and testosterone: anabolic effects on muscle. Horm Res Paediatr 76,81, 2011 [DOI] [PubMed] [Google Scholar]
- 193.Sculthorpe N., Solomon A.M., Sinanan A., Bouloux P.-M., Grace F., and Lewis M.Androgens affect myogenesis in vitro and increase local IGF-1 expression. Med Sci Sports Exerc 44,610, 2012 [DOI] [PubMed] [Google Scholar]
- 194.Kanno Y., Ota R., Someya K., Kusakabe T., Kato K., and Inouye Y.Selective androgen receptor modulator, YK11, regulates myogenic differentiation of C2C12 myoblasts by follistatin expression. Biol Pharm Bull 36,1460, 2013 [DOI] [PubMed] [Google Scholar]
- 195.Heemers H.V., and Tindall D.J.Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28,778, 2007 [DOI] [PubMed] [Google Scholar]
- 196.Claessens F., Denayer S., Tilborgh N., Kerkhofs S., Helsen C., and Haelens A.Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. J Nucl Recept Signal 6,1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dubois V., Laurent M., Boonen S., Vanderschueren D., and Claessens F.Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci 69,1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rahman F., and Christian H.Non-classical actions of testosterone: an update. Trends Endocrinol Metab 18,371, 2007 [DOI] [PubMed] [Google Scholar]
- 199.Fu R., Liu J., Fan J., Li R., Li D., Yin J., and Cui S.Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J Cell Physiol 227,98, 2012 [DOI] [PubMed] [Google Scholar]
- 200.McHenry J., Carrier N., Hull E., and Kabbaj M.Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol 2013. [Epub ahead of print]; DOI: 10.1016/j.yfrne.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bennett N.C., Gardiner R.A., Hooper J.D., Johnson D.W., and Gobe G.C.Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol 42,813, 2010 [DOI] [PubMed] [Google Scholar]
- 202.Cheng J., Watkins S.C., and Walker W.H.Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in Sertoli cells. Endocrinology 148,2066, 2007 [DOI] [PubMed] [Google Scholar]
- 203.Baron S., Manin M., Beaudoin C., Leotoing L., Communal Y., Veyssiere G., and Morel L.Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem 279,14579, 2004 [DOI] [PubMed] [Google Scholar]
- 204.Basualto-Alarcon C., Jorquera G., Altamirano F., Jaimovich E., and Estrada M.Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc 45,1712, 2013 [DOI] [PubMed] [Google Scholar]
- 205.White J.P., Gao S., Puppa M.J., Sato S., Welle S.L., and Carson J.A.Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol 365,174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tollefsen S.E., Lajara R., McCusker R.H., Clemmons D.R., and Rotwein P.Insulin-like growth factors (IGF) in muscle development. Expression of IGF-I, the IGF-I receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem 264,13810, 1989 [PubMed] [Google Scholar]
- 207.Manning B.D.Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 167,399, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Harrington L.S., Findlay G.M., and Lamb R.F.Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci 30,35, 2005 [DOI] [PubMed] [Google Scholar]
- 209.Sancak Y., Thoreen C.C., Peterson T.R., Lindquist R.A., Kang S.A., Spooner E., Carr S.A., and Sabatini D.M.PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25,903, 2007 [DOI] [PubMed] [Google Scholar]
- 210.Matheny R.W., and Nindl B.C.Loss of IGF-IEa or IGF-IEb impairs myogenic differentiation. Endocrinology 152,1923, 2011 [DOI] [PubMed] [Google Scholar]
- 211.Hribal M.L., Nakae J., Kitamura T., Shutter J.R., and Accili D.Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J Cell Biol 162,535, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tureckova J., Wilson E.M., Cappalonga J.L., and Rotwein P.Insulin-like growth factor-mediated muscle differentiation. J Biol Chem 276,39264, 2001 [DOI] [PubMed] [Google Scholar]
- 213.Anne Ulrike T., Angelika M., Daisy R., Joseph B., Shinji H., and David J.G.Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296,C1258, 2009 [DOI] [PubMed] [Google Scholar]
- 214.Valdés J.A., Flores S., Fuentes E.N., Osorio-Fuentealba C., Jaimovich E., and Molina A.IGF-1 induces IP3-dependent calcium signal involved in the regulation of myostatin gene expression mediated by NFAT during myoblast differentiation. J Cell Physiol 228,1452, 2013 [DOI] [PubMed] [Google Scholar]
- 215.Jiao S., Ren H., Li Y., Zhou J., Duan C., and Lu L.Differential regulation of IGF-I and IGF-II gene expression in skeletal muscle cells. Mol Cell Biochem 373,107, 2013 [DOI] [PubMed] [Google Scholar]
- 216.Crossland H., Kazi A.A., Lang C.H., Timmons J.A., Pierre P., Wilkinson D.J., Smith K., Szewczyk N.J., and Atherton P.J.Focal adhesion kinase is required for IGF-1-mediated growth of skeletal muscle cells via a TSC2-mTOR-S6K1-associated pathway. Am J Physiol Endocrinol Metab 305,E183, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Klossner S., Durieux A.-C., Freyssenet D., and Flueck M.Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 106,389, 2009 [DOI] [PubMed] [Google Scholar]
- 218.Goldspink G.Impairment of IGF-I gene splicing and MGF expression associated with muscle wasting. Int J Biochem Cell Biol 38,481, 2006 [DOI] [PubMed] [Google Scholar]
- 219.Barton E.R., DeMeo J., and Lei H.The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol 108,1069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Barton E.R.The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Meab 31,791, 2006 [DOI] [PubMed] [Google Scholar]
- 221.Brisson B.K., and Barton E.R.New modulators for IGF-I activity within IGF-I processing products. Front Endocrinol 4,1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang S.Y., and Goldspink G.Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522,156, 2002 [DOI] [PubMed] [Google Scholar]
- 223.Ates K., Yang S.Y., Orrell R.W., Sinanan A.C.M., Simons P., Solomon A., Beech S., Goldspink G., and Lewis M.P.The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Lett 581,2727, 2007 [DOI] [PubMed] [Google Scholar]
- 224.Wu J., Wu K., Lin F., Luo Q., Yang L., Shi Y., Song G., and Sung K.-L.P.Mechano-growth factor induces migration of rat mesenchymal stem cells by altering its mechanical properties and activating ERK pathway. Biochem Biophys Res Commun 441,202, 2013 [DOI] [PubMed] [Google Scholar]
- 225.Yin H.-N., Chai J.-K., Yu Y.-M., Shen C.-A., Wu Y.-Q., Yao Y.-M., Liu H., Liang L.-M., Tompkins R.G., and Sheng Z.-Y.Regulation of signaling pathways downstream of IGF-I/insulin by androgen in skeletal muscle of glucocorticoid-treated rats. J Trauma 66,1083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Piccirillo R., Demontis F., Perrimon N., and Goldberg A.L.Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev Dyn 2013. [Epub ahead of print]; DOI: 10.1002/dvdy.24036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Han H.Q., Zhou X., Mitch W.E., and Goldberg A.L.Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol 45,2333, 2013 [DOI] [PubMed] [Google Scholar]
- 228.Lee S.-J.Quadrupling muscle mass in mice by targeting TGF-β signaling pathways. PLoS One 2,e789, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gao F., Kishida T., Ejima A., Gojo S., and Mazda O.Myostatin acts as an autocrine/paracrine negative regulator in myoblast differentiation from human induced pluripotent stem cells. Biochem Biophys Res Commun 431,309, 2013 [DOI] [PubMed] [Google Scholar]
- 230.Tsuchida K., Nakatani M., Uezumi A., Murakami T., and Cui X.Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J 55,11, 2008 [DOI] [PubMed] [Google Scholar]
- 231.Fuentes E.N., Ruiz P., Valdes J.A., and Molina A.Catabolic signaling pathways, atrogenes, and ubiquitinated proteins are regulated by the nutritional status in the muscle of the fine flounder. PLoS One 7,e44256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jeong J., Conboy M.J., and Conboy I.M.Pharmacological inhibition of myostatin/TGF-β receptor/pSmad3 signaling rescues muscle regenerative responses in mouse model of type 1 diabetes. Acta Pharmacol Sin 34,1052, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Amthor H., Nicholas G., McKinnell I., Kemp C.F., Sharma M., Kambadur R., and Patel K.Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol 270,19, 2004 [DOI] [PubMed] [Google Scholar]
- 234.Ruas J.L., White J.P., Rao R.R., Kleiner S., Brannan K.T., Harrison B.C., Greene N.P., Wu J., Estall J.L., Irving B.A., Lanza I.R., Rasbach K.A., Okutsu M., Nair K.S., Yan Z., Leinwand L.A., and Spiegelman B.M.A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151,1319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rodino-Klapac L.R., Janssen P.M.L., Shontz K.M., Canan B., Montgomery C.L., Griffin D., Heller K., Schmelzer L., Handy C., Clark K.R., Sahenk Z., Mendell J.R., and Kaspar B.K.Micro-dystrophin and follistatin co-delivery restores muscle function in aged DMD model. Hum Mol Genet 22,4929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang L., Rajan V., Lin E., Hu Z., Han H.Q., Zhou X., Song Y., Min H., Wang X., Du J., and Mitch W.E.Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 25,1653, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Datta-Mannan A., Yaden B., Krishnan V., Jones B.E., and Croy J.E.An engineered human follistatin variant: insights into the pharmacokinetic and pharmocodynamic relationships of a novel molecule with broad therapeutic potential. J Pharmacol Exp Ther 344,616, 2012 [DOI] [PubMed] [Google Scholar]
- 238.Nikolic N., Skaret Bakke S., Tranheim Kase E., Rudberg I., Flo Halle I., Rustan A.C., Thoresen G.H., and Aas V.Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One 7,e33203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Flaibani M., Boldrin L., Cimetta E., Piccoli M., Coppi P.D., and Elvassore N.Muscle differentiation and myotubes alignment is influenced by micropatterned surfaces and exogenous electrical stimulation. Tissue Eng Part A 15,2447, 2009 [DOI] [PubMed] [Google Scholar]
- 240.Kawahara Y., Yamaoka K., Iwata M., Fujimura M., Kajiume T., Magaki T., Takeda M., Ide T., Kataoka K., Asashima M., and Yuge L.Novel electrical stimulation sets the cultured myoblast contractile function to ‘on’. Pathobiology 73,288, 2006 [DOI] [PubMed] [Google Scholar]
- 241.Kaji H., Ishibashi T., Nagamine K., Kanzaki M., and Nishizawa M.Electrically induced contraction of C2C12 myotubes cultured on a porous membrane-based substrate with muscle tissue-like stiffness. Biomaterials 31,6981, 2010 [DOI] [PubMed] [Google Scholar]
- 242.Sirivisoot S., and Harrison B.Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int J Nanomed 6,2483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sekine S., Ido Y., Miyake T., Nagamine K., and Nishizawa M.Conducting polymer electrodes printed on hydrogel. J Am Chem Soc 132,13174, 2010 [DOI] [PubMed] [Google Scholar]
- 244.Ido Y., Takahashi D., Sasaki M., Nagamine K., Miyake T., Jasinski P., and Nishizawa M.Conducting polymer microelectrodes anchored to hydrogel films. ACS Macro Lett 1,400, 2012 [DOI] [PubMed] [Google Scholar]
- 245.Mawad D., Stewart E., Officer D.L., Romeo T., Wagner P., Wagner K., and Wallace G.G.A single component conducting polymer hydrogel as a scaffold for tissue engineering. Adv Funct Mater 22,2692, 2012 [Google Scholar]
- 246.Ku S.H., Lee S.H., and Park C.B.Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials 33,6098, 2012 [DOI] [PubMed] [Google Scholar]
- 247.Fujita H., Nedachi T., and Kanzaki M.Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res 313,1853, 2007 [DOI] [PubMed] [Google Scholar]
- 248.Nedachi T., Fujita H., and Kanzaki M.Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab 295,E1191, 2008 [DOI] [PubMed] [Google Scholar]
- 249.Gregory P., Low R.B., and Stirewalt W.S.Changes in skeletal-muscle myosin isoenzymes with hypertrophy and exercise. Biochem J 238,55, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liao I.C., Liu J., Bursac N., and Leong K.Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell Mol Bioeng 1,133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yuge L., and Kataoka K.Differentiation of myoblasts is accelerated in culture in a magnetic field. In Vitro Cell Dev Biol Anim 36,383, 2000 [DOI] [PubMed] [Google Scholar]
- 252.Coletti D., Yang E., Marazzi G., and Sassoon D.TNFalpha inhibits skeletal myogenesis through a PW1-dependent pathway by recruitment of caspase pathways. EMBO J 21,631, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Charest J.L., Garcia A.J., and King W.P.Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials 28,2202, 2007 [DOI] [PubMed] [Google Scholar]
- 254.Hosseini V., Ahadian S., Ostrovidov S., Camci-Unal G., Chen S., Kaji H., Khademhosseini A.Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng Part A 18,2453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Discher D.E., Janmey P., andWang Y.-l., Tissue cells feel and respond to the stiffness of their substrate. Science 310,1139, 2005 [DOI] [PubMed] [Google Scholar]
- 256.Engler A.J., Griffin M.A., Sen S., Bonnemann C.G., Sweeney H.L., and Discher D.E.Myotubes differentiate optimally on substrates with tissue-like stiffness. J Cell Biol 166,877, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Collinsworth A.M., Zhang S., Kraus W.E., and Truskey G.A.Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol 283,C1219, 2002 [DOI] [PubMed] [Google Scholar]
- 258.Engler A.J., Rehfeldt F., Sen S., and Discher D.E.Microtissue elasticity: measurements by atomic force microscopy and its influence on cell differentiation. Methods Cell Biol 83,521, 2007 [DOI] [PubMed] [Google Scholar]
- 259.Hu X., Park S.-H., Gil E.S., Xia X.-X., Weiss A.S., and Kaplan D.L.The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials 32,8979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Brosig M., Ferralli J., Gelman L., Chiquet M., and Chiquet-Ehrismann R.Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int J Biochem Cell Biol 42,1717, 2010 [DOI] [PubMed] [Google Scholar]
- 261.Kuang W., Tan J., Duan Y., Duan J., Wang W., Jin F., Jin Z., Yuan X., and Liu Y.Cyclic stretch induced miR-146a upregulation delays C2C12 myogenic differentiation through inhibition of Numb. Biochem Biophys Res Commun 378,259, 2009 [DOI] [PubMed] [Google Scholar]
- 262.Kook S.-H., Son Y.-O., Choi K.-C., Lee H.-J., Chung W.-T., Hwang I.-H., and Lee J.-C.Cyclic mechanical stress suppresses myogenic differentiation of adult bovine satellite cells through activation of extracellular signal-regulated kinase. Mol Cell Biochem 309,133, 2008 [DOI] [PubMed] [Google Scholar]
- 263.Kook S.-H., Lee H.-J., Chung W.-T., Hwang I.-H., Lee S.-A., Kim B.-S., and Lee J.-C.Cyclic mechanical stretch stimulates the proliferation of C2C12 myoblasts and inhibits their differentiation via prolonged activation of p38 MAPK. Mol Cells 25,479, 2008 [PubMed] [Google Scholar]
- 264.Kumar A., Murphy R., Robinson P., Wei L.E.I., and Boriek A.M.Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-κB transcription factor. FASEB J 18,1524, 2004 [DOI] [PubMed] [Google Scholar]
- 265.Tatsumi R., Hattori A., Ikeuchi Y., Anderson J.E., and Allen R.E.Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 13,2909, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tatsumi R., Liu X., Pulido A., Morales M., Sakata T., Dial S., Hattori A., Ikeuchi Y., and Allen R.E.Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol 290,C1487, 2006 [DOI] [PubMed] [Google Scholar]
- 267.Tatsumi R.Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J 81,11, 2010 [DOI] [PubMed] [Google Scholar]
- 268.Akimoto T., Ushida T., Miyaki S., Tateishi T., and Fukubayashi T.Mechanical stretch is a down-regulatory signal for differentiation of C2C12 myogenic cells. Mater Sci Eng C 17,75, 2001 [Google Scholar]
- 269.Abe S., Rhee S., Iwanuma O., Hiroki E., Yanagisawa N., Sakiyama K., and Ide Y.Effect of mechanical stretching on expressions of muscle specific transcription factors MyoD, Myf-5, Myogenin and MRF4 in proliferated myoblasts. Anat Histol Embryol 38,305, 2009 [DOI] [PubMed] [Google Scholar]
- 270.Gomes A.R., Soares A.G., Peviani S., Nascimento R.B., Moriscot A.S., and Salvini T.F.The effect of 30 minutes of passive stretch of the rat Soleus muscle on the myogenic differentiation, myostatin, and atrogin-1 gene expressions. Arch Phys Med Rehabil 87,241, 2006 [DOI] [PubMed] [Google Scholar]
- 271.Bayati V., Sadeghi Y., Shokrgozar M.A., Haghighipour N., Azadmanesh K., Amanzadeh A., and Azari S.The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell 43,359, 2011 [DOI] [PubMed] [Google Scholar]
- 272.Powell C.A., Smiley B.L., Mills J., and Vandenburgh H.H.Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol 283,C1557, 2002 [DOI] [PubMed] [Google Scholar]
- 273.Zhan M., Jin B., Chen S.-E., Reecy J.M., and Li Y.-P.TACE release of TNF-α mediates mechanotransduction-induced activation of p38 MAPK and myogenesis. J Cell Sci 120,692, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ji G., Liu D., Liu J., Gao H., Yuan X., and Shen G.p38 mitogen-activated protein kinase up-regulates NF-κB transcriptional activation through RelA phosphorylation during stretch-induced myogenesis. Biochem Biophys Res Commun 391,547, 2010 [DOI] [PubMed] [Google Scholar]
- 275.MacIntosh B.R., Gardiner P.F., and McComas A.J.Skeletal Muscle: Form and Function. Champaign, IL: Human Kinetics, 2006 [Google Scholar]
- 276.Borg T.K., and Caulfield J.B.Morphology of connective tissue in skeletal muscle. Tissue Cell 12,197, 1980 [DOI] [PubMed] [Google Scholar]
- 277.Kühl U., Öcalan M., Timpl R., Mayne R., Hay E., and von der Mark K.Role of muscle fibroblasts in the deposition of type-IV collagen in the basal lamina of myotubes. Differentiation 28,164, 1984 [DOI] [PubMed] [Google Scholar]
- 278.Zou Y., Zhang R.-Z., Sabatelli P., Chu M.-L., and Bönnemann C.G.Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol 67,144, 2008 [DOI] [PubMed] [Google Scholar]
- 279.Frederick G.Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 13,264, 2003 [DOI] [PubMed] [Google Scholar]
- 280.Cooper S.T., Maxwell A.L., Kizana E., Ghoddusi M., Hardeman E.C., Alexander I.E., Allen D.G., and North K.N.C2C12 Co-culture on a fibroblast substratum enables sustained survival of contractile, highly differentiated myotubes with peripheral nuclei and adult fast myosin expression. Cell Motil Cytoskeleton 58,200, 2004 [DOI] [PubMed] [Google Scholar]
- 281.Mathew S.J., Hansen J.M., Merrell A.J., Murphy M.M., Lawson J.A., Hutcheson D.A., Hansen M.S., Angus-Hill M., and Kardon G.Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138,371, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ricotti L., Fujie T., Vazao H., Ciofani G., Marotta R., Brescia R., Filippeschi C., Corradini I., Matteoli M., Mattoli V., Ferreira L., and Menciassi A.Boron nitride nanotube-mediated stimulation of cell co-culture on micro-engineered hydrogels. PLoS One 8,e71707, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Levenberg S., Rouwkema J., Macdonald M., Garfein E.S., Kohane D.S., Darland D.C., Marini R., van Blitterswijk C.A., Mulligan R.C., D'Amore P.A., and Langer R.Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23,879, 2005 [DOI] [PubMed] [Google Scholar]
- 284.Koffler J., Kaufman-Francis K., Shandalov Y., Egozi D., Amiad Pavlov D., Landesberg A., and Levenberg S.Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci U S A 108,14789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bellamy L.M., Johnston A.P.W., De Lisio M., and Parise G.Skeletal muscle-endothelial cell cross talk through angiotensin II. Am J Physiol Cell Physiol 299,C1402, 2010 [DOI] [PubMed] [Google Scholar]
- 286.Germani A., Di Carlo A., Mangoni A., Straino S., Giacinti C., Turrini P., Biglioli P., and Capogrossi M.C.Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol 163,1417, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Boillée S., Vande Velde C., and Cleveland , Don W.ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52,39, 2006 [DOI] [PubMed] [Google Scholar]
- 288.Stavarachi M., Apostol P., Toma M., Cimponeriu D., and Gavrila L.Spinal muscular atrophy disease: a literature review for therapeutic strategies. J Med Life 3,3, 2010 [PMC free article] [PubMed] [Google Scholar]
- 289.Emery A.E.H.The muscular dystrophies. Lancet 359,687, 2002 [DOI] [PubMed] [Google Scholar]
- 290.Wu H., Xiong W.C., and Mei L.To build a synapse: signaling pathways in neuromuscular junction assembly. Development 137,1017, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Dutton E.K., Uhm C.S., Samuelsson S.J., Schaffner A.E., Fitzgerald S.C., and Daniels M.P.Acetylcholine receptor aggregation at nerve-muscle contacts in mammalian cultures: induction by ventral spinal cord neurons is specific to axons. J Neurosci 15,7401, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Merlie J.P., and Sanes J.R.Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature 317,66, 1985 [DOI] [PubMed] [Google Scholar]
- 293.Askanas V., Kwan H., Alvarez R.B., Engel W.K., Kobayashi T., Martinuzzi A., and Hawkins E.F.De novo neuromuscular junction formation on human muscle fibres cultured in monolayer and innervated by foetal rat spinal cord: ultrastructural and ultrastructural—cytochemical studies. J Neurocytol 16,523, 1987 [DOI] [PubMed] [Google Scholar]
- 294.Singhal N., and Martin P.T.Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol 71,982, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Patton B.L.Basal lamina and the organization of neuromuscular synapses. J Neurocytol 32,883, 2003 [DOI] [PubMed] [Google Scholar]
- 296.Wood S.J., and Slater R., C. Safety factor at the neuromuscular junction. Prog Neurobiol 64,393, 2001 [DOI] [PubMed] [Google Scholar]
- 297.Hughes B.W., Kusner L.L., and Kaminski H.J.Molecular architecture of the neuromuscular junction. Muscle Nerve 33,445, 2006 [DOI] [PubMed] [Google Scholar]
- 298.Nitkin R.M., Smith M.A., Magill C., Fallon J.R., Yao Y.M., Wallace B.G., and McMahan U.J.Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol 105,2471, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhang B., Luo S., Wang Q., Suzuki T., Xiong W.C., and Mei L.LRP4 serves as a coreceptor of Agrin. Neuron 60,285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kim N., Stiegler A.L., Cameron T.O., Hallock P.T., Gomez A.M., Huang J.H., Hubbard S.R., Dustin M.L., and Burden S.J.Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 135,334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Dobbins G.C., Luo S., Yang Z., Xiong W., and Mei L.Alpha-actinin interacts with rapsyn in agrin-stimulated AChR clustering. Mol Brain 1,18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Frank E., and Fischbach G.D.Early events in neuromuscular junction formation in vitro. Induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol 83,143, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Harper J.M., Krishnan C., Darman J.S., Deshpande D.M., Peck S., Shats I., Backovic S., Rothstein J.D., and Kerr D.A.Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci U S A 101,7123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rumsey J.W., Das M., Stancescu M., Bott M., Fernandez-Valle C., and Hickman J.J.Node of Ranvier formation on motoneurons in vitro. Biomaterials 30,3567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Li X.J., Du Z.W., Zarnowska E.D., Pankratz M., Hansen L.O., Pearce R.A., and Zhang S.C.Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 23,215, 2005 [DOI] [PubMed] [Google Scholar]
- 306.Wagner S., Dorchies O., Stoeckel H., Warter J.-M., Poindron P., and Takeda K.Functional maturation of nicotinic acetylcholine receptors as an indicator of murine muscular differentiation in a new nerve-muscle co-culture system. Pflügers Arch Eur J Physiol 447,14, 2003 [DOI] [PubMed] [Google Scholar]
- 307.Dhawan V., Lytle I.F., Dow D.E., Huang Y.-C., and Brown D.L.Neurotization improves contractile forces of tissue-engineered skeletal muscle. Tissue Eng 13,2813, 2007 [DOI] [PubMed] [Google Scholar]
- 308.Guo X., Das M., Rumsey J., Gonzalez M., Stancescu M., and Hickman J.Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng Part C Method 16,1347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Koning M., Harmsen M.C., van Luyn M.J.A., and Werker P.M.N.Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med 3,407, 2009 [DOI] [PubMed] [Google Scholar]
- 310.Schuurman W., Khristov V., Pot M.W., Weeren P.R.v., Dhert W.J.A., and Malda J.Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 3,1, 2011 [DOI] [PubMed] [Google Scholar]
- 311.Mauro A.Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9,493, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Yin H., Price F., and Rudnicki M.A.Satellite cells and the muscle stem cell niche. Physiol Rev 93,23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zammit P.S., Golding J.P., Nagata Y., Hudon V., Partridge T.A., and Beauchamp J.R.Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166,347, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kuang S., Kuroda K., Le Grand F., and Rudnicki M.A.Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129,999, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Cerletti M., Jurga S., Witczak C.A., Hirshman M.F., Shadrach J.L., Goodyear L.J., and Wagers A.J.Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell 134,37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Rossi C.A., Flaibani M., Blaauw B., Pozzobon M., Figallo E., Reggiani C., Vitiello L., Elvassore N., and De Coppi P.In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J 25,2296, 2011 [DOI] [PubMed] [Google Scholar]
- 317.Fishman J.M., Tyraskis A., Maghsoudlou P., Urbani L., Totonelli G., Birchall M.A., and De Coppi P.Skeletal muscle tissue engineering: which cell to use? Tissue Eng Part B Rev 19,503, 2013 [DOI] [PubMed] [Google Scholar]
- 318.LaBarge M.A., and Blau H.M.Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111,589, 2002 [DOI] [PubMed] [Google Scholar]
- 319.Dezawa M., Ishikawa H., Itokazu Y., Yoshihara T., Hoshino M., Takeda S.-i., Ide C., andNabeshima Y.-i. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309,314, 2005 [DOI] [PubMed] [Google Scholar]
- 320.Bianco P., Cao X., Frenette P.S., Mao J.J., Robey P.G., Simmons P.J., and Wang C.-Y.The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19,35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Penton C.M., Thomas-Ahner J.M., Johnson E.K., McAllister C., and Montanaro F.Muscle side population cells from dystrophic or injured muscle adopt a fibro-adipogenic fate. PLoS One 8,e54553, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Asakura A., Seale P., Girgis-Gabardo A., and Rudnicki M.A.Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159,123, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Meeson A.P., Hawke T.J., Graham S., Jiang N., Elterman J., Hutcheson K., DiMaio J.M., Gallardo T.D., and Garry D.J.Cellular and molecular regulation of skeletal muscle side population cells. Stem Cells 22,1305, 2004 [DOI] [PubMed] [Google Scholar]
- 324.Tanaka K.K., Hall J.K., Troy A.A., Cornelison D.D.W., Majka S.M., and Olwin B.B.Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell 4,217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Bachrach E., Li S., Perez A.L., Schienda J., Liadaki K., Volinski J., Flint A., Chamberlain J., and Kunkel L.M.Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A 101,3581, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mitchell K.J., Pannerec A., Cadot B., Parlakian A., Besson V., Gomes E.R., Marazzi G., and Sassoon D.A.Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol 12,257, 2010 [DOI] [PubMed] [Google Scholar]
- 327.Montarras D., L'Honoré A., and Buckingham M.Lying low but ready for action: the quiescent muscle satellite cell. FEBS J 280,4036, 2013 [DOI] [PubMed] [Google Scholar]
- 328.Usas A., and Huard J.Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials 28,5401, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Torrente Y., Tremblay J.-P., Pisati F., Belicchi M., Rossi B., Sironi M., Fortunato F., El Fahime M., D'Angelo M.G., Caron N.J., Constantin G., Paulin D., Scarlato G., and Bresolin N.Intraarterial injection of muscle-derived Cd34+Sca-1+ stem cells restores dystrophin in mdx mice. J Cell Biol 152,335, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Li H., Usas A., Poddar M., Chen C.-W., Thompson S., Ahani B., Cummins J., Lavasani M., and Huard J.Platelet-rich plasma promotes the proliferation of human muscle derived progenitor cells and maintains their stemness. PLoS One 8,e64923, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sampaolesi M., Blot S., D/' Antona G., Granger N., Tonlorenzi R., Innocenzi A., Mognol P., Thibaud J.-L., Galvez B.G., Barthelemy I., Perani L., Mantero S., Guttinger M., Pansarasa O., Rinaldi C., Cusella De Angelis M.G., Torrente Y., Bordignon C., Bottinelli R., and Cossu G.Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444,574, 2006 [DOI] [PubMed] [Google Scholar]
- 332.Meregalli M., Farini A., Belicchi M., Parolini D., Cassinelli L., Razini P., Sitzia C., and Torrente Y.Perspectives of stem cell therapy in Duchenne muscular dystrophy. FEBS J 280,4251, 2013 [DOI] [PubMed] [Google Scholar]
- 333.Dellavalle A., Sampaolesi M., Tonlorenzi R., Tagliafico E., Sacchetti B., Perani L., Innocenzi A., Galvez B.G., Messina G., Morosetti R., Li S., Belicchi M., Peretti G., Chamberlain J.S., Wright W.E., Torrente Y., Ferrari S., Bianco P., and Cossu G.Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9,255, 2007 [DOI] [PubMed] [Google Scholar]
- 334.Sirabella D., Angelis L., and Berghella L.Sources for skeletal muscle repair: from satellite cells to reprogramming. J Cachexia Sarcopenia Muscle 4,125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Torrente Y., Belicchi M., Sampaolesi M., Pisati F., Meregalli M., Antona G., Tonlorenzi R., Porretti L., Gavina M., Mamchaoui K., Pellegrino M.A., Furling D., Mouly V., Butler-Browne G.S., Bottinelli R., Cossu G., and Bresolin N.Human circulating AC133+ stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest 114,182, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Torrente Y., Belicchi M., Marchesi C., D'Antona G., Cogiamanian F., Pisati F., Gavina M., Giordano R., Tonlorenzi R., Fagiolari G., Lamperti C., Porretti L., Lopa R., Sampaolesi M., Vicentini L., Grimoldi N., Tiberio F., Songa V., Baratta P., Prelle A., Forzenigo L., Guglieri M., Pansarasa O., Rinaldi C., Mouly V., Butler-Browne G.S., Comi G.P., Biondetti P., Moggio M., Gaini S.M., Stocchetti N., Priori A., D'Angelo M.G., Turconi A., Bottinelli R., Cossu G., Rebulla P., and Bresolin N.Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant 16,563, 2007 [DOI] [PubMed] [Google Scholar]
- 337.Barberi T., Bradbury M., Dincer Z., Panagiotakos G., Socci N.D., and Studer L.Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med 13,642, 2007 [DOI] [PubMed] [Google Scholar]
- 338.Zheng J.K., Wang Y., Karandikar A., Wang Q., Gai H., Liu A.L., Peng C., and Sheng H.Z.Skeletal myogenesis by human embryonic stem cells. Cell Res 16,713, 2006 [DOI] [PubMed] [Google Scholar]
- 339.Yamanaka S.Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1,39, 2007 [DOI] [PubMed] [Google Scholar]
- 340.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S.Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131,861.2007 [DOI] [PubMed] [Google Scholar]
- 341.Okita K., Nakagawa M., Hyenjong H., Ichisaka T., and Yamanaka S.Generation of mouse induced pluripotent stem cells without viral vectors. Science 322,949, 2008 [DOI] [PubMed] [Google Scholar]
- 342.Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., Zhao T., Ye J., Yang W., Liu K., Ge J., Xu J., Zhang Q., Zhao Y., and Deng H.Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341,651, 2013 [DOI] [PubMed] [Google Scholar]
- 343.Park I.-H., Arora N., Huo H., Maherali N., Ahfeldt T., Shimamura A., Lensch M.W., Cowan C., Hochedlinger K., and Daley G.Q.Disease-specific induced pluripotent stem cells. Cell 134,877, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Darabi R., Arpke R.W., Irion S., Dimos J.T., Grskovic M., Kyba M., and Perlingeiro R.C.R.Human ES- and iPS-derived myogenic progenitors restore dystrophin and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10,610, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Tedesco F.S., Gerli M.F.M., Perani L., Benedetti S., Ungaro F., Cassano M., Antonini S., Tagliafico E., Artusi V., Longa E., Tonlorenzi R., Ragazzi M., Calderazzi G., Hoshiya H., Cappellari O., Mora M., Schoser B., Schneiderat P., Oshimura M., Bottinelli R., Sampaolesi M., Torrente Y., Broccoli V., and Cossu G.Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med 4,140ra89, 2012 [DOI] [PubMed] [Google Scholar]
- 346.Darabi R., and Perlingeiro R.C.R.A perspective on the potential of human iPS cell-based therapies for muscular dystrophies: advancements so far and hurdles to overcome. J Stem Cell Res Ther 3,1000e113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Adam C.Endogenous musculoskeletal tissue engineering—a focused perspective. Cell Tissue Res 347,489, 2012 [DOI] [PubMed] [Google Scholar]
- 348.Turner N., and Badylak S.Regeneration of skeletal muscle. Cell Tissue Res 347,759, 2012 [DOI] [PubMed] [Google Scholar]
- 349.Jakob F., Ebert R., Rudert M., Nöth U., Walles H., Docheva D., Schieker M., Meinel L., and Groll J.In situ guided tissue regeneration in musculoskeletal diseases and aging. Cell Tissue Res 347,725, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hutmacher D., Duda G., and Guldberg R.Endogenous musculoskeletal tissue regeneration. Cell Tissue Res 347,485, 2012 [DOI] [PubMed] [Google Scholar]
- 351.Krook A., Bjornholm M., Galuska D., Jiang X.J., Fahlman R., Myers M.G., Wallberg-Henriksson H., and Zierath J.R.Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49,284, 2000 [DOI] [PubMed] [Google Scholar]
- 352.Fujita H., Hatakeyama H., Watanabe T.M., Sato M., Higuchi H., and Kanzaki M.Identification of three distinct functional sites of insulin-mediated GLUT4 trafficking in adipocytes using quantitative single molecule imaging. Mol Biol Cell 21,2721, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Chiang S.-H., Baumann C.A., Kanzaki M., Thurmond D.C., Watson R.T., Neudauer C.L., Macara I.G., Pessin J.E., and Saltiel A.R.Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410,944, 2001 [DOI] [PubMed] [Google Scholar]
- 354.Hatakeyama H., and Kanzaki M.Molecular basis of insulin-responsive GLUT4 trafficking systems revealed by single molecule imaging. Traffic 12,1805, 2011 [DOI] [PubMed] [Google Scholar]
- 355.Kanzaki M.Insulin receptor signals regulating GLUT4 translocation and actin dynamics. Endocr J 53,267, 2006 [DOI] [PubMed] [Google Scholar]
- 356.Smith J.L., Patil P.B., Minteer S.D., Lipsitz J.R., and Fisher J.S.Possibility of autocrine β-adrenergic signaling in C2C12 myotubes. Exp Biol Med 230,845, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Shimokawa T., Kagami M., Kato M., Kurosaki E., Shibasaki M., and Katoh M.Effect of YM-126414 on glucose uptake and redistribution of glucose transporter isotype 4 in muscle cells. Eur J Pharmacol 410,1, 2000 [DOI] [PubMed] [Google Scholar]
- 358.Nedachi T., and Kanzaki M.Regulation of glucose transporters by insulin and extracellular glucose in C2C12 myotubes. Am J Physiol Endocrinol Metab 291,E817, 2006 [DOI] [PubMed] [Google Scholar]
- 359.Hayata K., Sakano K., and Nishinaka S.Establishment of new highly insulin-sensitive cell lines and screening of compounds to facilitate glucose consumption. J Pharmacol Sci 108,348, 2008 [DOI] [PubMed] [Google Scholar]
- 360.Kang L., Chung B.G., Langer R., and Khademhosseini A.Microfluidics for drug discovery and development: from target selection to product lifecycle management. Drug Discov Today 13,1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Ostrovidov S., Annabi N., Seidi A., Ramalingam M., Dehghani F., Kaji H., and Khademhosseini A.Controlled release of drugs from gradient hydrogels for high-throughput analysis of cell-drug interactions. Anal Chem 84,1302, 2012 [DOI] [PubMed] [Google Scholar]
- 362.Elliott N.T., and Yuan F.A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci 100,59, 2011 [DOI] [PubMed] [Google Scholar]
- 363.Ghaemmaghami A.M., Hancock M.J., Harrington H., Kaji H., and Khademhosseini A.Biomimetic tissues on a chip for drug discovery. Drug Discov Today 17,173, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.den Hertog J.Chemical genetics: drug screens in Zebrafish. Bioscience Rep 25,289, 2005 [DOI] [PubMed] [Google Scholar]
- 365.Guyon J.R., Steffen L.S., Howell M.H., Pusack T.J., Lawrence C., and Kunkel L.M.Modeling human muscle disease in zebrafish. Biochim Biophys Acta 1772,205, 2007 [DOI] [PubMed] [Google Scholar]
- 366.Catoire H., Pasco M.Y., Abu-Baker A., Holbert S., Tourette C., Brais B., Rouleau G.A., Parker J.A., and Neri C.Sirtuin inhibition protects from the polyalanine muscular dystrophy protein PABPN1. Hum Mol Genet 17,2108, 2008 [DOI] [PubMed] [Google Scholar]
- 367.Nagamine K., Kawashima T., Sekine S., Ido Y., Kanzaki M., and Nishizawa M.Spatiotemporally controlled contraction of micropatterned skeletal muscle cells on a hydrogel sheet. Lab Chip 11,513, 2011 [DOI] [PubMed] [Google Scholar]
- 368.Roses A.D.Pharmacogenetics in drug discovery and development: a translational perspective. Nat Rev Drug Discov 7,807, 2008 [DOI] [PubMed] [Google Scholar]
- 369.Shansky J., Ferland P., McGuire S., Powell C., DelTatto M., Nackman M., Hennessey J., and Vandenburgh H.H.Tissue engineering human skeletal muscle for clinical applications. In: Vunjak-Novakovic G., and Freshney R.I., eds. Culture of Cells for Tissue Engineering. Hoboken, NJ: John Wiley & Sons, Inc., 2006, pp. 239–257 [Google Scholar]
- 370.Webb S.iPS cell technology gains momentum in drug discovery. Nat Rev Drug Discov 8,263, 2009 [DOI] [PubMed] [Google Scholar]
- 371.Pouton C.W., and Haynes J.M.Embryonic stem cells as a source of models for drug discovery. Nat Rev Drug Discov 6,605, 2007 [DOI] [PubMed] [Google Scholar]
- 372.Thomson H.Bioprocessing of embryonic stem cells for drug discovery. Trends Biotechnol 25,224, 2007 [DOI] [PubMed] [Google Scholar]
- 373.Dennis R.G., Herr H., Parker K.K., Larkin L., Arruda E., and Baar K.Engineered Muscle Actuator: Cells and Tissues. Chapel Hill, NC: UNC, 2007, p. 24 [Google Scholar]
- 374.Neal D., and Asada H.Co-fabrication of live skeletal muscles as actuators in a millimeter scale mechanical system. International Conference on Robotics and Automation (ICRA), Shanghai, China, 2011, pp. 3251–3256 [Google Scholar]
- 375.Wang X.Piezoelectric nanogenerators—harvesting ambient mechanical energy at the nanometer scale. Nano Energy 1,13, 2012 [Google Scholar]
- 376.Olivo J., Carrara S., and De Micheli G.Energy harvesting and remote powering for implantable biosensors. IEEE Sens J 11,1573, 2011 [Google Scholar]
- 377.Ahmad R., and Hashim M.H.Development of energy harvesting device using piezoelectric material. 4th International Conference on Modeling, Simulation and Applied Optimization (ICMSAO), Kuala Lumpur, Malaysia, 2011, pp. 1–6 [Google Scholar]
- 378.Wang Z., and Yang R.Muscle-driven nanogenerators. US Patent, 2011
- 379.Ishisaka T., Sato H., Akiyama Y., Furukawa Y., and Morishima K.Bio-actuated power generator using heart muscle cells on a PDMS membrane. 14 th International Conference on Solid-State Sensors, Actuators and Microsystems, Lyon, France, 2007, pp. 903–906 [Google Scholar]
- 380.Vein J.Method for producing tissue engineered meat for consumption. US Patent, 2004
- 381.Post M.J.Cultured meat from stem cells: challenges and prospects. Meat Sci 92,297, 2012 [DOI] [PubMed] [Google Scholar]
- 382.Coghlan A.What's the beef? Cultured meat remains a distant dream. New Sci 219,10, 2013 [Google Scholar]
- 383.Eschenhagen T.Artificially Produced Three-Dimensional Muscle Tissue. United States patent US 7,618,452B2. 2009, Nov. 17, pp. 1–18
- 384.Przewozniak M., Czaplicka I., Czerwinska A.M., Markowska-Zagrajek A., Moraczewski J., Streminska W., Janczyk-Ilach K., Ciemerych M.A., and Brzoska E.Adhesion proteins—an impact on skeletal myoblast differentiation. PLoS One 8,e61760, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Sohn R.L., Huang P., Kawahara G., Mitchell M., Guyon J., Kalluri R., Kunkel L.M., and Gussoni E.A role for nephrin, a renal protein, in vertebrate skeletal muscle cell fusion. Proc Natl Acad Sci U S A 106,9274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Antigny F., Koenig S., Bernheim L., and Frieden M.During post-natal human myogenesis, normal myotube size requires TRPC1- and TRPC4-mediated Ca2+ entry. J Cell Sci 126,2525, 2013 [DOI] [PubMed] [Google Scholar]
- 387.Iannotti F.A., Barrese V., Formisano L., Miceli F., and Taglialatela M.Specification of skeletal muscle differentiation by repressor element-1 silencing transcription factor (REST)-regulated Kv7.4 potassium channels. Mol Biol Cell 24,274, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Leroy M.C., Perroud J., Darbellay B., Bernheim L., and Konig S.Epidermal growth factor receptor down-regulation triggers human myoblast differentiation. PLoS One 8,e71770, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Vitadello M., Gherardini J., and Gorza L.The stress protein/chaperone Grp94 counteracts muscle disuse atrophy by stabilizing subsarcolemmal nNOS. Antioxid Redox Signal 2013. [Epub ahead of print]; DOI: 10.1089/ars.2012.4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pavlath G.K.Spatial and functional restriction of regulatory molecules during mammalian myoblast fusion. Exp Cell Res 316,3067, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Kitamura T., Kitamura Y.I., Funahashi Y., Shawber C.J., Castrillon D.H., Kollipara R., DePinho R.A., Kitajewski J., and Accili D.A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest 117,2477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Rajakumar D., Alexander M., and Oommen A.Oxidative stress, NF-κB and the ubiquitin proteasomal pathway in the pathology of calpainopathy. Neurochem Res 38,2009, 2013 [DOI] [PubMed] [Google Scholar]
- 393.Hudson M.B., and Price S.R.Calcineurin: a poorly understood regulator of muscle mass. Int J Biochem Cell Biol 45,2173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Hindi S.M., Tajrishi M.M., and Kumar A.Signaling mechanisms in mammalian myoblast fusion. Sci Signal 6,re2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Hulmi J.J., Oliveira B.M., Silvennoinen M., Hoogaars W.M.H., Ma H., Pierre P., Pasternack A., Kainulainen H., and Ritvos O.Muscle protein synthesis, mTORC1/MAPK/Hippo signaling, and capillary density are altered by blocking of myostatin and activins. Am J Physiol Endocrinol Metab 304,E41, 2013 [DOI] [PubMed] [Google Scholar]
- 396.Kalish B.T., Kieran M.W., Puder M., and Panigrahy D.The growing role of eicosanoids in tissue regeneration, repair, and wound healing. Prostaglandins Other Lipid Mediat 104–105,130, 2013 [DOI] [PubMed] [Google Scholar]
- 397.Muñoz-Cánoves P., Scheele C., Pedersen B.K., and Serrano A.L.Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J 280,4131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Johns N., Stephens N.A., and Fearon K.C.H.Muscle wasting in cancer. Int J Biochem Cell Biol 45,2215, 2013 [DOI] [PubMed] [Google Scholar]
- 399.Fuentes E.N., Valdes J.A., Molina A., and Bjornsson B.T.Regulation of skeletal muscle growth in fish by the growth hormone—insulin-like growth factor system. Gen Comp Endocrinol 192,136, 2013 [DOI] [PubMed] [Google Scholar]
- 400.Schakman O., Kalista S., Barbe C., Loumaye A., and Thissen J.P.Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45,2163, 2013 [DOI] [PubMed] [Google Scholar]
- 401.Palma C., and Clementi E.Nitric oxide in myogenesis and therapeutic muscle repair. Mol Neurobiol 46,682, 2012 [DOI] [PubMed] [Google Scholar]