Abstract
Cannabis use is associated with an earlier age of onset of psychosis (AOP). However, the reasons for this remain debated. Methods: We applied a Cox proportional hazards model to 410 first-episode psychosis patients to investigate the association between gender, patterns of cannabis use, and AOP. Results: Patients with a history of cannabis use presented with their first episode of psychosis at a younger age (mean years = 28.2, SD = 8.0; median years = 27.1) than those who never used cannabis (mean years = 31.4, SD = 9.9; median years = 30.0; hazard ratio [HR] = 1.42; 95% CI: 1.16–1.74; P < .001). This association remained significant after controlling for gender (HR = 1.39; 95% CI: 1.11–1.68; P < .001). Those who had started cannabis at age 15 or younger had an earlier onset of psychosis (mean years = 27.0, SD = 6.2; median years = 26.9) than those who had started after 15 years (mean years = 29.1, SD = 8.5; median years = 27.8; HR = 1.40; 95% CI: 1.06–1.84; P = .050). Importantly, subjects who had been using high-potency cannabis (skunk-type) every day had the earliest onset (mean years = 25.2, SD = 6.3; median years = 24.6) compared to never users among all the groups tested (HR = 1.99; 95% CI: 1.50- 2.65; P < .0001); these daily users of high-potency cannabis had an onset an average of 6 years earlier than that of non-cannabis users. Conclusions: Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users.
Key words: psychotic disorders, age of onset, gender, cannabis, survival plots, drug use, high-potency cannabis
Introduction
Psychotic patients with a history of cannabis use have an earlier age of onset of psychosis (AOP) than those without such a history by almost 3 years, as summarized by Large et al (2011).1 However, many studies have shown that male gender is also associated with a younger age of onset of schizophrenia.2–4 Because males are more likely to use recreational drugs than females,5,6 this raises the question of whether the earlier AOP in cannabis users could be confounded by gender.
The age at starting cannabis and subsequent patterns of cannabis use may also be relevant as they influence risk of psychosis. For example, Arseneault et al (2002) reported that the risk of schizophreniform psychosis is greatest among those starting early in adolescence.7 A popular idea suggests that this is because the immature brain is especially susceptible to the adverse effects of cannabis.8–10 On the other hand, an Australian survey has suggested that AOP is determined by duration of use of cannabis. These investigators concluded that the apparent relationship with age of first use of cannabis is an artifact caused by those who started earlier having had a longer duration of use. Thus, the effect of cannabis use on risk of developing psychotic disorders and on AOP might be conditional on the cumulative effect of cannabis on the individual.11
Evidence compatible with this latter suggestion comes from our previous work showing that frequency of cannabis use is important in determining risk of psychosis, and that use of high potency types of cannabis such as “skunk” carries a higher risk of psychosis.12,13 This is of public health importance since high potency types are increasing available.14
Given that the current factors, which are influencing risk, might also influence AOP, we set out to investigate (1) whether the association between cannabis use and younger AOP could be explained by the confounding effect of male gender and if not, (2) which aspects of the pattern of cannabis use—(a) frequency of use, (b) type of cannabis used, and (c) the age at first use—determine the association with earlier AOP.
Methods
Participants
As part of the Genetics and Psychosis (GAP) case-control study,12,13 we approached all patients aged 18–65 years who presented with their first episode of psychosis to the adult in-patient units of the South London and Maudsley NHS Foundation Mental Health Trust between 2005 and 2010. Patients who met ICD10 criteria (WHO 1992) for a diagnosis of nonaffective (F20-F29) and affective (F30-F33) psychosis, validated by administering the Schedules for Clinical Assessment in Neuropsychiatry (SCAN),15 were invited to participate in the study. The SCAN interview was used to elucidate symptoms present in the month before assessment. Those cases meeting criteria for organic psychosis (F09) were excluded. If cases were too unwell to cooperate, they were recontacted following initiation of treatment.
Of the 606 approached, 145 (23%) refused to take part. Refusers were more likely to be of black [χ2 (3) = 52.9; P < .01] and of male gender [χ2 (1) = 75.3; P < .01] compared to those who consented. Therefore, in all the analyses, we tested for the potential confounding effect of ethnicity and gender. In addition, as part of a qualitative project nested in the study, 12 refusers were asked why they did not take part in the study; the main reasons were lack of interest in the research and the length of our assessment.
Thus, 461 first-episode patients were recruited. However, the data we present here are based on the 410 patients (89.1%) for whom we had complete data on both exposure to cannabis and on date of first contact with psychiatric services for psychosis. Ninety-two (22.4%) of the sample met criteria for Affective Psychosis, the rest having Non-Affective Psychosis.
Measures
Sociodemographic data were collected using a standard schedule.16 From March 2006, a more detailed history of cannabis use was taken by adding to the assessment the Cannabis Experience Questionnaire modified version, CEQmv.12 This later addition meant that there were data missing on detailed pattern of cannabis use of those participants recruited before this (10.9%).
From the CEQmv, we derived detailed information on history of use of tobacco, alcohol, cannabis, and other illicit drugs. Those who reported that they had used cannabis were asked about their age at first use and their duration of use (expressed in years).
Cannabis use was recorded as (1) lifetime history of cannabis use, ie, had the subject ever used cannabis at any point in their lifetime prior to illness onset? (no = 0; yes = 1); (2) lifetime frequency of cannabis use, ie, the frequency that characterized the subject’s most consistent pattern of use (none = 0; at week ends or less frequently = 1; everyday = 2); among the cannabis users, a small proportion of subjects (0.5%) reported having used cannabis more than 4 days a week, and they were included in the everyday category; and (3) type of cannabis used, ie, the type preferentially used by the subject (none = 0; low potency (hash-type) = 1; high potency (skunk-type = 2). This variable was grouped according to the characteristics of the cannabis samples seized by the Police in South East London.17 In that study, skunk-type cannabis was found to have an average delta-9-tetrahydrocannabinol (THC) content of 16% compared with 4% for hash-type cannabis. In addition, skunk-type cannabis may have a greater psychotogenic effect than hash also because it contains less cannabidiol (CBD), which may have antipsychotic properties.18–21 In the London survey, the hash samples had a THC/CBD ratio = 1 while the skunk samples had virtually no CBD.17
AOP was defined as the date of first contact with psychiatric services for psychosis. This definition of onset was chosen as it is clearly defined, represents the time when not just one but a cluster of psychotic symptoms had reached the illness threshold (according to ICD10 criteria), and is the most commonly reported definition of illness onset in studies of schizophrenia.4
Statistical Analysis
Stata 12 was used for all analyses. An initial analysis included all participants on whom we had information on lifetime cannabis use, and the association between any lifetime cannabis use, pattern of use, and AOP was investigated.
Since these were longitudinal data, with time to AOP as the outcome, survival analyses were used. The results are shown graphically in Kaplan-Meier (KM) plots, and Cox regression was used for statistical modeling. In the survival analyses, the variable AOP was calculated as the time from birth to first contact with psychiatric services for psychosis.
It is only possible to make a meaningful distinction between “age” and “cohort” in studies that have recruited over long periods, ie, decades. In this study, the patients were recruited between 2005 and 2010, so age and cohort are almost identical; the subjects’ age coincides with the AOP (the outcome) in a first-episode psychosis cohort. Therefore, in the analyses, we do not adjust for “cohorts = age” since this would mean actually adjusting for the outcome.
Finally, the association between the age at which participants first used cannabis and their AOP was considered. The mean age at first cannabis use was 16.04 years (SD = 4.3) and the median 16 years; 95% and 99% of the users had started, respectively, by age 17 and 24 years. Although it might be thought more appropriate to consider age at first use as a continuous predictor, examination of its effect using several dummy variables by partitioning the variable at the quartile points showed that the effect of age at first use was not linear with the log-hazard rate of psychosis onset. The group starting <15 years was associated with an earlier age at onset, while the other 3 quartiles were similar; therefore, we dichotomized age at first use into 2 categories: 1 = younger than 15 years (25th percentile) and 0 = 15 years or older. This cutoff also reflected the existing literature that indicates an age at first use of cannabis younger than 15 years as a critical window of exposure associated with a greater risk of developing psychosis.8,22–26
KM graphs of survival functions and Nelson-Aalen (NA) plots as well a formal numerical test were used to assess the assumption of proportionality required for a Cox regression. Associations between potential confounders with both AOP and cannabis use were assessed using chi-squared tests. Unadjusted Cox proportional hazard (PH) regressions were run for each exposure of interest (cannabis use, age at first use, cannabis type or frequency of use). These models were then adjusted for any significant covariates, along with gender, which was specified as a covariate a priori. Cox regression is the appropriate technique to use since these are time-to-event data. A hazard ratio (HR) of greater than 1 was interpreted as corresponding to a younger AOP (higher probability of experiencing a psychotic disorder at a particular time point).
Results
Sample Characteristics
Our sample was predominantly male (66%). Totally, 62% had used cannabis at some point in their lives. The distribution of the AOP of the whole sample was skewed, with the majority of onsets occurring at younger ages (supplementary figure 1). Therefore, we report the AOP with both its mean and median values. The mean AOP was 29.58 (SD = 9.04) years and the median was 27.6 years; 10% of people had an onset aged 20 years or less, and 10% (the 90th centile) aged 42 years or older.
Ethnicity [χ2 (3) = 17.3; P = .001], other illicit drugs use [χ2 = 98.6 (1); P < .001], and tobacco use [χ2 (1) = 128.35; P < .001] were associated with cannabis use (table 1). However, we found no association between ethnicity [χ2 (3) = 1.92; P = .59], tobacco use (z = 1.64; P = .10) or other illicit drug use (z = −0.29; P = .76), and AOP.
Table 1.
Demographics of Patients, According to History of Cannabis Use
| History of Cannabis Use (n = 256) |
No History of Cannabis Use (n = 154) |
Test Statistic | P Value | |
|---|---|---|---|---|
| Mean (SD); median age at onset of psychosis (y) | 28.2 (8.0); 27.1 | 31.4 (9.9); 30.0 | z = 3.13 | .001 |
| Gender, n (%) | ||||
| Male | 194 (75.9) | 81 (52.5) | χ2 (1) = 24.5 | <.001 |
| Female | 62 (24.1) | 73 (47.5) | ||
| Ethnicity | ||||
| White | 88 (34.1) | 42 (26.9) | χ2 (3) = 17.3 | .001 |
| Black African | 93 (36.4) | 39 (25.6) | ||
| Black Caribbean | 47 (18.2) | 56 (35.9) | ||
| Other | 28 (11.2) | 17 (11.6) | ||
| Other illicit drugs use | ||||
| Never | 131 (51.3) | 148 (96.7) | χ2 (1) = 98.6 | <.001 |
| Yes | 125 (48.7) | 6 (3.3) | ||
| Lifetime tobacco smoking | ||||
| Never | 25 (10.1) | 100 (65.0) | χ2 (1) = 128.4 | <.001 |
| Yes | 231 (89.9) | 54 (35.0) | ||
Note: P values from Mann-Whitney U and chi-squared tests.
Gender and Cannabis Use
Males had AOP significantly younger (mean years = 28.8, SD = 8.7; median years = 27.3) than females (mean years = 31.2, SD = 9.4; median years = 29.3; HR = 1.26; 95% CI: 1.02–1.56; P = .028). Furthermore, males were more likely to have a history of cannabis use [χ2 (1) = 24.5; P < .001] than females.
An unadjusted Cox proportional hazards regression (n = 410) found a significant relationship between AOP and history of cannabis use. Subjects who used cannabis had a significantly higher probability to present with their first episode of psychosis at a younger age (mean years = 28.2 y, SD = 8.0; median years = 27.1 y) than those who never used it (mean years = 31.4 y, SD = 9.9; median years = 30.0 y; HR = 1.42; 95% CI: 1.16–1.74; P < .001). This was only slightly attenuated when adjusting for gender and remained significant (HR = 1.39; 95% CI: 1.11–1.68; P < .001) table 2. This finding reflects the fact that within each gender, cannabis users had an earlier onset than nonusers. Moreover, males who used cannabis had an earlier onset (mean years = 26.2, SD = 7.4; median years = 25.7) than females of the same cannabis using status (mean years = 28.7, SD = 9.3; median years = 27.9; HR = 1.23; 95% CI: 0.996–1.52) figure 1. This effect, however, marginally fell short of being statistically significant (P = .054). The interaction between gender and history of cannabis use was not significant (P = .28).
Table 2.
Effects of Pattern of Cannabis Use on the Hazard for Experiencing Onset of Psychotic Disorder at Any Given Time
| Mean (SD); Median (y) Age of Onset of Psychosis | Adjusted Hazard Ratioa | 95% CI | P Value | |
|---|---|---|---|---|
| Cannabis use | ||||
| Never | 31.4 (9.9); 30.0 | — | ||
| Yes | 28.2 (8.0); 27.1 | 1.39 | 1.11–1.68 | <.001 |
| Type used independent of frequency of use | ||||
| Hash-like (low potency) | 30.1 (9.6); 28.9 | — | ||
| Skunk-like (high potency) | 26.7 (6.3) 25.2 | 1.68 | 1.08–2.63 | .002 |
| Frequency of use independent of type used | ||||
| Less than daily | 29.8 (9.2); 28.5 | — | ||
| Daily | 27.1 (7.8); 25.9 | 1.60 | 1.11–2.31 | .011 |
| Age at first use of cannabis | ||||
| >15 y | 29.1 (8.5); 27.8 | — | ||
| ≤15 y | 27.0 (6.2); 26.9 | 1.36 | 1.04–1.80 | .050 |
| Age at first use of cannabis independent of type used and frequency of use | ||||
| >15 y | 29.1 (8.5); 27.8 | — | ||
| ≤15 y | 28.9 (8.2); 27.4 | 1.18 | 0.81–1.73 | .37 |
| Hazard ratio for the effect of degree of exposure to cannabis | ||||
| Never used cannabis | 31.4 (9.9); 30.0 | — | ||
| Hash less than weekly | 31.1 (9.5); 29.8 | 0.90 | 0.49–1.28 | .357 |
| Hash everyday | 29.5 (8.9); 27.4 | 1.15 | 0.64–2.09 | .624 |
| Skunk less than weekly | 26.5 (7.6); 25.3 | 1.48 | 1.17–2.04 | .015 |
| Skunk everyday | 25.2 (6.3); 24.6 | 1.99 | 1.50–2.65 | <.0001 |
Note: aAll the above HR adjusted for gender.
Fig. 1.
This graph illustrates that cannabis use is associated with an earlier age of onset of psychosis in both males and females. Among those who never used cannabis, males are still younger than females when they experience their onset of psychosis. SD = standard deviation. As the age of onset is not normally distributed, we also report the median age in years (M = median).
NA survival probability plots of age of onset, gender, and history of cannabis use were used to assess the proportionality assumption required for a Cox PH model, and no violations of this assumption were found.
Type of Cannabis Used and Pattern of Use
Among users, associations were found between gender and type of cannabis used (χ2 (1) = 13.33; P < .001) as well as frequency of use [χ2 (1) = 24.51; P < .001], male subjects being more likely than females to have used cannabis daily and to prefer high-potency (skunk-type) cannabis. Male subjects were also more likely to have started using cannabis at a younger age compared to females (males: mean years = 16.1, SD = 4.0; median years = 15.5; females: mean years = 18.4, SD = 6.8; median years = 16.5; z = −2.7; P = .007). Among those with a history of cannabis use, the mean duration of use was 9.5 years (SD = 7.1); median = 8.1 years. There was no association between gender and duration of use (z = 0.26; P = .79).
We investigated associations between the type, and frequency of cannabis use, with AOP. Adjusting for gender, and frequency of use (n = 154), patients who preferentially used high-potency (skunk-type) cannabis had a significantly earlier onset (mean years = 26.7, SD = 6.3; median years = 25.2; HR = 1.68; 95% CI: 1.08–2.63; P = .002), than patients who used low potency (hash-type) cannabis (mean years = 30.1, SD = 9.6; median years = 28.9). In the same model, daily cannabis use was associated with an earlier AOP (mean years = 27.1, SD = 7.8; median years = 25.9; HR = 1.60; 95% CI: 1.11–2.31; P = .011) compared to that of occasional cannabis users (mean years = 29.8, SD = 9.2; median years = 28.5) table 2. This implies that once gender and the type or frequency of cannabis use are taken into account (1) use of high-potency (skunk-type) cannabis and (2) daily use are independently associated with earlier AOP.
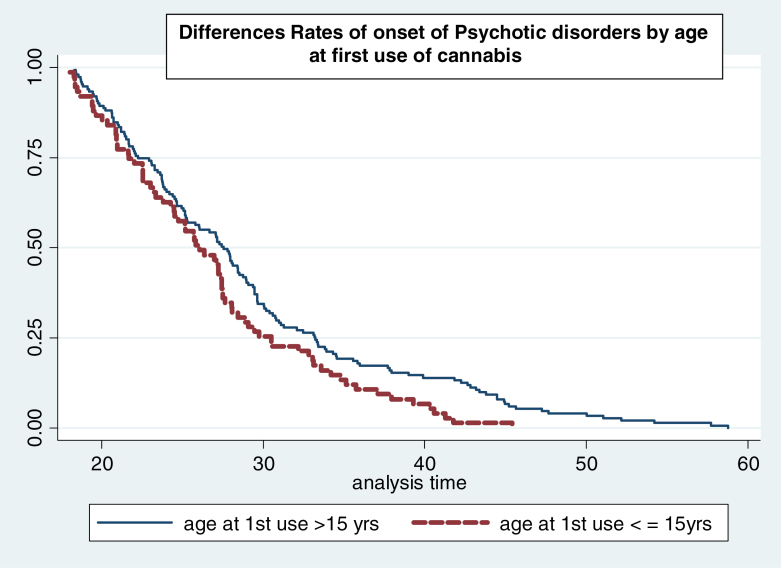
Those who first used cannabis earlier than age 15 reported more frequent use [χ2 (1) = 12.5; P < .001] and preferential use of high-potency (skunk-type) cannabis [χ2 (1) = 5.4; P = .002] than those who began using at age 15 or older. Earlier age at first use was found to be associated with an earlier AOP (HR = 1.40; 95% CI: 1.06–1.83; P = .002). When adjusting for gender and duration, this association remained significant (HR = 1.36; 95% CI: 1.04–1.80; P = .050) table 2. Thus, among cannabis users of the same age, gender, and duration of use, those who began using cannabis earlier than 15 years experienced psychosis onset at a significantly younger age (figure 2).
Fig. 2.
Kaplan–Meier survival curves showing rate (y axis) of onset for participants grouped by age at first use of cannabis. Subjects who started using cannabis at age 15 years or younger experience their onset of psychosis (x axis in years) earlier compared with those who started using cannabis older than 15 years of age.
However, when gender, type of cannabis used, and frequency of use were controlled for (n = 143), the association between age at first use and AOP was no longer significant (HR = 1.18; 95% CI: 0.81–1.73) table 2.
Formal tests of the models described above showed no violations of the proportional hazards assumption.
The Effect of Degree of Exposure to Cannabis
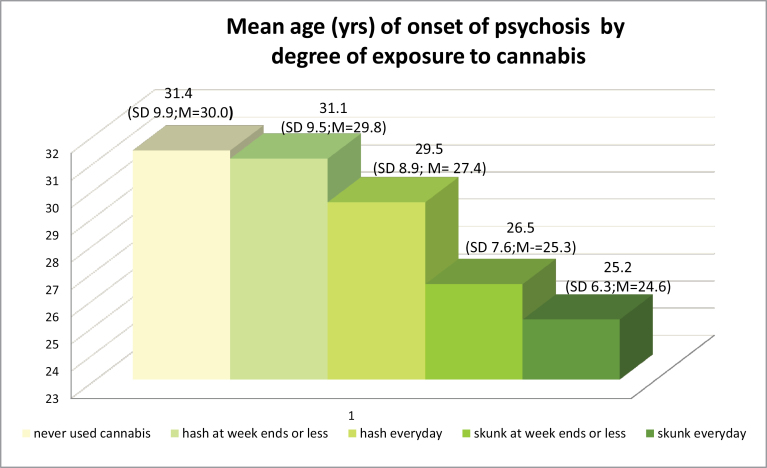
Using Kruskal-Wallis test, the median age of onset of psychotic disorders significantly varied [χ2 (4) = 19.99; P < .001] when we compared those who never used cannabis with the following 4 groups (representing a graded degree of exposure to cannabis): 1 = low potency (hash-type) at weekends or less; 2 = low potency (hash-type) every day; 3 = high potency (skunk-type) at week ends or less; 4 = high potency (skunk) every day. Figure 3 shows a step-wise reduction in mean AOP from those who never used cannabis to those who had used high-potency type cannabis, every day. Those patients who used high-potency cannabis daily were on average over 6 years younger at time of onset (mean age = 25.2, SD = 6.3; median years = 24.6) compared to those who never used cannabis (mean years = 31.4, SD = 9.9; median years = 30.0).
Fig. 3.
This graph illustrates that subjects who never used cannabis experience their first episode of psychosis at the oldest ages (mean ages in years) compared to those who used cannabis. In addition, the greater is the degree of exposure to cannabis (cannabis exposure scale expressed in type x frequency), the youngest is the mean age of onset of psychotic disorders. SD = standard deviation. As the age of onset is not normally distributed, we also report the median age in years (M = median).
In a final Cox PH model adjusted for gender, we found the effect of cannabis use on AOP was driven by a dose effect with those who had been using high-potency cannabis every day having an earlier onset of developing a psychotic disorder at any given time when compared to non-cannabis users of the same age and gender (HR = 1.99; 95% CI: 1.50–2.65; P < .0001). Thus, they had the earliest onset among all the groups tested (table 2).
Discussion
The mean age of onset of our sample is consistent with those previous first-episode psychosis studies, which included subjects who presented up to 65 years of age and both nonaffective and affective psychosis, the latter known to have an onset at an older age.4
Moreover, our survival analyses confirm the findings of Large et al (2011) that patients with a history of cannabis use have an AOP on average 3 years younger than those who never used cannabis.1 In contrast with of Large et al (2011), we found no association between having used other drugs or tobacco, or any confounding sociodemographic variables, and AOP.1,27 However, consistent with the literature, males not only had an AOP significantly younger than females but were also more likely to have used cannabis. So, if males have an earlier onset and are also more likely to use cannabis, is the association between cannabis use and early onset just an artifact of gender?
The data reported in figure 1 and in table 2 clearly show that the subjects with the earliest AOP are male cannabis users. Indeed, the Cox regression analysis, adjusted for gender, indicates that subjects with a history of cannabis use have a 39% increased hazard of experiencing a psychosis onset at any given time compared to never users of the same gender and age. Thus, in line with a Dutch study,28 our data suggest an independent effect of both gender and cannabis use on AOP and identify male cannabis users as the group with the earliest AOP.
Moreover, consistent with evidence that the magnitude of the risk is dependent upon the potency of the cannabis used and the frequency of use,12,13 we found a differential effect of pattern of cannabis use on AOP. Daily use and use of high-potency (skunk) cannabis had independent effects in bringing further forward the AOP compared to those who used infrequently and or low potency (hash) cannabis. This indicates that the effect of cannabis use on the timing of onset of a psychotic illness is dose dependent, an effect consistent with the dose-dependent association between cannabis use and risk of developing psychosis.29
The association between cannabis use and risk of psychosis is reported to be greater in those who started using cannabis in early adolescence when sensitive brain developmental processes are taking place.8,10,30,31 Therefore, it is plausible that starting cannabis in early adolescence might also impact on AOP. Indeed, our findings show that patients who had started using cannabis before 15 years of age had a greater probability of experiencing their onset of psychotic disorder at any given time than someone of the same age who started use later (figure 2). Interestingly, this association remained significant when taking into account gender and duration of use but disappeared when we controlled for the type of cannabis used and the frequency of use. Because patients who started using cannabis before age 15 were much more likely to be daily users, our data cannot entirely separate the effects of age of first cannabis use and frequency of use, or, for that matter, age of first use, frequency and total accumulated dose. It could be that the more cannabis one is exposed to, the greater the risk of psychosis (and the earlier the onset) and that the earlier one starts using cannabis, the greater scope there is to be exposed.
This latter would be consistent with the data of Stefanis et al (2013).11 Nevertheless, unlike them, we did not find that duration of cannabis use explained the association between early age at first use and AOP. Rather, it was how frequently an individual used cannabis and what type they chose to use which best predicted the probability of an earlier AOP. Thus, our data are the first to clearly indicate that rather than just measuring the duration of cannabis use, it is important to establish how often and what type of cannabis has been used. This provides a better estimate of the effect of cannabis use on risk of psychosis and on AOP. This is similar to the situation among subjects reporting 10 years of drinking alcohol; here the risk of onset of liver disease is more accurately estimated by how often and what individuals drink rather than simply their duration of alcohol consumption.
Limitations
One possible limitation is that we used the date of first contact with psychiatric services for a psychotic disorder as the estimate for AOP. Many studies have considered such contact as a proxy for date of onset of psychotic disorders.1,4 Estimating the date of onset of the first psychotic symptom from patients’ self-report is liable to recall bias; in contrast, the date of first contact with psychiatric services is clearly defined and is the most commonly used definition of age at onset, allowing comparisons with other studies.
However, it has been suggested by Compton et al (2009) that an earlier age of contact in cannabis users may just mean that those who have used cannabis become visible to services more quickly (ie, have a shorter duration of untreated psychosis [DUP]) than those who never used cannabis, though the actual age of onset of symptoms may not differ. Furthermore, the same authors have suggested that during the period of untreated psychosis, before coming in contact with services, some individuals may intensify their cannabis use and thus precipitate the onset of full-blown psychosis.32 We, therefore, examined these hypotheses using information that we collected from a subgroup (N = 270) using the Nottingham DUP assessment.33 We found no difference in the mean (P = .220) and median (P = .839) lengths of DUP, estimated as number of weeks of untreated psychosis, between patients who had used cannabis and those who never did. Moreover, there was no association between length of DUP and frequency of use (P = .420) or type of cannabis used (P = .634). Our results are consistent with those of Myles et al (2012), which indicate that cannabis use does not significantly affect DUP.34 Furthermore, Barnett et al (2007) showed that prodromal patients are more likely to stop using cannabis if they experience psychotic symptoms rather than continuing or increasing their use.35
Another possible source of recall bias derives from using information on cannabis use based on self-reported data. Therefore, in a random sample of 56 cases, we carried out a urine drug screen to test the reliability of the data on current use (up to 4 weeks prior the assessment). Of the 56 cases tested, 34 had reported they were not current users; 32 of these (94%) had a negative urinary drug screening; only 2 tested positive and these were excluded from the analyses.13 Moreover, any such bias is likely to underestimate the strength of the relationship we report between cannabis use and age of onset. Other evidence has suggested that asking psychotic patients about their use of cannabis is, at least in some situations, more accurate than urine or blood testing.36
Finally, our sample is representative of subjects accessing the adult psychiatric services who, therefore, experience their AOP between age 18 and 65 years. Thus, we are excluding those who have their illness onset in the early teens, a time when many adolescents are likely to first try cannabis.37
Implications
Many of those who make contact with services for the first time and receive a diagnosis of a psychotic disorder are still in education or attempting to enter the job market and perhaps not yet in a long-term relationship, ie, without important sources of social and emotional stability. Consequently, experiencing an earlier AOP may impact negatively on their likelihood of achieving their optimum level of function. If, by avoiding cannabis use, an individual’s AOP can be delayed up to 6 years, this could enable him/her to finish education and learn some professional skills, thus possibly facilitating later recovery.
The greater effect of high-potency cannabis is of substantial public health importance, as use of such types is becoming commoner in many countries.14,38 Indeed, it is critical that educational campaigns are developed informing young adolescents, particularly males, of their risk when using cannabis, especially daily and of the high potency types.
Conclusions
We confirm an association between cannabis use and earlier AOP and further show this to be independent of gender. Moreover, daily cannabis use and the use of high-potency cannabis are independently associated with a significantly higher hazard to make contact with services for psychosis at any given time. Finally, a younger age at first cannabis use (≤15 years) is associated with a younger AOP only in those who had used cannabis daily. All these findings support a true effect of cannabis use on AOP, which is dose dependent, similar to its effect on risk of developing a psychotic disorder.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Funding was provided by the UK National Institute of Health Research Specialist Biomedical Research Centre for Mental Health, SLAM, the Institute of Psychiatry at King’s College London, The Psychiatry Research Trust, the Maudsley Charity Research Fund, and by the European Community’s Seventh Framework Program (HEALTH-F2-2009–241909, Project EU-GEI).
Supplementary Material
Acknowledgments
This project was carried out in collaboration with The GAP and PUMP study teams and The South London and Maudsley (SLaM) NHS Foundation Trust. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561 [DOI] [PubMed] [Google Scholar]
- 2. Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28 (suppl 2):17–54 [DOI] [PubMed] [Google Scholar]
- 3. Castle D, Sham P, Murray R. Differences in distribution of ages of onset in males and females with schizophrenia. Schizophr Res. 1998;33:179–183 [DOI] [PubMed] [Google Scholar]
- 4. Eranti SV, Maccabe JH, Bundy H, Murray RM. Gender difference in age at onset of schizophrenia: a meta-analysis. Psychol Med. 2013;43:155–167 [DOI] [PubMed] [Google Scholar]
- 5. Chen KW, Banducci AN, Guller L, et al. An examination of psychiatric comorbidities as a function of gender and substance type within an inpatient substance use treatment program. Drug Alcohol Depend. 2011;118:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schepis TS, Desai RA, Cavallo DA, et al. Gender differences in adolescent marijuana use and associated psychosocial characteristics. J Addict Med. 2011;5:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monshouwer K, Smit F, de Graaf R, van Os J, Vollebergh W. First cannabis use: does onset shift to younger ages? Findings from 1988 to 2003 from the Dutch National School Survey on Substance Use. Addiction. 2005;100:963–970 [DOI] [PubMed] [Google Scholar]
- 9. Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–385 [DOI] [PubMed] [Google Scholar]
- 10. Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35:1779–1787 [DOI] [PubMed] [Google Scholar]
- 11. Stefanis NC, Dragovic M, Power BD, Jablensky A, Castle D, Morgan VA. Age at initiation of cannabis use predicts age at onset of psychosis: the 7- to 8-year trend. Schizophr Bull. 2013;39:251–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Forti M, Iyegbe C, Sallis H, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72:811–816 [DOI] [PubMed] [Google Scholar]
- 14. Sheila Hardwick LK. Home office cannabis paper potency study: http//science.homeoffice.gov.uk/hosdb/; 2008
- 15. World Health Organization. “Schedules for Clinical Assessment in Neuropsychiatry (SCAN).” Geneva, Switzerland: WHO; 1992 [Google Scholar]
- 16. Mallett R, Leff J, Bhugra D, Pang D, Zhao JH. Social environment, ethnicity and schizophrenia. A case-control study. Soc Psychiatry Psychiatr Epidemiol. 2002;37:329–335 [DOI] [PubMed] [Google Scholar]
- 17. Potter DJ, Clark P, Brown MB. Potency of delta 9-THC and other cannabinoids in cannabis in England in 2005: implications for psychoactivity and pharmacology. J Forensic Sci. 2008;53:90–94 [DOI] [PubMed] [Google Scholar]
- 18. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19–27 [DOI] [PubMed] [Google Scholar]
- 20. Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–1616 [DOI] [PubMed] [Google Scholar]
- 22. Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160:511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galvez-Buccollini JA, Proal AC, Tomaselli V, et al. Association between age at onset of psychosis and age at onset of cannabis use in non-affective psychosis. Schizophr Res. 2012;139:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griffith-Lendering MF, Wigman JT, Prince van Leeuwen A, et al. Cannabis use and vulnerability for psychosis in early adolescence–a TRAILS study. Addiction. 2013;108:733–740 [DOI] [PubMed] [Google Scholar]
- 25. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67:440–447 [DOI] [PubMed] [Google Scholar]
- 26. Schubart CD, van Gastel WA, Breetvelt EJ, et al. Cannabis use at a young age is associated with psychotic experiences. Psychol Med. 2011;41:1301–1310 [DOI] [PubMed] [Google Scholar]
- 27. Power BD, Dragovic M, Jablensky A, Stefanis NC. Does accumulating exposure to illicit drugs bring forward the age at onset in schizophrenia? Aust N Z J Psychiatry. 2013;47:51–58 [DOI] [PubMed] [Google Scholar]
- 28. Dekker N, Meijer J, Koeter M, et al. GROUP Investigators. Age at onset of non-affective psychosis in relation to cannabis use, other drug use and gender. Psychol Med. 2012;42:1903–1911 [DOI] [PubMed] [Google Scholar]
- 29. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572 [DOI] [PubMed] [Google Scholar]
- 30. Konings M, Henquet C, Maharajh HD, Hutchinson G, Van Os J. Early exposure to cannabis and risk for psychosis in young adolescents in Trinidad. Acta Psychiatr Scand. 2008;118:209–213 [DOI] [PubMed] [Google Scholar]
- 31. Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18 [DOI] [PubMed] [Google Scholar]
- 32. Compton MT, Kelley ME, Ramsay CE, et al. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009;166:1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh SP, Cooper JE, Fisher HL, et al. Determining the chronology and components of psychosis onset: The Nottingham Onset Schedule (NOS). Schizophr Res. 2005;80:117–130 [DOI] [PubMed] [Google Scholar]
- 34. Myles N, Newall H, Nielssen O, Large M. The association between cannabis use and earlier age at onset of schizophrenia and other psychoses: meta-analysis of possible confounding factors. Curr Pharm Des. 2012;18:5055–5069 [DOI] [PubMed] [Google Scholar]
- 35. Barnett JH, Werners U, Secher SM, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007;190:515–520 [DOI] [PubMed] [Google Scholar]
- 36. Hjorthøj CR, Fohlmann A, Larsen AM, Arendt M, Nordentoft M. Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clinical trial. Addiction. 2012;107:1123–1131 [DOI] [PubMed] [Google Scholar]
- 37. Perkonigg A, Lieb R, Höfler M, Schuster P, Sonntag H, Wittchen HU. Patterns of cannabis use, abuse and dependence over time: incidence, progression and stability in a sample of 1228 adolescents. Addiction. 1999;94:1663–1678 [DOI] [PubMed] [Google Scholar]
- 38. UNODC. World Drug Report 2013. Crime UNOoDa, UNODC 2013. United Nation on Drugs and Crime. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.