Abstract
Background: The period preceding the first psychotic episode is regarded as a promising period for intervention. We aimed to develop an optimized prediction model of a first psychosis, considering different sources of information. The outcome of this model may be used for individualized risk estimation. Methods: Sixty-one subjects clinically at high risk (CHR), participating in the Dutch Prediction of Psychosis Study, were assessed at baseline with instruments yielding data on neuropsychology, symptomatology, environmental factors, premorbid adjustment, and neurophysiology. The follow-up period was 36 months. Results: At 36 months, 18 participants (29.5%) had made a transition to psychosis. Premorbid adjustment (P = .001, hazard ratio [HR] = 2.13, 95% CI = 1.39/3.28) and parietal P300 amplitude (P = .004, HR = 1.27, 95% CI = 1.08/1.45) remained as predictors in the Cox proportional hazard model. The resulting prognostic score (PS) showed a sensitivity of 88.9% and a specificity of 82.5%. The area under the curve of the PS was 0.91 (95% CI = 0.83–0.98, cross-validation: 0.86), indicating an outstanding ability of the model to discriminate between transition and nontransition. The PS was further stratified into 3 risk classes establishing a prognostic index. In the class with the worst social-personal adjustment and lowest P300 amplitudes, 74% of the subjects made a transition to psychosis. Furthermore, transition emerged on average more than 17 months earlier than in the lowest risk class. Conclusions: Our results suggest that predicting a first psychotic episode in CHR subjects could be improved with a model including premorbid adjustment and information-processing variables in a multistep algorithm combining risk detection and stratification.
Key words: clinical high risk, psychosis prediction, P300 event-related potential, premorbid adjustment, prognostic index
Introduction
First prodromal signs and symptoms of a developing nonaffective psychosis, including impairment of social and role functioning, can occur several years prior to the overt clinical manifestation. This period, retrospectively referred to as the prodrome, is characterized by various features such as negative, basic and depressive symptoms as well as mild, subthreshold psychotic symptoms.1,2 Ultrahigh risk (UHR) criteria have been defined to pros pectively identify people in this prodromal phase.3–6 In a recent meta-analysis,7 the average 1-year transition rate to first episode psychosis in UHR subjects was 21.7%, increasing to 31.5% after 3 years of follow-up.
Another approach for risk prediction focuses on basic symptoms, ie, self-perceived disturbances in several domains including cognition and perception, resulting in transition rates to psychosis of 34.9% within 3 years and 70% within 10 years.8,9
Several clinical models have been proposed to further increase the validity of prediction of transition to psychosis in samples preselected by one or both of these approaches, yielding mixed results (for review, see Ruhrmann et al10). Neuropsychological tests and biomarkers have been proposed as most promising candidates for improving clinical risk estimation.11–16 Riecher-Rössler et al17 were the first to identify a risk profile for transition to psychosis including not only clinical but also a neurocognitive variable: suspiciousness, negative symptoms (anhedonia/asociality) and a high false alarm rate of the neuropsychological test “TAP Go/NoGo.” With these variables combined into one model, sensitivity was found to be 83.3% and specificity was 79.3%. Based on a small sample (n = 28, 46% transitions) followed up for at least 4 years, the same group subsequently proposed a model combining negative symptoms and an electroencephalographic parameter, theta absolute power, resulting in a sensitivity of 92% and a specificity of 87%.18
In the North American Prodrome Longitudinal Study (NAPLS),19 a combination of 3 baseline variables (genetic risk with recent functional decline, higher levels of unusual thoughts or suspiciousness, and more severe social impairment) resulted in a marked increase of the positive predictive power. The European Prediction of Psychosis Study (EPOS) introduced a prognostic index (PI) based on a 6-variable prediction model including global functioning within the previous year.20
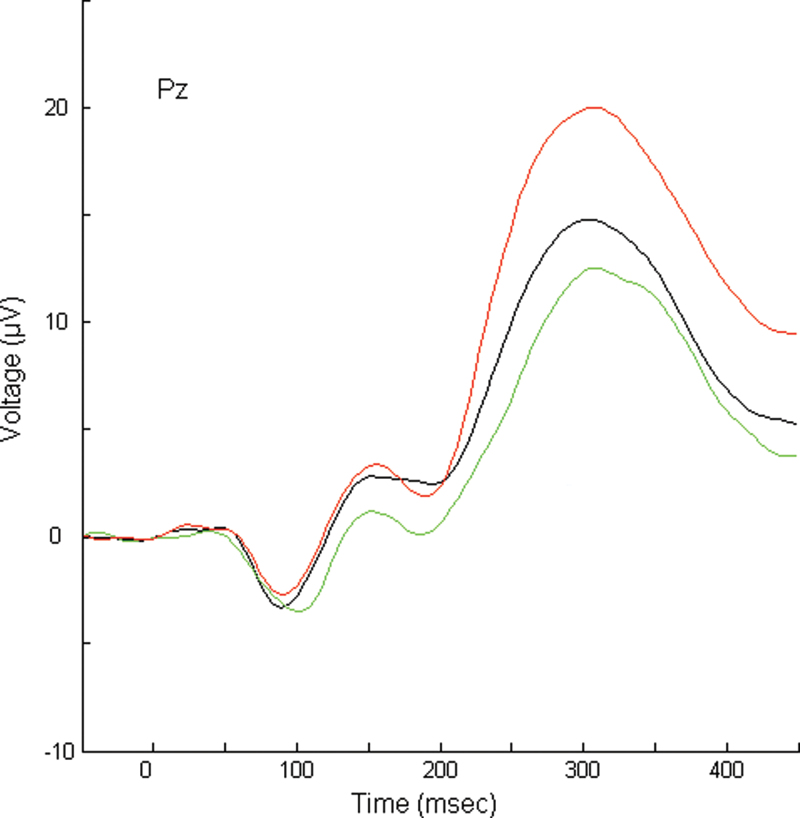
In the Dutch Prediction of Psychosis Study (DUPS), subjects clinically at high risk (CHR) were assessed with a comprehensive assessment battery covering 5 domains (neuropsychology, psychopathology, social and role functioning, environmental factors and premorbid adjustment, and neurophysiology) and were followed up for 3 years.21 Transition to first episode psychosis was found to be significantly associated with (table 1) (1) reduced semantic verbal fluency,22 (2) increased social anhedonia and withdrawal,23 (3) urbanicity,24 (4) poor premorbid adjustment,24 and (5) reduced amplitude of the midline parietal (Pz) P300 event-related potential (ERP)25 (figure 1). The P300 is a cognition related wave, closely associated with attention and memory and a reduced P300 amplitude is one of the most reported potential biomarkers of schizophrenia.26–28 Several papers showed a reduced P300 amplitude in CHR subjects.29–34 Yet, none of these reports included (enough) converted cases to enable a predictor analysis except for the DUPS study.25
Table 1.
Predictive Variables in the Domains Investigated in the DUPS Project
| Domain | Potentially Predictive Variables | Most Predictive Variable |
|---|---|---|
| Neuropsychology | Sustained attention, verbal learning and memory, semantic and phonological fluency, spatial working memory, and motor speed22 | Semantic verbal fluency |
| Clinical variables | All items of the SIPS23 | Item “social anhedonia and withdrawal” |
| Environmental factors | Ethnicity, urbanicity, head trauma, CNS infection, intrauterine or perinatal complications, unemployment, and receiving state benefits24 | Urbanicity |
| Premorbid adjustment | All items of the PAS24 | Social-sexual aspects of life during early adolescence (age: 12–15) and premorbid social-personal adjustment (highest level ever attained) |
| Neurophysiology | N100, N200, N2b, P200, and P30025 | Parietal P300 amplitude |
Note: DUPS, Dutch Prediction of Psychosis Study; SIPS, Structured Interview for Prodromal Syndromes; CNS, central nervous system; PAS, Premorbid Adjustment Scale.
Fig. 1.
Grand average target waveforms for each group at Pz. Subjects clinically at high risk (CHR) with transition to psychosis = green, lower line; CHR subjects without transition = black, middle line; and control group= red, upper line. Pz, midline parietal.
While all factors mentioned above contribute to the prediction of a first psychosis, their relative contribution is small. As suggested by first reports combining the psychopathological14 and the neurocognitive12–15,18 or the neurophysiological level,18 respectively, prediction of psychosis may be significantly improved by integrative models, considering variables from different levels, including neurocognition and neurobiology.
The aim of the current report was, therefore, to evaluate whether prediction could be improved by combining the various individual psychosis predictors identified in the DUPS project. We hypothesized that using only the best predictors in a second step PI could lead to individualized risk estimation in CHR subjects both with respect to transition rate as well as time to transition. Individualized risk estimation would open a new avenue to targeted prevention, ie, tailoring the intervention to the actual needs of the patient.
Methods
Recruitment
Between August 2002 and July 2009, data were collected from help-seeking individuals (age: 12–35 y) who met ultrahigh risk and/or basic symptoms criteria and agreed to participate in the DUPS.21
Subjects were referred to the Academic Medical Center (AMC) mainly by professionals in secondary mental health services because of a suspected prepsychotic development. Eligible for the study were subjects who met at least one of the following criteria (for a more elaborate description, see Nieman et al21):
Attenuated positive symptoms
Brief limited intermittent psychotic symptoms
Genetic risk in combination with reduced functioning (as assessed with the Global Assessment of Functioning Scale-Modified [GAF-M]35)
Basic symptoms
Exclusion criteria were the following: a low estimated verbal IQ (IQ < 85) as assessed by the Dutch National Adult Reading test,36 past or present psychotic episode lasting longer than 1 week (ie, fulfilling Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM-IV]37 criteria of a brief psychotic episode for at least 7 days, assessed by the Structured Clinical Interview for DSM-IV38), and symptoms relevant for inclusion arising from a known general medical disorder or drugs or alcohol dependency as defined by the Comprehensive International Diagnostic Interview (CIDI39). On account of the naturalistic design of the present study, (prior) use of antipsycho tics was not considered an exclusion criterion.
The investigation was carried out in accordance with the latest version of the Declaration of Helsinki. The study design was approved by the medical ethics committee of the AMC. Informed written consent from participants was obtained after the procedure had been fully explained. Written informed consent was also obtained from parents or guardians if the participant was below the age of 18 years.
Instruments
Psychopathology.
The Structured Interview for Prodromal Syndromes (SIPS 3.040 including GAF-M) and its rating scale The Scale of Prodromal Symptoms (SOPS) were employed to determine the presence, severity, and type of ultrahigh risk criteria.
The DUPS investigators received extensive training from Dr Tandy Miller, one of the SIPS authors, including a reliability check after approximately 6 months. The pair-wise interrater concordance of the SIPS was 77% and determined acceptable by the training team.
The Bonn Scale for the Assessment of Basic Symptoms-Prediction list (BSABS-P41), an abbreviated version of the Schizophrenia Proneness Instrument,42 was used to assess basic symptoms. The investigators received repeated training by the scale’s first author (Dr F. Schultze-Lutter). Concordance rate with expert rating (F. Schultze-Lutter) was 87.9%.
Environmental Factors and Premorbid Adjustment.
Urbanicity was defined as living in a city with more than 100 000 inhabitants. Urbanicity
variables were birth place population and current living place population.
Furthermore, premorbid adjustment was assessed with the Premorbid Adjustment Scale (PAS)43 in all age periods if applicable (<11, 12–15, 16–18, >19 y). Start of the CHR (early morbid) phase was assessed with the positive items of the SIPS. The PAS was scored by trained research assistants with as much information as possible, ie, from the patient, parents or guardians, and medical records. In the current predictive model, only the mean scores of the PAS items “social-sexual aspects of life during early adolescence (age: 12–15)” and “premorbid social-personal adjustment” were included (see table 1). The latter PAS item gives an indication of social-personal adjustment in both the social and academic domain in the period of best functioning that was ever attained.
Neuropsychology.
The verbal fluency test is used to measure the quality and quantity of verbal output generation. In the semantic verbal fluency test,22 subjects were asked to name as many words within 1 min in the semantic category “animals.” The dependent variable for this task was the mean number of acceptable words in 1 min.44
Neurophysiology.
ERPs were assessed at baseline using an active auditory oddball paradigm in the DUPS CHR subjects and a matched healthy control group, as described by van Tricht et al.25 The P300 results of the control group are only used in figure 1 as a reference. A total of 300 tones (80% nontargets of 1000 Hz, 20% targets of 2000 Hz, sequence randomized, interstimulus interval of 1480ms, ie, a stimulation frequency of .67 Hz) were binaurally presented for 100 ms through headphones at an intensity of 50 dB above hearing threshold. The subjects were instructed to count the targets and respond to them with a button press. The electroencephalogram was recorded with a band-pass filter of 0.04–300 Hz, with a sampling rate of 1000 Hz. Twenty-one silver-silver chloride disk electrodes (impedances < 5 kΩ) were attached to electrode sites according to the international 10–20 system, with a reference electrode on linked mastoids and a ground electrode on the forehead. To register eye movements and blinks, 4 additional electrodes were attached at the outer canthi of both eyes and above and below the left eye.
Digitized data for each subject were analyzed offline with BrainVision Analyzer (Brain products: http://www.brainproducts.com). After baseline correction, signals were digitally filtered with a low-pass filter of 30 Hz and a high-pass filter of .10 Hz (24 dB/octave) and were epoched at 50 ms before stimulus and 450 ms after stimulus. The maximum allowed absolute difference between 2 values in 1 segment was 200 µV, and the maximum allowed voltage step was 50 µV. Segments in which these values were exceeded were removed. Both vertical and horizontal eye movements were detected and removed with eye-movement detection measures developed by Gratton et al.45 Epochs were averaged separately for nontarget and target tones. If the number of artifact free trials was below 26, the recording was excluded from further analyses.
Peak amplitudes were semiautomatically detected and calculated relative to prestimulus baseline of 50 ms.46 The P300 component was calculated as the waveform generated by target tones and defined as the largest positive value between 250 and 450 ms after stimulus presentation. Peak amplitudes and peak latencies were calculated with a computer algorithm. All peaks were visually inspected. If necessary, adjustments were made hereafter.
Procedure
After referral to the AMC, subjects were invited for a first interview with a psychiatrist and
a psychologist. In this face-to-face interview, which lasted approximately 2 h, subjects were asked about their lifetime history of complaints, family history of psychiatric disorders, as well as drug and medicine use. Subsequently, the SIPS was administered. Simultaneously, in another interview, parents or guardians of all patients were separately asked about the lifetime development of their child.
All the diagnostic information for each subject was discussed in a staff meeting. Those patients considered to meet CHR criteria were asked if they would like to participate in the DUPS project. They were referred back to their referring mental health professionals. Some received treatment while others were only monitored. Patients, their parents or caretakers, and the referring instances were asked to contact the DUPS project in case of increasing symptoms. In addition, a SIPS interview was carried out at 9, 18, 24, and 36 months. The follow-up SIPS interview covered the period since the previous SIPS interview. With this interview, we scored the SOPS.
Transition to psychosis was operationalized as any single item on the positive subscale of SIPS (SIPS-Positive) with a score of 6 for more than 7 days.47,48 The respective DSM-IV diagnosis was assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders.38
Statistical Analysis
Statistical analyses were performed using SPSS statistical software (version 20). Six subjects had a missing value on the PAS. We were able to impute 3 of these missing values on the item “social-sexual aspects of life” and 1 missing value on the item “social-personal adjustment” with the multiple imputations function of SPSS. For the remaining 3 missing values, there was no data available per subject on other items from the same section.
To evaluate possible differences in general characteristics between the transition and no transition group, we used t tests and chi-square tests. For developing the reported prediction model, we used variables that we found to be predictive of a first psychotic episode in the DUPS project in previous research (see table 1). We employed stepwise Cox proportional hazard analysis for model development. Applying the regression equation derived from the final Cox model to each subject, we generated individual prognostic scores (PSs).20 The PS is calculated as {0.757 × social-personal adjustment score} + {−0.231 × P300 amplitude}. The indices 0.757 and −0.231 are the β values of the Cox model as specified in table 3. Based on the resulting PS, we generated a PI with 3 risk classes.20 Subsequently, a log rank test was calculated to compare the survival distributions of these risk classes.
Table 3.
Predictor Variables (Cox Proportional Hazard Model)
| B | SE | Wald | df | P Value | Hazard Ratio | 95% CI for Hazard Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| PAS social-personal adjustment | 0.757 | 0.219 | 11.900 | 1 | .001 | 2.131 | 1.387 | 3.277 |
| Pz P300 amplitude | −0.231 | 0.080 | 8.442 | 1 | .004 | 1.269 | 1.077 | 1.472 |
As clinical outcome was known for all subjects, logistic regression analysis could be applied for calculating prognostic accuracy. To calculate sensitivity and specifi city of the PS, a binary logistic regression analysis was employed.
For the individual PSs, the area under the receiver operating characteristic curve (AUC) was estimated as a threshold independent measure of the ability to discriminate between transition and nontransition; discrimination is considered “acceptable” for 0.7 ≤ AUC < 0.8, “excellent” for 0.8 ≤ AUC < 0.9, and “outstanding” for AUC ≥0.9.49 In addition, positive and negative likelihood ratios (LRs), and positive (PPV) and negative (NPV) predictive values were calculated.
Bootstrap techniques were used to internally validate the PS and to reduce overfit bias. In bootstrap, random samples with replacement are drawn from the original data set. These resamples have the same size as the original cohort but due to replacement, their composition is different. The process is repeated multiple times (in our study, 100 times), and the model derived from the original data set is tested in these bootstrap resamples. The average of the performance index (the AUC) is considered the bias-corrected estimate of how well the model would perform in the future. The software R version 2.13.2 (The R Foundation for Statistical Computing) was used in the validation with bootstrap techniques.
Results
A total of 61 participants were assessed at baseline. General characteristics and study measures are shown in table 2. The sample used in the current study consists of the DUPS subjects who were assessed with all the measurements described in the previously published articles in table 1. At the end of the 36-month follow-up period, 18 participants (29.5%) had made the transition to psychosis. These subjects received the following DSM-IV diagnoses: schizophrenia (n = 12), schizophreniform disorder (n = 3), schizoaffective disorder (n = 2), and brief psychotic disorder (n = 1).
Table 2.
General Characteristics
| Transition (n = 18) | No Transition (n = 43) | Total CHR Group (n = 61) | Statistic | P Value | |
|---|---|---|---|---|---|
| Mean age (SD) in years | 20.3 (4.0) | 19 (3.8) | 19.9 (3.9) | t = −1.18 | .24 |
| Gender (male/female) | 13/5 | 27/16 | 40/21 | χ2 = 0.50 | .56 |
| Mean premorbid IQ (SD) | 105.35 (11.1) | 107.94 (9.6) | 106.11 (10.7) | t = −0.86 | .38 |
| Medication | χ2 = 0.99 | .80 | |||
| None | 10 | 25 | 35 | ||
| Antipsychotics | 5 | 8 | 13 | ||
| Antidepressants | 1 | 5 | 6 | ||
| Other | 2 | 5 | 7 | ||
| Inclusion category | χ2 = 4.80 | .73 | |||
| APS | 5 | 9 | 14 | ||
| APS + BS | 10 | 26 | 36 | ||
| APS + BS + BLIPS | 1 | 3 | 4 | ||
| APS + GR + BS | 1 | 1 | 2 | ||
| BLIPS | 0 | 1 | 1 | ||
| BLIPS + APS | 1 | 0 | 1 | ||
| GR + BS | 0 | 1 | 1 | ||
| GR + BS + BLIPS | 0 | 2 | 2 | ||
| Cannabis use (in past year) | 5 (27.8%) | 20 (46.5%) | 25 (41%) | χ2 = 1.85 | .45 |
| SIPS social anhedonia and withdrawal | 3.83 (1.50) | 2.40 (2.04) | 2.82 (2.00) | t = −3.14 | .003 |
| Pz P300 amplitude | 10.49 (1.92) | 16.47 (5.96) | 14.71 (5.78) | t = 5.90 | <.0001 |
| Semantic verbal fluency | 18.76 (4.22) | 21.09 (5.25) | 20.43 (5.06) | t = 1.63 | .11 |
| Urbanicity | 1.78 (.94) | 2.33 (1.50) | 2.16 (1.11) | t = 1.94 | .06 |
| PAS social-sexual aspects (12–15y) | 2.72 (1.57) | 1.42 (1.53) | 1.80 (1.64) | t = −3.01 | .004 |
| PAS social-personal adjustment | 3.44 (1.50) | 2.15 (.92) | 2.55 (1.16) | t = −4.60 | <.0001 |
Note: APS, attenuated positive symptoms; BS, basic symptoms; BLIPS, brief limited intermittent psychotic symptoms; GR, genetic risk plus reduced functioning; CHR, clinically at high risk; Pz, midline parietal. Abbreviations are explained in the footnote to table 1.
With respect to general characteristics at baseline, the CHR groups with and without a transition to psychosis did not differ in age, premorbid intelligence, gender, cannabis and medication use, or the distribution of inclusion criteria (table 2).
Prediction Model
Because of 3 missing values in the PAS, 58 subjects were included into the Cox regression analysis. In the Cox model, poor social-personal adjustment and reduced P300 parietal (Pz) amplitude predicted transition to a first psychotic episode (see table 3). The relative risk of developing a psychosis doubles with the increase of 1 (signifying a worse score) on the social-personal adjustment item score of the PAS. Furthermore, with a decrease of the Pz P300 amplitude of 1 µV, the relative risk of developing a first psychotic disorder increases by 27%.
Entering the PSs in the logistic regression with the default probability threshold of 0.5, equaling a PS of −0.38, sensitivity was 0.72 and specificity was 0.88. Furthermore, the PPV was 0.72, NPV 0.88, positive LR 5.78 and negative LR 0.32. To the aim of obtaining a high sensitivity (thereby avoiding false negatives), a second explorative logistic regression was calculated with a PS of −0.57 as cutoff (equaling a probability threshold of 0.4), resulting in a sensitivity of 88.9% and a specifi city of 82.5%, a PPV of 0.70, NPV of 0.94, positive LR of 5.08, and negative LR of 0.13. The overall accuracy of the model was 84.5%.
Receiver Operating Characteristic Curve
The AUC was 0.91 (95% CI: 0.83–0.98). The number of events in our sample precluded splitting for generating a training and a validation sample. Therefore, we applied a bootstrap procedure generating 100 samples for cross-validation of the logistic model. Receiver operating characteristic curve analysis was used again as a conservative method testing the cutoff independent discriminative ability of our model. The resulting AUC of 0.86 was still in the middle of the range of ≤0.80 to <0.90, indicating an “excellent” discriminative ability according to Hosmer and Lemeshow.49
PI Classes
The PSs were stratified into 3 classes, thus establishing a PI for risk classification. Table 4 lists the transition rates per class.
Table 4.
PI for Risk Classification
| Risk Class of PI | Prognostic Scorea | Number of Subjectsb | Transition (%) | Estimated Time to Transition (Months) | |
|---|---|---|---|---|---|
| Mean (SE) | 95% CI | ||||
| I | <−1.51 | 27 | 1 (3.7) | 35.46 (0.53) | 34.42–36.50 |
| II | −1.51/−0.39 | 12 | 3 (25) | 31.86 (2.39) | 27.18–36.54 |
| III | >−0.39 | 19 | 14 (73.7) | 18.04 (12.09) | 12.09–24.00 |
Note: PI, prognostic index.
aThe individual prognostic scores are calculated as {0.757 × social-personal adjustment score} + {−0.231 × P300 amplitude}.
bOf the 58 subjects that were included in the final Cox model.
Figure 2 shows the transition rates for the 3 classes. With regard to the survival curves, class I differed significantly from class II (χ2 = 4.03, P < .045) and class III (χ2 = 29.28, P < .0001). Furthermore, class II differed significantly from class III (χ2 = 7.44, P < .006). The mean time to transition of class III differed from class II by more than 1 year and from class I by more than 17 months, with no overlap of the lower CI 95 limit of class II and the upper limit of the adjacent class. Nine patients (47.4%) made the transition to psychosis within a year in risk class III. One subject (8.3%) in risk class II and none of the subjects in risk class I made a transition within 1 year.
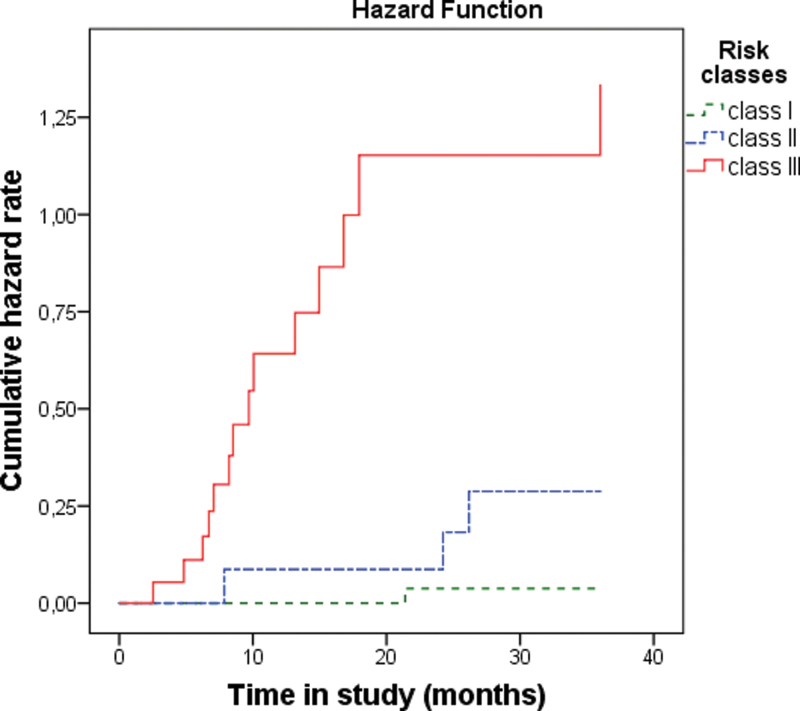
Fig. 2.
Kaplan-Meier survival analysis of risk classes of the prognostic index. 36-month hazard rate: class I = 0.038, class II = 0.288, and class III = 1.335.
Discussion
We considered several variables from different domains for developing a model aimed at improving the predictive validity of clinical risk in subjects included in accordance with UHR criteria and the basic symptom approach. Parietal P300 amplitude and social-personal adjustment (during the premorbid period of best functioning ever attained) were included into the final model, which showed a sensitivity of 88.9% and a specificity 82.5%. Furthermore, according to the literature,49 the AUC of 0.91 demonstrated an “outstanding” ability of the model to discriminate between transition and nontransition. The NPV of 94.3 and the negative LR of 0.13 indicate that the model was able to rule out a transition to psychosis within the underlying observation period of 36 months with a very high probability.50 The positive LR of 5.1 indicates that a positive classification makes it 5 times more likely to develop a psychosis than a negative test. Like the PPV of 70%, this indicates that the model has a moderate value for a correct detection of a person at risk if it was only used for a dichotomous classification. Yet, a further stratification provides a superior opportunity for using a PS as a tool for individualized risk estimation, towards a targeted intervention.51
Individualized Risk Estimation
We were able to separate 3 statistically distinct risk classes. The transition rate in the highest class was almost 20 times higher than in the lowest class and almost 3 times higher than in class II. Thus, compared to the general transition rate of 29.5% predicted by the inclusion criteria, applying our model as a second step of risk stratification51 led to an important improvement of individual risk estimation. This included the ability to predict not only the magnitude of risk but also the time to transition, which differed in class III markedly from the other classes; the mean difference to the lowest class was more than 17 months. In addition, in the lowest risk class, none of the subjects transitioned within a year, while in the highest risk class, 47.4% of the subjects transitioned within this time frame, which should have a significant impact on interventional measures.
P300 and Premorbid Adjustment
The observed “time to event” differences are even more pronounced than in the first proposal of a PI for psychosis prediction,20 which was based on psychopathological, demographical, and functional variables. This may be caused by the inclusion of the P300 variable: introducing a parameter directly reflecting a disturbance of cerebral information processing can be expected to be a more precise measure of the pathophysiological process and, thus, the probability to develop a more severe course. This notion is supported by a study demonstrating a clear distinction of times to event by another ERP, the mismatch negativity.52 Thus, the identified index may be a further step forward on the way to the desired individual risk classification, opening a new avenue to targeted prevention, ie, tailoring the measures of intervention to the needs of the subject.10,20
In addition to reduced P300 amplitude, poor premorbid social-personal adjustment remained as a predictor in the model, indicating that this variable can not be explained by information-processing deficits alone. The main predictive PAS item in the final model was “premorbid social-personal adjustment.” This item is scored over the period of best functioning that was ever attained, including childhood. It is, therefore, unlikely that the score on the “premorbid social-personal adjustment” item of the PAS reflects adjustment in the prodromal period, in which social and role functioning often declines further19,20,47,54 but rather reflects adjustment in the preceding premorbid period.
Poor premorbid adjustment is characterized in a substantial proportion of schizophrenia patients by early and progressive deterioration in social functioning, which is present years before the onset of psychosis53,55 and rarely improves over time.56,57
In the seminal work of Strauss and Carpenter58 on prediction of outcome in first-episode schizophrenia subjects, frequency of social contacts remained relatively stable over an 11-year follow-up period, indica ting that subjects with poor social functioning at baseline showed poor social functioning at 11-year follow-up. This domain was only loosely correlated with the other outcome domains (ie, duration of hospitalization in the previous year, time spent employed during the past year, and symptom severity during the past month), suggesting a relatively independent status.
Our data reveal that the combination of information-processing deficits and reduced premorbid social-personal adjustment may be associated with the highest risk of transition to the first psychotic episode in help-seeking individuals meeting UHR or basic symptom criteria.
Limitations
In spite of the outlined strengths of our study, some critical issues regarding our data need to be addressed. First, a methodological issue must be considered. Because fitted models always perform in an “optimistic manner”49 in the model-development data, replication in an independent, larger sample is needed to control for tailor-made modeling. In theory, sample splitting is an option for model validation in large samples. However, the limited number of transitions did not allow this for statistical reasons. Although a bootstrapping analysis confirmed our results with regard to the discriminative ability of the score derived from our Cox model, existing or future samples of comparable risk definition and larger size are required to validate our findings.
Several variables that have shown to be predictive of a first psychotic episode were not assessed in our sample, eg, baseline volume loss of the temporal lobes.59 However, this variable may not be an independent predictor of a first psychotic episode in our model because reduced P300 amplitude has frequently been linked to superior temporal gyrus volume loss.60 Otherwise, temporal volume loss may turn out to be an independent predictor instead of reduced P300 amplitude in a model that would include magnetic resonance imaging results. Future studies in larger samples, encompassing further predictive variables, are warranted.
Third, van Mastrigt and Addington61 reported that some items of the general scale of the PAS may be unfavorably biased against young patients. For example, at the item E1 covering education, young patients may receive an unfavorable score because they did not yet complete their education. However, the 2 PAS items we used in our analysis were not subject to this bias because one of these items concerned the age period of 12–15 years (social-sexual aspects), and the other concerned the highest level ever attained (social-personal adjustment).
Conclusions
To the best of our knowledge, the present study is the first in which the predictive value of neuropsychological, psychopathological, environmental, functional, and neurophysiological variables has been tested in an integrative approach. Our results demonstrate that such a procedure is essential to differentiate between redundant and nonredundant variables. Furthermore, the resulting model enabled a stratification of risk estimation with regard to the 2 important dimensions of risk, magnitude and time to transition. Thus, we demonstrated that predicting a first psychotic episode in a help-seeking sample meeting UHR or basic symptom criteria may be improved by applying the suggested model as a second step for risk stratification. However, transferring our approach into clinical practice requires validation in an independent sample. A successful transfer would provide new opportunities for developing targeted intervention strategies based on a subjects’ individual risk index.
Funding
The Netherlands Organisation for Health Research and Development, Zon-Mw (2630.0001) for the Dutch Prediction of Psychosis Study; European Commission in Brussels, Belgium for the European Prediction of Psychosis Study (QLGU-CT-2001-01081).
Acknowledgments
The authors thank the EEG technicians of the Clinical Neurophysiology Unit for their assistance with the P300 recordings. We also thank T. Boerée for his technical assistance. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Häfner H, Maurer K, Löffler W, et al. The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol. 1998;33:380–386 [DOI] [PubMed] [Google Scholar]
- 2. Schultze-Lutter F, Ruhrmann S, Berning J, Maier W, Klosterkötter J. Basic symptoms and ultrahigh risk criteria: symptom development in the initial prodromal state. Schizophr Bull. 2010;36:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32 [DOI] [PubMed] [Google Scholar]
- 4. Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Early detection and intervention in the initial prodromal phase of schizophrenia. Pharmacopsychiatry. 2003;36(suppl 3):S162–S167 [DOI] [PubMed] [Google Scholar]
- 5. McGlashan T, Walsh B, Woods SW. The Psychosis-Risk Syndrome. Handbook for Diagnosis and Follow-Up. New York, NY: Oxford University Press; 2010 [Google Scholar]
- 6. Schultze-Lutter F, Schimmelmann BG, Ruhrmann S, Michel C. ‘A rose is a rose is a rose’, but at-risk criteria differ. Psychopathology. 2013; 46:75–87 [DOI] [PubMed] [Google Scholar]
- 7. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229 [DOI] [PubMed] [Google Scholar]
- 8. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164 [DOI] [PubMed] [Google Scholar]
- 9. Schultze-Lutter F, Klosterkötter J, Picker H, Steinmeyer E, Ruhrmann S. Predicting first-episode psychosis by basic symptom criteria. Clin Neuropsychiatry. 2007;4:11–22 [Google Scholar]
- 10. Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Sub-threshold of psychosis- a challenge to diagnosis and treatment. Clin Neuropsychiatry. 2010;7:72–87 [Google Scholar]
- 11. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193 [DOI] [PubMed] [Google Scholar]
- 12. Seidman LJ, Giuliano AJ, Meyer EC, et al. ; North American Prodrome Longitudinal Study (NAPLS) Group. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fusar-Poli PG Deste R Smieskova R et al. Cognitive functioning in prodromal psychosis: a meta-analysis of cognitive functioning in prodromal psychosis. Arch Gen Psychiatry. 2012;69:62–71 [DOI] [PubMed] [Google Scholar]
- 14. Cannon TD. Clinical and genetic high-risk strategies in understanding vulnerability to psychosis. Schizophr Res. 2005;79:35–44 [DOI] [PubMed] [Google Scholar]
- 15. Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkötter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr Res. 2007;92:116–125 [DOI] [PubMed] [Google Scholar]
- 16. Addington J, Cadenhead KS, Cornblatt BA, et al. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riecher-Rössler A, Pflueger MO, Aston J, et al. Efficacy of using cognitive status in predicting psychosis: a 7-year follow-up. Biol Psychiatry. 2009;66:1023–1030 [DOI] [PubMed] [Google Scholar]
- 18. Zimmermann R, Gschwandtner U, Wilhelm FH, Pflueger MO, Riecher-Rössler A, Fuhr P. EEG spectral power and negative symptoms in at-risk individuals predict transition to psychosis. Schizophr Res. 2010;123:208–216 [DOI] [PubMed] [Google Scholar]
- 19. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European Prediction of Psychosis Study. Arch Gen Psychiatry. 2010;67:241–251 [DOI] [PubMed] [Google Scholar]
- 21. Nieman DH, Rike WH, Becker HE, et al. Prescription of antipsychotic medication to patients at ultra high risk of developing psychosis. Int Clin Psychopharmacol. 2009;24:223–228 [DOI] [PubMed] [Google Scholar]
- 22. Becker HE, Nieman DH, Dingemans PM, van de Fliert JR, de Haan L, Linszen DH. Verbal fluency as a possible predictor for psychosis. Eur Psychiatry. 2010;25:105–110 [DOI] [PubMed] [Google Scholar]
- 23. Velthorst E, Nieman DH, Becker HE, et al. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr Res. 2009;109:60–65 [DOI] [PubMed] [Google Scholar]
- 24. Dragt S, Nieman DH, Veltman D, et al. Environmental factors and social adjustment as predictors of a first psychosis in subjects at ultra high risk. Schizophr Res. 2011;125:69–76 [DOI] [PubMed] [Google Scholar]
- 25. van Tricht MJ, Nieman DH, Koelman JH, et al. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol Psychiatry. 2010;68:642–648 [DOI] [PubMed] [Google Scholar]
- 26. Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329 [DOI] [PubMed] [Google Scholar]
- 27. Nieman DH, Koelman JH, Linszen DH, Bour LJ, Dingemans PM, Ongerboer de Visser BW. Clinical and neuropsychological correlates of the P300 in schizophrenia. Schizophr Res. 2002;55:105–113 [DOI] [PubMed] [Google Scholar]
- 28. Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701 [DOI] [PubMed] [Google Scholar]
- 29. van der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr Res. 2005;77:309–320 [DOI] [PubMed] [Google Scholar]
- 30. Frommann I, Brinkmeyer J, Ruhrmann S, et al. Auditory P300 in individuals clinically at risk for psychosis. Int J Psychophysiol. 2008;70:192–205 [DOI] [PubMed] [Google Scholar]
- 31. Bramon E, Shaikh M, Broome M, et al. Abnormal P300 in people with high risk of developing psychosis. Neuroimage. 2008;41:553–560 [DOI] [PubMed] [Google Scholar]
- 32. Ozgürdal S, Gudlowski Y, Witthaus H, et al. Reduction of auditory event-related P300 amplitude in subjects with at-risk mental state for schizophrenia. Schizophr Res. 2008;105:272–278 [DOI] [PubMed] [Google Scholar]
- 33. Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2012;42:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71:98–104 [DOI] [PubMed] [Google Scholar]
- 35. Hall RC. Global Assessment of Functioning. A modified scale. Psychosomatics. 1995;36:267–275 [DOI] [PubMed] [Google Scholar]
- 36. Schmand B Bakker D Saan R Louman J The Dutch Reading Test for Adults: a measure of premorbid intelligence level. Tijdschr Gerontol Geriatr. 1991;22:15–19 [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 38. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Arlington, VA: American Psychiatric Publishing; 1997 [Google Scholar]
- 39. Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Soc Psychiatry Psychiatr Epidemiol. 1998;33:80–88 [DOI] [PubMed] [Google Scholar]
- 40. McGlashan TH Miller TJ Woods SW Rosen JH Hoffman RE Davdison L Structured Interview for Prodromal Syndromes. New Haven, CT: PRIME Research Clinic Yale School of Medicine; 2001 [Google Scholar]
- 41. Schultze-Lutter F, Klosterkötter J. Bonn Scale for the Assessment of Basic Symptoms - Prediction List, BSABS-P. Cologne, Germany: University of Cologne; 2002 [Google Scholar]
- 42. Schultze-Luttter F, Addington J, Ruhrmann S, Klosterkötter J. Schizophrenia Proneness Instrument, Adult version (SPI-A). Rome, Italy: Giovanni Fioriti Editore s.r.l.: 2007 [Google Scholar]
- 43. Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull. 1982;8:470–484 [DOI] [PubMed] [Google Scholar]
- 44. Lezak MD. Neuropsychological Assessment. New York, N: Y: Oxford University Press; 1995 [Google Scholar]
- 45. Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484 [DOI] [PubMed] [Google Scholar]
- 46. Duncan CC, Barry RJ, Connolly JF, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120:1883–1908 [DOI] [PubMed] [Google Scholar]
- 47. Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142 [DOI] [PubMed] [Google Scholar]
- 48. Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17 [DOI] [PubMed] [Google Scholar]
- 49. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed New York, NY: John Wiley & Sons; 2000 [Google Scholar]
- 50. Haynes RB, Sackett DL, Guyatt GH, Tugwell P. Clinical Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Williams; 2006 [Google Scholar]
- 51. Ruhrmann S, Klösterkotter J, Bodatsch M, et al. Chances and risks of predicting psychosis. Eur Arch Psych Clin Neurosci. 2012;262(suppl 2):85–90 [DOI] [PubMed] [Google Scholar]
- 52. Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966 [DOI] [PubMed] [Google Scholar]
- 53. Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cornblatt BA, Carrión RE, Addington J, et al. Risk factors for psychosis: impaired social and role functioning. Schizophr Bull. 2012;38:1247–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–1335 [DOI] [PubMed] [Google Scholar]
- 56. Haas GL, Sweeney JA. Premorbid and onset features of first-episode schizophrenia. Schizophr Bull. 1992;18:373–386 [DOI] [PubMed] [Google Scholar]
- 57. Kelley ME, Gilbertson M, Mouton A, van Kammen DP. Deterioration in premorbid functioning in schizophrenia: a developmental model of negative symptoms in drug-free patients. Am J Psychiatry. 1992;149:1543–1548 [DOI] [PubMed] [Google Scholar]
- 58. Strauss JS Carpenter WT Jr Prediction of outcome in schizophrenia. III. Five-year outcome and its predictors. Arch Gen Psychiatry. 1977;34:159–163 [DOI] [PubMed] [Google Scholar]
- 59. Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288 [DOI] [PubMed] [Google Scholar]
- 60. McCarley RW, Salisbury DF, Hirayasu Y, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331 [DOI] [PubMed] [Google Scholar]
- 61. van Mastrigt S, Addington J. Assessment of premorbid function in first-episode schizophrenia: modifications to the Premorbid Adjustment Scale. J Psychiatry Neurosci. 2002;27:92–101 [PMC free article] [PubMed] [Google Scholar]