Abstract
Oxytocin (OT) and arginine vasopressin (AVP) exert robust influence on social affiliation and specific cognitive processes in healthy individuals. Abnormalities in these neuroendocrine systems have been observed in psychotic disorders, but their relation to impairments in behavioral domains that these endocrines modulate is not well understood. We compared abnormalities of OT and AVP serum concentrations in probands with schizophrenia (n = 57), schizoaffective disorder (n = 34), and psychotic bipolar disorder (n = 75); their first-degree relatives without a history of psychosis (n = 61, 43, 91, respectively); and healthy controls (n = 66) and examined their association with emotion processing and cognition. AVP levels were lower in schizophrenia (P = .002) and bipolar probands (P = .03) and in relatives of schizophrenia probands (P = .002) compared with controls. OT levels did not differ between groups. Familiality estimates were robust for OT (h 2 = 0.79, P = 3.97e−15) and AVP (h 2 = 0.78, P = 3.93e−11). Higher levels of OT were associated with better emotion recognition (β = 0.40, P < .001) and general neuropsychological function (β = 0.26, P = .04) in healthy controls as expected but not in any proband or relative group. In schizophrenia, higher OT levels were related to greater positive symptom severity. The dissociation of OT levels and behavioral function in all proband and relative groups suggests that risk and illness factors associated with psychotic disorders are not related to reduced OT levels but to a disruption in the ability of physiological levels of OT to modulate social cognition and neuropsychological function. Decreased AVP levels may be a marker of biological vulnerability in schizophrenia because alterations were seen in probands and relatives, and familiality was high.
Key words: oxytocin, vasopressin, schizophrenia, bipolar disorder, emotion recognition
Introduction
Two phylogenetically ancient neuropeptides, oxytocin (OT) and arginine vasopressin (AVP), regulate specific social and cognitive processes that are abnormal in schizophrenia. Although OT is commonly known for its role in birth and lactation and AVP as an antidiuretic, both have widespread influences on emotion and cognition in healthy individuals.1–5 Reciprocal interactions between OT and AVP facilitate shifts between positive socially oriented and defensive states. OT attenuates the stress response, improves emotion processing, and can have negative effects on verbal learning and memory in healthy individuals.1–3,6–8 In contrast, AVP amplifies reactivity to stressors and under some conditions can have beneficial effects on attention, verbal learning, and memory.1–3,6–8
Several lines of evidence suggest that alterations in these endocrine systems may be involved in the pathophysiology of schizophrenia.9,10 First, some studies have reported differences in cerebrospinal fluid and peripheral levels of OT and AVP in schizophrenia.11–17 Similar results have been reported recently in bipolar disorder.18 Second, there is notable overlap between the neurobehavioral functions modulated by OT and AVP in healthy adults with those that are abnormal in schizophrenia, such as social cognition and memory.19–22 For example, AVP 1b knockout mice exhibit impairments in memory and social behavior.23 Third, these hormones both regulate physiological activity in brain regions that have established functional and structural alterations in the disorder.24–28 OT receptor concentrations are highest within the hippocampus, septum, and amygdala29 and the highest level of brain AVP 1b receptor expression is in the hippocampus,30 which is an important brain structure for memory31 and is abnormal in schizophrenia.25,32 In preclinical models, haploinsufficient reeler mice, an animal model of neural deficits in schizophrenia, show reductions in OT receptors especially in the hippocampus and piriform cortex.33 AVP-deficient Brattleboro rats also show several phenotypes of schizophrenia including sensorimotor gaiting and social cognitive deficits, some of which are reversed by antipsychotic drugs.34–36 Fourth, postmortem AVP concentrations are reduced in the temporal cortex of schizophrenia patients.37
Although OT and AVP have been implicated in schizophrenia,14,38 the degree to which alterations in these hormone systems contribute to neurobehavioral deficits in schizophrenia and other psychotic disorders has not been systematically examined. Although supraphysiologic doses of OT modulate emotional and cognitive processes in healthy individuals20,21 and in some small-sample schizophrenia studies,39–46 it remains unclear whether there are altered physiological protein levels across these disorders and whether physiological levels of OT and AVP modulate neurobehavioral processes in schizophrenia and other psychotic disorders as in healthy individuals. Studies of endocrine levels and their neurobehavioral associations are needed to address these questions.
Different assay methods may contribute to the considerable variability in findings across previous studies. Enzyme immunoassay (EIA) without extraction provides greater sensitivity and specificity for OT and AVP47 than commercially available radioimmunoassay. With regard to endocrine—neurobehavioral associations, 2 small-sample studies reported positive associations between peripheral OT levels and emotion processing in schizophrenia11,48 and trust-related interpersonal interactions have been reported to modulate peripheral OT levels in healthy individuals49,50 but not in schizophrenia.50 In schizophrenia, disruptions in peripheral levels of AVP have been linked to poorer cognitive functioning and increased positive symptom severity.14 In addition to questions about abnormalities in peripheral hormone levels and their clinical associations, other questions of interest include whether alterations in endocrine levels and their associations are present across psychotic disorders and whether they are familial.
This study was designed to examine alterations in OT and AVP levels and the relation of endocrine levels to behavior and cognition in patients with schizophrenia, schizoaffective disorder, and psychotic bipolar disorder and in their first-degree family members. Our first study aim was to compare peripheral OT and AVP levels across the patient groups and their relatives. We hypothesized that patients and their relatives would show atypical OT and AVP levels, compared with controls. Our second aim was to evaluate whether peripheral OT and AVP levels were associated with clinical symptoms, emotion processing, and cognitive functioning. We predicted that higher physiologic levels of OT, which we found previously,51 would be associated with less severe symptoms and better emotion processing and cognitive functioning, whereas higher AVP levels would be associated with more severe symptoms and poorer emotion processing and cognitive functioning.
Methods
Participants
This study included participants from the Chicago site of the Bipolar and Schizophrenia Network on Intermediate Phenotypes (BSNIP) consortium.52 Written informed consent was provided by all participants (participant assent and parental consent for those <18 years). Participants included probands with schizophrenia (n = 57), schizoaffective disorder (n = 34, 76% bipolar type), psychotic bipolar disorder (n = 75), their first-degree relatives without a history of psychosis (n = 61, 43, and 91, respectively), and healthy controls (n = 66; tables 1 and 2). DSM-IV diagnoses were made at consensus meetings using all available information including findings from the Structured Clinical Interview for DSM-IV Axis I Disorders.54 First-degree relatives were administered the Structured Interview for DSM-IV Personality (SIDP-IV).55 Healthy comparison subjects had no history of a psychotic disorder or recurrent depression and no known immediate family history of these disorders.
Table 1.
Demographics and Clinical Characteristics for Health Comparison Subjects and Probands with Schizophrenia, Schizoaffective Disorder, and Psychotic Bipolar Disorder
| Schizophrenia Groupa (n = 57) | Schizoaffective Groupb (n = 34) | Psychotic Bipolar Groupc (n = 75) | Healthy Comparison Groupd (n = 66) | Post Hoce | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| Age (y) | 35.31 (12.92) | 37.50 (14.12) | 32.97 (13.61) | 37.18 (12.65) | — |
| Education (y) | 12.86 (2.49) | 13.67 (2.89) | 14.11 (2.58) | 15.36 (2.44) | d > a, b, c; c > a |
| WRAT | 94.24 (15.67) | 100.00 (16.63) | 103.93 (15.09) | 103.33 (12.37) | b, c, d > a |
| BACS composite | −1.39 (1.42) | −1.11 (1.20) | −0.77 (1.31) | 0.14 (1.14) | d > a, b, c; c > a |
| PENN ER-40 (% correct) | 76.75 (11.43) | 79.77 (9.85) | 80.25 (8.46) | 82.64 (7.37) | d, c > a |
| SFS | 126.07 (19.74) | 125.53 (19.81) | 140.05 (20.21) | 162.57 (14.65) | d > a, b, c; c > a, b |
| PANSS | |||||
| Positive subscale | 19.41 (5.66) | 19.59 (4.71) | 12.84 (3.89) | — | a, b > c |
| Negative subscale | 20.21 (7.02) | 17.38 (6.60) | 13.68 (4.53) | — | a, b > c |
| YMRS | 7.86 (6.48) | 8.26 (5.57) | 5.62 (6.21) | — | a, b > c |
| MADRS | 9.80 (7.26) | 15.32 (9.85) | 10.83 (9.31) | — | b > a, c |
| n (%) | n (%) | n (%) | n (%) | ||
| Male | 35 (61) | 15 (44) | 24 (32) | 28 (42) | a > b, c, d |
| Race | |||||
| Caucasian | 25 (44) | 18 (53) | 58 (77) | 37 (56) | c > a, b, d |
| African American | 24 (42) | 15 (44) | 11 (15) | 18 (27) | a, b > c, d |
| Other | 8 (14) | 1 (3) | 6 (8) | 11 (17) | d > b |
| Blood draw ≤ noon | 49 (86) | 32 (94) | 67 (89) | 63 (95) | — |
| Medication classf | |||||
| Antipsychotic | |||||
| First generation | 12 (21) | 5 (15) | 3 (4) | — | a, b > c |
| Second generation | 43 (75) | 25 (73) | 51 (68) | — | — |
| Anticonvulsant/ mood stabilizer | 10 (17) | 22 (65) | 53 (71) | — | b, c > a |
| Lithium | — | 2 (6) | 22 (29) | — | c > a, b |
| Valproic acid | 6 (10) | 7 (20) | 13 (17) | — | — |
| Antidepressant | 20 (35) | 21 (62) | 35 (47) | — | b, c > a |
| Sedative/anxiolytic | 15 (26) | 13 (38) | 22 (29) | — | — |
| Stimulant | 5 (9) | 2 (6) | 11 (15) | — | — |
| Anticholinergic | 5 (9) | 1 (3) | 4 (5) | — | — |
Note: WRAT, Wide-Range Achievement Test-IV: reading test; BACS, Brief Assessment of Cognition in Schizophrenia race-adjusted composite (higher = better functioning); PENN-ER-40, Penn Emotion Recognition-40 test; SFS, Social Functioning scale (higher = better functioning); PANSS, Positive and Negative Syndrome scale; YMRS, Young Mania Rating scale; MADRS, Montgomery Asberg Depression Rating scale.
a, b, c and d—all differences noted are at P < .05.
ePost hoc tests were computed when the omnibus F test was significant at P < .05.
Table 2.
Demographics, History of Psychosis, and Personality Traits for Healthy Comparison Subjects, First-Degree Relatives of Probands With Schizophrenia, Schizoaffective Disorder, and Psychotic Bipolar Disorder
| Relatives of Schizophrenia Probandsa (n = 61) | Relatives of Schizoaffective Probandsb (n = 43) | Relatives of Bipolar Probandsc (n = 91) | Healthy Comparison Groupd (n = 66) | Post Hoce | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| Age (y) | 42.77 (15.83) | 39.79 (16.39) | 41.01 (15.47) | 37.18 (12.65) | — |
| Education (y) | 13.97 (2.38) | 14.40 (2.86) | 15.19 (2.64) | 15.36 (2.44) | c, d > a, b |
| WRAT | 98.82 (14.67) | 105.29 (15.37) | 104.15 (13.88) | 103.33 (12.37) | — |
| BACS composite | −0.11 (1.03) | 0.26 (1.02) | 0.17 (1.12) | 0.14 (1.14) | — |
| PENN ER-40 (% correct) | 81.08 (6.22) | 81.18 (7.05) | 82.27 (7.52) | 82.64 (7.37) | — |
| SFS | 150.84 (18.33) | 148.11 (20.55) | 152.46 (18.41) | 162.57 (14.65) | d > a, b, c |
| n (%) | n (%) | n (%) | n (%) | ||
| Male | 15 (24) | 14 (33) | 32 (35) | 28 (42) | — |
| Race | |||||
| Caucasian | 33 (54) | 27 (63) | 78 (86) | 37 (56) | c>a, b, d |
| African American | 22 (36) | 13 (30) | 10 (11) | 18 (27) | a, b, d > c |
| Other | 6 (10) | 3 (7) | 3 (3) | 11 (17) | d > b |
| Blood draw ≤ noon | 53 (87) | 42 (98) | 79 (87) | 63 (95) | — |
| Medications | |||||
| No medications | 53 (87) | 33 (77) | 62 (68) | — | — |
| Antipsychotic | |||||
| Second generation | 2 (3) | 2 (4) | 4(4) | — | — |
| Anticonvulsant/ mood stabilizer | — | 4 (9) | 7 (7) | — | — |
| Lithium | — | 1 (2) | 1 (1) | — | — |
| Valproic acid | 1 (2) | 2 (5) | 1 (1) | — | — |
| Antidepressant | 7 (11) | 7 (16) | 21 (23) | — | — |
| Sedative/anxiolytic | 3 (5) | 2 (5) | 8 (9) | — | — |
| Stimulant | — | 3 (7) | 4 (4) | — | — |
Note: WRAT, Wide-Range Achievement Test-IV: reading test; PENN-ER-40, Penn Emotion Recognition-40 test; SFS, Social Functioning scale (higher = better functioning); BACS, Brief Assessment of Cognition in Schizophrenia race-adjusted composite score (higher = better functioning); medication class consistent with Tamminga et al 52 and Hill et al. 53
a, b, c and d—all differences noted are at P < .05.
ePost hoc tests were computed when the omnibus F test was significant at P < .05.
Exclusion criteria included history of head injury with loss of consciousness >10 minutes; pregnancy; positive urine toxicology screen for common drugs of abuse on the day of testing; diagnosis of substance abuse in the past 30 days or substance dependence in the past 6 months; history of systemic medical or neurological disorder affecting mood or cognition; or age-corrected Wide-Range Achievement test reading test standard score ≤ 65. A full description of subject recruitment and clinical assessment is available.52
Measures
Clinical Symptoms and Global Functional Status.
Clinical symptom assessments included the Positive and Negative Syndrome scale,56 Montgomery Asberg Depression Rating scale,57 and Young Mania Rating scale.58 Community functioning was assessed with the Birchwood Social Functioning scale.59
Emotion Processing and Cognition.
The Penn Emotion Recognition-40 test (PENN ER-40)60–62 assesses the ability to accurately recognize specific emotions conveyed in pictures of faces. Stimuli are 40 faces (half female) expressing 1 of 4 emotions (happiness, sadness, anger, fear) or a neutral expression (8 faces per expression). The primary outcome measure was the accuracy of affect identification. The Brief Assessment of Cognition in Schizophrenia (BACS) battery assessed global neuropsychological function via a composite score.63,64
Serum Hormone Assays.
Blood samples were drawn in the morning when possible (89% blood draws before noon). The percentage of participants drawn before noon did not differ across groups (P = .21). Samples were stored in plain tubes, spun at 4°C, divided into 300 ul aliquots, and stored at −80°C. Samples were batched, diluted in an assay buffer to give reliable results within the linear portion of the standard curve (OT 1:4; AVP 1:2), and completed in duplicate using unextracted plasma. OT and AVP were quantified with an EIA kit (Enzo Life Sciences/Assay Designs).47 These EIAs are highly sensitive (minimal detection levels < 12 pg/ml OT; 4 pg/ml AVP) and specific with cross-reactivity between OT and AVP < 0.04%. Samples were assayed blind to subject information. All samples from a given subject were run at the same time. OT values > 2000 pg/ml and AVP values > 400 pg/ml were re-run to confirm sample accuracy. OT samples consistently remaining higher (10 samples) were set at 2000 pg/ml, and AVP samples constantly remaining higher (6 samples) were set at 400 pg/ml for statistical analyses. Intra-assay coefficients of variation were <10% for both assays.
Statistical Analyses
Hierarchical linear modeling (HLM) with SAS (version 9.2, SAS Institute Inc, Cary, NC) was used to examine group differences in peripheral hormone levels, with family membership treated as a random effect because participants were nested within families. To test hypotheses most firmly supported by prior studies, planned comparisons were performed to test for group differences starting with schizophrenia patients, their relatives, and healthy controls. To determine if pedigrees with psychotic bipolar disorder and schizoaffective disorder probands showed similar patterns to schizophrenia families, planned comparisons were performed to test for differences between these groups. We evaluated whether hormonal effects varied depending on whether patients were taking first- or second-generation antipsychotic medications or anticonvulsant/mood stabilizers; associations were minimal (supplementary table 1). Next, multivariable linear regression modeling was used to examine the degree to which endocrine levels were associated with emotion processing, cognition, clinical symptoms, and global functional status. When investigating hormone-behavior associations, OT and AVP were examined in the same multivariable models, so we could measure the contribution of OT controlling for AVP and vice versa because OT and AVP levels were modestly correlated across groups (r’s ranged between .25 and .43; supplementary table 2). Because these exploratory correlational analyses were performed for heuristic purposes to examine the potential clinical significance of group differences and clinical associations, we did not correct for multiple comparisons. All analyses were adjusted for age, race, and sex. Significance was set at P < .05; trends at P ≥ .05 and P < .10 are reported.
Estimates of familiality (h 2) represent the portion of the phenotypic variance accounted for by family membership and were obtained using sequential oligogenic linkage analysis routine.65 To test for the significance of familiality, a maximum likelihood ratio test compared a model in which phenotypic variation was explained by family membership to one not considering variation explained by familial factors. Because families were recruited through the identification of a psychotic proband and not as a representative community sample, a correction was applied to account for this ascertainment bias.66
Results
Baseline Differences in Cognition, Social Functioning, and Emotion Recognition
Consistent with multisite BSNIP consortium findings,52,53 there were significant group differences on the BACS composite and in community functioning (P’s < .001; Tables 1 and 2). All proband groups performed worse than healthy controls on both measures (P’s < .001). Schizophrenia probands also performed worse than bipolar probands on both measures (P’s < .05). On the PENN ER-40, schizophrenia probands (P < .001) performed worse than healthy controls and bipolar probands (P = .04). Differences between relatives and healthy controls were not significant.
Group Differences and Familiality Estimates for Peripheral Concentrations of OT and AVP
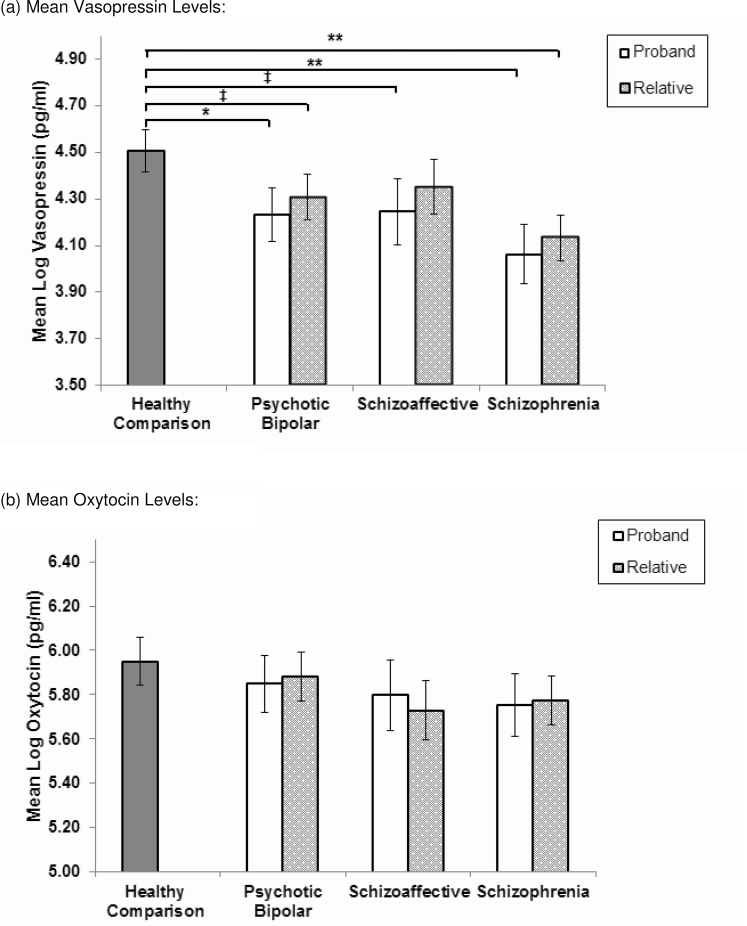
HLM analyses yielded significant group differences in AVP for schizophrenia probands and their relatives compared to controls, but not OT levels (figure 1). Schizophrenia probands (B = −0.44, SE = 0.14, P = .002) and their relatives (B = −0.37, SE = 0.12, P = .002) showed lower AVP but not OT levels, compared with controls. Bipolar probands (B = −0.27, SE = 0.13, P = .03) also had lower levels of AVP but not OT, compared with controls. Relatives of bipolar probands showed a trend for lower levels of AVP but not OT, compared with controls (B = −0.20, SE = 0.11, P = .07). Among the smaller sample of schizoaffective families, probands (M = 4.24, SE = 0.14) showed a trend toward lower levels of AVP, compared with controls (M = 4.51, SE = 0.09; B = −0.26, SE = 0.16, P = .09), similar in magnitude to the reduction seen in bipolar probands. Post hoc comparisons yielded no significant differences in peptides between proband groups or first-degree relative groups. All proband and relative groups showed a higher proportion of individuals with lower levels of AVP (ie, <1 SD from controls), compared with controls using the Lambda67,68 statistic (supplementary table 3). Additionally, there were more schizophrenia probands than bipolar probands with low levels of AVP (P = .04). The same pattern was demonstrated when comparing relatives of schizophrenia to relatives of bipolar probands (P = .05).
Fig. 1.
Comparison of serum vasopressin (a) and oxytocin levels (b) between the healthy comparison group, psychotic probands, and their first-degree relatives. **P < .01; *P < .05; ‡ P ≤ .09. Serum values were adjusted for age, race, sex, and first and second generation antipsychotic medication use.
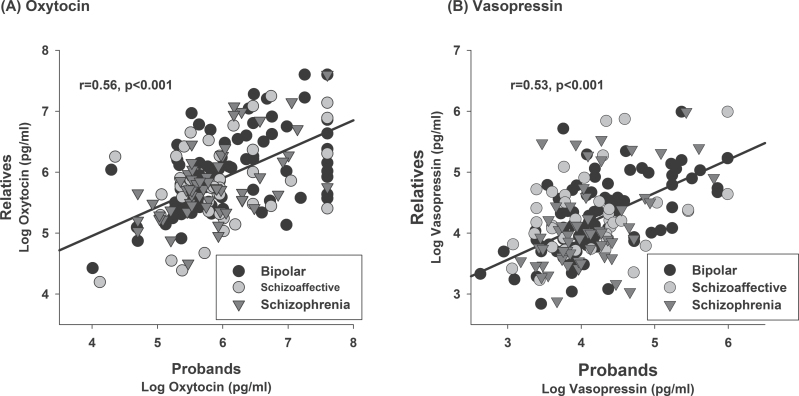
Familiality estimates were highly significant for both OT (h 2 = 0.79, SE = 0.08, P = 3.97e−15) and AVP (h 2 = 0.78, SE = 0.10, P = 3.93e−12) in the pooled data of proband families. Correlations between relative and proband’s OT and AVP levels were also robust (figure 2). Comparable familiality estimates were observed in schizophrenia and bipolar pedigrees (supplementary table 4). In probands, chlorpromazine equivalents of antipsychotic treatment69 were not associated with hormone levels. Excluding first-degree relatives taking psychiatric medications from the HLM analysis did not change the pattern of results.
Fig. 2.
Relationship between oxytocin and vasopressin levels in relatives and probands. Values for each relative are plotted relative to their family member proband. Data represents 203 pedigrees and 144 probands. Maximum levels for oxytocin and vasopressin were used as described in the text.
Hormone-Behavior Associations
Emotion Processing.
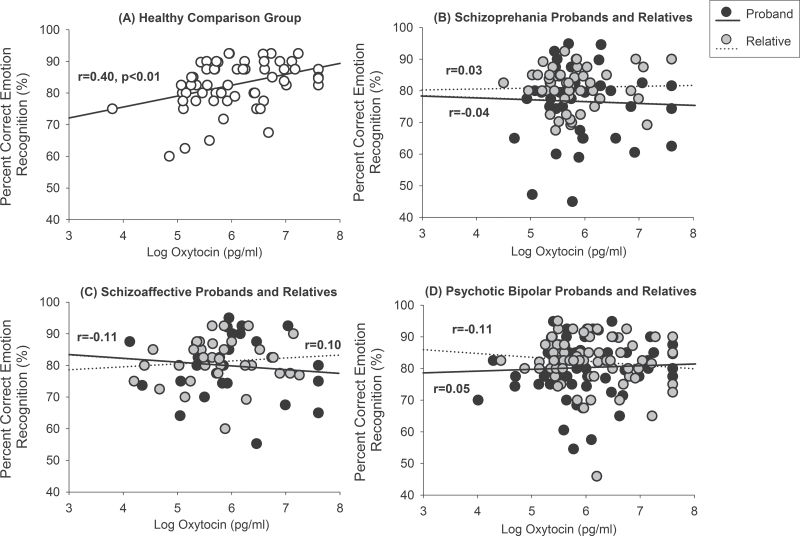
Higher levels of OT were associated with better emotion recognition accuracy in healthy controls (β = 0.40, B = 3.76, SE = 1.13, P < .001) but not in patients with schizophrenia or their relatives (Figure 3). Higher levels of OT in healthy individuals were associated with better emotion recognition of neutral (β = 0.35, B = 7.17, SE = 2.64, P < .01), happy, (β = 0.27, B = 1.44, SE = 0.69, P = .04), and sad faces (β = 0.26, B = 6.03, SE = 2.96, P = .04). OT levels also were not associated with emotion recognition in bipolar or schizoaffective probands or their relatives (figure 3). All associations were significantly reduced relative to controls (Fisher’s r to z transformations, P’s < .05) except for the smaller schizoaffective relative group for whom the difference was at the trend level (P = .10). AVP levels were not significantly associated with emotion recognition in any group.
Fig. 3.
Raw correlations showing (A) Higher serum levels of oxytocin are associated with better performance on a facial emotion recognition test in the healthy comparison group but not in (B) schizophrenia probands and their first-degree relatives, (C) schizoaffective probands and their first-degree relatives, or (D) psychotic bipolar and their first-degree relatives.
Cognition.
Similar to the emotion recognition results, higher levels of OT were associated with better global neuropsychological performance in healthy controls (β = 0.26, B = 0.36, SE = 0.17, P = .04) but not in any proband or relative group. AVP levels were not associated with overall neuropsychological performance in any group.
Clinical Symptoms.
In schizophrenia, higher levels of OT, but not AVP, were associated with more severe positive symptoms (β = 0.32, B = 2.67, SE = 1.17, P = .02). Because the sample was clinically stable for at least 1 month at the time of the study, this may reflect a relation between higher OT levels and the presence of more significant residual or treatment nonresponsive symptoms. There was a trend for higher levels of OT to be associated with more severe negative (β = 0.31, B = 2.36, SE = 1.33, P = .08) and manic symptoms (β = 0.32, B = 2.03, SE = 1.06, P = .06) in schizoaffective probands. In schizoaffective probands, those with lower AVP levels had more severe manic (β = −0.50, B = −4.06, SE = 1.29, P = .002) and positive symptoms (β = −0.40, B = −2.68, SE = 1.12, P = .02). Levels of OT and AVP were not related to symptom severity in bipolar probands. Across all relatives, regardless of proband diagnosis, those with a Cluster A (psychosis spectrum) personality disorder had lower levels of AVP than those without a Cluster A diagnosis (β = −0.17, B = 0.55, SE = 0.23, P = .02).
Community Functioning and Sex Differences.
OT and AVP were not associated with community functioning in any group. There were no significant sex by group interactions in hormone levels, and there were no sex differences in any hormone-behavior relationships.
Discussion
The results of this study demonstrate abnormalities of serum AVP and in the modulatory function of OT on emotion processing and cognitive functioning among individuals with psychotic disorders and their first-degree relatives. Both schizophrenia patients and their relatives showed decreased levels of AVP compared with the healthy comparison group; similar findings were seen in bipolar patients and their relatives albeit to a less prominent degree, and AVP levels were highly familial. Additionally, relatives with a psychosis spectrum personality disorder showed lower AVP levels than other relatives. Thus, reduced AVP levels have several characteristics of a promising endophenotype across psychotic disorders.
Findings with OT were notably different. OT levels were not reduced in any proband or relative group even though familiality was highly significant across psychotic disorders. Rather, findings with OT were most notable in their association with clinical symptoms and reduced association with neurobehavioral functions. First, OT levels in schizophrenia and schizoaffective probands were associated with higher rather than lower clinical symptom levels. Second, the positive association between OT levels and better social cognition and general neuropsychological function observed in our healthy controls and widely reported in the literature3,4 was not seen in any proband or relative group. This finding suggests a dissociation between OT levels and behavioral function in both probands with psychotic disorders and their unaffected relatives, such that hormone levels appear to have a reduced capacity to modulate affective and neuropsychological functioning. This pattern suggests alterations in downstream processes at the level of OT receptors, signal transduction, or in the neurophysiological integrity of brain regions dense with OT receptors that may be impacted by illness and risk rather than an abnormality in OT peptide levels.
Our findings are consistent with previous studies demonstrating AVP dysregulation in schizophrenia.14,15,17,38,70 Of note, however, the direction of effects in prior work has been mixed, perhaps in relation to state of illness and assays used. For example, in contrast to our clinically stable patients with schizophrenia showing lower levels of AVP, studies of unmedicated acutely ill first-episode patients show higher levels of AVP, compared with healthy controls in 2 of 3 studies.14,15,17 Higher AVP levels in acutely ill patients maybe the result of a dysregulated biological stress response system.71
The present findings extend prior work by demonstrating a similar pattern of abnormality in psychotic bipolar disorder and schizoaffective probands. There was a similar trend in the relatives of bipolar probands, and all relative groups had a higher percent of individuals with AVP levels 1 SD below healthy controls. Also, we observed that findings in family members were greater in those with psychosis spectrum personality traits. These findings suggest that alterations in AVP occur across psychotic disorders rather than selectively in schizophrenia. Medications could be confounding factors in our proband data because they varied based on clinical indication. However, statistical associations of effects with medication treatments were not significant, and AVP alterations in untreated relatives were similar to those seen in the patient probands. This pattern of effects is more consistent with disease-related rather than treatment-induced abnormalities.
In contrast to effects seen in serum AVP, we did not find significant alterations in OT levels in probands with psychotic disorders or their first-degree relatives. This is consistent with our previous studies of schizophrenia patients.14,51 The only other study to date using the same assay methodologies showed lower levels of peripheral OT in schizophrenia patients with polydipsia hyponatremia.11
In contrast to our previous findings,51 OT levels were associated with higher clinical symptom levels. At least 3 factors might have contributed to these differences. First, schizophrenia probands in this study were recruited from the community rather than a clinic and exhibited higher levels of positive symptoms than our previous study of chronic patients.51 Second, given our larger sample sizes we were able to examine OT-symptom associations separately for schizophrenia and schizoaffective probands. Analyses in our previous study combined both disorders. Third, OT levels were higher in probands in the present study. One possibility is that OT may have a protective or beneficial effect when the range of OT values is lower, but detrimental effects when OT values are higher. Alternatively, it is possible that higher OT levels in probands may have been a compensatory response and thus be mitigating symptoms that might otherwise be even higher.
With respect to clinical associations, relationships between OT and clinical symptoms were stronger in schizophrenia spectrum disorders than bipolar probands. Thus, the clinical significance of OT levels may be greater in psychotic disorders with higher levels of persistent positive and negative symptoms and poorer community functioning. The implications of this study for the use of exogenous OT as a treatment are indirect but worthy of note. While treatment-induced supraphysiological levels may have direct positive effects or reset responsivity of brain systems to OT modulation,40,44 the absence of abnormal physiological OT levels, the lack of association between OT levels and social cognition, and the positive relationship between OT levels and symptom severity do not in themselves offer support for this therapeutic strategy.
Concentrations of both peripheral hormone levels were highly familial, and recent investigations suggest that variations in genes for OT (OXTR) and AVP (V1aR) show associations with schizophrenia, symptom severity, and treatment response to clozapine in schizophrenia patients.72–75 Hence, the presence of alteration in family members and its familiality suggest that OT and AVP genes or other factors influencing the impact of these hormones on brain and behavioral function may contribute to psychosis vulnerability.
Limitations of this study include a cross-sectional study design, and a single measurement of hormone levels. The study was designed to assess trait-like alterations rather than clinical state relationships, so the range of symptom severity was limited by design. Thus, relations of hormones to active symptoms may be underestimated, or different patterns of alterations might be seen during acute episodes of illness. Experimental approaches, such as administration of intranasal OT or agents that affect the AVP system are needed to assess therapeutic implications and complement studies of peripheral hormone levels. In addition, it has been suggest that perturbations of the hypothalamic-pituitary-adrenal axis may be a risk factor for the development of psychotic disorders.71 This and other hormones merit study in order to examine patterns and specificity of hormone alterations associated with psychotic disorders. Finally, although neuropeptides in the peripheral circulation do not readily cross the blood-brain barrier, most peripheral OT and AVP are synthesized within the brain and released into the periphery via the posterior pituitary. Animal data indicate that central and peripheral levels of OT are correlated.76 While central nervous system implications of peripheral measures are uncertain, peripheral hormone levels, both at baseline and in reaction to social stimuli have been associated in numerous studies with multiple behavioral parameters that indicate the relevance of peripheral measures of neurobehavioral functions.77,78
Overall, our findings extend previous work in schizophrenia to suggest that OT and AVP may be involved in pathophysiology across psychotic disorders and that they may be involved in the vulnerability to schizophrenia as indicated by findings in first-degree relatives. The reduced modulation of relevant neurobehavioral functions by OT even in the context of normal peripheral hormone levels suggests that a downstream alteration in OT receptors or relevant limbic and neocortical systems where the receptors are densely expressed, rather than an alteration in hormone levels, may contribute to social cognition deficits in psychotic disorders.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
2012 NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation to L.H.R.; National Institute of Health (K12HD055892, K08MH083888, MH083126, MH077851, MH078113, MH077945, MH077852, MH077862).
Supplementary Material
Acknowledgments
Dr Bishop has served as an advisory board member for Physician Choice Laboratory Services. Dr Sweeney has been on advisory boards for Bristol-Myers Squibb, Eli Lilly, Roche, and Takeda and with Dr Bishop received grant support from Janssen. Dr Keshavan received research support from Sunovion and GlaxoSmithKline. Dr Tamminga has served on a drug development advisory board for Intracellular Therapies, as an expert witness for Kaye Scholer LLP and has served as a consultant for Astellas, Eli Lilly, Lundbeck, and Puretech Ventures/Karuna. The other authors have nothing to disclose. We thank Dr Gunvant Thaker for his collaboration and leadership in the BSNIP project. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fehm-Wolfsdorf G, Born J. Behavioral effects of neurohypophyseal peptides in healthy volunteers: 10 years of research. Peptides. 1991;12:1399–1406 [DOI] [PubMed] [Google Scholar]
- 2. Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336 [DOI] [PubMed] [Google Scholar]
- 3. Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557 [DOI] [PubMed] [Google Scholar]
- 4. Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538 [DOI] [PubMed] [Google Scholar]
- 5. Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176 [DOI] [PubMed] [Google Scholar]
- 6. Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818 [DOI] [PubMed] [Google Scholar]
- 7. Strupp B, Weingartner H, Goodwin FK, Gold PW. Neurohypophyseal hormones and cognition. Pharmacol Ther. 1983;23:267–279 [DOI] [PubMed] [Google Scholar]
- 8. Ferris CF. Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. Prog Brain Res. 2008;170:305–320 [DOI] [PubMed] [Google Scholar]
- 9. Feifel D. Oxytocin as a potential therapeutic target for schizophrenia and other neuropsychiatric conditions. Neuropsychopharmacology. 2012;37:304–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feifel D. Is oxytocin a promising treatment for schizophrenia? Expert Rev Neurother. 2011;11:157–159 [DOI] [PubMed] [Google Scholar]
- 11. Goldman M, Marlow-O’Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res. 2008;98:247–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ. Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci. 1984;234:162–165 [DOI] [PubMed] [Google Scholar]
- 13. Beckmann H, Lang RE, Gattaz WF. Vasopressin–oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10:187–191 [DOI] [PubMed] [Google Scholar]
- 14. Rubin LH, Carter CS, Bishop JR, et al. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr Res. 2013;146:138–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raskind MA, Weitzman RE, Orenstein H, Fisher DA, Courtney N. Is antidiuretic hormone elevated in psychosis? A pilot study. Biol Psychiatry. 1978;13:385–390 [PubMed] [Google Scholar]
- 16. Legros JJ, Gazzotti C, Carvelli T, et al. Apomorphine stimulation of vasopressin- and oxytocin-neurophysins. Evidence for increased oxytocinergic and decreased vasopressinergic function in schizophrenics. Psychoneuroendocrinology. 1992;17:611–617 [DOI] [PubMed] [Google Scholar]
- 17. Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naïve patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–1070 [DOI] [PubMed] [Google Scholar]
- 18. Turan T, Uysal C, Asdemir A, Kılıç E. May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology. 2013;38:2890–2896 [DOI] [PubMed] [Google Scholar]
- 19. Pinkham AE, Gur RE, Gur RC. Affect recognition deficits in schizophrenia: neural substrates and psychopharmacological implications. Expert Rev Neurother. 2007;7:807–816 [DOI] [PubMed] [Google Scholar]
- 20. Rocca CC, Heuvel Ev, Caetano SC, Lafer B. Facial emotion recognition in bipolar disorder: a critical review. Rev Bras Psiquiatr. 2009;31:171–180 [DOI] [PubMed] [Google Scholar]
- 21. Gur RE, Calkins ME, Gur RC, et al. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20 [DOI] [PubMed] [Google Scholar]
- 23. DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS, 3rd, Eichenbaum H. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci. 2009;29:2676–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seidman LJ, Pantelis C, Keshavan MS, et al. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr Bull. 2003;29:803–830 [DOI] [PubMed] [Google Scholar]
- 25. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193 [DOI] [PubMed] [Google Scholar]
- 26. Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109:891–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blumberg HP, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208 [DOI] [PubMed] [Google Scholar]
- 28. Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–1316 [DOI] [PubMed] [Google Scholar]
- 29. Argiolas A, Gessa GL. Central functions of oxytocin. Neurosci Biobehav Rev. 1991;15:217–231 [DOI] [PubMed] [Google Scholar]
- 30. Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol. 2000;10:205–210 [DOI] [PubMed] [Google Scholar]
- 32. Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528 [DOI] [PubMed] [Google Scholar]
- 33. Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/-) reeler mice. Neurol Res. 2005;27:339–345 [DOI] [PubMed] [Google Scholar]
- 34. Feifel D, Priebe K. Vasopressin-deficient rats exhibit sensorimotor gating deficits that are reversed by subchronic haloperidol. Biol Psychiatry. 2001;50:425–433 [DOI] [PubMed] [Google Scholar]
- 35. Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology. 2009;34:2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;181:278–286 [DOI] [PubMed] [Google Scholar]
- 37. Frederiksen SO, Ekman R, Gottfries CG, Widerlöv E, Jonsson S. Reduced concentrations of galanin, arginine vasopressin, neuropeptide Y and peptide YY in the temporal cortex but not in the hypothalamus of brains from schizophrenics. Acta Psychiatr Scand. 1991;83:273–277 [DOI] [PubMed] [Google Scholar]
- 38. Goldman MB. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res Rev. 2009;61:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feifel D, Macdonald K, Nguyen A, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680 [DOI] [PubMed] [Google Scholar]
- 40. Pedersen CA, Gibson CM, Rau SW, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132:50–53 [DOI] [PubMed] [Google Scholar]
- 41. Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2011;42:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davis MC, Lee J, Horan WP, et al. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res. 2013;147:393–397 [DOI] [PubMed] [Google Scholar]
- 43. Modabbernia A, Rezaei F, Salehi B, et al. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia: an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs. 2013;27:57–65 [DOI] [PubMed] [Google Scholar]
- 44. Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl). 2011;216:101–110 [DOI] [PubMed] [Google Scholar]
- 45. Feifel D, Macdonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res. 2012;139:207–210 [DOI] [PubMed] [Google Scholar]
- 46. Fischer-Shofty M, Shamay-Tsoory SG, Levkovitz Y. Characterization of the effects of oxytocin on fear recognition in patients with schizophrenia and in healthy controls. Front Neurosci. 2013;7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carter CS, Pournajafi-Nazarloo H, Kramer KM, et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–322 [DOI] [PubMed] [Google Scholar]
- 48. Rubin LH, Carter CS, Drogos L, et al. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res. 2011;130:266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527 [DOI] [PubMed] [Google Scholar]
- 50. Kéri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Soc Neurosci. 2009;4:287–293 [DOI] [PubMed] [Google Scholar]
- 51. Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res. 2010;124:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the bipolar and schizophrenia network on intermediate phenotypes (B-SNIP). Am J Psychiatry. 2013;170:–1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hill SK, Reilly JL, Keefe RS, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the bipolar and schizophrenia network on intermediate phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P). New York, NY: New York State Psychiatric Institute; 1995 [Google Scholar]
- 55. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. Washington, DC: American Psychiatric Press; 1997 [Google Scholar]
- 56. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389 [DOI] [PubMed] [Google Scholar]
- 58. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435 [DOI] [PubMed] [Google Scholar]
- 59. Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859 [DOI] [PubMed] [Google Scholar]
- 60. Kohler CG, Anselmo-Gallagher G, Bilker W, Karlawish J, Gur RE, Clark CM. Emotion-discrimination deficits in mild Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:926–933 [DOI] [PubMed] [Google Scholar]
- 61. Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143 [DOI] [PubMed] [Google Scholar]
- 62. Pinkham AE, Sasson NJ, Calkins ME, et al. The other-race effect in face processing among African American and Caucasian individuals with schizophrenia. Am J Psychiatry. 2008;165:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keefe RS, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102:108–115 [DOI] [PubMed] [Google Scholar]
- 64. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297 [DOI] [PubMed] [Google Scholar]
- 65. Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Beaty TH, Liang KY, Seerey S, Cohen BH. Robust inference for variance components models in families ascertained through probands: II. Analysis of spirometric measures. Genet Epidemiol. 1987;4:211–221 [DOI] [PubMed] [Google Scholar]
- 67. Egan MF, Goldberg TE, Gscheidle T, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107 [DOI] [PubMed] [Google Scholar]
- 68. Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316 [DOI] [PubMed] [Google Scholar]
- 69. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Walsh P, Spelman L, Sharifi N, Thakore JH. Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide-induced AVP release. Psychoneuroendocrinology. 2005;30:431–437 [DOI] [PubMed] [Google Scholar]
- 71. Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V. Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocrinology. 2012;37:1600–1613 [DOI] [PubMed] [Google Scholar]
- 72. Souza RP, de Luca V, Meltzer HY, Lieberman JA, Kennedy JL. Schizophrenia severity and clozapine treatment outcome association with oxytocinergic genes. Int J Neuropsychopharmacol. 2010;13:793–798 [DOI] [PubMed] [Google Scholar]
- 73. Souza RP, Ismail P, Meltzer HY, Kennedy JL. Variants in the oxytocin gene and risk for schizophrenia. Schizophr Res. 2010;121:279–280 [DOI] [PubMed] [Google Scholar]
- 74. Teltsh O, Kanyas-Sarner K, Rigbi A, Greenbaum L, Lerer B, Kohn Y. Oxytocin and vasopressin genes are significantly associated with schizophrenia in a large Arab-Israeli pedigree. Int J Neuropsychopharmacol. 2011;15:1–11 [DOI] [PubMed] [Google Scholar]
- 75. Montag C, Schoene-Bake JC, Wagner J, et al. Volumetric hemispheric ratio as a useful tool in personality psychology. Neurosci Res. 2013;75:157–159 [DOI] [PubMed] [Google Scholar]
- 76. Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176 [DOI] [PubMed] [Google Scholar]
- 77. Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav. 2012;61:359–379 [DOI] [PubMed] [Google Scholar]
- 78. Macdonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. 2013;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.