Abstract
Introduction: ABT-288 is a highly potent histamine-3 receptor antagonist that has demonstrated pro-cognitive effects in preclinical models relevant to schizophrenia. This study evaluated the efficacy and safety of two doses of ABT-288 in the treatment of cognitive impairment associated with schizophrenia. Methods: A randomized, double-blind, placebo-controlled, parallel-group 12-week study was conducted at 23 centers in the United States. Clinically stable subjects with schizophrenia were randomized in an equal ratio to ABT-288 10mg, ABT-288 25mg, or placebo once daily while continuing their antipsychotic regimen. The primary efficacy measure was the change from baseline to day 84 evaluation on the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB) composite score vs placebo. Secondary measures included cognitive functioning and psychiatric scales. Safety assessments and sparse pharmacokinetic sampling were also conducted. Results: A total of 214 subjects were randomized. The mean baseline MCCB composite score was 28.4. Approximately 80% of subjects completed the study. The MCCB composite score mean change from baseline to day 84 was numerically worse for both the 10mg (1.90, P = .618) and 25mg (0.64, P = .946) doses of ABT-288 vs placebo (2.19). Results from the secondary measures were consistent with the primary analysis. Subjects’ schizophrenia symptoms remained stable throughout the study as evidenced by stable Positive and Negative Syndrome Scale scores. Overall, study medication was tolerated; however, an increased incidence of psychosis-related and sleep-related adverse events was associated with ABT-288. Discussion: Neither dose of ABT-288 resulted in cognitive improvement in clinically stable adults with schizophrenia.
Key words: histamine-3 receptor, cognition disorders, cognitive dysfunction, humans, therapy
Introduction
Cognitive impairment associated with schizophrenia (CIAS) is a central component of the disease,1 and the treatment of CIAS has become a principal focus of schizophrenia research. These impairments are evident prior to other clinical aspects of the disease,2 are lifelong,3 and are accompanied by unfavorable outcomes in education, work, and social relationships.1,4 Research and development activity to identify agents that improve cognitive functioning in this population has recently intensified.
The histamine-3 (H3) receptor regulates the release of histamine and other pro-cognitive neurotransmitters such as acetylcholine, norepinephrine, and dopamine through an inhibitory process. H3 receptors are highly expressed in brain regions implicated in attention, learning, and memory such as the cerebral cortex and hippocampus. Patients with schizophrenia may have a histaminergic system that is different than those without the disease. In a study of cerebrospinal fluid from patients with chronic schizophrenia and controls, histamine metabolite levels were 2.6-fold higher in those with schizophrenia, an increase that could not be attributed to concomitant medications.5 A radioligand binding study of brain samples found significant increases in H3 receptor binding in patients with schizophrenia compared with normal controls, suggesting H3 receptors in the prefrontal cortex may be implicated in cognition.6
H3 receptor antagonists promote the release of the aforementioned neurotransmitters.7 Improved performance in preclinical models of attention, working memory, and memory consolidation has been observed for H3 antagonists,8–11 making H3 antagonism a promising mechanism for the treatment of cognitive disorders.7,9 Several H3 antagonists have been investigated in proof-of-concept studies in patients with Alzheimer’s dementia (AD),12,13 attention-deficit hyperactivity disorder (ADHD),14,15 and schizophrenia16,17 but affirmative results have not been reported to date.
ABT-288 is a highly selective and potent H3 receptor antagonist. Detailed characteristics and the pro-cognitive effects of ABT-288 in preclinical models relevant to cognitive disorders have been reported previously.18 In a phase 1 multiple dose study of subjects with schizophrenia, doses of ABT-288 up to 60mg once daily were administered and doses up to 45mg once daily were safe and well tolerated.19 Frequently reported adverse events such as abnormal dreams, insomnia, headache, and dizziness were similar to those observed in phase 1 studies of healthy volunteers and elderly subjects.20 Subjects with schizophrenia in the phase 1 study tolerated higher daily doses and plasma exposures of ABT-288 than healthy adults and elderly subjects, where the maximum tolerated dose was 3mg once daily. In subjects with schizophrenia, ABT-288 mean elimination half-life was 34–38 hours in the majority of evaluated dose groups.19
The objective of this study was to assess the efficacy and safety of ABT-288 when added to an antipsychotic regimen for 12 weeks (84 d) in treating cognitive impairment in clinically stable subjects with schizophrenia. The doses selected for this study were the highest well-tolerated dose from phase 1 (ABT-288 25mg once daily) and a lower dose (10mg once daily) that provided plasma exposures covering the efficacious levels in preclinical models of cognition. ABT-288 25mg once daily provided an approximately 2-fold margin based on the maximum tolerated dose tested in phase 1 (45mg once daily).
Methods
Study Design
This phase 2, multicenter, randomized, double-blind, placebo-controlled, parallel-group proof-of-concept study was conducted from March 2010 to July 2011 at 23 sites in the United States (NCT 01077700). Protocols, amendments, and informed consent forms were approved by institutional review boards, and written informed consent was obtained prior to any study procedures. An independent data monitoring committee reviewed unblinded safety data during the study.
The study had two screening visits during the 28- to 42-day period prior to randomization to ensure subject eligibility and stability (Supplementary Figure). Approximately 210 subjects (70 per group) were to be randomized at the baseline (day −1) visit in a 1:1:1 ratio to receive ABT-288 10mg, ABT-288 25mg, or placebo once daily for 12 weeks. The sponsor’s Department of Clinical Statistics provided the randomization schedule. To maintain the blind, ABT-288 and placebo capsules were identical in appearance. Subjects returned for study visits at days 7, 14, 28, 42, 56, and 84, and telephone contacts were made at days 21, 35, and 70. Subjects were to continue their antipsychotic treatment regimen for the entire study and were followed for 14 days after their last dose of study drug.
Subjects
Eligible subjects were clinically stable males and females with schizophrenia age 20–55 years who were being treated with 1–2 atypical antipsychotics. Subjects were to have a current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, text revision21 diagnosis of schizophrenia confirmed by the Mini-International Neuropsychiatric Interview22 version 6.0.0. Subjects had to have been diagnosed and/or treated for schizophrenia for at least 2 years. The main inclusion criteria included Positive and Negative Syndrome Scale23 (PANSS) item scores of ≤4 for delusions, conceptual disorganization, hallucinatory behavior, and excitement; no clinically significant extrapyramidal symptoms (EPS) at the first visit; a Severity of Abnormal Movements item score of ≤2 on the Abnormal Involuntary Movement Scale (AIMS)24 on day −1; a Global Clinical Rating of Akathisia item score of ≤ 2 on the Barnes Akathisia Rating Scale25 on day −1; and a Calgary Depression Scale for Schizophrenia26 total score of ≤10 at screening.
Subjects with a current or past diagnosis of schizoaffective disorder, bipolar disorder, manic episode, dementia, post-traumatic stress disorder, obsessive-compulsive disorder, substance dependence disorder, mental retardation, or medical or neurological disorder that could confound cognitive testing results were excluded. Subjects who were experiencing a current major depressive episode or had received electroconvulsive therapy within 6 months prior to screening were not allowed to enroll in the study.
Subjects were also excluded from the study if they were taking clozapine or first-generation antipsychotic agents or had taken mood stabilizers or antiepileptic drugs within 8 weeks of screening. Stable doses of antidepressants (except buproprion, tricyclics, and monoamine oxidase inhibitors) were permitted as long as no dosing changes had been made within 4 weeks of day −1. The use of agents for the treatment of EPS (eg, benztropine, trihexyphenidyl) was not allowed prior to randomization. Anxiolytic and hypnotic medications were allowed if their use had been stable since prior to the first screening visit. Subjects could be enrolled regardless of smoking status, but were excluded if substances of abuse were detected in blood or urine prior to randomization.
After randomization, changes in antipsychotic, antidepressant, anxiolytic, and EPS medications were permitted when necessary to maintain clinical stability. When as needed dosing of anxiolytic, hypnotic, or EPS medications was required, the timing of cognitive testing was to be adjusted to occur at least 12 hours afterwards. Regularly dosed agents were to be administered according to schedule without regard to cognitive testing.
Assessments
The Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery27,28 (MCCB) was conducted during screening, at day −1 (baseline), and at days 42 and 84. The primary efficacy variable was the MCCB composite score change from baseline to the day 84 evaluation. Secondary efficacy variables included the following: the 7 domain scores of the MCCB; the University of California San Diego Performance-based Skills Assessment-229 (UPSA-2) total score, conducted at baseline and day 84; the 16-item version of the Negative Symptom Assessment Scale30 (NSA-16), conducted at baseline and days 14, 42, and 84; and the Cambridge Neuropsychological Test Automated Battery (CANTAB, Cambridge Cognition, Ltd), administered during the initial and second screening visits and on days 28 and 56. Subjects who could not complete cognitive testing during screening or baseline were excluded from further participation in the study.
Clinical symptoms were assessed throughout the study using the PANSS and the Clinical Global Impression-Severity24 (CGI-S). Vital signs, 12-lead electrocardiograms (ECGs), physical examinations, clinical laboratory tests, Columbia-Suicide Severity Rating Scale31 (C-SSRS), and adverse event collection were performed throughout the study to evaluate safety. A blood sample was taken at study visits on days 7, 14, 28, 42, 56, and 84/premature discontinuation to determine plasma study drug concentrations, for a total of 6 planned samples per subject. Subject compliance with study medication was determined via pill counts of returned blister packs.
Statistical Analyses
Sample Size.
This was the first study to assess the efficacy of ABT-288 on cognition in subjects with schizophrenia using the MCCB, so it was not possible to estimate a projected effect size. Nonetheless, a sample size of 63 subjects per group would provide a power of 62%, 72%, 80%, and 87% to detect an effect size of 0.35, 0.40, 0.45, and 0.50 on the MCCB, respectively, for a 1-sided test at α = 0.05. These were believed to be clinically meaningful effect sizes for cognition in a therapeutic area where there are no known effective treatments. The approximately 70 subjects per treatment group planned for this study included an assumption that approximately 10% of subjects would not have postrandomization efficacy assessments. The 1-sided test was selected because ABT-288 had to demonstrate improvement compared with placebo to be considered effective.
Primary and Secondary Analyses.
All subjects who took at least 1 dose of study drug were included in the intent-to-treat (ITT) data set. Treatment group differences between each ABT-288 dose group and placebo were evaluated using a 1-sided test at the significance level of 0.05. Because this was a proof-of-concept study, no adjustments for multiple comparisons were made. When “site” was included as a factor in a statistical model, sites lacking at least 2 ITT subjects per treatment group were combined according to a pre-specified algorithm. Baseline was defined as the last observation prior to the first dose of study drug, and the final evaluation was the last observation occurring between the first dose and within 3 days after the last dose of study drug.
The primary efficacy analysis evaluated treatment group differences between each ABT-288 dose group and placebo for the change from baseline to day 84 on the MCCB composite score. This analysis was performed using a mixed-effects model, repeated-measures (MMRM) analysis that included fixed, categorical effects for treatment, site, visit, and treatment-by-visit interaction, with continuous fixed covariates for baseline score and the baseline score-by-visit interaction.
Secondary analysis of the MCCB composite score was performed using an ANCOVA with factors of treatment and site with baseline score as a covariate on change from baseline to the final evaluation of MCCB for the ITT data set. The change from baseline to final evaluation for the MCCB domain scores, NSA-16, PANSS, and CGI-S scores was analyzed using an ANCOVA model with factors of treatment and site, with baseline score as a covariate. To evaluate the change from baseline to each post-baseline observation, the MMRM analysis used for primary efficacy measure was conducted for the MCCB domains, NSA-16, PANSS, CGI-S, and CANTAB scores. Cigarette and alcohol use over the previous week was also summarized for ITT subjects who were current smokers and drinkers, respectively.
Pharmacokinetic Analyses.
Plasma concentrations of ABT-288 were determined using a validated liquid chromatography method with tandem mass spectrometric detection as previously described.20 ABT-288 plasma concentrations for each dose level were combined across all visits and summarized statistically by dose group and time since the previous dose of ABT-288.
Safety Analyses.
Treatment-emergent adverse events were summarized using the Medical Dictionary for Regulatory Activities (MedDRA) version 14.0.32 Treatment group differences in the percentage of subjects reporting adverse events were analyzed by Fisher’s exact test. Changes from baseline in laboratory, vital sign, body mass index, and ECG measurements were analyzed using a 1-way ANOVA with treatment as the main effect; potentially clinically significant values in these parameters were also identified. Subjects in each category of the C-SSRS were summarized by treatment group, and the PWC-20 total score was analyzed using a 2-way ANOVA with the terms of treatment and study site.
Results
Subjects
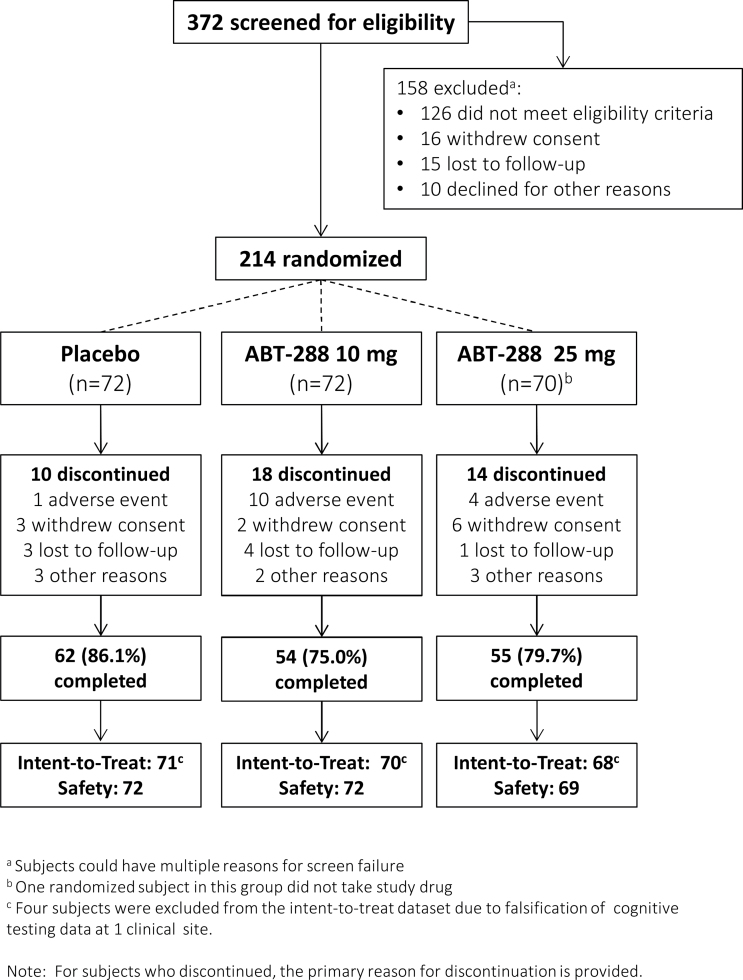
Subject disposition is illustrated in figure 1. A total of 171 treated subjects (80.3%) completed the study. The overall mean ± SD duration of treatment was 74.0±23.5 days (range 1–105). Baseline demographic characteristics are presented in table 1. The number of male subjects, and mean baseline weight and height were higher in the placebo group compared with both ABT-288 dose groups, leading to statistically significant differences among treatment groups for gender (P = .032), weight (P = .048), and height (P = .050).
Fig. 1.
Subject disposition.
Table 1.
Baseline Demographic Characteristics
| Characteristic (Safety Population) | Placebo, N = 72 | ABT-288 10mg, N = 72 | ABT-288 25mg, N = 69 | Total, N = 213 |
|---|---|---|---|---|
| Age, mean (SD), y | 43.0 (9.16) | 43.9 (9.51) | 42.3 (9.77) | 43.1 (9.46) |
| Age distribution, N (%) | ||||
| ≤40 y | 28 (38.9) | 20 (27.8) | 29 (42.0) | 77 (36.2) |
| >40 y | 44 (61.1) | 52 (72.2) | 40 (58.0) | 136 (63.8) |
| Gender, N (%) | ||||
| Male | 55 (76.4) | 40 (55.6) | 46 (66.7) | 141 (66.2) |
| Female | 17 (23.6) | 32 (44.4) | 23 (33.3) | 72 (33.8) |
| Race, N (%) | ||||
| Black | 43 (59.7) | 44 (61.1) | 40 (58.0) | 127 (59.6) |
| White | 28 (38.9) | 24 (33.3) | 27 (39.1) | 79 (37.1) |
| Asian | 0 | 3 (4.2) | 2 (2.9) | 5 (2.3) |
| Hawaiian native | 1 (1.4) | 0 | 0 | 1 (0.5) |
| Multirace | 0 | 1 (1.4) | 0 | 1 (0.5) |
| Hispanic ethnicity*, N (%) | 4 (5.6) | 3 (4.2) | 12 (17.4) | 19 (8.9) |
| Height**, mean (SD), cm | 176.3 (8.02) | 172.4 (9.35) | 173.3 (11.99) | 174.0 (9.99) |
| Body weight**, mean (SD), kg | 97.0 (15.01) | 89.8 (18.61) | 92.0 (19.75) | 92.9 (18.05) |
| BMI, mean (SD), kg/m2 | 31.3 (4.62) | 30.1 (5.52) | 30.5 (5.28) | 30.6 (5.15) |
| Tobacco use | ||||
| User | 49 (68.1) | 51 (70.8) | 50 (72.5) | 150 (70.4) |
| Nonuser | 19 (26.4) | 13 (18.1) | 12 (17.4) | 44 (20.7) |
| Ex-user | 4 (5.6) | 8 (11.1) | 7 (10.1) | 19 (8.9) |
| Current alcohol use | 18 (25.0) | 19 (26.8)a | 17 (24.6) | 54 (25.5) |
| Characteristic (ITT Population) | Placebo, N = 71 | ABT-288 10mg, N = 70 | ABT-288 25mg, N = 68 | Total, N = 209 |
| Age at onset of psychotic symptoms, mean (SD), y | 23.0 (8.37) | 23.1 (8.51) | 22.0 (7.81) | 22.7 (8.21) |
| Age at schizophrenia diagnosis, mean (SD), y | 27.8 (9.35) | 26.8 (9.19) | 26.0 (8.23) | 26.9 (8.93) |
| Age at first psychiatric hospitalization, mean (SD), y | 26.0 (9.82) | 24.6 (9.17) | 26.1 (8.47) | 25.6 (9.13) |
| Age when antipsychotic drugs first prescribed, mean (SD), y | 27.9 (9.89) | 25.9 (8.91) | 25.3 (8.06) | 26.4 (9.02) |
| Months on stable antipsychotic treatment, mean (SD) | 19.9 (20.27) | 24.1 (44.03) | 28.6 (36.77) | 24.1 (35.07) |
| Baseline PANSS total score, mean (SD) | 65.4 (11.14) | 62.8 (12.25) | 65.1 (12.21) | 64.4 (11.87) |
Note: BMI, body mass index; ITT, intent-to-treat; PANSS, Positive and Negative Syndrome Scale.
a N = 71 subjects.
*P ≤ .05 for differences across treatment groups from Fisher’s exact test.
**P ≤ .05 for differences across treatment groups from 1-way ANOVA.
In the ITT population, the mean ± SD baseline PANSS total score was 64.4±11.87 and the mean ± SD baseline MCCB composite score was 28.4 ± 12.13; both were similar across treatment groups. All subjects in the safety population (n = 213) reported taking concomitant antipsychotic drugs. Risperidone (33.8%), quetiapine (26.3%), olanzapine (19.2%), aripiprazole (15.5%), paliperidone (8.5%), and ziprasidone (8.0%) were the most frequently reported. Investigators considered 79.3% of subjects to have been treatment compliant during the entire study (ie, took at least 70% of study medication), as estimated by pill counts and investigator judgment.
Efficacy
The primary analysis included 188/214 randomized subjects. Twenty-six randomized subjects (12.1%) were excluded because they lacked a baseline or on-treatment MCCB composite score (n = 21), they did not take a dose of study drug (n = 1), or their data were not analyzed due to the discovery of cognitive testing data falsification at 1 clinical site (n = 4).
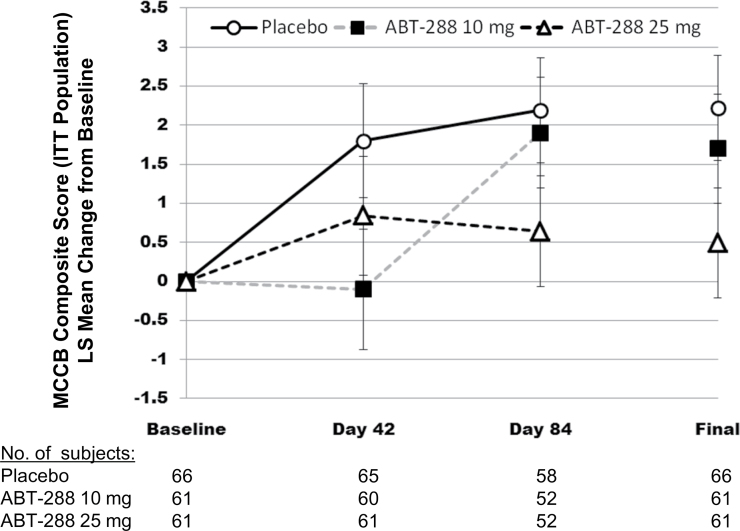
The mean MCCB composite scores (±SD) at baseline and the final evaluation were as follows: ABT-288 10mg once daily baseline 29.48±10.93, final 31.10±12.02; ABT-288 25mg once daily baseline 25.95±12.47, final 26.61±11.31; and placebo baseline 28.77±12.23, final 30.79±12.56. Mean increases (improvement) from baseline to day 84 were observed for all treatment groups. Least-squares mean increases in the MCCB composite score for ABT-288 10mg (1.90, P = .618 vs placebo) and ABT-288 25mg (0.64, P = .946 vs placebo), however, were less than the placebo group (2.19), indicating no meaningful improvement in cognition. The difference in the change from baseline to day 84 least-squares means (±SE) vs placebo was −0.29±0.950 for ABT-288 10mg and −1.55±0.949 for ABT-288 25mg. Results from the secondary ANCOVA analysis of the MCCB composite score concurred with the primary analysis (figure 2). The results from a post hoc 2-tailed analysis were consistent with the primary analysis (2-sided P = .762 and .108 for ABT-288 10mg and 25mg, respectively).
Fig. 2.
MCCB composite score: LS mean change from baseline over time (ITT population). ITT, intent-to-treat; LS, least squares; MCCB, MATRICS Consensus Cognitive Battery. Mixed-effect model repeated-measures analysis of change from baseline to each visit and the secondary ANCOVA of change from baseline to the final evaluation for the MCCB composite score. Error bars represent the SE of the least squares means.
Results of other secondary efficacy analyses generally agreed with the primary analysis. There were no significant treatment group differences between each ABT-288 dose group and placebo in the ANCOVA analyses on any of the MCCB domains, or the NSA-16, PANSS, UPSA-2, and CGI-S scores (table 2). There were no meaningful differences on change from baseline to final scores on the PANSS total (−1.46, −2.41, −3.80), positive (−0.72, −0.97, −1.05), negative (−0.60, −1.09, −0.88), or general psychopathology (−0.20, −0.50, −1.91) scores for the ABT-288 25mg, ABT-288 10mg, and placebo treatment groups, respectively. Sporadic changes were observed at various time points on the CANTAB battery, but these changes were not accompanied by corresponding changes on the MCCB.
Table 2.
Secondary Efficacy Results, ANCOVA (ITT Population)
| Assessment | Placebo | ABT-288 10 mg | ABT-288 25 mg |
|---|---|---|---|
| MCCB composite score | N = 66 | N = 61 | N = 61 |
| Baseline mean (SD) | 28.77 (12.23) | 29.48 (10.93) | 25.95 (12.47) |
| LS mean change to final (SE), P | 2.22 (0.67) | 1.70 (0.70), .712 | 0.49 (0.70), .966 |
| MCCB domains speed of processing | |||
| Baseline mean (SD) | 33.36 (11.82) | 34.33 (9.77) | 30.59 (10.55) |
| LS mean change to final (SE), P | 2.28 (0.88) | 3.02 (0.93), .276 | 3.18 (0.93), .238 |
| Verbal learning | |||
| Baseline mean (SD) | 38.20 (7.54) | 38.51 (7.70) | 35.39 (7.55) |
| LS mean change to final (SE), P | 0.78 (0.75) | 0.75 (0.79), .514 | −0.49 (0.79), .883 |
| Working memory | |||
| Baseline mean (SD) | 36.02 (12.38) | 37.07 (10.62) | 33.28 (11.83) |
| LS mean change to final (SE), P | 1.68 (0.76) | 1.59 (0.81), .533 | 0.36 (0.80), .889 |
| Reasoning and problem solving | |||
| Baseline mean (SD) | 41.09 (10.04) | 39.72 (8.14) | 40.11 (9.70) |
| LS mean change to final (SE), P | 2.34 (0.69) | 2.04 (0.72), .623 | 0.18 (0.72), .987 |
| Visual learning | |||
| Baseline mean (SD) | 38.47 (11.47) | 37.23 (11.21) | 36.64 (12.06) |
| LS mean change to final (SE), P | 1.96 (1.16) | 1.44 (1.21), .625 | −0.21 (1.21), .907 |
| Attention/vigilance | |||
| Baseline mean (SD) | 37.85 (12.29) | 37.03 (10.71) | 36.49 (12.03) |
| LS mean change to final (SE), P | 0.17 (1.11) | 1.14 (1.16), .267 | −0.04 (1.16), .553 |
| Social cognition | |||
| Baseline mean (SD) | 34.05 (13.09) | 37.80 (13.43) | 34.30 (12.82) |
| LS mean change to final (SE), P | 1.22 (1.14) | −1.07 (1.20), .922 | −2.12 (1.19), .981 |
| UPSA-2 total score | N = 57 | N = 55 | N = 53 |
| Baseline mean (SD) | 89.95 (13.75) | 89.05 (15.03) | 86.02 (17.47) |
| LS mean change to final (SE), P | 3.29 (1.34) | 2.23 (1.39), .720 | 0.25 (1.38), .949 |
| NSA-16 total score | N = 69 | N = 65 | N = 64 |
| Baseline mean (SD) | 44.51 (10.08) | 43.80 (11.99) | 44.41 (10.60) |
| LS mean change to final (SE), P | −1.59 (0.70) | −1.28 (0.73), .624 | 0.22 (0.73), .967 |
| PANSS total score | N = 68 | N = 66 | N = 63 |
| Baseline mean (SD) | 65.12 (11.19) | 63.21 (12.42) | 65.30 (12.17) |
| LS mean change to final (SE), P | −3.80 (0.91) | −2.41 (0.95), .861 | −1.46 (0.96), .965 |
| CGI-S | N = 69 | N = 65 | N = 64 |
| Baseline mean (SD) | 3.61 (0.57) | 3.49 (0.71) | 3.63 (0.55) |
| LS mean change to final (SE), P | −0.22 (0.05) | −0.16 (0.06), .776 | 0.01 (0.06), .998 |
| Cigarettes/day over past week* | N = 41 | N = 46 | N = 42 |
| Baseline mean (SD) | 12.23 (7.06) | 13.50 (9.91) | 12.29 (7.72) |
| LS mean change to final (SE), P | −0.86 (0.87) | −0.37 (0.83), .661 | 0.93 (0.84), .936 |
| Number of days drinking during the past weeka | N = 15 | N = 16 | N = 14 |
| Baseline mean (SD) | 0.40 (0.91) | 0.56 (0.81) | 0.36 (0.84) |
| LS mean change to final (SE), P | −0.06 (0.43) | 0.41 (0.42), .785 | −0.44 (0.44), .257 |
Note: CGI-S, Clinical Global Impression-Severity; ITT, intent-to-treat; LS, least squares; MCCB, MATRICS Consensus Cognitive Battery; NSA-16, 16-item Negative Symptom Assessment Scale; PANSS, Positive and Negative Syndrome Scale; UPSA-2, University of California San Diego Performance-based Skills Assessment-2.
P-values (italicized) are one-sided specified a priori.
aCurrent smokers and drinkers, respectively, in the ITT.
Pharmacokinetics
ABT-288 mean plasma exposures were 40%–50% lower than previously observed in subjects with stable schizophrenia in a phase 1 setting, suggesting low study drug compliance. Examination of the individual ABT-288 plasma concentrations at the different visits suggested that approximately 40% of the subjects overall were non-compliant, at least occasionally. Approximately 20% of the subjects were consistently non-compliant (ie, in ≥3 visits) and 30% of the subjects appeared to be non-compliant at the final visit. Approximately 15% of subjects had no measurable ABT-288 levels from at least one visit. However, approximately 80% of the subjects overall had plasma concentrations within or higher than the than preclinical efficacious plasma concentrations at the final visit.
Safety
Approximately 60% of subjects (n = 127) experienced an adverse event and 11 subjects (5.2%) experienced a serious adverse event, with rates similar across treatment groups (table 3). Compared with placebo, higher percentages of subjects in both ABT-288 dose groups reported adverse events assessed by the investigator as probably or possibly related to study drug. Serious adverse events of anxiety and paranoia in the ABT-288 10mg group and one event of suicidal ideation in the ABT-288 25mg group were classified by the investigator as possibly related to ABT-288.
Table 3.
Summary of Treatment-Emergent Adverse Events (Safety Population)
| Overall, N (%) | Placebo, N = 72 | ABT-288 10mg, N = 72 | ABT-288 25mg, N = 69 | Total, N = 213 |
|---|---|---|---|---|
| Any adverse event | 41 (56.9) | 48 (66.7) | 38 (55.1) | 127 (59.6) |
| Discontinued due to an adverse event* | 1 (1.4) | 10 (13.9) | 6 (8.7) | 17 (8.0) |
| Possibly or probably drug relateda,** | 16 (22.2) | 28 (38.9) | 27 (39.1) | 71 (33.3) |
| Severe adverse event | 3 (4.2) | 5 (6.9) | 2 (2.9) | 10 (4.7) |
| Moderate adverse eventb | 15 (20.8) | 20 (27.8) | 19 (27.5) | 54 (25.4) |
| Mild adverse eventc | 23 (31.9) | 23 (31.9) | 17 (24.6) | 63 (29.6) |
| Serious adverse event | 3 (4.2) | 5 (6.9) | 3 (4.3) | 11 (5.2) |
| Adverse events reported by for 4 or more subjects taking ABT-288 | ||||
| MedDRA preferred term, N (%) | ||||
| Insomnia | 4 (5.6) | 12 (16.7) | 9 (13.0) | 25 (11.7) |
| Headache | 3 (4.2) | 9 (12.5) | 3 (4.3) | 15 (7.0) |
| Nausea | 3 (4.2) | 8 (11.1) | 4 (5.8) | 15 (7.0) |
| Dizziness | 2 (2.8) | 2 (2.8) | 8 (11.6) | 12 (5.6) |
| Dry mouth | 4 (5.6) | 2 (2.8) | 6 (8.7) | 12 (5.6) |
| Hypertension | 2 (2.8) | 4 (5.6) | 3 (4.3) | 9 (4.2) |
| Psychotic disorder | 3 (4.2) | 3 (4.2) | 3 (4.3) | 9 (4.2) |
| Abnormal dreams | 1 (1.4) | 4 (5.6) | 3 (4.3) | 8 (3.8) |
| Cough | 3 (4.2) | 2 (2.8) | 3 (4.3) | 8 (3.8) |
| Nasopharyngitis | 2 (2.8) | 2 (2.8) | 4 (5.8) | 8 (3.8) |
| Anxiety | 0 | 4 (5.6) | 3 (4.3) | 7 (3.3) |
| Diarrhoea | 2 (2.8) | 2 (2.8) | 3 (4.3) | 7 (3.3) |
| Depression | 0 | 4 (5.6) | 2 (2.9) | 6 (2.8) |
| Agitation | 1 (1.4) | 2 (2.8) | 2 (2.9) | 5 (2.3) |
| Hot flush | 0 | 2 (2.8) | 3 (4.3) | 5 (2.3) |
| Adverse events of special interestd | ||||
| Psychosis and psychotic disorders | 4 (5.6) | 8 (11.1) | 11 (15.9) | 23 (10.8) |
| Hostility and aggression | 6 (8.3) | 8 (11.1) | 8 (11.6) | 22 (10.3) |
| Sleep disorders and disturbances* | 5 (6.9) | 15 (20.8) | 10 (14.5) | 30 (14.1) |
| Arrhythmia-related investigations, signs, and symptoms | 1 (1.4) | 2 (2.8) | 2 (2.9) | 5 (2.3) |
| Suicide/self-injury | 0 | 2 (2.8) | 3 (4.3) | 5 (2.3) |
Note: MedDRA, Medical Dictionary for Regulatory Activities.
aInvestigator believed the event was possibly or probably related to study drug.
bSubjects whose most severe adverse event was rated as “moderate” by the investigator.
cSubjects whose most severe adverse event was rated as “mild” by the investigator.
dStandardized MedDRA Query or MedDRA High Level Group Term.
*P < .05 for ABT-288 10mg and both doses of ABT-288 combined vs placebo (Fisher’s exact test).
**P < .05 for ABT-288 10mg, ABT-126 25mg, and both doses of ABT-288 vs placebo (Fisher’s exact test).
Compared with placebo, statistically significantly greater proportions of subjects had adverse events in the “psychiatric disorders” System Organ class in the ABT-288 10mg group (P = .010 vs placebo) and in both ABT-288 doses combined (ABT-288 overall; P = .011 vs placebo). A significantly greater number of subjects had adverse events related to sleep disorders and sleep disturbances in the ABT-288 10mg group (P = .028 vs placebo) and in the ABT-288 overall group (P = .037 vs placebo; table 3).
There was a statistically significant treatment difference among treatment groups in the percentage of subjects whose primary reason for study discontinuation was an adverse event (P = .012) or given as any reason for discontinuation (P = .011). MedDRA preferred terms psychotic disorder (n = 4) and anxiety (n = 2) were the most frequent adverse events leading to discontinuation (n = 17 total). No clinically meaningful changes or trends related to ABT-288 treatment were identified in laboratory values, vital signs, ECG measurements, or C-SSRS and PWC-20 ratings.
Discussion
Neither dose of ABT-288 demonstrated pro-cognitive efficacy in subjects with schizophrenia in this study. While the composite score of the MCCB in all 3 treatment groups increased (improved) from baseline to day 84, the change from baseline in both ABT-288 treatment groups was not statistically different from placebo. Results from secondary efficacy measures were consistent with findings from the primary analysis.
Two safety signals were detected in this study. There was a higher incidence of psychosis-related adverse events in the active treatment groups. An increase in psychosis-related events were observed in the phase 1 study in subjects with schizophrenia, but the inflection point appeared to occur at doses greater than those used in this study.19 Higher rates of psychosis were not reported in previous schizophrenia studies with H3 antagonists.16,17 However, doses in all of these trials were comparable to those used in other populations, not the 10-fold increase administered in this study. Although an increase in locomotor activity was not observed in animal models, these findings suggest ABT-288 may be activating in the schizophrenia population at high doses. There was also a higher rate of sleep-related adverse events associated with ABT-288 treatment. This is consistent with observations from our phase 1 studies and reports of other H3 antagonists16,17 and is not unexpected given the release of the pro-wake neurotransmitter histamine that is central to the mechanism of H3 antagonists.19
This study was designed largely in accordance with the MATRICS guidelines, and several lines of evidence suggest the hypothesis was adequately tested. The overall subject completion rate of 80.3% was comparable to other trials of CIAS in clinically stable patients.16,17 The placebo response on the MCCB (which may include practice and trial effects) was approximately 2 points, an effect similar to that reported in previous trials of 8–12 weeks in duration in the stable schizophrenia population.33,34 The statistical variability on the primary endpoint as measured by the SD of the change score (5.6) was lower than those values reported in similar trials.33,34 The plasma concentrations achieved in the clinical trial were within or generally greater than those that produced pro-cognitive effects in animal models. There was ample evidence of target engagement, given the higher incidence of sleep-related adverse events in individuals receiving ABT-288 compared with placebo. The sample size of this trial was based on a 1-sided test and would have only 62% power to detect a 0.35 effect size. The improvement of both ABT-288 doses on the primary efficacy endpoint was numerically lower than that of the placebo group. It is, therefore, unlikely that a larger trial would have produced positive results.
These negative clinical findings were in contrast to the consistent and reproducible preclinical pro-cognitive effects of ABT-288 observed across several domains relevant to schizophrenia.18 These findings underscore a broader challenge in neuropsychiatric drug development, as preclinical behavior models do not fully recapitulate the human disease.
Published results of clinical trials with other H3 antagonists have shown mixed results, with some success in wake promotion but an absence of an effect in cognition.35–37 MK-0249 had no positive effects on cognition in patients with mild-to-moderate AD,12 schizophrenia,16 or in adults with ADHD.14 Other publicly available data indicate that GSK239512 failed to demonstrate an effect in subjects with schizophrenia17 and mild-to-moderate AD,13 as did the H3 antagonist PF-03654746 in studies of CIAS, AD, and ADHD.9,38,39 In over 400 adults with ADHD, bavisant had no significant improvement ADHD symptoms, in contrast to the active controls.15
Additionally, a 12-week multicenter, randomized placebo-controlled trial of ABT-288 in 242 patients with mild-to-moderate AD did not demonstrate a pro-cognitive effect.40 This study replicates the same finding of a lack of pro-cognitive effects of ABT-288 in subjects with schizophrenia. Despite their differences, the doses evaluated in the AD and present trial are comparable when the limits of ABT-288 tolerability in the respective populations are considered.
While previous research has implicated the histaminergic system in the pathophysiology of schizophrenia,5,6,41 ABT-288 did not demonstrate pro-cognitive effects in stable subjects with schizophrenia. In our opinion, the lack of effect in this and other studies of other H3 antagonists published in peer reviewed journals or presented at scientific symposia lead us to conclude that this is not a viable target to elicit cognitive improvement in CIAS or other cognition-related indications.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Abbott Laboratories; AbbVie.
Supplementary Material
Acknowledgments
G Haig, E Bain, W Robieson, and A Othman are employees of AbbVie and hold Abbott and/or AbbVie stock. R Lenz and J Baker are former employees of Abbott and hold Abbott and/or AbbVie stock. Abbott/AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. The authors were assisted in the preparation of this manuscript by Muriel Cunningham, a professional medical writer compensated by AbbVie.
References
- 1. Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr Clin North Am. 2005;28:613–633 [DOI] [PubMed] [Google Scholar]
- 2. Bartók E, Berecz R, Glaub T, Degrell I. Cognitive functions in prepsychotic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:621–625 [DOI] [PubMed] [Google Scholar]
- 3. Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32 [DOI] [PubMed] [Google Scholar]
- 4. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51 [DOI] [PubMed] [Google Scholar]
- 5. Prell GD, Green JP, Kaufmann CA, et al. Histamine metabolites in cerebrospinal fluid of patients with chronic schizophrenia: their relationships to levels of other aminergic transmitters and ratings of symptoms. Schizophr Res. 1995;14:93–104 [DOI] [PubMed] [Google Scholar]
- 6. Jin CY, Anichtchik O, Panula P. Altered histamine H3 receptor radioligand binding in post-mortem brain samples from subjects with psychiatric diseases. Br J Pharmacol. 2009;157:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol. 2008;154:1166–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altenbach RJ, Liu H, Banfor PN, et al. Synthesis, potency, and in vivo profiles of quinoline containing histamine H3 receptor inverse agonists. J Med Chem. 2007;50:5439–5448 [DOI] [PubMed] [Google Scholar]
- 9. Brioni JD, Esbenshade TA, Garrison TR, Bitner SR, Cowart MD. Discovery of histamine H3 antagonists for the treatment of cognitive disorders and Alzheimer’s disease. J Pharmacol Exp Ther. 2011;336:38–46 [DOI] [PubMed] [Google Scholar]
- 10. Fox GB, Esbenshade TA, Pan JB, et al. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther. 2005;313:176–190 [DOI] [PubMed] [Google Scholar]
- 11. Medhurst AD, Atkins AR, Beresford IJ, et al. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer’s disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther. 2007;321:1032–1045 [DOI] [PubMed] [Google Scholar]
- 12. Egan M, Yaari R, Liu L, et al. Pilot randomized controlled study of a histamine receptor inverse agonist in the symptomatic treatment of AD. Curr Alzheimer Res. 2012;9:481–490 [DOI] [PubMed] [Google Scholar]
- 13. GlaxoSmithKline. Studysummary: A randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of the H3 receptor antagonist, GSK239512 in subjects with mild to moderate Alzheimer’s disease. http://www.gsk-clinicalstudyregister.com/result_comp_list.jsp?phase=All&studyType=All&population=Adult&marketing=All&compound=GSK239512 Accessed August 13, 2012
- 14. Herring WJ, Wilens TE, Adler LA, et al. Randomized controlled study of the histamine H3 inverse agonist MK-0249 in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2012;73:e891–e898 [DOI] [PubMed] [Google Scholar]
- 15. Weisler RH, Pandina GJ, Daly EJ, Cooper K, Gassmann-Mayer C. Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs. 2012;26:421–434 [DOI] [PubMed] [Google Scholar]
- 16. Egan M, Zhao X, Gottwald R, et al. Randomized crossover study of the histamine H3 inverse agonist MK-0249 for the treatment of cognitive impairment in patients with schizophrenia. Schizophr Res. 2013;146:224–230 [DOI] [PubMed] [Google Scholar]
- 17. GlaxoSmithKline. Studysummary:a randomized double-blind, placebo controlled, parallel group study to evaluate the cognitive enhancing effect of GSK239512 in stable patients with schizophrenia. http://www.gsk-clinicalstudyregister.com/result_comp_list.jsp?phase=All&studyType=All&population=Adult&marketing=All&compound=GSK239512 Accessed August 23, 2012
- 18. Esbenshade TA, Browman KE, Miller TR, et al. Pharmacological properties and procognitive effects of ABT-288, a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther. 2012;343:233–245 [DOI] [PubMed] [Google Scholar]
- 19. Othman AA, Haig G, Florian H, et al. The H3 antagonist ABT-288 is tolerated at significantly higher exposures in subjects with schizophrenia than in healthy volunteers [published online ahead of print 2013]. Br J Clin Pharmacol. 10.1111/bcp.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Othman AA, Haig G, Florian H, Locke C, Zhang J, Dutta S. Safety, tolerability and pharmacokinetics of the histamine H3 receptor antagonist, ABT-288, in healthy young adults and elderly volunteers. Br J Clin Pharmacol. 2013;75:1299–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 22. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33; quiz 34–57 [PubMed] [Google Scholar]
- 23. Kay S, Fiszbein A, Opler L. ThePositive andNegativeSyndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;261:76. [DOI] [PubMed] [Google Scholar]
- 24. Guy W. ECDEUAssessment Manual forPsychopharmacology,Revised. Vol Publication ADM 76–338. Bethesda, MD: United States Department of Health, Education, and Welfare; 1976 [Google Scholar]
- 25. Barnes T. A rating scale for drug-induced akathisia. Br J Psychiatry . 1989;154:672–676 [DOI] [PubMed] [Google Scholar]
- 26. Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208 [DOI] [PubMed] [Google Scholar]
- 27. Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220 [DOI] [PubMed] [Google Scholar]
- 28. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213 [DOI] [PubMed] [Google Scholar]
- 29. Patterson T, Goldman S. The UCSD performance-based skills assessment (UPSA-2). Copy written material R-10-06. 2005.
- 30. Alphs LD, Summerfelt A, Lann H, Muller RJ. The negative symptom assessment: a new instrument to assess negative symptoms of schizophrenia. Psychopharmacol Bull. 1989;25:159–163 [PubMed] [Google Scholar]
- 31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MedDRA Maintenance and Support Services Organization. Medical Dictionary for Regulatory Activities (MedDRA). Version 14.0. Chantilly, VA: MedDRA Maintenance and Support Services Organization [Google Scholar]
- 33. Umbricht D, Murray S, Lowe D, et al. TheEffect of thePartial Nicotinic Alpha7 Receptor Agonist R3487 onCognitive Deficits inSchizophrenia. Hollywood,FL: ACNP; 2009 [Google Scholar]
- 34. Dunbar G, Hosford D, Lieberman J, Segreti A. The alpha7 nicotinic receptor agonist TC-5619 had beneficial effects with favorable tolerability in a phase 2 trial in cognitive dysfunction in schizophrenia. In: 13th International Congress on Schizophrenia Research; April 2– 6; Colorado Springs, Colorado [Google Scholar]
- 35. Anruhlf I. Results of clinical trials of tripolisant in narcolepsy and Parkinson’s disease. Eur Neuropsychopharm. 2009;19:S204 [Google Scholar]
- 36. Iannone R, Palcza J, Renger JJ, et al. Acute alertness-promoting effects of a novel histamine subtype-3 receptor inverse agonist in healthy sleep-deprived male volunteers. Clin Pharmacol Ther. 2010;88:831–839 [DOI] [PubMed] [Google Scholar]
- 37. Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163:713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Center for Advancing Translational Sciences, National Institutes of Health. Pfizer Inc., PF-03654746. http://www.ncats.nih.gov/files/PF-03654746.pdf Accessed September 04, 2012
- 39. Boyer S, Palumbo D, Chappell P, Li B, Pinter G. Results of an H3 receptor antagonist clinical trial in adults diagnosed with ADHD. In: International Society for CNS Clinical Trials and Methodology; February 22–24; Washington DC [Google Scholar]
- 40. Haig G, Meier A, Pritchett Y, et al. Evaluation of efficacy and safety of the H3 antagonist ABT-288 in mild-to-moderate Alzheimer’s disease. In: Alzheimer’s Association International Conference; July 14–19; British Columbia, Canada [Google Scholar]
- 41. Nuutinen S, Panula P. Histamine in neurotransmission and brain diseases. Adv Exp Med Biol. 2011;709:95–107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.