Abstract
A growing body of literature suggests that exposure to infections, particularly maternal infections, during pregnancy confers risk for later development of psychotic disorder. Though brain development proceeds throughout childhood and adolescence, the influence of infections during these ages on subsequent psychosis risk is insufficiently examined. The aim of this study was to investigate the potential association between infections during childhood and nonaffective psychoses in a large population-based birth cohort with follow up long enough to include peak incidence of nonaffective psychosis. We included all individuals born in Sweden between 1973 and 1985, (N = 1172879), with follow up on first time inpatient care with nonaffective psychosis from age 14 years until 2006, (N = 4638). Following adjustment for differences in sex, socioeconomic status, family history of psychosis, and hospital admissions involving noninfectious, nonpsychiatric care, we observed a small but statistically significant association between hospital admissions for infections, in general, throughout childhood (0–13 years) and a later diagnosis of nonaffective psychosis, hazard ratio (HR) = 1.10 (95% CI 1.03–1.18), and this association seemed to be driven by bacterial infection, HR = 1.23 (95% CI 1.08–1.40). Bacterial infections and central nervous system infections during preadolescence (10–13 years) conferred the strongest risk, HR 1.57 (95% CI 1.21–2.05) and HR 1.96 (95% CI 1.05–3.62), respectively. Although preadolescence appeared to be a vulnerable age period, and bacterial infection the most severe in relation to psychosis development, the present findings can also indicate an increased susceptibility to hospital admission for infections among children who will later develop nonaffective psychosis due to social or familial/genetic factors.
Key words: psychosis, prenatal, schizophrenia, epidemiology, cohort study
Introduction
While the etiology of schizophrenia and other nonaffective psychoses remain elusive, they are currently believed to be caused by a genetic predisposition/vulnerability interacting with environmental exposures to interfere with brain development.1 While no major risk alleles for these disorders have been identified, genetic risk has been consistently reported in the major histocompatibility complex (MHC) region on chromosome 62 enriched in genes involved in immune functions. Among various environmental factors, exposures to prenatal infections have been identified as risk factors, reviewed by Brown & Derkits.3 Although fetal life is a crucial period of brain development, the brain development does proceed throughout childhood and adolescence,4 and thus, the critical period for different exposures can potentially span a wider window than the fetal period alone. Accordingly, disturbances in brain maturation during childhood have also been proposed to contribute to the etiology of schizophrenia.5 However, few studies have examined the risks associated with infections during childhood for developing psychotic illness, reviewed by Khandaker et al,6 and hence important knowledge gaps remain. To date, we and others have concentrated only on infections in the central nervous system (CNS). It is therefore not known if CNS infections contribute to the development of psychosis later in life or if individuals who develop psychosis are more likely to have been diagnosed with such infections due to differences in susceptibility and vulnerability to infections.7 Indeed, infections can potentially directly be involved in the later development of psychosis through mechanisms that are common to all, or certain groups of pathogens (such as viruses or bacteria), or unique to a specific pathogen. In fact, altered systemic levels of cytokines, immune modulating molecules, induced by a wide range of infections, are often reported in patients with schizophrenia.8 In addition, the shared components as part of the immune response could implicate a dose-response effect by successive infections. Moreover, specific ontogenetic events occur at different ages throughout childhood, implying various consequences depending on the timing of the insult.4 However, to the best of our knowledge, exposure to infection during specific age periods has not been investigated so far.
Hence, in light of the increasing indices of associations between various infections, and immunologic processes during brain development, and psychotic illness, we investigate the effect of any severe infection during childhood on the risk of developing psychotic disorder later in life. We further assess whether the association is general or specific for bacterial, or viral infection, or for CNS, or non-CNS infection. To identify potential windows of vulnerability, we explore exposure to severe infections during 5 age periods and risk for adult psychotic illness.
In addition, we investigate a possible dose-response relationship between multiple episodes of infection and psychotic illness.
Methods
Registers
This study is based on linkages to several registers held by Statistics Sweden and the National Board of Health and Welfare. The National Patient Register (NPR) includes virtually all inpatient care in Sweden since 19739 and was used to follow up the study population regarding hospitalization with infection and nonaffective psychoses. Data on perinatal variables were retrieved from the Medical Birth Register (MBR). MBR was initiated in 1973 and includes data from the prenatal, delivery, and neonatal periods from about all deliveries in Sweden (0.5%–3% missing data).10 The Population and Housing Census (PHC) was administered every 5 years and included, by law, all individuals registered and living in Sweden with information on demographic data.11 For the current study, we obtained data on socioeconomic status (SES) from the PHC of 1985, and 1990. The Total Population Register was initiated in 1968.11 This basic register includes the entire Swedish population and its distribution and was used for data on year of migration.
Study Population
All children born in Sweden between 1973 and 1985 who were alive on their 14th birthday were identified in the NPR. Adopted individuals and individuals who emigrated before 14 years of age were excluded.
Assessment of Exposure
All diagnoses that arose from infection were identified in ICD-8, -9, and -10 (s upplementary table 1). We omitted all codes “sequel” and “post.” Somatic inpatient care with a primary diagnose of infection was identified and arranged according to age of exposure: first year of life, 1–4 years, 5–9 years, and 10–13 years. The age categories were chosen according to the timing and bulk of neurobiological processes in the brain.4
Psychotic Disorder
Data on subsequent inpatients care with a diagnosis of nonaffective psychoses (ICD-10 F20-29 and ICD-9 295, 297 and 298 except 298A and B) from 14 years of age were extracted from the Patient Register.
Assessment of Confounders
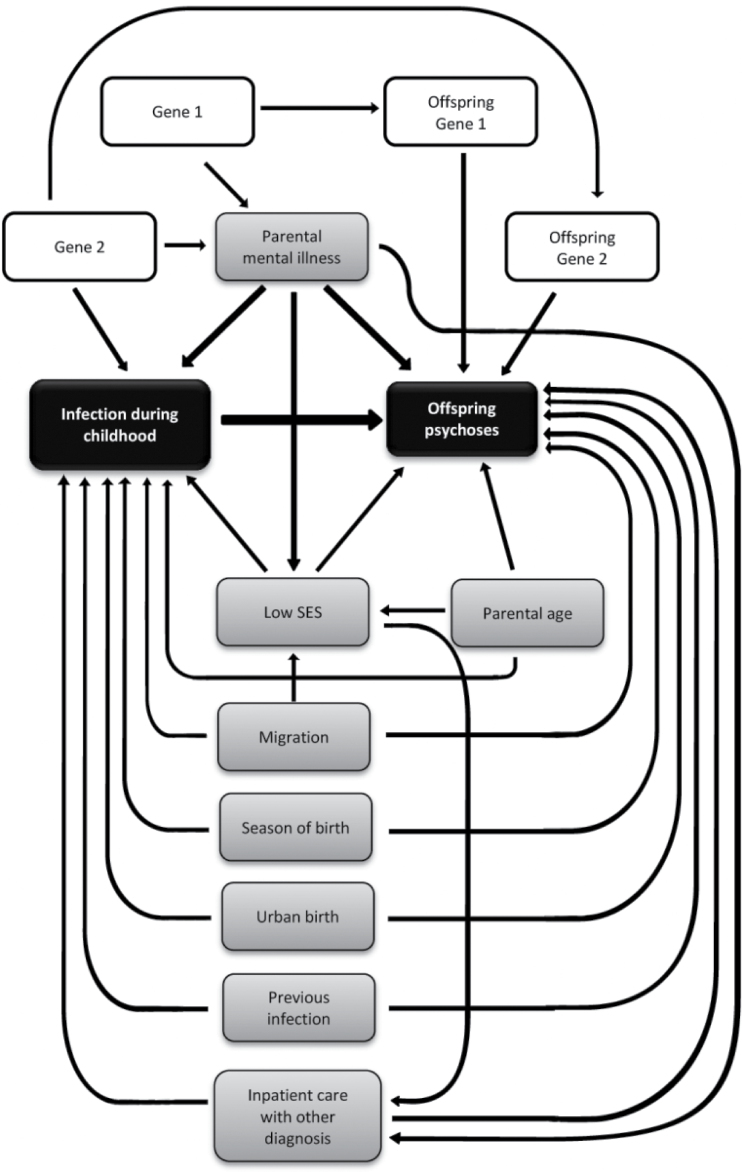
Considered covariates are presented in figure 1; male sex,12 season at time of birth (December-May),13 urban birth (born in municipality with ≥200000 inhabitants 1980),14 advanced parental age (≥35 years at time of birth, separately for mother and father),15 migration status (parent born outside Sweden, separately for mother and father),16 low SES (one parent unemployed, and household receiving social welfare benefits),17 parental psychiatric history (ICD-8 290–315, ICD-9 280–319, and ICD-10 F 00-99, separately for mother and father),18 hospitalization with other diagnoses (other than of infectious, or psychiatric origin), and maternal hospitalization with infection during pregnancy19 (pregnancy was defined as first day of last menstruation before pregnancy up until day of delivery).
Fig. 1.
Covariates associated with infection and nonaffective psychosis (arrows indicate direction of a theoretic causal influence between 2 variables).
The association between these covariates and both the exposure and the outcome were examined (table 1). To decrease the bias due to confounding, but not to lose precision in the estimates, only covariates with an effect of hazard ratio/odds ratio (HR/OR) ≥1.2 on exposure and outcome, and with a prevalence ≥5%, were adjusted for in the analyses.
Table 1.
Prevalences of Potential Confounders and Their Association With Nonaffective Psychoses, and Childhood Infection
| Nonaffective Psychosis HR (95% CI) | Childhood Infection OR (95% CI) | Prevalence (%) | ||
|---|---|---|---|---|
| Male sex | 1.4 (1.3–1.4) | 1.3 (1.3–1.3) | 51 | |
| Born December–May | 1.1 (1.0–1.1) | 1.0 (0.9–1.0) | 52 | |
| Urban birth | 1.4 (1.3–1.5) | 1.2 (1.2–1.3) | 14 | |
| Parental migration | 2.0 (1.8–2.1) | 1.2 (1.2–1.2) | 9 | |
| Parental age ≥35 | Maternal | 1.4 (1.3–1.5) | 1.0 (1.0–1.0) | 8 |
| Paternal | 1.4 (1.3–1.5) | 1.0 (1.0–1.0) | 18 | |
| Parental psychiatric illness | Maternal | 3.1 (2.9–3.4) | 1.5 (1.5–1.5) | 8 |
| Paternal | 2.6 (2.4–2.8) | 1.3 (1.2–1.3) | 9 | |
| Socioeconomic status | Parent unemployed | 2.4 (2.2–2.7) | 1.3 (1.3–1.4) | 3 |
| Household receiving social welfare benefits | 1.5 (1.4–1.6) | 1.1 (1.1–1.1) | 35 | |
| Inpatient care with other diagnosesa | 1.3 (1.2–1.4) | 2.1 (2.1–2.1) | 34 | |
| Maternal infection during pregnancy | 1.6 (1.2–2.0) | 1.8 (1.7–1.9) | 0.9 | |
Note: CI, confidence interval; HR, hazard ratio; OR, odds ratio.
aHospital admission with all diagnoses except a diagnosis of infection or a psychiatric diagnosis.
Statistical Methods
Several analyses were conducted: The main association investigated was the relationship between a registered diagnosis of infection during childhood, and diagnosis of nonaffective psychosis in adult life. In addition, we explored the effect of exposure at 4 different age periods and, moreover, subdivided the infection diagnoses into bacterial, viral, and other infection (“other infection” included all nonbacterial and nonviral infections, and unknown or not specified infection), and into CNS and non-CNS infection, to explore if the risk is specific. Next, we investigated whether the risk of nonaffective diagnosis increased with number of admissions with infection during childhood. To avoid double registration because of multiple visits with the same infection, we required >21 days between admissions for the same type of infection for it to be considered as a new episode.
Based on the theoretical causation model illustrated in figure 1, and the covariates’ association with both the exposure and the outcome (table 1) male sex, urban birth, parental history of psychiatric disorder, parental migration, and admission with other diagnoses were the potential confounders finally included in the adjusted models. In addition, we included admission with any infection during previous time periods in the model to take preceding infections into account.
Survival time was calculated in years, and the analyses included a single endpoint defined as the following: starting from 14 years of age until what came first of nonaffective psychosis, death, emigration, or the December 31, 2006. Hazard ratios and 95% CI were calculated by Cox regression. OR and 95% CI were calculated by logistic regression. All statistical analyses were made by IBM SPSS statistics 21.lnk.
Approval
The study was approved by the Regional Ethics Committee of Stockholm, EPN. Dnr 2010/1185-31/5.
Results
A total of 1 172 879 children were followed up in the registers. Altogether, 4638 (0.4%) of the children were subsequently diagnosed with nonaffective psychoses. As expected, children who later developed nonaffective psychosis tended to be male, have older parents, immigrated parents, and parents with diagnoses of mental disorder (table 2). They were more often born in urban environment, brought up in a family with low socioeconomic status, and were more often admitted to hospital with diagnoses other than those involving infections or psychoses (table 2)
Table 2.
Characteristics of the Study Population
| All Children Born in Sweden 1973–1985 (N = 1172879) | ||||
|---|---|---|---|---|
| No diagnose of nonaffective psychosisa (n = 1168 241, 99.6%) | Diagnose of nonaffective psychosisa (n = 4638, 0.4%) | |||
| n (%) | n | % | ||
| Male | 599673 (51.3) | 2 728 | 58.8 | |
| Urban birthb | 162793 (13.9) | 835 | 18.0 | |
| Born December–May | 609938 (52.2) | 2 495 | 53.8 | |
| Parental age ≥35 | Maternal | 94928 (8.1) | 466 | 10.0 |
| Paternal | 213553 (18.3) | 1 031 | 22.2 | |
| Parental psychiatric illnessc | Maternal | 90278 (7.7) | 972 | 21.0 |
| Paternal | 102304 (8.8) | 928 | 20.0 | |
| Parental immigrationd | Maternal | 108559 (9.3) | 761 | 16.4 |
| Paternal | 114204 (9.8) | 801 | 17.3 | |
| Socioeconomic status | Parent unemployed | 38080 (3.2) | 358 | 7.7 |
| Household receiving social welfare benefits | 407759 (34.9) | 2 138 | 46.1 | |
| Hospital admission with infectione | First year | 66955 (5.7) | 304 | 6.6 |
| 1–4 y | 133282 (11.4) | 603 | 13.0 | |
| 5–9 y | 57130 (4.9) | 297 | 6.4 | |
| 10–13 y | 33395 (2.8) | 163 | 3.5 | |
| 0–13 y | 246510 (21.1) | 1140 | 24.6 | |
| Inpatient care with other diagnosesf | 399014 (34.2) | 1785 | 38.5 | |
| Percent with ≥4 hospital admissions with infectione during childhood, 0–13 y | 0.75 | 1.27 | ||
aICD-10 F20-29.
b>200 000 habitants 1980.
cICD-10 F00-99.
dBorn outside Sweden.
eDiagnoses (s upplementary table 1).
fHospital admission with all diagnoses except a diagnosis of infection or a psychiatric diagnosis.
Of the potential confounders included in the final model, individual-analyses (not shown) identified the strongest confounding effects by hospital admission during childhood with other diagnoses, and by parental psychiatric history on the association between childhood infection and psychosis risk. Adjusting for parental history of more narrow psychiatric diagnoses such as schizophrenia, nonaffective psychosis, or affective psychosis, did not have any substantial influence on the results. Neither did adjustments for maternal infection during pregnancy.
There was a statistically significantly increased risk of 10% (fully adjusted) of developing nonaffective psychoses among children who had suffered any severe infection during childhood (table 3) The risk did not differ by age at time of infection, (table 3).
Table 3.
Hazard Ratio (HR) of Nonaffective Psychoses Among Individuals Born in Sweden 1973–1985 After Hospital Admission With Various Infectious Agents During Childhood
| Type of Infection | Hospital Admission With Infection | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–13 y | First Year | 1–4 y | 5–9 y | 10–13 y | |||||||||||
| Cases N | Crude HR (95% CI) | Adjusteda HR (95% CI) | Cases N | Crude HR (95% CI) | Adjusteda HR (95% CI) | Cases N | Crude HR (95% CI) | Adjustedb HR (95% CI) | Cases N | Crude HR (95% CI) | Adjustedb HR (95% CI) | Cases N | Crude HR (95% CI) | Adjustedb HR (95% CI) | |
| Any infection | 1114 | 1.27 (1.19–1.36) | 1.10 (1.03–1.18) | 300 | 1.29 (1.15–1.45) | 1.05 (0.93–1.18) | 586 | 1.22 (1.12–1.33) | 1.05 (0.96–1.14) | 290 | 1.31 (1.16–1.47) | 1.17 (1.04–1.32) | 157 | 1.19 (1.01–1.39) | 1.09 (0.93–1.28) |
| Bact infection | 240 | 1.41 (1.24–1.60) | 1.23 (1.08–1.40) | 47 | 1.17 (0.88–1.56) | 0.99 (0.74–1.32) | 92 | 1.51 (1.23–1.86) | 1.30 (1.06–1.60) | 52 | 1.08 (0.82–1.41) | 0.97 (0.74–1.28) | 56 | 1.76 (1.35–2.29) | 1.57 (1.21–2.05) |
| Viral infection | 443 | 1.14 (1.04–1.26) | 0.99 (0.90–1.10) | 108 | 1.18 (0.97–1.42) | 0.98 (0.81–1.19) | 236 | 1.04 (0.91–1.18) | 0.90 (0.79–1.03) | 117 | 1.33 (1.10–1.59) | 1.15 (0.96–1.39) | 33 | 1.17 (0.83–1.65) | 1.01 (0.72–1.43) |
| Other infection | 633 | 1.31 (1.20–1.42) | 1.12 (1.03–1.22) | 168 | 1.41 (1.21–1.65) | 1.11 (0.95–1.29) | 325 | 1.32 (1.18–1.48) | 1.11 (0.99–1.24) | 140 | 1.41 (1.19–1.67) | 1.28 (1.08–1.51) | 82 | 1.06 (0.86–1.32) | 1.00 (0.80–1.25) |
| CNS infection | 45 | 1.38 (1.03–1.86) | 1.22 (0.91–1.64) | 5 | 1.06 (0.44–2.56) | 0.91 (0.38–2.19) | 14 | 1.27 (0.75–2.14) | 1.08 (0.64–1.83) | 17 | 1.36 (0.84–2.18) | 1.22 (0.76–1.96) | 10 | 2.14 (1.15–3.97) | 1.96 (1.05–3.62) |
| Non-CNS infection | 1089 | 1.27 (1.18–1.36) | 1.10 (1.02–1.18) | 299 | 1.29 (1.15–1.45) | 1.06 (0.94–1.19) | 576 | 1.22 (1.11–1.33) | 1.04 (0.96–1.14) | 278 | 1.31 (1.16–1.48) | 1.17 (1.04–1.32) | 150 | 1.17 (0.99–1.37) | 1.07 (0.91–1.26) |
Note: CI, confidence interval.
aAdjusted for male sex, urban birth, parental migration, parental psychiatric history, and hospital admission with other diagnoses.
bAdjusted for male sex, urban birth, parental migration, parental psychiatric history, hospital admission with other diagnoses, and infection during previous time periods.
The association was strongest between hospital admission with bacterial infection, HR 1.23 (95% CI 1.08–1.40), and the later development of nonaffective psychoses. Exposure to bacterial infection during preadolescence (10–13 years) was associated with the highest risk increase, HR 1.57 (95% CI 1.21–2.05) compared with viral or other infections.
There was no major difference in risk increase between CNS and non-CNS infection with one exception, again, during preadolescence as the risk associated with CNS infection increased to HR 1.96 (95% CI 1.05–3.62).
Multiple admissions with infection during childhood increased the risk of nonaffective psychoses to HR 1.37 (95% CI 1.06–1.78) for ≥4 infections (table 4).
Table 4.
Adjusted HRa of Nonaffective Psychoses Among Children With Hospital Admission for Infection During Childhood Compared With Children Who Were Not
| No. of Hospital Admissions During Childhood | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 (N = 802) | 2 (N = 187) | 3 (N = 66) | ≥4 (N = 59) | |||||
| Crude HR (95% CI) | Adjusteda HR (95% CI) |
Crude HR (95% CI) | Adjusteda HR (95% CI) |
Crude HR (95% CI) | Adjusteda HR (95% CI) |
Crude HR (95% CI) | Adjusteda HR (95% CI) |
|
| Nonaffective psychosis | 1.23 (1.14–1.32) | 1.10 (1.02–1.19) | 1.27 (1.10–1.47) | 1.04 (0.90–1.20) | 1.55 (1.21–1.98) | 1.20 (0.94–1.53) | 1.90 (1.47–2.46) | 1.37 (1.06–1.78) |
Note: CI, confidence interval; HR, hazard ratio.
aAdjusted for male sex, urban birth, parental migration, parental psychiatric history, and hospital admission with other diagnoses.
To test the proportional hazard assumption, we included an interaction term between exposure to infection age 0–13 and follow-up time in the model. The interaction term was not significant (P = .474).
Discussion
In the present study, we observed a small but significant association between hospital admission for infections during childhood and the later development of nonaffective psychoses. This association was strongest for bacterial infections, particularly during preadolescence. Although “other infections” is a large group of unknown origin, the results suggest that the risk increase is not mediated by factors common to all infections but rather by factors specific for bacterial infections. However, to explore the specific role of different pathogens, further analyses of register data in combination with serological and experimental studies are needed.
In this first study to investigate non-CNS infections as well as infections in the CNS during childhood, we found that these infections were equally associated with the later development of nonaffective psychosis with the exception of exposure during the preadolescence period. As mentioned earlier, previous studies on the association between childhood infections and psychoses, have with few exceptions only investigated CNS infections. Out of these, only 2 fulfill basic methodological requirements as found by Khandaker et al, our previous study,20 and the study by Koponen et al.21 Both these studies reported associations with viral CNS infection. Notably, our previous study had only 23 cases exposed to CNS infection, 19 to viral, and 4 to bacterial, whereas the Finnish study had 6 cases exposed to CNS infection, 4 to viral, and 2 to bacterial infection. Thus, the somewhat contradicting results with the present study may be due to insufficient numbers in the previous studies.
Preadolescence seems to be a vulnerable age period in the present study; CNS infections and bacterial infections in general were more strongly associated with future psychosis risk during this period compared with other age periods. This finding is in line with a recent meta-analysis, suggesting that Neisseria Meningitidis, a common cause of bacterial meningitis, is more prevalent among adolescents than among younger children or older individuals.22 Long-term sequelae of bacterial meningitis relating to both cognitive and behavioral problems are being increasingly recognized,23 suggesting a causal relation between certain infections during adolescence and the later diagnosis of psychosis. We here verify the dose-response relation between consecutive infections and nonaffective psychosis in boys aged 0–3 recently reported by Liang & Chikritzhs and extend it to include also girls and ages 0–13.24
While our findings suggest that infections can contribute to the development of psychosis, it is possible that these hospital admissions reflect an increased exposure, susceptibility or sensitivity to infections among individuals with other kinds of vulnerability to psychosis. Indeed, our risk estimates were attenuated by adjustment for admission with other diagnoses and parental psychiatric disease, suggesting that social factors related to exposure, as well as familial factors related to psychiatric disease, to some extent, contributed to the present findings. A Danish study recently reported that hospital treatment for infections is more common also among parents of probands in Denmark suggestive of a genetic liability to hospital admission among these individuals.7 Moreover, we recently reported lower levels of several acute phase proteins, involved in the innate, first-line defense against microbes, in neonatal blood from individuals diagnosed with nonaffective psychosis compared with matched control subjects.25 Taken together with an increasing literature consistently reporting genetic risk for psychiatric disorders in the MHC regions,2 these findings suggest that individuals who will later develop nonaffective psychosis might exhibit subtle immune deficiencies that render them more susceptible or vulnerable to early-life infections. The key question is obviously if some of these infections are causally involved in the development of psychosis. While this remains to be established, it is interesting to note that infections of neonatal wild-type mice and mice genetically deficient in MHC presentation suggest that immune function is critically involved in determining the long-term outcome of the infection in terms of cognitive abilities and sensorimotor gating in adult mice.26
Limitations
In register-based studies, incomplete reporting and crude exposure information is a limitation. However, registered diagnoses of psychotic disorders, including schizophrenia have proved to be reliable.27,28
The validity of infectious disease diagnoses in the NPR is high. Among diagnoses that include false positive registrations, the majority are anyhow a diagnosis of infection.29 Nevertheless, considering this misclassification risk, the association between specific infections and subsequent psychotic disorder has to be interpreted with caution.
The exposure included hospital treated infections, hence, probably only severe infections. The majority of infections do not require hospital care, resulting in misclassification and a plausible underestimation of the association.
Conclusion
We here report a small association between hospital admissions for infections in general throughout childhood and a later diagnosis of nonaffective psychosis. While bacterial infection and CNS infections during preadolescence appeared to confer the strongest risk, we cannot exclude that our present findings indicate an increased susceptibility to hospital admission for infections among children who will later develop nonaffective psychosis.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
The Swedish Research Council (2006-3002); the regional agreement on medical training and clinical research (20090401).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432 [DOI] [PubMed] [Google Scholar]
- 2. Stefansson H, Ophoff RA, Steinberg S; Genetic Risk and Outcome in Psychosis (GROUP). Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266 [DOI] [PubMed] [Google Scholar]
- 5. Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull. 2001;27:443–455 [DOI] [PubMed] [Google Scholar]
- 6. Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen PR, Laursen TM, Mortensen PB. Association between parental hospital-treated infection and the risk of schizophrenia in adolescence and early adulthood. Schizophr Bull. 2013;39:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808 [DOI] [PubMed] [Google Scholar]
- 9. Kvalitet och innehåll i patientregistret. www.socialstyrelsen.se Accessed July 10, 2013.
- 10. TheNational Board of Health and Welfare. The medical birth register. 2011. http://www.socialstyrelsen.se/register/halsodataregister/medicinskafodelseregistret Accessed June 8, 2013.
- 11. SCB-data för forskning 2011. In: SCBr, ed. Innehållsbeskrivning av olika register. Örebro, Sweden: SCB, registerenhet; 2011 [Google Scholar]
- 12. Mitford E, McCabe K, Reay R, Turkington D. Inclusion criteria in epidemiological psychosis research: the importance of reporting outpatient data, gender and affective psychoses. Acta Psychiatr Scand. 2011;124:412–3 [DOI] [PubMed] [Google Scholar]
- 13. Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29:587–593 [DOI] [PubMed] [Google Scholar]
- 14. Harrison G, Fouskakis D, Rasmussen F, Tynelius P, Sipos A, Gunnell D. Association between psychotic disorder and urban place of birth is not mediated by obstetric complications or childhood socio-economic position: a cohort study. Psychol Med. 2003;33:723–731 [DOI] [PubMed] [Google Scholar]
- 15. Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry. 2003;60:673–678 [DOI] [PubMed] [Google Scholar]
- 16. Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24 [DOI] [PubMed] [Google Scholar]
- 17. Wicks S, Hjern A, Dalman C. Social risk or genetic liability for psychosis? A study of children born in Sweden and reared by adoptive parents. Am J Psychiatry. 2010;167:1240–1246 [DOI] [PubMed] [Google Scholar]
- 18. Dean K, Stevens H, Mortensen PB, Murray RM, Walsh E, Pedersen CB. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Arch Gen Psychiatry. 2010;67:822–829 [DOI] [PubMed] [Google Scholar]
- 19. Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dalman C, Allebeck P, Gunnell D, Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am J Psychiatry. 2008;165:59–65 [DOI] [PubMed] [Google Scholar]
- 21. Koponen H, Rantakallio P, Veijola J, Jones P, Jokelainen J, Isohanni M. Childhood central nervous system infections and risk for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254:9–13 [DOI] [PubMed] [Google Scholar]
- 22. Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–861 [DOI] [PubMed] [Google Scholar]
- 23. Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(suppl 2):B3–B9 [DOI] [PubMed] [Google Scholar]
- 24. Liang W, Chikritzhs T. Early childhood infections and risk of schizophrenia. Psychiatry Res. 2012;200:214–217 [DOI] [PubMed] [Google Scholar]
- 25. Gardner RM, Dalman C, Wicks S, Lee BK, Karlsson H. Neonatal levels of acute phase proteins and later risk of non-affective psychosis. Transl Psychiatry. 2013;3:e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S. Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1-/- mice. Int J Neuropsychopharmacol. 2010;13:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalman Ch, Broms J, Cullberg J, Allebeck P. Young cases of schizophrenia identified in a national inpatient register–are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol. 2002;37:527–531 [DOI] [PubMed] [Google Scholar]
- 28. Ekholm B, Ekholm A, Adolfsson R, Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59:457–464 [DOI] [PubMed] [Google Scholar]
- 29. Ludvigsson JF, Andersson E, Ekbom A, External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.