Abstract
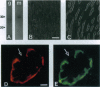
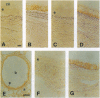
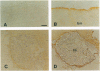
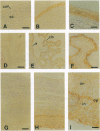
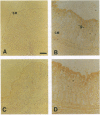
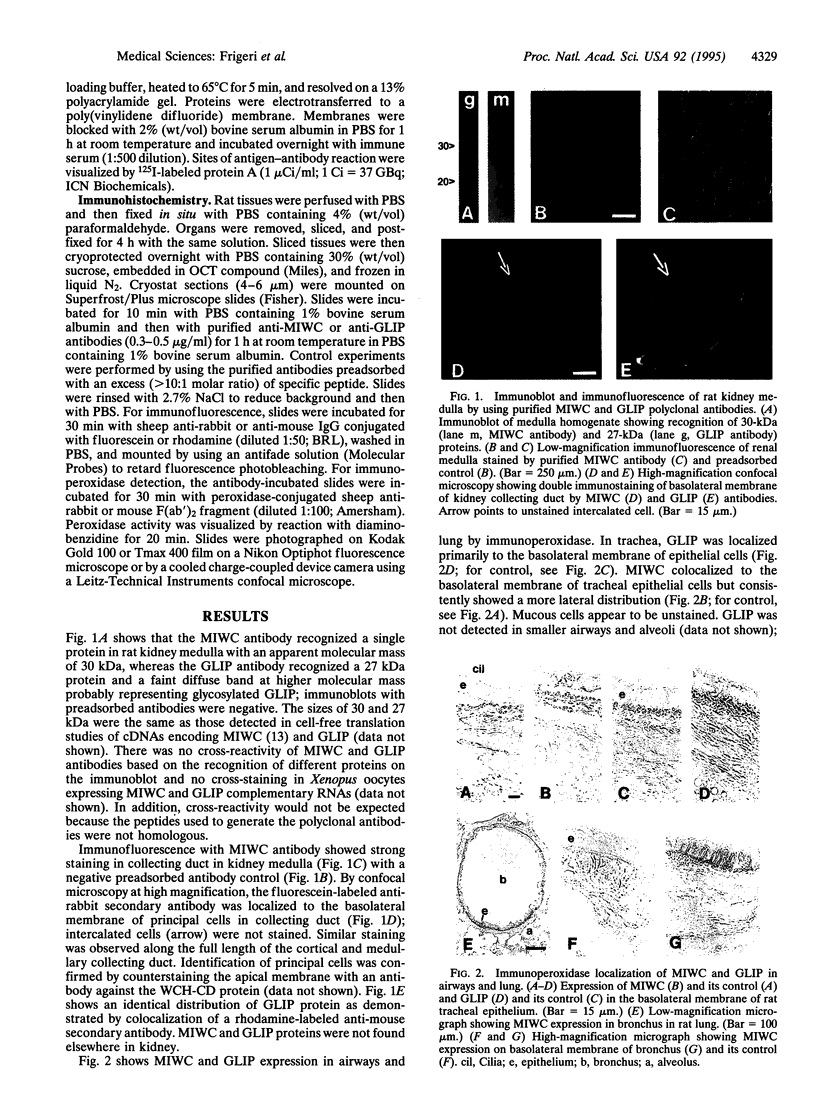
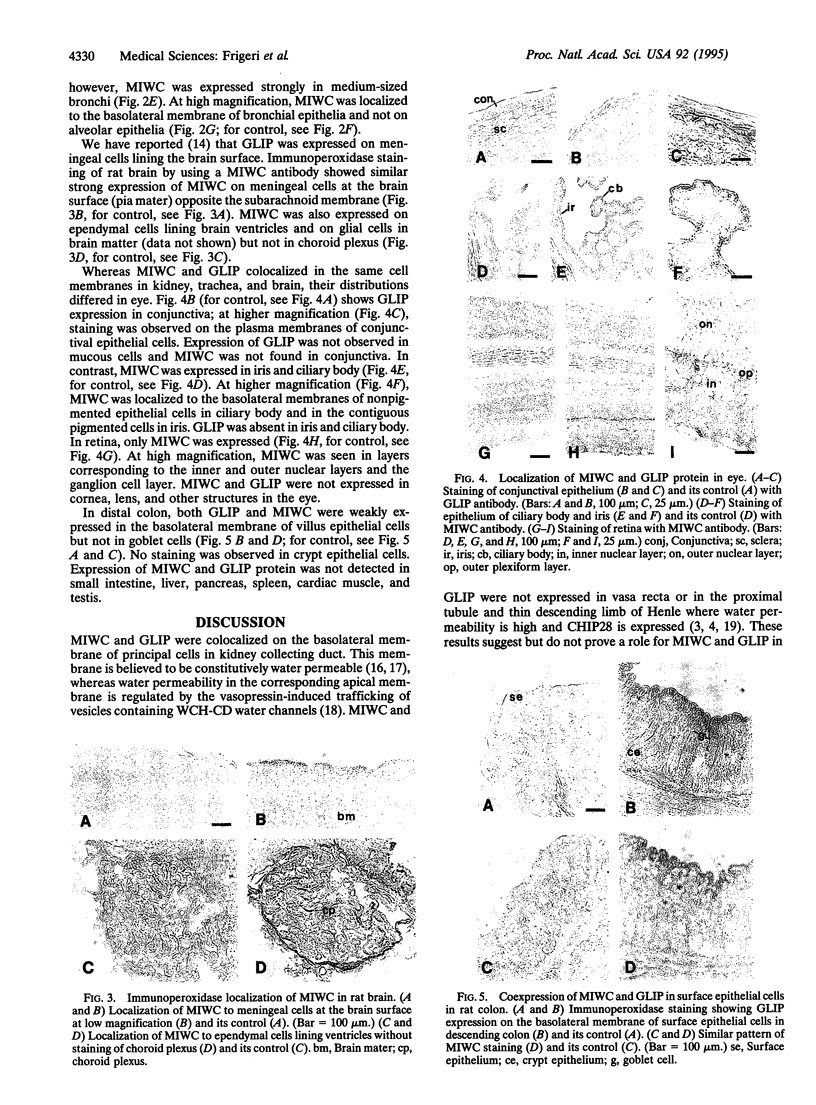
Two water channel homologs were cloned recently from rat kidney, mercurial-insensitive water channel (MIWC) and glycerol intrinsic protein (GLIP). Polyclonal antibodies were raised against synthetic C-terminal peptides and purified by affinity chromatography. MIWC and GLIP antibodies recognized proteins in rat kidney with an apparent molecular mass of 30 and 27 kDa, respectively, and did not cross-react. By immunofluorescence, MIWC and GLIP were expressed together on the basolateral plasma membrane of collecting duct principal cells in kidney. By immunohistochemistry, MIWC and GLIP were expressed on tracheal epithelial cells with greater expression of GLIP on the basal plasma membrane and MIWC on the lateral membrane; only MIWC was expressed in bronchial epithelia. In eye, GLIP was expressed in conjunctival epithelium, whereas MIWC was found in iris, ciliary body, and neural cell layers in retina. MIWC and GLIP colocalized on the basolateral membrane of villus epithelial cells in colon and brain ependymal cells. Expression of MIWC and GLIP was not detected in small intestine, liver, spleen, endothelia, and cells that express water channels CHIP28 or WCH-CD. These studies suggest water/solute transporting roles for MIWC and GLIP in the urinary concentrating mechanism, cerebrospinal fluid absorption, ocular fluid balance, fecal dehydration, and airway humidification. The unexpected membrane colocalization of MIWC and GLIP in several tissues suggests an interaction at the molecular and/or functional levels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deen P. M., Verdijk M. A., Knoers N. V., Wieringa B., Monnens L. A., van Os C. H., van Oost B. A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994 Apr 1;264(5155):92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Folkesson H. G., Matthay M. A., Hasegawa H., Kheradmand F., Verkman A. S. Transcellular water transport in lung alveolar epithelium through mercury-sensitive water channels. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4970–4974. doi: 10.1073/pnas.91.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993 Feb 11;361(6412):549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Strange K., Zeidel M. L. Current understanding of the cellular biology and molecular structure of the antidiuretic hormone-stimulated water transport pathway. J Clin Invest. 1991 Jul;88(1):1–8. doi: 10.1172/JCI115263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Lian S. C., Finkbeiner W. E., Verkman A. S. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am J Physiol. 1994 Apr;266(4 Pt 1):C893–C903. doi: 10.1152/ajpcell.1994.266.4.C893. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Ma T., Skach W., Matthay M. A., Verkman A. S. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994 Feb 25;269(8):5497–5500. [PubMed] [Google Scholar]
- Hasegawa H., Zhang R., Dohrman A., Verkman A. S. Tissue-specific expression of mRNA encoding rat kidney water channel CHIP28k by in situ hybridization. Am J Physiol. 1993 Jan;264(1 Pt 1):C237–C245. doi: 10.1152/ajpcell.1993.264.1.C237. [DOI] [PubMed] [Google Scholar]
- Ishibashi K., Sasaki S., Fushimi K., Uchida S., Kuwahara M., Saito H., Furukawa T., Nakajima K., Yamaguchi Y., Gojobori T. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Frigeri A., Hasegawa H., Verkman A. S. Cloning of a water channel homolog expressed in brain meningeal cells and kidney collecting duct that functions as a stilbene-sensitive glycerol transporter. J Biol Chem. 1994 Aug 26;269(34):21845–21849. [PubMed] [Google Scholar]
- Ma T., Frigeri A., Skach W., Verkman A. S. Cloning of a novel rat kidney cDNA homologous to CHIP28 and WCH-CD water channels. Biochem Biophys Res Commun. 1993 Dec 15;197(2):654–659. doi: 10.1006/bbrc.1993.2529. [DOI] [PubMed] [Google Scholar]
- Marmor M. F. Control of subretinal fluid: experimental and clinical studies. Eye (Lond) 1990;4(Pt 2):340–344. doi: 10.1038/eye.1990.46. [DOI] [PubMed] [Google Scholar]
- McKie A. T., Powrie W., Naftalin R. J. Mechanical aspects of rabbit fecal dehydration. Am J Physiol. 1990 Mar;258(3 Pt 1):G391–G394. doi: 10.1152/ajpgi.1990.258.3.G391. [DOI] [PubMed] [Google Scholar]
- Nielsen S., DiGiovanni S. R., Christensen E. I., Knepper M. A., Harris H. W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Smith B. L., Zeidel M. L., Moulds J. J., Agre P. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science. 1994 Sep 9;265(5178):1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- Priver N. A., Rabon E. C., Zeidel M. L. Apical membrane of the gastric parietal cell: water, proton, and nonelectrolyte permeabilities. Biochemistry. 1993 Mar 16;32(10):2459–2468. doi: 10.1021/bi00061a002. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Valenti G., Verbavatz J. M., Van Hoek A. N., Verkman A. S., Ausiello D. A., Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol. 1992 Dec;263(6 Pt 1):C1225–C1233. doi: 10.1152/ajpcell.1992.263.6.C1225. [DOI] [PubMed] [Google Scholar]
- Strange K., Spring K. R. Cell membrane water permeability of rabbit cortical collecting duct. J Membr Biol. 1987;96(1):27–43. doi: 10.1007/BF01869332. [DOI] [PubMed] [Google Scholar]
- Verbavatz J. M., Brown D., Sabolić I., Valenti G., Ausiello D. A., Van Hoek A. N., Ma T., Verkman A. S. Tetrameric assembly of CHIP28 water channels in liposomes and cell membranes: a freeze-fracture study. J Cell Biol. 1993 Nov;123(3):605–618. doi: 10.1083/jcb.123.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Pisano M. M., Chepelinsky A. B. Tandem sequence repeats in transmembrane channel proteins. Trends Biochem Sci. 1991 May;16(5):170–171. doi: 10.1016/0968-0004(91)90065-4. [DOI] [PubMed] [Google Scholar]
- Zhang R., Skach W., Hasegawa H., van Hoek A. N., Verkman A. S. Cloning, functional analysis and cell localization of a kidney proximal tubule water transporter homologous to CHIP28. J Cell Biol. 1993 Jan;120(2):359–369. doi: 10.1083/jcb.120.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeswijk M. P., van Os C. H. Osmotic water permeabilities of brush border and basolateral membrane vesicles from rat renal cortex and small intestine. J Membr Biol. 1986;92(2):183–193. doi: 10.1007/BF01870707. [DOI] [PubMed] [Google Scholar]