Abstract
Stress has been linked to the pathogenesis of schizophrenia. Genetic variation in neuregulin 1 (NRG1) increases the risk of developing schizophrenia and may help predict which high-risk individuals will transition to psychosis. NRG1 also modulates sensorimotor gating, a schizophrenia endophenotype. We used an animal model to demonstrate that partial genetic deletion of Nrg1 interacts with stress to promote neurobehavioral deficits of relevance to schizophrenia. Nrg1 heterozygous (HET) mice displayed greater acute stress-induced anxiety-related behavior than wild-type (WT) mice. Repeated stress in adolescence disrupted the normal development of higher prepulse inhibition of startle selectively in Nrg1 HET mice but not in WT mice. Further, repeated stress increased dendritic spine density in pyramidal neurons of the medial prefrontal cortex (mPFC) selectively in Nrg1 HET mice. Partial genetic deletion of Nrg1 also modulated the adaptive response of the hypothalamic-pituitary-adrenal axis to repeated stress, with Nrg1 HET displaying a reduced repeated stress-induced level of plasma corticosterone than WT mice. Our results demonstrate that Nrg1 confers vulnerability to repeated stress-induced sensorimotor gating deficits, dendritic spine growth in the mPFC, and an abberant endocrine response in adolescence.
Key words: schizophrenia, neuregulin 1, PPI, dendritic morphology, adolescence, stress
Introduction
Current theories of schizophrenia pose that the disorder arises due to a complex interaction between genetic and environmental factors in early development that disturbs brain maturation.1–3 Stress may be the common denominator linking several environmental factors that increase the risk of developing schizophrenia.2 Childhood, adolescent, and adult stressful life events trigger the onset of schizophrenia symptoms.4 Further, alterations in hypothalamic-pituitary-adrenal (HPA) axis function such as blunted stress-induced levels of cortisol have been observed in schizophrenia patients.5,6 Studies on adopted identical twins living in different environments suggest that genetic predisposition to schizophrenia interacts with stressful upbringing to cause schizophrenia.7 Animal studies have provided evidence that schizophrenia susceptibility genes such as disturbed in schizophrenia 1 and pituitary adenylate cyclase-activating peptide may confer vulnerability to the effects of stress,8,9 but more studies are needed to observe whether additional genes play a role.
Human genetic studies have isolated neuregulin 1 (NRG1) as a putative schizophrenia susceptibility gene, and schizophrenia patients show altered expression of neuregulin 1-ErbB receptor components.10–13 Genetic variation in NRG1 has recently been shown to offer a potential genetic marker in the prediction of which ultrahigh-risk individuals transition to psychosis.14 NRG1 is involved in various central nervous system developmental processes including synapse formation and the plasticity of dendrites and particularly dendritic spines.15–17 Schizophrenia patients and individuals with single nucleotide polymorphisms (SNPs) in NRG1 display deficits in prepulse inhibition (PPI) of startle.18,19 PPI is a highly heritable form of sensorimotor gating in which the organism shows a weaker startle response to a startling stimulus that is preceded by a lower magnitude, nonstartling stimulus.
Nrg1 HET mice provide a viable animal model to explore gene-environment interactions of relevance to understanding schizophrenia. These mice show altered neurobehavioral sensitivity to drugs of abuse that activate the HPA axis and are linked to triggering psychotic reactions in humans.20–28 Our work on Nrg1-cannabinoid interactions has been translated in human studies because NRG1 SNPs increase cannabis dependence risk and magnify cannabinoid effects on attentional processing.29,30 Nrg1 HET mice show impairments in PPI12,23,24,31,32; however, this phenotype is unstable and difficult to reproduce.22,33–35 Here, we will examine if stress is necessary for unmasking PPI deficits in Nrg1 HET mice, a finding that would reconcile the inconsistent reports of this phenotype in the literature. We used environmentally enriched, group-housed control mice to support normal cognitive and sensorimotor development as a means to clearly characterize whether Nrg1 and stress interact to promote PPI deficits.36
The medial prefrontal cortex (mPFC) and hippocampus are strongly implicated in the neurobiology of schizophrenia, stress, and PPI.37–40 Both human and animal studies have shown that the mPFC regulates sensorimotor gating function.41,42 Infusion of drugs that modulate neurotransmission in the mPFC and deletion of N-methly-D-aspartic acid (NMDA) receptors selectively from pyramidal neurons in layers II/III of the mPFC promotes PPI deficits.43–46 The mPFC undergoes significant changes in neuronal connectivity in adolescence, which may confer greater vulnerability to the effects of stress on dendritic morphology, including the plasticity of dendritic spines that harbor the majority of excitatory synapses in the brain.16,38,47 Adolescent mice also display a greater response to stress than adult mice due to prolonged activation of the HPA axis.48,49 Glucocorticoids also modulate dendritic morphology in the mPFC50,51 and exert higher level negative feedback on the HPA axis.52,53 Recent evidence demonstrates that the loss of dendritic spines in layers II/III of the mPFC promoted by chronic stress exposure reduces the inhibitory influence of the mPFC on HPA axis activity.54 In this study, we hypothesize that Nrg1 hypomorphism confers vulnerability to the effects of stress on sensorimotor gating function, dendritic spine density in the mPFC, and regulation of the HPA axis.
Material and Methods
Mice
Adolescent (postnatal day [PND] 35–49) male and female Nrg1 HET mice (C57BL/6JArc background strain) and wild-type (WT) littermates were used. Nrg1 transmembrane domain knockout mice were generated by Prof Richard Harvey (Victor Chang Cardiac Research Institute, Sydney) using a targeting vector in which most of exon 11, which encodes the transmembrane domain, was replaced by a neomycin resistance gene cassette.12 Homozygous Nrg1 knockout mice die as embryos due to cardiac defects; however, Nrg1 HET mice grow to be healthy and fertile. Nrg1 HET mice exhibit various schizophrenia-relevant behavioral and neurobiological phenotypes. They display locomotor hyperactivity that can be reversed by clozapine, PPI deficits, a context-dependent increase in aggressive behavior, diminished social and novel object recognition memory, and impaired contextual fear conditioning.12,55–58 They also exhibit NMDA receptor hypofunction and increased expression of serotonin 5-HT2A receptors and serotonin transporters.59,60 Further details of mice housing conditions are described in the supplementary material. All research and animal care procedures were approved by the University of Sydney’s Animal Ethics Committee and were in agreement with the Australian Code of Practice for the Care and use of Animals for Scientific Purposes.
Experimental Design
For an overview of the experimental design see figure 1A. Male and female mice were randomly allocated to 4 experimental groups: (1) WT-no stress (WT NS; n = 17, 10 female, 7 male); (2) WT-stress (WT S; n = 19, 9 female, 10 male); (3) Nrg1 HET-no stress (Nrg1 HET NS; n = 18, 7 female and 11 male); and (4) Nrg1 HET-stress (Nrg1 HET S; n = 17, 10 female, 7 male).
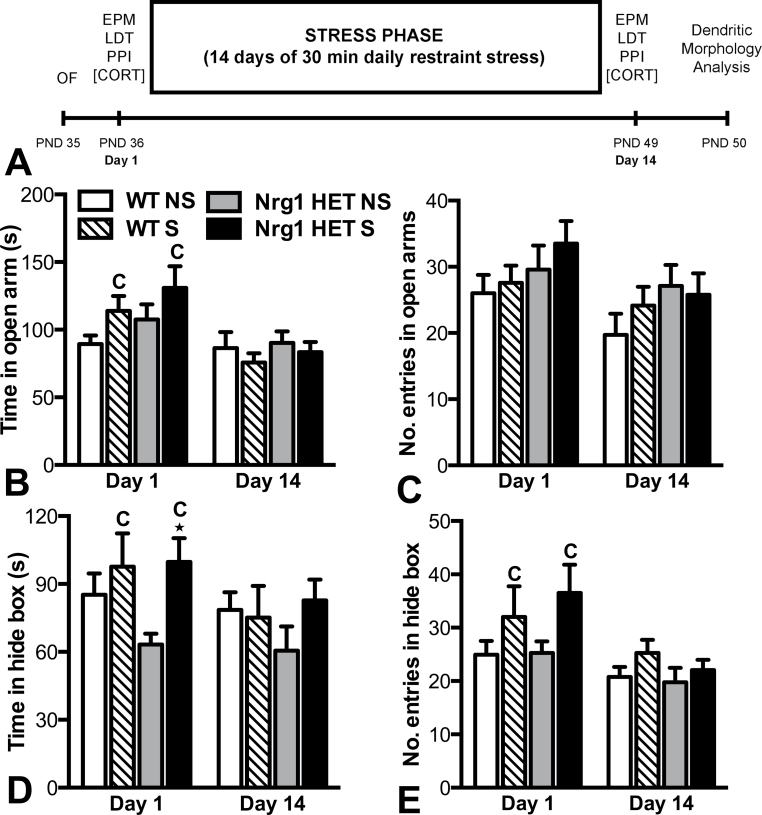
Fig. 1.
Examination of the acute and repeated effects of restraint stress on anxiety-related behaviors in adolescent WT and Nrg1 HET mice. (A) Overview of the experimental design. (B) Time in the open arms, and (C) entries in the open arms in the elevated plus maze. (D) Time in the hide box and (E) entries into hide box in the light-dark test. Acute restraint stress significantly increased time in the hide box of Nrg1 HET mice compared with nonstressed Nrg1 HET mice *P < .05. C represents a significant main effect of stress condition in the two factor ANOVA. Data are presented as means ± SEM. WT, wild-type mice; Nrg1 HET, neuregulin 1 heterozygous mice; NS, nonstressed homecage controls; S, mice subjected to restraint stress.
On PND 35, baseline locomotor activity was measured in the open field for 20 minutes to confirm that Nrg1 HET mice displayed a hyperactive phenotype (supplementary figure S1). From PND 36 (day 1) onward, mice were subjected to 30min/day of restraint stress for 14 days until PND 49 (day 14). Restraint stress was chosen because it is a well-characterized psychological stressor in rodents, which activates the HPA axis, increases anxiety-related behavior, and reduces PPI in rodents.61–64 Furthermore, it is well established that repeated restraint stress induces morphological changes in the medial prefrontal cortex and hippocampus, structures implicated in the pathophysiology of schizophrenia and involved in circuits regulating PPI.65–69 A study using the psychosocial stressor, chronic social defeat stress, demonstrated greater stress-induced working memory impairments in adolescent Nrg1 HET mice compared with WT mice; however, this study could not discern any greater effects of stress on PPI in Nrg1 HET mice.32 Social defeat studies require control animals to undergo social isolation, which we hypothesize led to a floor effect on PPI in control Nrg1 HET mice that precluded the delineation of any Nrg1-stress interaction on PPI. Therefore, in this study, we utilized restraint stress, which allowed comparison with control mice that were group housed under environmentally enriched conditions. Environmentally enriched housing is beneficial when exploring gene and environment interactions (G × E) in mice because it better approximates human cognitive and sensorimotor development than standard housing.36
Immediately following the 30 minute restraint stress session on days 1 and 14, all animals underwent three consecutive tests in a battery of minimally stressful behavioral tests to reduce the impact of stress on our control mice: first, the elevated plus maze (EPM; 10min), followed by the light-dark test (LDT; 10min), and finally the PPI paradigm (30min). The EPM and LDT, both animal models of anxiety, were included to confirm that our restraint stress paradigm was an effective stressor and to provide a contrast to the observations made in the PPI paradigm, which is more relevant to the pathophysiology of schizophrenia. A 5-minute rest period occurred between the behavioral tests that were conducted in the same sequence on both day 1 and day 14. Blood and brains were extracted (for corticosterone and dendritic morphology analysis respectively) immediately following the 30 minute restraint session or 24h following the final restraint stress episode, respectively. In addition, mice were examined for their recovery from the stressor by sampling plasma 90 minute after the completion of an acute restraint stress episode. A detailed description of restraint stress, behavioral testing, corticosterone assays, and dendritic morphology quantification procedures can be found in the supplementary material.
The mPFC and the CA1 region of the hippocampus were chosen for dendritic morphology analysis because they are both involved in the regulation of PPI, are stress sensitive, and are implicated in the pathophysiology of schizophrenia.39,70,71 We chose to focus on layers II/III of the dorsal anterior cingulate cortex and prelimbic cortex because these areas are vulnerable to dendritic atrophy following restraint stress in both rats and mice.38,47,67,72–74 The dorsal anterior cingulate and prelimbic cortices are difficult to distinguish in Golgi-stained tissue, therefore they were analyzed together as previously described.51 Another reason for focusing on layers II/III is that there is a preponderance of morphological and neurobiological abnormalities that have been observed in homologous regions of the schizophrenia brain.75 In particular, pyramidal neurons of layer III of the dorsolateral prefrontal cortex of schizophrenia brain show reductions in dendritic spine density.76,77 We chose the CA1 region of the hippocampus because this region also undergoes loss of dendritic spines following repeated restraint stress.47,78 Furthermore, in adolescent mice, dendritic spines exhibit greater plasticity in the CA1 region than in the CA3 region in response to genetic differences in stress reactivity.79
Statistical Analysis
Statistical analyses were performed using SPSS (IBM) or Statview (SAS Institute Inc) software. All data were analyzed using 3- or 4-factor ANOVA (between subjects or mixed model analyses) with, where appropriate, genotype, condition, or day as between-subject factors and day or prepulse intensity as within-subject factors. Two-factor ANOVA for day 1 or day 14 data separately were also performed. PPI data were analyzed by 2-factor ANOVA with repeated measures (for prepulse intensity) on days 1 and 14. Planned Bonferroni-corrected comparisons were conducted to further analyze differences between experimental groups on all measures using the following analyses (WT-no stress vs Nrg1 HET-no stress, WT-stress vs Nrg1 HET-stress, WT-no stress vs WT-stress, and Nrg1 HET-no stress vs Nrg1 HET-stress). The results of all analyses were deemed significant at P < .05.
Results
Acute but Not Repeated Restraint Stress Increased Anxiety-Related Behavior in a Task-Specific Manner Selectively in Nrg1 HET Mice
Data for the EPM and LDT paradigms is given in figure 1. Three-factor mixed model ANOVA of time spent on the open arms of the EPM data showed a significant effect of day (F 1,63 = 11.23, P < .01) and trend for a genotype by condition by day interaction (F 1,63 = 3.81, P = .07). Two-factor ANOVA of day 1 data revealed no effect of genotype or a genotype by condition interaction for time spent on the open arms (figure 1B). There was a significant effect of condition (F 1,64 = 4.27, P < .05), indicating stress significantly increased time spent on the open arm following acute stress. On day 14, no effect of genotype, condition, or a genotype by condition interaction was observed. Three-factor mixed model ANOVA of entries into the open arms of the EPM showed a significant effect of day (F 1,62 = 9.92, P < .01) only, with mice entering the open arms less on day 14 than on day 1 (figure 1C). Two-factor ANOVA showed no effect of genotype, condition, or a significant genotype by condition interaction on day 1 or day 14. Planned Bonferroni comparisons of time spent in the open arms and number of entries in the open arms revealed no significant differences between any of the groups on day 1 or day 14.
Three-factor mixed model ANOVA of LDT data (see figures 1D and 1E) revealed a trend toward an increased time spent in the hide box and a significant increase in the number of entries into the hide box following the stress condition (F 1,66 = 3.27, P = .07; F 1,66 = 5.20, P < .05, respectively). There was also a significant effect of day for both of these measures (F 1,66 = 4.89, P < .05; F 1,66 = 12.96, P < .001, respectively). Two-factor ANOVA of day 1 data revealed a significant effect of condition on the time spent and entries into the hide box (F 1,66 = 5.14, P < .05; F (1,66) = 3.62, P < .05, respectively), demonstrating that restraint stress significantly increased anxiety-like behaviors in the LDT. However, there was no effect of genotype or a genotype by condition interaction for both these measures. Planned Bonferroni comparisons showed that stressed Nrg1 HET mice spent significantly more time in the hide box than nonstressed Nrg1 HET mice (P < .05). No significant comparisons were evident when comparing WT-stress with WT nonstressed animals, nor when comparing homecage control Nrg1 HET and WT mice for either hide time or entries into the hide box on day 1. For day 14, 2-factor ANOVA showed no main effects of genotype or condition, and there was no significant genotype by condition interaction. Bonferrroni comparisons showed no significant differences between any of the groups on day 14. The effects observed on anxiety-related behavior in the EPM and LDT cannot be explained by changes observed in locomotor activity in these tests as assessed by total entries and total distance travelled data collected in these models, respectively (see supplementary figure S2).
Adolescent Nrg1 HET Mice but Not WT Mice Exposed to Repeated Restraint Stress Failed to Normally Develop PPI
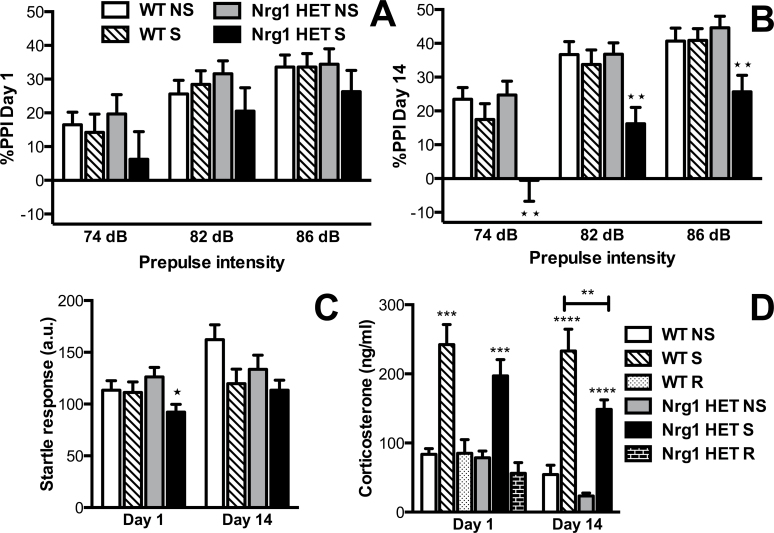
Data for the PPI paradigm is given in figure 2. Four-factor mixed model ANOVA found a significant main effect of condition (F 1,67 = 6.64, P < .05) and a significant genotype by condition interaction (F 1,67 = 4.75, P < .05). In addition, we observed a significant effect of prepulse intensity (F 2,66 = 123.93, P < .0001). While there was no overall significant effect of day in the 4-factor mixed model ANOVA, nonstressed Nrg1 HET mice and WT mice (both nonstressed and stressed) together displayed a developmental increase in PPI (effect of day, 2-factor repeated measures ANOVA: F 1,53 = 4.86, P < .05) that was not evident in the repeatedly stressed Nrg1 HET mice. Three-factor mixed model ANOVA of day 1 data similarly showed a significant effect of prepulse intensity (F 1,67 = 101.99, P < .001), highlighting that %PPI increased with the prepulse intensity (figure 2A). No other effects were observed on day 1. On day 14 (figure 2B), 3-factor mixed model ANOVA revealed a significant effect of prepulse intensity (F 1,67 = 95.67, P < .001), a trend for an effect of genotype (F 1,67 = 3.90, P = .05) and a significant effect of condition (F 1,67 = 10.15, P < .01). Importantly, there was a significant genotype by condition interaction (F 1,67 = 5.91, P < .05) because stressed Nrg1 HET mice selectively showed reduced %PPI unlike stressed WT mice. Bonferroni-corrected planned contrasts indicated that restraint stress on day 14 significantly reduced %PPI in Nrg1 HET mice at prepulse intensities of 74 (P < .01), 82 (P < .01), and 86 dB (P < .01). No such effects were observed in WT mice.
Fig. 2.
Partial genetic deletion of Nrg1 conferred vulnerability to repeated stress-induced prepulse inhibition (PPI) deficits and a blunted HPA axis response to stress in adolescence. (A) %PPI following acute stress, and (B) repeated stress. Repeated restraint stress significantly reduced %PPI in Nrg1 HET mice compared with nonstressed Nrg1 HET mice at 74, 82, and 86 dB prepulse intensities **Ps < .01. (C) Startle response to a 120 dB stimulus. Acute stress significantly reduced the startle response in Nrg1 HET mice compared with nonstressed Nrg1 HET mice *P < .05. (D) Plasma corticosterone concentrations sampled immediately after restraint stress and following recovery from stress when sampled 90 minutes after the restraint stress episode. Acute and repeated stress significantly increased corticosterone compared with respective nonstressed mice in both WT and Nrg1 HET mice ***Ps < .001, ****P < .0001. Following repeated stress, stress-induced plasma corticosterone concentrations were significantly lower in Nrg1 HET mice than WT mice **P < .01. Data are represented as means ± SEM. a.u., arbitrary units; WT, wild-type mice; Nrg1 HET, neuregulin 1 heterozygous mice; NS, nonstressed homecage controls; S, mice subjected to restraint stress; R, mice after 90-minute recovery from acute stress.
The PPI deficit observed in Nrg1 HET mice following repeated restraint stress cannot be confounded by stress-induced changes in body weight (supplementary table S1) or the startle response because we observed no concomitant effects on these measures. Three-factor mixed model ANOVA of startle response data showed a significant main effect of condition (F 1,67 = 7.66, P < .01), a significant effect of day (F 1,67 = 9.47, P < .01), and trend for a genotype by condition by day interaction (F 1,67 = 3.81, P = .05) (figure 2C). On days 1 and 14, 2-factor ANOVA revealed no effect of genotype or a genotype by condition interaction for startle response; however, a trend toward an effect of condition was observed on both days (F 1,67 = 3.94, P = .05; F 1,67 = 5.69, P < .05, respectively). On day 1, unlike WT mice, stress exposure significantly reduced the startle response in Nrg1 HET mice compared with nonstressed controls, as highlighted by Bonferroni-corrected planned comparisons (P < .05).
Repeated Restraint Stress Induced Increased Corticosterone Levels in Adolescent WT Mice Relative to Nrg1 HET Mice
Data for plasma corticosterone levels is given in figure 2D. Three-factor between-subject ANOVA revealed a significant effect of genotype (F 1,66 = 6.66, P < .05), condition (F 1,66 = 110.47, P < .0001), and day (F 1,66 = 4.67, P < .05) but no significant interactions. A 2-factor ANOVA on day 1 data revealed a significant effect of condition (F 2,45 = 36.55, P < .001) because both WT and Nrg1 HET mice exhibited an equivalent elevation in plasma corticosterone levels in response to an acute restraint stress session. This was further supported by planned Bonferroni comparisons that showed acutely stressed WT and Nrg1 HET mice had significantly increased corticosterone levels compared with their respective homecage controls (P < .001) and that the levels of stressed WT and Nrg1 HET mice did not significantly differ. Nrg1 HET and WT mice both displayed an equivalent complete recovery to baseline plasma corticosterone levels 90 minutes after the acute restraint stress episode (P > .05).
Two-factor ANOVA of animals subjected to 14 days of daily 30-minute restraint stress showed a significant effect of genotype (F 1,32 = 9.86, P < .01) and stress (F 1,32 = 67.95, P < .001) on plasma corticosterone levels but no genotype by stress interaction. Planned Bonferroni comparisons indicated that repeated restraint stress maintained an elevated plasma corticosterone level in both WT and Nrg1 HET mice compared with their respective homecage controls; however, stressed WT mice had a significantly increased plasma corticosterone concentration compared with stressed Nrg1 HET mice (P < .001). No significant differences were observed between nonstressed WT and Nrg1 HET groups on day 14 (P > .05).
Repeated Restraint Stress Increased Apical Dendritic Spine Density and Decreased Apical Dendritic Lengths and Complexity in Layer II/III Pyramidal Neurons of the mPFC in Adolescent Nrg1 HET mice but not in WT Mice
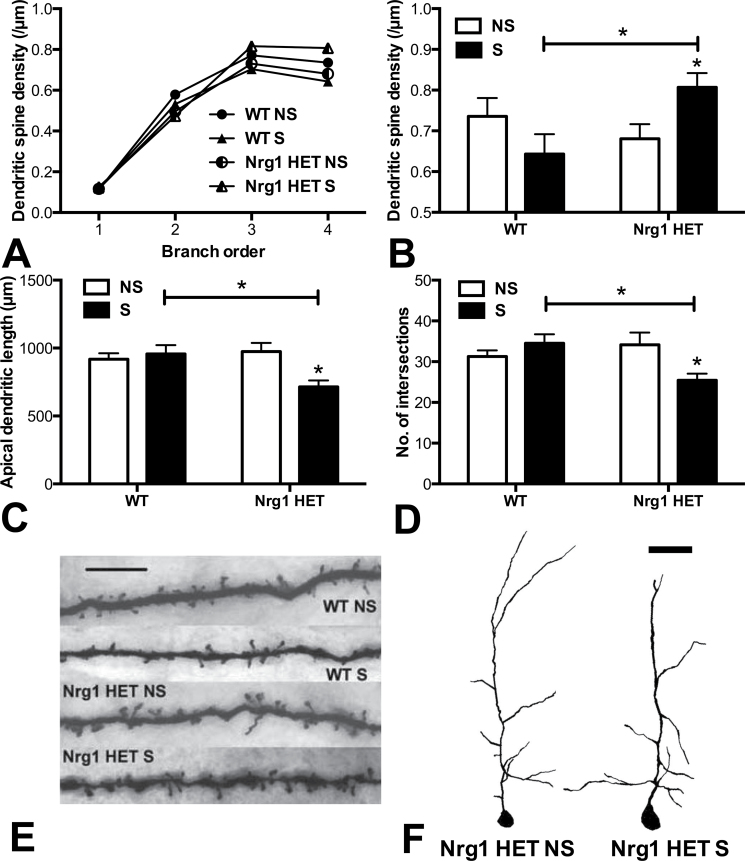
Data for dendritic spine densities, dendritic lengths, and dendritic complexity in the mPFC is given in figure 3. There was a significant effect of dendritic branching order (F 1,3 = 323.35, P < .0001) and importantly significant interactions of genotype by condition (F 1,24 = 6.15, P < .05), genotype by order (F 3,72 = 2.86, P < .05) and a trend toward a genotype by condition by order interaction (F 3,72 = 2.53, P = .06; figure 3A). When analyzing all of the branch orders separately using 2-factor ANOVA, there were no significant effects for apical dendritic spine density in the mPFC for orders 1–3. Two-factor ANOVA of fourth order dendrites in the mPFC showed no effect of genotype or condition but a significant genotype by condition interaction (F 1,23 = 6.94, P < .05) (figure 3B; see figure 3E for photomicrographs of representative dendritic spine segments). Planned Bonferroni comparisons of fourth order apical dendrite spine densities in the mPFC revealed that repeatedly stressed Nrg1 HET mice exhibited a significantly increased dendritic spine density compared with homecage Nrg1 HET controls (P < .05) and repeatedly stressed WT mice (P < .05). Repeatedly stressed WT mice showed a nonsignificant trend toward a reduced dendritic spine density compared with WT homecage controls.
Fig. 3.
Partial genetic deletion of Nrg1 promoted a repeated stress-induced increase in apical dendritic spine density and a decrease in dendritic lengths and complexity in layers II/III of the medial prefrontal cortex (mPFC). (A) Dendritic spine density per µm of apical dendrites with increasing dendritic branch order. (B) Dendritic spine density on fourth order dendrites. (C) Cumulative apical dendritic lengths and (D) no. of intersections as determined by Sholl analysis. Repeatedly stressed Nrg1 HET mice exhibited significantly increased dendritic spine density and decreased dendritic lengths and dendritic complexity, in the mPFC compared with nonstressed Nrg1 HET mice or stressed WT mice, *Ps < .05. (E) Representative photomicrographs of Golgi-stained dendritic spines on fourth order apical dendrites in the mPFC, scale bar = 5 µm. (F) Representative tracings of Golgi-stained mPFC apical dendrites in nonstressed vs stressed Nrg1 HET mice, scale bar = 50 µm. WT, wild-type mice; Nrg1 HET, neuregulin 1 heterozygous mice; NS, nonstressed homecage controls; S, mice subjected to restraint stress.
Two-factor ANOVA revealed a significant genotype by condition interaction for dendritic lengths (F 1,24 = 7.34, P < .05) and dendritic complexity as supported by the number of intersections determined using Sholl analysis (F 1,24 = 7.45, P < .05) in the mPFC (figures 3C and 3D, respectively, also see figure 3F for representative traced apical dendrites). Planned Bonferroni comparisons showed that repeatedly stressed Nrg1 HET mice had significantly lower cumulative dendritic lengths and number of intersections than Nrg1 HET control mice (Ps < .05, respectively) and repeatedly stressed WT mice (Ps < .05, respectively).
Nrg1 HET Mice Exhibit Significantly Shorter Apical Dendritic Lengths and Reduced Dendritic Complexity Than WT Mice in the CA1 Region of the Hippocampus
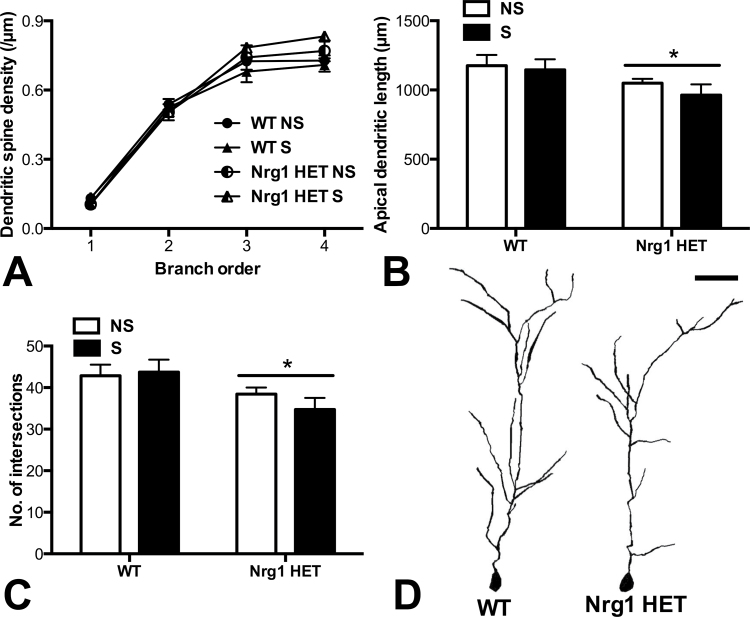
Partial genetic deletion of Nrg1 or stress did not alter dendritic spine density in the CA1 region of the hippocampus (figure 4A). There was a significant effect of dendritic branching order (F 1,3 = 418.89, P < .0001) and a significant interaction between branch order and genotype (F 3,72 = 3.11, P < .05) for dendritic spine density. Two-factor ANOVA of each of the branch orders separately revealed no significant differences in apical spine density in the CA1 region of adolescent Nrg1 HET and WT mice.
Fig. 4.
Partial genetic deletion of Nrg1 decreased apical dendritic lengths and complexity in pyramidal neurons of the CA1 region of the hippocampus. (A) Dendritic spine density per µm of apical dendrites with increasing dendritic branch order. (B) Cumulative apical dendritic lengths and (C) no. of intersections as determined by Sholl analysis. Nrg1 HET mice exhibited shorter apical dendritic lengths and less dendritic complexity than WT mice in the CA1 region, *P < .05 represents main effect of genotype. (D) Representative tracings of Golgi-stained apical dendrites of pyramidal neurons in the CA1 region of the hippocampus, scale bar = 50 µm. WT, wild-type mice; Nrg1 HET, neuregulin 1 heterozygous mice; NS, nonstressed homecage controls; S, mice subjected to restraint stress.
Two-factor ANOVA of apical dendrite lengths and complexity of pyramidal neurons of the CA1 region of the hippocampus revealed significant effects of genotype (F 1,24 = 5.05, P < .05; F 1,24 = 6.73, P < .05, respectively) because Nrg1 HET mice exhibited significantly reduced cumulative apical dendritic lengths and number of intersections compared with WT mice irrespective of their stress condition (figures 4B and 4C, respectively, see figure 4D for representative traced apical dendrites). No effect of condition or a genotype by condition interaction was observed. Planned Bonferroni comparisons revealed no significant differences between specific experimental groups for dendritic length or number of intersections.
Discussion
Our results show that partial genetic deletion of the schizophrenia susceptibility gene Nrg1 coupled with repeated restraint stress impairs normal sensorimotor gating development, correlating with altered dendritic morphology of pyramidal neurons in the mPFC and a reduced repeated stress-induced plasma concentration of corticosterone. Repeated restraint stress in adolescence disrupted the normal development of PPI selectively in Nrg1 HET mice but not in WT mice. We then examined the neuronal basis for this effect by examining dendritic morphology in the mPFC, a structure involved in higher order regulation of sensorimotor gating.80,81 We found that repeated stress selectively enhanced dendritic spine density while also decreasing dendritic length and complexity in the mPFC of Nrg1 HET mice. As glucocorticoid systems are involved in the plasticity of dendritic spines82,83 and pyramidal neurons in layers II/III of the mPFC exerts an inhibitory influence on HPA activity,54,84 we also measured plasma corticosterone levels and observed that Nrg1 HET mice displayed a blunted stress-induced corticosterone response following repeated stress compared with WT mice.
PPI provides a useful endophenotype to improve our understanding of the pathophysiology of schizophrenia, which is complex in its symptomology and genetic and environmental determinants. The study of PPI also has its advantages because it can be reproduced in animals enabling controlled studies to clearly dissect gene-environment interactions and more detailed neurobiological assessments. Individuals with NRG1 variants display deficits in PPI18,19 although another publication could not demonstrate such a relationship.85 Here, we demonstrated PPI deficits were only penetrant following repeated stress exposure in mice with a partial genetic deletion of Nrg1. Our results suggest that the interaction of Nrg1 and stress does not trigger a PPI deficit per se, but rather derails the normal developmental increase in PPI that occurs from adolescence to adulthood.86,87 These findings further clarify the interactive effects of genetic modulation of Nrg1 and stress on neurobehavioral phenotypes in animals32,62,88,89 and humans.90 Johnson and Adler91 observed that stress promoted sensorimotor gating deficits in humans; however, there was a large variability in this response that might be explained by genetic factors. Our findings suggest that variation in NRG1 confer vulnerability to repeated stress-induced PPI deficits in humans, particularly if stress exposure occurs prior to the complete development of PPI in adulthood.
Providing a neurobiological correlate for the abnormal development of PPI in stressed Nrg1 HET mice, we observed that repeated restraint stress selectively increased dendritic spine densities and decreased dendritic lengths and complexity in the mPFC of Nrg1 HET mice but not in WT mice. The dorsolateral PFC, the human homolog of the rodent mPFC, has been strongly associated with abnormal sensorimotor gating in schizophrenia patients,42 and animal studies show that the mPFC exerts higher order control over the striato-pallido-tegmento-pontine pathway that mediates sensorimotor gating. Indeed, infusions of agents that affect dopaminergic, glutamatergic, and cannabinoid neurotransmission in the mPFC modulate PPI.43–45 Further, deletion of NMDA receptor obligatory subunits selectively from pyramidal neurons in layers II/III of the mPFC triggers PPI deficits.46 Altered dendritic morphology in the mPFC of stressed Nrg1 HET mice might impair the normal developmental increase of PPI from adolescence to adulthood by increasing activation of the ventral striatum via direct excitatory glutamatergic inputs to presynaptic dopamine terminals in the nucleus accumbens.92 Although layers II/III of the mPFC only modestly innervate the ventral striatum, the predominant cortico-cortical connections stemming from these mPFC layers might also play a role in mediating the interaction of Nrg1 and stress on the development of PPI.93–96
It is not entirely surprising that Nrg1 and stress interact to modulate dendritic morphology given both Nrg1 and stress alone have been shown to modulate the growth and development of dendrites and dendritic spines.15–17,38,47,65 However, future research is needed to delineate the precise molecular mechanisms subserving the interaction of Nrg1 and stress in the regulation of dendritic morphology in the mPFC. The increased dendritic spine densities in the mPFC of stressed Nrg1 HET mice may be mediated by inflammatory cytokines because Nrg1 HET mice exposed to chronic social defeat stress show reduced expression of interleukin 1β (IL-1β) in the PFC.32 IL-1β has been shown to decrease dendritic spine size and dendritic complexity, and schizophrenia patients with SNPs in IL-1β show increased brain activation in the PFC.97,98 It was surprising to observe here that increased dendritic spine density occurred with a concomitant reduction in dendritic lengths and complexity. More intensive chronic restraint stress paradigms in adult rodents trigger the retraction of dendrites and diminution of dendritic spine densities.38,47,69 However, there are instances of stress inducing opposing effects on dendritic lengths/complexity and dendritic spines, particularly when stress is applied prenatally.99–101 How these apparently conflicting stress-induced changes on dendritic morphology alters the functional connectivity of the mPFC needs to be addressed in future work.
Our findings of a blunted stress-induced corticosterone response in Nrg1 HET mice, a genetic animal model of schizophrenia, accord with studies showing reduced cortisol release following stress in medication-naive schizophrenia patients with first-episode psychosis.6 The mPFC exerts inhibitory control over the HPA axis under conditions of stress. Recent evidence shows that repeated stress-induced loss of dendritic spines in layers II/III of the prelimbic cortex promotes overactivity of the mPFC-anterior bed nucleus of the stria terminalis (aBNST) circuit that normally exerts inhibitory control over the paraventricular nucleus (PVN) of the hypothalamus and ultimately plasma levels of corticosterone under acute stress conditions.54,102,103 Therefore, it is possible that the increased dendritic spine densities in layers II/III of mPFC observed in Nrg1 HET mice following repeated stress may have dampened repeated stress-induced HPA axis activation in these animals via enhancing activation of the inhibitory mPFC-aBNST-PVN circuit.
Since Stefansson et al 12 first reported PPI deficits in Nrg1 HET mice, many studies have failed to replicate this phenomenon highlighting that it is unreliable and subject to the influence of environmental, neurodevelopmental, and methodological factors.33–35 In our experience, adult Nrg1 HET animals (>5 months of age) are more likely to exhibit PPI deficits23,25 than adolescent mice, which at best only display a very mild PPI deficit.22,24 A recent article reported adolescent Nrg1 HET mice displayed marked PPI deficits and that chronic social defeat stress did not accentuate these deficits.32 We propose that the baseline adolescent PPI deficits in the homecage controls observed by Desbonnet et al 32 are explained by the unintended stress exposure experienced through single housing. Therefore, no greater effect of chronic social defeat stress on PPI could be discerned as they had already induced a significant PPI deficit in their control Nrg1 HET mice. Here, we reconcile the many discordant findings made in the literature by demonstrating in environmentally enriched, adolescent Nrg1 HET mice that stress is necessary for unmasking PPI deficits. Our work complements the findings of Desbonnet et al 32 who demonstrated that adolescent stress promoted selective impairments in working memory function in Nrg1 HET mice.
Adolescent Nrg1 HET mice displayed a selective acute stress-induced increase in anxiety-related behavior in the LDT, unlike their stressed WT counterparts. This effect disappeared with repeated stress exposure and was model specific because no differential effect of stress was observed in the EPM. Our prior research in adult Nrg1 HET mice revealed that these animals display a marginally greater acute restraint stress-induced rise in plasma corticosterone levels compared with WT mice62; however, such a difference in adolescent animals was not apparent here. A prolonged behavioral stress response was evident in our Nrg1 HET mice because the differential effect on anxiety-related behavior in the LDT was observed after testing in the EPM. Taylor et al 89 showed that type-II Nrg1 hypomorphic rats displayed an impaired recovery to basal plasma corticosterone levels following restraint stress, which might explain our finding. However, here we showed no genotypic differences in the recovery of plasma corticosterone levels following acute stress.
Homecage control Nrg1 HET mice had shorter apical dendrite lengths and lower dendritic complexity in the CA1 region of the hippocampus compared with WT mice. One allele of the transmembrane domain of Nrg1 is deleted in Nrg1 HET mice, which would reduce all 6 Nrg1 isoforms.12 Nrg1 has neurotrophic effects on hippocampal neurons in primary culture, and depletion of ErbB4 decreases the number of primary neurites, whereas stimulation of ErbB4 using a soluble form of NRG1 results in exuberant dendritic arborization.104,105 Our results are consistent with the notion that Nrg1 HET mice harbor reduced levels of synaptic Nrg1 compared with WT mice in the hippocampus, leading to diminished dendritic lengths and complexity of the apical pyramidal neurons in the CA1 region.
There is conflicting evidence for the role of the Nrg1-ErbB system in schizophrenia, with overactivity and underactivity of this system suggested by human and animal studies. Postmortem studies provided evidence of increased expression of NRG1 isoforms in the PFC and hippocampus of schizophrenia patients.106–108 However, other studies reported a decrease in NRG1 isoform expression in the PFC.109 Indeed, NRG1 isoform expression may be simultaneously increased and decreased in the PFC, dependent on the isoform.110 Brain area–specific alterations in NRG1 cleavage products in postmortem tissue have also been demonstrated, with increased expression of N-terminal NRG1 fragments in the PFC and decreased levels of soluble 50kDa NRG1 fragments in the hippocampus being measured from the same postmortem samples.111 Simply a change in neuregulin expression (whether it be increased or decreased) may be sufficient to increase schizophrenia-related phenotypes, as animal studies show that underexpression (like in Nrg1 HET mice) and overexpression of Nrg1 increased schizophrenia-relevant behaviors such as hyperactivity, PPI deficits, and cognitive impairments.17,25,32,112 Reconciling how bidirectional changes in Nrg1-ErbB signaling may increase schizophrenia-relevant phenotypes and response to stress will be an important aim for future research. Studies may examine the role of NMDA receptors in Nrg1-stress interactions given evidence that both increased or decreased Nrg1-ErbB4 signaling may promote NMDA receptor hypofunction.113
In conclusion, we showed that partial genetic deletion of Nrg1 and repeated stress interact in adolescence to impair the development of sensorimotor gating, blunt the HPA axis response to stress, and alter apical dendritic morphology in the mPFC.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
University of Sydney Bridging Grant (to J.C.A.); Brain & Behavior Research Foundation Young Investigator Award (to J.C.A.); Schizophrenia Research Institute; NSW Ministry of Health grant (1003886 to T.K.); Motor Neuron Disease Research Institute of Australia (Mick Rodger Benalla MND Research Grant); National Health and Medical Research Council (1045643 to T.K.).
Supplementary Material
Acknowledgments
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432 [DOI] [PubMed] [Google Scholar]
- 2. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212 [DOI] [PubMed] [Google Scholar]
- 3. Van Winkel R, Esquivel G, Kenis G, et al. REVIEW: genome-wide findings in schizophrenia and the role of gene-environment interplay. CNS Neurosci Ther. 2010;16:e185–e192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tessner KD, Mittal V, Walker EF. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophr Bull. 2011;37:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2012;135:170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Venrooij JA, Fluitman SB, Lijmer JG, et al. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr Bull. 2012;38:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thapar A, Harold G, Rice F, Langley K, O’donovan M. The contribution of gene-environment interaction to psychopathology. Dev Psychopathol. 2007;19:989–1004 [DOI] [PubMed] [Google Scholar]
- 8. Haque FN, Lipina TV, Roder JC, Wong AH. Social defeat interacts with Disc1 mutations in the mouse to affect behavior. Behav Brain Res. 2012;233:337–344 [DOI] [PubMed] [Google Scholar]
- 9. Ishihama T, Ago Y, Shintani N, et al. Environmental factors during early developmental period influence psychobehavioral abnormalities in adult PACAP-deficient mice. Behav Brain Res. 2010;209:274–280 [DOI] [PubMed] [Google Scholar]
- 10. Alaerts M, Ceulemans S, Forero D, et al. Support for NRG1 as a susceptibility factor for schizophrenia in a northern Swedish isolated population. Arch Gen Psychiatry. 2009;66:828–837 [DOI] [PubMed] [Google Scholar]
- 11. Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res. 2008;100:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatzimanolis A, McGrath JA, Wang R, et al. Multiple variants aggregate in the neuregulin signaling pathway in a subset of schizophrenia patients. Transl Psychiatry. 2013;3:e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bousman CA, Yung AR, Pantelis C, et al. Effects of NRG1 and DAOA genetic variation on transition to psychosis in individuals at ultra-high risk for psychosis. Transl Psychiatry. 2013;3:e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barros CS, Calabrese B, Chamero P, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol. 2011;95:275–300 [DOI] [PubMed] [Google Scholar]
- 17. Chen YJ, Johnson MA, Lieberman MD, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roussos P, Giakoumaki SG, Adamaki E, Bitsios P. The influence of schizophrenia-related neuregulin-1 polymorphisms on sensorimotor gating in healthy males. Biol Psychiatry. 2011;69:479–486 [DOI] [PubMed] [Google Scholar]
- 19. Hong LE, Wonodi I, Stine OC, Mitchell BD, Thaker GK. Evidence of missense mutations on the neuregulin 1 gene affecting function of prepulse inhibition. Biol Psychiatry. 2008;63:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnold JC, Boucher AA, Karl T. The yin and yang of cannabis-induced psychosis: the actions of Δ(9)-tetrahydrocannabinol and cannabidiol in rodent models of schizophrenia. Curr Pharm Des. 2012;18:5113–5130 [DOI] [PubMed] [Google Scholar]
- 21. Boucher AA, Hunt GE, Karl T, Micheau J, McGregor IS, Arnold JC. Heterozygous neuregulin 1 mice display greater baseline and Delta(9)-tetrahydrocannabinol-induced c-Fos expression. Neuroscience. 2007;149:861–870 [DOI] [PubMed] [Google Scholar]
- 22. Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. Transmembrane domain Nrg1 mutant mice show altered susceptibility to the neurobehavioural actions of repeated THC exposure in adolescence. Int J Neuropsychopharmacol. 2013;16:163–175 [DOI] [PubMed] [Google Scholar]
- 23. Long LE, Chesworth R, Huang XF, et al. Distinct neurobehavioural effects of cannabidiol in transmembrane domain neuregulin 1 mutant mice. PLoS One. 2012;7:e34129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spencer JR, Darbyshire KM, Boucher AA, Arnold JC. Adolescent neuregulin 1 heterozygous mice display enhanced behavioural sensitivity to methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:376–381 [DOI] [PubMed] [Google Scholar]
- 25. Boucher AA, Hunt GE, Micheau J, et al. The schizophrenia susceptibility gene neuregulin 1 modulates tolerance to the effects of cannabinoids. Int J Neuropsychopharmacol. 2011;14:631–643 [DOI] [PubMed] [Google Scholar]
- 26. Spencer JR, Darbyshire KM, Boucher AA, et al. Novel molecular changes induced by Nrg1 hypomorphism and Nrg1-cannabinoid interaction in adolescence: a hippocampal proteomic study in mice. Front Cell Neurosci. 2013;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karl T, Arnold JC. What does a mouse tell us about neuregulin 1-cannabis interactions? Front Cell Neurosci. 2013;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spencer JR, Chohan TW, Karl T, Arnold JC. Female neuregulin 1 heterozygous mice require repeated exposure to Delta9-tetrahydrocannabinol to alter sensorimotor gating function. Pharmacopsychiatry. 2013;46:286–291 [DOI] [PubMed] [Google Scholar]
- 29. Han S, Yang BZ, Kranzler HR, et al. Linkage analysis followed by association show NRG1 associated with cannabis dependence in African Americans. Biol Psychiatry. 2012;72:637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stadelmann AM, Roser P, Arning L, Gallinat J, Epplen JT, Juckel G. Acute effects of Delta9-tetrahydrocannabinol on the auditory evoked mismatch negativity are modulated by the NRG1 gene. Pharmacopsychiatry. 2010;43:194–195 [DOI] [PubMed] [Google Scholar]
- 31. Boucher AA, Hunt GE, Micheau J, et al. The schizophrenia susceptibility gene neuregulin 1 modulates tolerance to the effects of cannabinoids. Int J Neuropsychopharmacol. 2011;14:631–643 [DOI] [PubMed] [Google Scholar]
- 32. Desbonnet L, O’Tuathaigh C, Clarke G, et al. Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene × environment interaction. Brain Behav Immun. 2012;26:660–671 [DOI] [PubMed] [Google Scholar]
- 33. van den Buuse M, Wischhof L, Lee RX, Martin S, Karl T. Neuregulin 1 hypomorphic mutant mice: enhanced baseline locomotor activity but normal psychotropic drug-induced hyperlocomotion and prepulse inhibition regulation. Int J Neuropsychopharmacol. 2009;12:1383–1393 [DOI] [PubMed] [Google Scholar]
- 34. Boucher AA, Arnold JC, Duffy L, Schofield PR, Micheau J, Karl T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of Delta9-tetrahydrocannabinol. Psychopharmacology (Berl). 2007;192:325–336 [DOI] [PubMed] [Google Scholar]
- 35. Karl T, Burne TH, Van den Buuse M, Chesworth R. Do transmembrane domain neuregulin 1 mutant mice exhibit a reliable sensorimotor gating deficit? Behav Brain Res. 2011;223:336–341 [DOI] [PubMed] [Google Scholar]
- 36. Burrows EL, McOmish CE, Hannan AJ. Gene-environment interactions and construct validity in preclinical models of psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1376–1382 [DOI] [PubMed] [Google Scholar]
- 37. Alfarez DN, De Simoni A, Velzing EH, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19:828–836 [DOI] [PubMed] [Google Scholar]
- 38. Radley JJ, Rocher AB, Rodriguez A, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl). 2008;199:331–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hazlett EA, Buchsbaum MS, Haznedar MM, et al. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198 [PubMed] [Google Scholar]
- 42. Tregellas JR, Davalos DB, Rojas DC, et al. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valsamis B, Chang M, Typlt M, Schmid S. Activation of mGluR2/3 receptors in the ventro-rostral prefrontal cortex reverses sensorimotor gating deficits induced by systemic NMDA receptor antagonists. Int J Neuropsychopharmacol. 2013:1–10 [DOI] [PubMed] [Google Scholar]
- 44. Shoemaker JM, Saint Marie RL, Bongiovanni MJ, Neary AC, Tochen LS, Swerdlow NR. Prefrontal D1 and ventral hippocampal N-methyl-D-aspartate regulation of startle gating in rats. Neuroscience. 2005;135:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wegener N, Kuhnert S, Thüns A, Roese R, Koch M. Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology (Berl). 2008;198:375–385 [DOI] [PubMed] [Google Scholar]
- 46. Rompala GR, Zsiros V, Zhang S, Kolata SM, Nakazawa K. Contribution of NMDA receptor hypofunction in prefrontal and cortical excitatory neurons to schizophrenia-like phenotypes. PLoS One. 2013;8:e61278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kassem MS, Lagopoulos J, Stait-Gardner T, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47:645–661 [DOI] [PubMed] [Google Scholar]
- 48. Vázquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23:663–700 [DOI] [PubMed] [Google Scholar]
- 49. Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–145 [DOI] [PubMed] [Google Scholar]
- 50. Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006 [DOI] [PubMed] [Google Scholar]
- 52. Czéh B, Perez-Cruz C, Fuchs E, Flügge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter? Behav Brain Res. 2008;190:1–13 [DOI] [PubMed] [Google Scholar]
- 53. Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180 [DOI] [PubMed] [Google Scholar]
- 54. Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J Neurosci. 2013;33:14379–14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O’Tuathaigh CM, O’Connor AM, O’Sullivan GJ, et al. Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous ‘knockout’ of the schizophrenia risk gene neuregulin-1. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:462–466 [DOI] [PubMed] [Google Scholar]
- 56. O’Tuathaigh CM, Babovic D, O’Sullivan GJ, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27 [DOI] [PubMed] [Google Scholar]
- 57. Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes Brain Behav. 2007;6:677–687 [DOI] [PubMed] [Google Scholar]
- 58. Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domain neuregulin 1 mutant mice. Neuroscience. 2010;170:800–807 [DOI] [PubMed] [Google Scholar]
- 59. Dean B, Karl T, Pavey G, Boer S, Duffy L, Scarr E. Increased levels of serotonin 2A receptors and serotonin transporter in the CNS of neuregulin 1 hypomorphic/mutant mice. Schizophr Res. 2008;99:341–349 [DOI] [PubMed] [Google Scholar]
- 60. Bjarnadottir M, Misner DL, Haverfield-Gross S, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/- knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sutherland JE, Conti LH. Restraint stress-induced reduction in prepulse inhibition in Brown Norway rats: role of the CRF2 receptor. Neuropharmacology. 2011;60:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chesworth R, Yulyaningsih E, Cappas E, Arnold J, Sainsbury A, Karl T. The response of neuregulin 1 mutant mice to acute restraint stress. Neurosci Lett. 2012;515:82–86 [DOI] [PubMed] [Google Scholar]
- 63. Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22:82–91 [DOI] [PubMed] [Google Scholar]
- 64. Sutherland JE, Burian LC, Covault J, Conti LH. The effect of restraint stress on prepulse inhibition and on corticotropin-releasing factor (CRF) and CRF receptor gene expression in Wistar-Kyoto and Brown Norway rats. Pharmacol Biochem Behav. 2010;97:227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722 [DOI] [PubMed] [Google Scholar]
- 66. Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30:6726–6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320 [DOI] [PubMed] [Google Scholar]
- 68. Martínez-Téllez RI, Hernández-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804 [DOI] [PubMed] [Google Scholar]
- 69. Magariños AM, Li CJ, Gal Toth J, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Darbra S, Mòdol L, Pallarès M. Allopregnanolone infused into the dorsal (CA1) hippocampus increases prepulse inhibition of startle response in Wistar rats. Psychoneuroendocrinology. 2012;37:581–585 [DOI] [PubMed] [Google Scholar]
- 71. Kusljic S, van den Buuse M. Functional dissociation between serotonergic pathways in dorsal and ventral hippocampus in psychotomimetic drug-induced locomotor hyperactivity and prepulse inhibition in rats. Eur J Neurosci. 2004;20:3424–3432 [DOI] [PubMed] [Google Scholar]
- 72. Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6 [DOI] [PubMed] [Google Scholar]
- 75. Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology. 2010;35:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73 [DOI] [PubMed] [Google Scholar]
- 77. Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pawlak R, Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci U S A. 2005;102:18201–18206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pillai AG, de Jong D, Kanatsou S, et al. Dendritic morphology of hippocampal and amygdalar neurons in adolescent mice is resilient to genetic differences in stress reactivity. PLoS One. 2012;7:e38971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862 [DOI] [PubMed] [Google Scholar]
- 81. Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. Adv Neurol. 1995;66:21–34; discussion 34–26. [PubMed] [Google Scholar]
- 82. Jafari M, Seese RR, Babayan AH, Gall CM, Lauterborn JC. Glucocorticoid receptors are localized to dendritic spines and influence local actin signaling. Mol Neurobiol. 2012;46:304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108:16074–16079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Quednow BB, Schmechtig A, Ettinger U, et al. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol Psychiatry. 2009;66:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Uehara T, Sumiyoshi T, Seo T, et al. Long-term effects of neonatal MK-801 treatment on prepulse inhibition in young adult rats. Psychopharmacology (Berl). 2009;206:623–630 [DOI] [PubMed] [Google Scholar]
- 87. Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcohol Clin Exp Res. 2008;32:2062–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Taylor SB, Taylor AR, Koenig JI. The interaction of disrupted Type II Neuregulin 1 and chronic adolescent stress on adult anxiety- and fear-related behaviors. Neuroscience. 2013;249:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Taylor SB, Taylor AR, Markham JA, Geurts AM, Kanaskie BZ, Koenig JI. Disruption of the neuregulin 1 gene in the rat alters HPA axis activity and behavioral responses to environmental stimuli. Physiol Behav. 2011;104:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kéri S, Kiss I, Seres I, Kelemen O. A polymorphism of the neuregulin 1 gene (SNP8NRG243177/rs6994992) affects reactivity to expressed emotion in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:418–420 [DOI] [PubMed] [Google Scholar]
- 91. Johnson MR, Adler LE. Transient impairment in P50 auditory sensory gating induced by a cold-pressor test. Biol Psychiatry. 1993;33:380–387 [DOI] [PubMed] [Google Scholar]
- 92. Dai H, Okuda H, Iwabuchi K, et al. Social isolation stress significantly enhanced the disruption of prepulse inhibition in mice repeatedly treated with methamphetamine. Ann N Y Acad Sci. 2004;1025:257–266 [DOI] [PubMed] [Google Scholar]
- 93. Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177 [DOI] [PubMed] [Google Scholar]
- 94. Hammer TB, Oranje B, Skimminge A, et al. Structural brain correlates of sensorimotor gating in antipsychotic-naive men with first-episode schizophrenia. J Psychiatry Neurosci. 2013;38:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Campbell LE, Hughes M, Budd TW, et al. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci. 2007;26:2327–2333 [DOI] [PubMed] [Google Scholar]
- 96. Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:463–477 [DOI] [PubMed] [Google Scholar]
- 97. Fatjó-Vilas M, Pomarol-Clotet E, Salvador R, et al. Effect of the interleukin-1β gene on dorsolateral prefrontal cortex function in schizophrenia: a genetic neuroimaging study. Biol Psychiatry. 2012;72:758–765 [DOI] [PubMed] [Google Scholar]
- 98. Goshen I, Avital A, Kreisel T, Licht T, Segal M, Yirmiya R. Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neurosci. 2009;29:3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109 [DOI] [PubMed] [Google Scholar]
- 100. Bock J, Murmu MS, Biala Y, Weinstock M, Braun K. Prenatal stress and neonatal handling induce sex-specific changes in dendritic complexity and dendritic spine density in hippocampal subregions of prepubertal rats. Neuroscience. 2011;193:34–43 [DOI] [PubMed] [Google Scholar]
- 101. Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. MPTP neurotoxicity and testosterone induce dendritic remodeling of striatal medium spiny neurons in the C57Bl/6 mouse. Parkinsons Dis. 2011;2011:138471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Radley JJ. Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front Behav Neurosci. 2012;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–9695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Krivosheya D, Tapia L, Levinson JN, et al. ErbB4-neuregulin signaling modulates synapse development and dendritic arborization through distinct mechanisms. J Biol Chem. 2008;283:32944–32956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004;27:379–393 [DOI] [PubMed] [Google Scholar]
- 106. Weickert CS, Tiwari Y, Schofield PR, Mowry BJ, Fullerton JM. Schizophrenia-associated HapICE haplotype is associated with increased NRG1 type III expression and high nucleotide diversity. Transl Psychiatry. 2012;2:e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307 [DOI] [PubMed] [Google Scholar]
- 108. Law AJ, Lipska BK, Weickert CS, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5’ SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bertram I, Bernstein HG, Lendeckel U, et al. Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann N Y Acad Sci. 2007;1096:147–156 [DOI] [PubMed] [Google Scholar]
- 110. Parlapani E, Schmitt A, Wirths O, et al. Gene expression of neuregulin-1 isoforms in different brain regions of elderly schizophrenia patients. World J Biol Psychiatry. 2010;11:243–250 [DOI] [PubMed] [Google Scholar]
- 111. Marballi K, Cruz D, Thompson P, Walss-Bass C. Differential neuregulin 1 cleavage in the prefrontal cortex and hippocampus in schizophrenia and bipolar disorder: preliminary findings. PLoS One. 2012;7:e36431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yin DM, Chen YJ, Lu YS, et al. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron. 2013;78:644–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pan B, Huang XF, Deng C. Antipsychotic treatment and neuregulin 1-ErbB4 signalling in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:924–930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.