Abstract
Altered peripheral carbonyl stress markers, high levels of serum pentosidine, which accumulates following carbonyl stress, and low levels of pyridoxal (vitamin B6), which detoxifies reactive carbonyl compounds, have been reported in a cross-sectional study of chronic schizophrenia. However, changes in the levels of these compounds in patients with schizophrenia have not been investigated in a longitudinal study. To clarify whether these markers may be biological markers that reflect the clinical course of the disease, the serum levels of these compounds were investigated in a cross-sectional and a longitudinal study. One hundred and thirty-seven acute-stage Japanese patients were enrolled. Among these, 53 patients were followed from the acute stage to remission. A portion of patients in the acute stage (14 cases, 10.2%) showed extremely high pentosidine levels. These levels were not associated with the severity of symptoms but were associated with antipsychotic dose amounts. Pyridoxal levels were lower in schizophrenia and increased according to the clinical course of the illness. Furthermore, 18 patients with decreased pyridoxal levels according to the clinical course showed that the greater the decrease in pyridoxal levels, the lesser the improvement in symptoms. Thus, extremely high pentosidine levels in a portion of patients may be caused by higher daily antipsychotic doses, whereas pyridoxal levels were lower in schizophrenia and increased according to the clinical course. Patients with decreasing pyridoxal levels during the clinical course showed less improvement in symptoms. Carbonyl stress markers may also be therapeutic biological markers in some patients with schizophrenia.
Key words: clinical course, pentosidine, pyridoxal, vitamin B6
Introduction
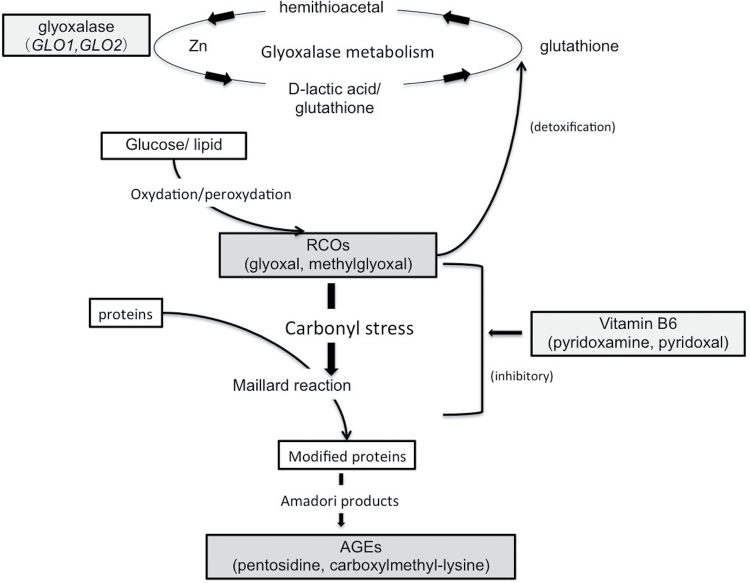
Oxidative stress, a possible pathophysiological mechanism in schizophrenia,1–3 and in its first-episode psychosis,4 converts glucose and lipids to reactive carbonyl compounds (RCOs), and excess RCOs are converted to advanced glycation end products (AGEs) and advanced lipoxidation end products. Accumulation of these products is called carbonyl stress and is considered to be related to the pathogenesis of several diseases, including diabetes mellitus, chronic renal failure, and mental illness.5 Recently, interesting results were reported showing that pentosidine, an AGE, is significantly increased in the peripheral blood of patients with schizophrenia compared with controls. In addition, a subpopulation with high pentosidine levels also showed low levels of vitamin B6, which detoxifies RCOs.6 Patients with high pentosidine levels also shared certain clinical features, such as a family history of psychiatric illness, severity of certain symptoms and genetic features on glyoxalase I (GLO1), a rate-limiting enzyme for the detoxification of RCOs.6 If genetic factors decrease GLO1 activity, RCOs can accumulate and cause further decreases (depletion and/or low ingestion) in vitamin B6 levels. This results in insufficient detoxification of the accumulated RCOs, thereby leading to continuous accumulation of AGEs (figure 1). The disruption of this pathway can lead to elevated protein modification by RCOs, which can lose their normal function and contribute to the development of the disease.5 A recent study with a larger number of patients with chronic schizophrenia (n = 157) and detailed clinical investigations showed that patients with apparent carbonyl stress may be resistant to treatment during the chronic stage,7 supporting the establishment of carbonyl stress markers in chronic schizophrenia. However, regarding the acute stage and clinical course, no reports have been published that followed patients from the acute stage to the remission stage. Thus, whether pentosidine and vitamin B6 reflect the clinical condition and can be used as “therapeutic” biological markers for patients with schizophrenia according to their clinical course remains unknown.
Fig. 1.
Mechanism of carbonyl stress and its detoxification with vitamin B6 and glyoxalase. Reactive carbonyl compounds (RCOs), which cause carbonyl stress, are detoxified by degradation into lactic acid and glutathione by glyoxalase enzymes. Glyoxalase 1 and 2 (GLO1 and GLO2) are the rate-limiting enzymes in this metabolic pathway. Inhibition of RCO generation and the Maillard reaction by vitamin B6 results in the suppression of AGE accumulation. This figure is adapted from 2 previous publications, with partial modification.5,18
In the present study, we (1) reinvestigated whether carbonyl stress status can serve as a biological marker in patients with schizophrenia during the acute stage and (2) investigated the levels of these carbonyl stress markers twice as paired samples in peripheral blood from patients with schizophrenia during the acute stage and the remission stage. Finally, we discuss whether these compounds are stable markers that can be used as diagnostic and therapeutic biological markers in schizophrenia according to symptoms and genetic status.
Materials and Methods
Patients
One hundred and thirty-seven Japanese patients with schizophrenia (paranoid, disorganized, or catatonic type) that met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of schizophrenia according to clinical interviews by at least 3 experienced psychiatrists were admitted to the Juntendo Koshigaya Hospital (Saitama) or Juntendo Hospital (Tokyo) due to worsening of their symptoms. No first-episode, drug-naive patients were included in our study population. Eight patients were medication free at the time of admission due to disease recurrence; ie, they were not taking any antipsychotics (duration of drug discontinuation before admission: mean ± SD, 18.0±23.2 mo; range, 2–72 mo). Among the 137 patients, 53 patients could be followed from the time of admission and discharge (table 2). Thus, clinical data for these 53 patients, including serum measurements, were considered paired samples. Of the remaining 84 patients, 3 remained hospitalized (just admitted) and 81 were discharged without the doctors ordering examinations, due to short notice from patients and their families because of improvement in symptoms, or simple carelessness in not ordering examinations at discharge. Patients with other schizophrenia spectrum disorders, including schizophreniform disorder, schizoaffective disorder, psychosis not otherwise specified, and schizoid personality disorder, were excluded. In addition, according to blood tests at admission including glucose, glycohemoglobin A1C, creatinine, and urea nitrogen, patients were excluded if they had diabetes mellitus and/or chronic renal disease, which can increase AGEs. Glomerular filtration rates (normal >60ml/min) and urinalysis were also used to evaluate renal function. The time of discharge was thoroughly discussed with the patients and their families and was determined according to whether the patient had improved sufficiently to be treated on an outpatient basis. Forty-seven healthy controls (table 1) were also included in the study. The healthy controls did not meet current or past criteria for any Axis I disorder of DSM-IV. All participants met the following criteria: (1) no systemic or neurologic disease; (2) no past head trauma with loss of consciousness; and (3) no lifetime history of alcohol or substance dependence. No healthy controls had diabetes mellitus and/or chronic renal disease.
Table 2.
Changes in Characteristics and Test Scores in the 53 Patients With Schizophrenia That Were Followed Up
| Variables | Paired-sample Patients With Schizophrenia (n = 53) | |||
|---|---|---|---|---|
| Sex, M/F | 27/26 | |||
| Age, mean ± SD, y | 38.4±14.5 (17–76) | |||
| Onset (range), y | 25.6±10.5 (12–53) | |||
| Duration of illness (range), y | 14.3±12.6 (0.1–48) | |||
| DUP (range), mo | 20.3±28.1 (0.1–120) | |||
| Duration of hospitalization (range), days | 108.5±86.5 (2–411) | |||
| Wilcoxon test | ||||
| At Admission | At Discharge | Z | P | |
| CP dose, mg/day | 836.7±677.4 (0-2625) | 937.4±453.9 (150–2475) | 1.60 | .110 |
| BPRS scores (Total) | 60.7±14.0 (41–96) | 38.9±9.4 (18–63) | −5.91 | <.001 |
| (Positive) | 16.5±4.6 (7–24) | 9.9±3.2 (3–18) | −5.82 | <.001 |
| (Negative) | 10.5±3.2 (3–18) | 8.5±3.2 (3–18) | −4.66 | <.001 |
| Pentosidine, ng/ml | 37.1±20.6 (14.1–135.6) | 38.2±18.2 (14.1–128.4) | 0.85 | .397 |
| Pyridoxal, ng/ml | 8.2±6.3 (2.5–36.1) | 9.3±4.9 (0.1–27.4) | 2.00 | .046 |
P values with statistical significance are in bold.
Abbreviations are explained in the first footnote to table 1.
Table 1.
Clinical Variables and Serum Pentosidine and Pyridoxal Levels in Healthy Controls and Patients With Schizophrenia at Admission
| Variables | Controls | Patients With Schizophrenia | Statistical Test and P Value | |
|---|---|---|---|---|
| (n = 47) | (n = 137) | Mann-Whitney U | ||
| χ2 | P | |||
| Sex, M/F | 17/30 | 68/69 | 2.55 | .11 |
| Age, mean (y) | 31.0±5.0 (22–48) | 38.9±13.9 (16–76) | 12.8 | <.001 |
| Onset (y) | NA | 23.8±9.0 (12–53) | ||
| Duration of education (y) | NA | 12.4±2.5 (9–20) | ||
| Family history (y/n) | NA | 46/91 | ||
| Duration of illness (y) | NA | 17.9±14.2 (0–56) | ||
| DUP (mo) | NA | 16.7±32.2 (0–300) | ||
| Number of admissions | NA | 3.1±2.3 (1–10) | ||
| CP dose (mg/day) | NA | 735.7±577.9 (0-2625) | ||
| BPRS (Total) | NA | 61.5±14.1 (34–96) | ||
| (Positive) | NA | 16.6±4.6 (7–25) | ||
| (Negative) | NA | 10.8±3.5 (3–19) | ||
| Pentosidine, ng/ml | 36.2±10.2 (10.7–60.0) | 36.2±17.4 (11.6–135.6) | −1.44 | .151 |
| Pyridoxal, ng/ml | 11.7±6.4 (3.7–29.8) | 8.8±6.9 (0.1–40.7) | −3.71 | <.001 |
Note: Data are the mean ± SD (range).
P values with statistical significance are in bold.
BPRS, Brief Psychiatric Rating Scale; CP dose, chlorpromazine equivalent dose; DUP, duration of untreated psychosis; NA, not applicable.
Evaluation of Clinical Symptoms
Clinical symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS) (each item was rated on a scale of 1–7).8 The BPRS scores included direct interviews that were independently evaluated by well-trained experienced psychiatrists. The overall total rating and scores dealing with positive and negative symptom clusters were used.9 The presence of a family history of psychiatric disease was defined as having a first- or second-degree relative with any neuropychiatric disorder.
A top priority of the Juntendo University Schizophrenia Projects (JUSP)10–12 is to improve patients’ symptoms in the most effective manner. Accordingly, the use of drug therapy was not controlled due to ethical considerations. The Ethics Committee of the Juntendo University School of Medicine approved the present study (2012083). All participants gave their written informed consent prior to participating in the study.
Measurements of Carbonyl Stress Markers
Measurements for pentosidine using a competitive enzyme-linked immunosorbent assay kit (FSK Pentosidine; Fushimi Pharmaceutical Co, Ltd),13,14 and for vitamin B6 (pyridoxine, pyridoxal, and pyridoxamine) using high-performance liquid chromatography were described in detail at elsewhere (supplementary methods). Serum levels of pyridoxine and pyridoxamine were expected to be low in vivo, and indeed, serum levels of pyridoxine and pyridoxamine were below the lower limit of detection (3.0ng/ml and 0.6ng/ml, respectively). Thus, we used serum pyridoxal levels to represent serum vitamin B6 levels.
Genotyping of Functional Polymorphisms in GLO1
A relatively common missense mutation (rs4746, Glu111Ala, minor allele frequency; schizophrenia = .28, controls = .13) in exon 4 of GLO1 causes a decrease in enzyme activity, resulting in the accumulation of pentosidine.6 The influence of this mutation on levels of pentosidine was also investigated by TaqMan genotyping methods (see s upplemental m ethods).11
Statistical Analysis
Chi-square tests were used to assess differences in the distribution of frequencies (eg, gender). The differences in the serum pentosidine and pyridoxal levels between the unpaired groups were examined using the 2-tailed Mann-Whitney U test for 2-group comparisons and the Kruskal-Wallis test for comparison of 3 or more groups. The differences in the pentosidine and pyridoxal levels in paired samples of patient serum between the time of admission and the time of discharge were examined using the Wilcoxon matched-pairs signed-rank test. The correlations between clinical features, such as duration of hospitalization, and measured serum substance levels were analyzed using Pearson’s correlation test. The correlations between the BPRS scores and measured serum substances were analyzed using Spearman’s correlation test.
Results
Carbonyl Stress Markers in Patients With Schizophrenia at the Acute Stage
The characteristics of all participants are given in table 1. No significant differences were found in the gender distribution between healthy controls and schizophrenic patients (table 1). Significant differences were noted in the age between patients with schizophrenia and controls (table 1). Thus, the correlation between age and the main measurements, levels of serum pentosidine and pyridoxal, were first analyzed. Serum levels of pentosidine and pyridoxal did not show significant correlations with age in either patients (r = .04 and −.13, P > .05) or controls (r = .02 and −.20, P > .05).
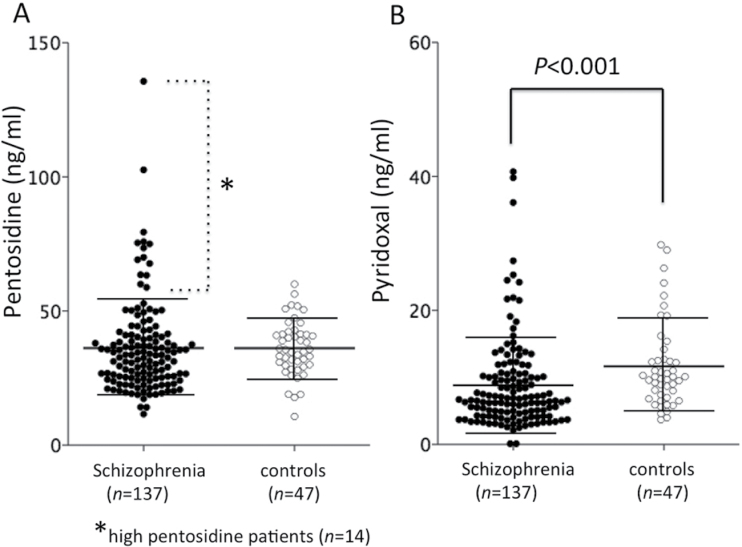
No significant differences were found between serum pentosidine levels in patients at admission and healthy controls (table 1), but as previously reported,6 extremely high pentosidine levels (>2 SD higher than the mean in controls, >57.6ng/ml) were more frequently found in patients with schizophrenia (14 cases, 10.2%) than in controls (1 case, 2.1%) (table 1, figure 2A). Pyridoxal levels were significantly lower in patients with schizophrenia than in controls (table 1, figure 2B). Serum pentosidine and pyridoxal levels were not correlated at the time of admission in schizophrenia (r = −.153, P = .074) and well as in normal controls (r = .204, P = .169). With respect to clinical symptoms, the severity of symptoms was not significantly correlated with levels of pentosidine or pyridoxal. Interestingly, serum pentosidine levels from patients with schizophrenia at admission showed a significant positive association with daily chlorpromazine (CP) dose amount (r = .361, P < .001), whereas pyridoxal levels did not (r = −.028, P = .748). We established a speculative “total accumulation of dose amounts of antipsychotics” as “[(duration of illness)−(duration of untreated psychosis)]×(daily CP dose amount at admission).” The “total accumulation of dose amounts of antipsychotics” also showed a strong positive association with serum pentosidine levels (r = .490, P < .001) but not with pyridoxal levels (r = −.05, P = .569).
Fig. 2.
Serum levels of carbonyl stress markers in normal controls and patients with schizophrenia at admission. A. Pentosidine. B. Pyridoxal. Fourteen patients with high pentosidine (>2 SD higher than the mean in controls, >57.6ng/ml) are indicated with an asterisk. Values were compared with the 2-tailed Mann-Whitney U test. Error bars indicate mean and standard deviations.
The schizophrenic patients in this study included 8 medication-free patients at admission. The levels of pentosidine in these 8 patients were lower than those in the 129 medicated patients, but the difference was not significant (unmedicated, 27.0±9.3ng/ml; medicated, 36.6±17.7ng/ml; χ2 = 1.62; P = .10). Pyridoxal levels were also not significantly different (unmedicated, 8.1±4.0ng/ml; medicated, 8.8±7.1ng/ml; χ2 = −0.32; P = .75). All clinical variables, such as age, duration of illness, duration of untreated psychosis, and numbers of admissions (table 1), did not show any significant correlations with levels of any carbonyl stress markers. Comparison of carbonyl stress markers between patients with and without a family history did not show a significant difference. Additionally, nutrition variables (body mass index, hemoglobin A1C, creatinine, glucose, total cholesterol, triacylglycerol and total protein) and lifestyle factors (smoking and alcohol habit) were not associated with carbonyl stress markers (supplementary table 1).
Clinical Features of Patients With High Pentosidine Levels at the Acute Stage
Fourteen cases showed quite high pentosidine levels that were over 2 SD of control levels. A previous study suggested that these patients may be associated with development of a certain subtype of schizophrenia, carbonyl stress schizophrenia.6 Thus, clinical variables were compared between patients with high pentosidine levels (>57.6ng/ml) and patients with normal levels (<57.6ng/ml) (in figure 2A, patients with high pentosidine levels are indicated with an asterisk). No significant differences in clinical symptoms or variables were found between patients with high pentosidine levels compared with those with normal pentosidine levels, except for the duration of illness and the daily CP dose amount (supplementary table 2). The duration of illness was significantly longer in high pentosidine patients (22.4±9.4 y vs 15.3±11.4 y, χ2 = 2.221, P = .026). Furthermore, the daily CP dose amount was approximately 2-fold higher in patients with elevated pentosidine (1291.8±636.2mg/day) than in patients with normal pentosidine (671.4±537.5mg/day, χ2 = 3.598, P < .001, supplementary table 2).
Change in Pentosidine and Pyridoxal Levels According to the Clinical Course of Schizophrenia
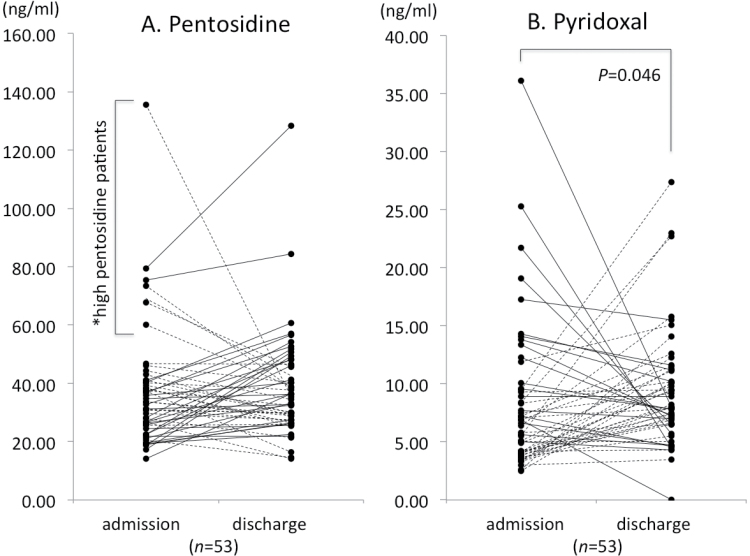
To account for potential bias, the clinical variables in table 1 were compared between the 53 discharged patients with paired samples and 81 discharged patients with admission data only. There were no significant differences in the clinical variables, including BPRS scores at discharge (data were not shown). Fifty-three patients with schizophrenia could be followed from the time of admission to discharge, enabling paired comparisons of their serum biomarkers (table 2, figure 3A and 3B). As expected, total BPRS and positive and negative symptoms were significantly improved from the time of admission to the time of discharge. The daily CP dose amount did not significantly change in these patients. The paired samples showed a marginally significant increase in pyridoxal levels from the time of admission to discharge, but this was not seen for pentosidine. Although the levels increased, the pyridoxal in patients with schizophrenia at discharge (9.3±4.9ng/ml) were still lower than those in controls (11.7±6.4ng/ml), and the difference was close to statistical significance (Mann-Whitney U, χ2 = −1.96, P = .050). Among the 53 cases with paired samples, the pentosidine were decreased in approximately half of the patients [26 cases (49%); 27 cases (51%) were increased] from the time of admission to discharge (figure 3A). Pyridoxal were increased in more than half of patients (34 cases [63%]; 18 cases [37%] were decreased) (figure 3B). Interestingly, only patients with decreased pyridoxal levels (18 cases, figure 3B) from the time of admission to discharge showed a significant correlation between their change in pyridoxal and change in total BPRS scores (r = −.542, P = .025) and positive symptom scores (r = −.528, P = .029). The greater the decrease in pyridoxal levels, the lesser the improvement in symptoms (supplementary figure 1). The remaining 3 subgroups (patients with an increase and/or decrease in pentosidine, and an increase in pyridoxal levels) did not show any correlations between changes in markers and changes in clinical symptoms (all P > .05). Among the 14 patients with high pentosidine (figure 2A and supplementary table 2), 7 patients provided paired samples. Of these, 5 patients showed a decrease in serum pentosidine levels, and the other 2 patients showed an increase (figure 3A, asterisk). One patient with a decrease in serum pentosidine levels showed no change in clinical symptoms, but the other 6 patients, including 2 with increased pentosidine levels, showed improvement in total, positive, and negative symptom scores from BPRS.
Fig. 3.
Changes in serum carbonyl stress markers in paired-sample patients with schizophrenia (n = 53) who were followed from the time of admission to discharge. Dotted lines indicate a decrease in carbonyl stress (decrease in pentosidine and increase in pyridoxal), and solid lines indicate an increase in carbonyl stress (increase in pentosidine and decrease in pyridoxal) over time.
The changes [(value at “discharge” − value on “admission”) / value at “admission”] in pentosidine and pyridoxal levels were not significantly correlated with changes in clinical symptoms (total, positive, and negative symptom cluster scores of BPRS: r = −.092 to .016, all P > .05). Δpentosidine and Δpyridoxal levels also did not show any significant correlation with Δdaily CP dose amount (r = .105 and −.131, respectively, all P > .05). In addition, Δpentosidine and Δpyridoxal levels did not show any correlation with each other (r = .032, P = 0,821).
At the time of discharge, nutrition variables did not show associations with carbonyl stress markers (supplementary table 1). In addition, at the time of discharge when accurate compliance was confirmed, there was no influence among the types of antipsychotics used (first-generation antipsychotics, FGA; second-generation antipsychotics, SGA; and concomitant use of both types) on carbonyl stress markers (supplementary table 3).
Classification of Patients With Schizophrenia by the Comprehensive Status of Carbonyl Stress
To investigate differences in clinical severity and improvement in symptoms, patients at admission and paired-sample patients were classified according to their comprehensive carbonyl stress status at the time of admission, discharge and during hospitalization (detailed categories, see supplementary table 4). Again, the degree of clinical symptoms and their improvements showed no significant differences among the groups categorized according to carbonyl stress status (supplementary table 5).
Genetic Influence of Functional Polymorphisms in GLO1 on Pentosidine and Pyridoxal Levels
The rs4746 was genotyped in 74 patients with schizophrenia at admission, and 47 of these provided a sample at discharge and were considered as providing usable paired samples for comparison of a change in carbonyl stress markers. No patients had the homozygous minor allele (Ala/Ala). No significant differences in carbonyl stress marker levels were found between patients with Glu/Glu genotypes and Glu/Ala genotypes. In addition, Δcarbonyl stress markers were not different between these genotyped patients (supplementary table 6).
Discussion
This study reproduced a previous study by Arai et al6 of schizophrenia in the chronic state. We also investigated whether the levels of serum carbonyl stress markers were altered in patients with schizophrenia even at the acute stage, and if they changed according to the clinical course with multifarious parameters.
The pentosidine levels were not significantly altered in patients with schizophrenia and did not change according to the clinical course. The pentosidine levels did not show any correlation with physical factors, including nutrition status and lifestyle. However, as a previous study suggested,6 some patients at admission (14 cases, 10.2%) indeed showed extremely high pentosidine (figure 2A). We expected that these patients can be referred to as having “carbonyl stress schizophrenia” with severe symptoms, resistance to treatment, and genetic features.6,7 However, these 14 cases with high pentosidine showed no association with symptom severity, except for the duration of illness and daily CP dose amounts. Interestingly, the patient with the highest pentosidine (135ng/ml) showed the highest daily CP dose amount (2625mg/day). Furthermore, pentosidine levels from all patients with schizophrenia (n = 137) at admission showed a significant positive association with the daily CP dose amount, but not with the duration of illness. Thus, we speculate that accumulation of antipsychotics (dose × duration) may elevate the serum pentosidine levels. Indeed, analysis of the correlation between accumulation of antipsychotics before admission and pentosidine at admission showed a strong association. In addition, although the number of medication-free patients was small (n = 8), they showed a tendency for lower pentosidine levels than medicated patients. For patients with paired samples (n = 53) from admission to discharge, because their daily CP dose amount did not significantly increase (table 2), we found no significant association between Δpentosidine and Δdaily CP dose amounts. It may require only about 3 months (108.5 days) without an increase in the daily CP dose amount to increase pentosidine levels. However, these findings should be interpreted carefully, because drug-naive patients with an at-risk mental state show high pentosidine levels,15 and the patients in this study with the highest pentosidine levels (135ng/ml) showed a drastic decrease in pentosidine (35.3ng/ml) that was accompanied by accumulation of the total CP dose amount (2475mg/day and 245 days) and improvement in symptoms (Δtotal BPRS; −48.8%). Although the antipsychotic dose amount is likely a major factor in increasing pentosidine levels, other factors may also contribute.
The findings for pyridoxal were as follows: (1) levels were lower in schizophrenia compared with normal controls; (2) levels increased according to the clinical course, although the increased levels were still lower than those of normal controls (not statistically significant); and (3) 18 patients who showed a decrease in pyridoxal levels according to the clinical course showed that the greater the decrease in pyridoxal, the less improvement in symptoms. These findings were consistent with a previous study that also showed significantly lower serum pyridoxal in schizophrenia.6 The present study reproduced these previous data, and we also speculate that these lower levels increased according to the clinical course from a worse state to a better state. Carbonyl stress may be an aspect of the pathophysiology of schizophrenia, because pyridoxal levels increased as symptoms improved. A major limitation of the present study is that we could not infer what the lower serum pyridoxal and the increase in clinical course directly reflected, because we could not show either a correlation between pyridoxal and the severity of symptoms at admission, or between a change in pyridoxal levels and the degree of improvement in symptoms according to the clinical course. It is difficult to presume that lower pyridoxal levels were caused by poor nutrition status at admission, because at the time of discharge, the nutrition status of all patients was good due to the hospital diets, and the pyridoxal levels were still lower than those in controls, as with previous study.6 Of importance regarding the lower pyridoxal levels in schizophrenia, the details of the mechanism (eg, higher consumption or lower absorption) are unknown, but the lower levels are almost certainly involved in the pathophysiology of schizophrenia. Interestingly, some patients with schizophrenia showed that the greater the decrease in pyridoxal during their clinical course, the worse carbonyl stress likely was and the lowest the improvement in symptoms, especially positive symptoms, was observed. Even in chronic schizophrenia, lower pyridoxal levels show correlation with more severe symptoms, especially positive symptoms.6 Thus, our present findings for pyridoxal in the acute stage could be interrelated with pyridoxal in the chronic stage in patients with treatment-resistant schizophrenia.6 Pyridoxal is a candidate for augmentation therapy, perhaps not for all patients, but for treatment-resistant patients with lower pyridoxal levels and/or in cases where levels decrease during the clinical course. Taking supplemental vitamin B6 from the early stage of the disease, not from the late stage, may also be beneficial.16
We determined that there were no clinical features in relation to an assumed severe carbonyl stress status using a combination of pentosidine and pyridoxal levels in a cross-sectional and longitudinal study (supplementary tables 4 and 5). We found no important features, including related major genetic factors (supplementary table 6). Although some patients experienced carbonyl stress, this was not reflected in the severity of symptoms or other clinical features at the acute stage. The mechanism responsible for high pentosidine levels could be partly due to an accumulation of the daily dose of antipsychotics until the long-term clinical course, but other unknown factors are likely involved in schizophrenia. Only a decrease in pyridoxal during the clinical course reflected the low improvement of symptoms relatively early in the disease. The observation that altered pyridoxal and pentosidine levels in schizophrenia indicate only the existence of carbonyl stress in these patients and these levels did not directly contribute to the development of the disease. If anything, the identification of RCO-modified proteins might reveal more direct associations with the disease or its severity.
The limitations of the methodology of the present study were (1) healthy controls were younger; (2) the interval of the 2 measurements of biological markers and the duration of hospitalization were not controlled; and (3) the type of drug therapy was not controlled. Factors (1) and (2) are not likely to be major factors associated with carbonyl stress marker levels because correlations with these variables were not observed. For factor (3), there were no differences in the levels of carbonyl stress markers among the types of antipsychotics (FGA and SGA). However, these factors, in particular the duration of illness and individual antipsychotics should be controlled to properly assess the potential of carbonyl stress markers as “therapeutic” biological markers for schizophrenia. From a genetic point of view, we only investigated one major functional polymorphism in GLO1. Other gene-gene interactions, such as 22q11.2 deletion, paired-like homeobox 2b, and GLO1, may cause an increase in carbonyl stress.17
Conclusion
From the point of view of the carbonyl stress status, we can classify patients with schizophrenia as patients (1) with extremely high pentosidine levels that may be caused by higher antipsychotic dose amounts; (2) with lower pyridoxal levels that increased according to the clinical course; and (3) with pyridoxal levels that decreased according to the clinical course and that were accompanied by less improvement in symptoms. The role of carbonyl stress in schizophrenia is gradually being elucidated as a diagnostic and therapeutic biological marker.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Juntendo Institute of Mental Health (201109, 201209).
Supplementary Material
Acknowledgments
All authors contributed to the conceptualization, design, and writing of this manuscript. No authors have any conflicts of interest to disclose pertaining to this article.
References
- 1. Marchbanks RM, Ryan M, Day IN, Owen M, McGuffin P, Whatley SA. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res. 2003;65:33–38 [DOI] [PubMed] [Google Scholar]
- 2. Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–97, 643 [DOI] [PubMed] [Google Scholar]
- 3. Yao JK, Reddy RD, van Kammen DP. Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs. 2001;15:287–310 [DOI] [PubMed] [Google Scholar]
- 4. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaisson S, Gillery P. Evaluation of nonenzymatic posttranslational modification-derived products as biomarkers of molecular aging of proteins. Clin Chem. 2010;56:1401–1412 [DOI] [PubMed] [Google Scholar]
- 6. Arai M, Yuzawa H, Nohara I, et al. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch Gen Psychiatry. 2010;67:589–597 [DOI] [PubMed] [Google Scholar]
- 7. Miyashita M, Arai M, Kobori A, et al. Clinical features of schizophrenia with enhanced carbonyl stress. Schizophr Bull. September 23, 2013. 10.1093/schbul/sbt129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Overall JE, Gorham DR. The Brief Psychiatry Rating Scale. Psychol Rep. 1962;10:799–812 [Google Scholar]
- 9. Bech P, Kastrup M, Rafaelsen OJ. Mini-compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand Suppl. 1986;326:1–37 [PubMed] [Google Scholar]
- 10. Hatano T, Ohnuma T, Sakai Y, et al. Plasma alanine levels increase in patients with schizophrenia as their clinical symptoms improve-Results from the Juntendo University Schizophrenia Projects (JUSP). Psychiatry Res. 2010;177:27–31 [DOI] [PubMed] [Google Scholar]
- 11. Maeshima H, Ohnuma T, Sakai Y, et al. Increased plasma glutamate by antipsychotic medication and its relationship to glutaminase 1 and 2 genotypes in schizophrenia – Juntendo University Schizophrenia Projects (JUSP). Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1410–1418 [DOI] [PubMed] [Google Scholar]
- 12. Ohnuma T, Sakai Y, Maeshima H, et al. Changes in plasma glycine, L-serine, and D-serine levels in patients with schizophrenia as their clinical symptoms improve: results from the Juntendo University Schizophrenia Projects (JUSP). Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1905–1912 [DOI] [PubMed] [Google Scholar]
- 13. Jinno M, Takeuchi M, Watanabe A, et al. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum Reprod. 2011;26:604–610 [DOI] [PubMed] [Google Scholar]
- 14. Sanaka T, Funaki T, Tanaka T, et al. Plasma pentosidine levels measured by a newly developed method using ELISA in patients with chronic renal failure. Nephron. 2002;91:64–73 [DOI] [PubMed] [Google Scholar]
- 15. Arai M, Koike S, Oshima N, et al. Idiopathic carbonyl stress in a drug-naive case of at-risk mental state. Psychiatry Clin Neurosci. 2011;65:606–607 [DOI] [PubMed] [Google Scholar]
- 16. Lerner V, Miodownik C, Kaptsan A, Cohen H, Loewenthal U, Kotler M. Vitamin B6 as add-on treatment in chronic schizophrenic and schizoaffective patients: a double-blind, placebo-controlled study. J Clin Psychiatry. 2002;63:54–58 [DOI] [PubMed] [Google Scholar]
- 17. Toyosima M, Maekawa M, Toyota T, et al. Schizophrenia with the 22q11.2 deletion and additional genetic defects: case history. Br J Psychiatry. 2011;199:245–246 [DOI] [PubMed] [Google Scholar]
- 18. Monnier VM, Sell DR, Saxena A, et al. Measurement of oxidative stress: Technology, biomarkers and applications. Glycoxidative and carbonyl stress in aging and age-related diseases. In: Cutler RG, Rodriguez H, eds. Critical Reviews of Oxidative Stress and Aging.Advances in Basic Science, Diagnostics and Intervention, Vol 2 Singapore: World Scientific; 2003:414–426 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.