Abstract
Background: Antipsychotic-induced metabolic adversities are often difficult to manage. Using concomitant medications to counteract these adversities may be a rational option. Objective: To systematically determine the effectiveness of medications to counteract antipsychotic-induced metabolic adversities in patients with schizophrenia. Data Sources: Published articles until November 2013 were searched using 5 electronic databases. Clinical trial registries were searched for unpublished trials. Study Selection: Double-blind randomized placebo-controlled trials focusing on patients with schizophrenia were included if they evaluated the effects of concomitant medications on antipsychotic-induced metabolic adversities as a primary outcome. Data Extraction: Variables relating to participants, interventions, comparisons, outcomes, and study design were extracted. The primary outcome was change in body weight. Secondary outcomes included clinically relevant weight change, fasting glucose, hemoglobin A1c, fasting insulin, insulin resistance, cholesterol, and triglycerides. Data Synthesis: Forty trials representing 19 unique interventions were included in this meta-analysis. Metformin was the most extensively studied drug in regard to body weight, the mean difference amounting to −3.17 kg (95% CI: −4.44 to −1.90 kg) compared to placebo. Pooled effects for topiramate, sibutramine, aripiprazole, and reboxetine were also different from placebo. Furthermore, metformin and rosiglitazone improved insulin resistance, while aripiprazole, metformin, and sibutramine decreased blood lipids. Conclusion: When nonpharmacological strategies alone are insufficient, and switching antipsychotics to relatively weight-neutral agents is not feasible, the literature supports the use of concomitant metformin as first choice among pharmacological interventions to counteract antipsychotic-induced weight gain and other metabolic adversities in schizophrenia.
Key words: antipsychotic, meta-analysis, metabolic, concomitant, PRISMA, schizophrenia
Introduction
Antipsychotics can cause numerous side effects, including weight gain and metabolic derangements that are often difficult to manage; using concomitant medications to counteract these adversities may be a rational option. However, data are still limited regarding effective medications to counteract antipsychotic-induced metabolic adversities in schizophrenia. In an early report, Faulkner et al1 reviewed 18 randomized trials, which assessed the effects of adjunctive medications to counter weight gain in patients with schizophrenia. Despite identifying trials showing positive results for reboxetine and topiramate, and mixed results for sibutramine, nizatidine, and amantadine, based on the paucity of data, the authors concluded that adjunctive medications should be reserved for patients in which nonpharmacological strategies alone are inadequate. In a more recent report, Maayan et al2 performed a systematic review and meta-analysis on the effectiveness of add-on medications used to attenuate antipsychotic-induced weight gain and metabolic abnormalities. The authors concluded that metformin, D-fenfluramine, sibutramine, topiramate, and reboxetine significantly attenuated weight gain. Of note, this important work had some limitations: Study populations were heterogeneous regarding psychiatric diagnosis, combinations of antipsychotics aiming at the reduction of metabolic adversities were excluded, and unpublished trials were not included. In the latest systematic review and meta-analysis on pharmacological interventions for antipsychotic-induced and mood stabilizer–induced weight gain, Fiedorowicz et al3 concluded that metformin and topiramate were superior to placebo. Although this work updated the available evidence and reviewed a wider range of medications including combinations of antipsychotics, similar limitations can be pointed out: Study populations were heterogeneous regarding diagnosis, unpublished trials were not searched, and the number of trials included was limited to 32.
To our best knowledge, following the review by Fiedorowicz et al,3 10 double-blind randomized controlled trials (total n = 578) using concomitant drugs to counteract antipsychotic-induced metabolic adversities in patients with schizophrenia have been published, including first reports on reboxetine-betahistine combinations4 and zonisamide.5 To update the available evidence regarding this clinical relevant topic, we undertook a systematic review and meta-analysis regarding the effectiveness of add-on medications to counteract a wide range of antipsychotic-induced metabolic derangements, with a specific focus on patients with schizophrenia.
Methods
A study protocol was prepared before commencing data collection (supplementary appendix). The PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses)6 was followed to ensure transparent and complete reporting. Two independent authors (Y.M. and H.U.) undertook the search, assessed eligibility, and extracted data. Any discrepancies during these procedures were resolved through discussion.
Search
Published articles from 1950 to November 5, 2013 were searched without language restrictions using EMBASE, MEDLINE, PsycINFO, PubMed, and the Cochrane Library. Search terms included synonyms of schizophrenia, combination treatment, metabolic derangements, and the names of medications previously reviewed by Maayan et al.2 (supplementary appendix). Limits were set for “clinical trials” and “humans” where applicable. References of relevant articles were hand-searched for additional articles. Furthermore, unpublished studies were searched in clinical trial registries (http://clinicaltrials.gov/) using the term “schizophrenia” and synonyms of combination treatment, with a limit to “interventional studies.”
Selection Criteria
Studies were included if (1) they were double-blind randomized placebo-controlled trials using concomitant medications to counteract antipsychotic-induced metabolic adversities, (2) a majority of subjects had a diagnosis of schizophrenia or related psychotic disorders according to study diagnoses, and (3) they reported on changes of metabolic adversities as a primary outcome. We included combinations of antipsychotics if a second antipsychotic was used specifically to treat a metabolic adversity of the primary antipsychotic drug. If several publications were found from the same investigators using overlapping samples, we included data with the longest duration, the most detailed information, and/or data that were most relevant to our primary outcome (ie, weight gain).
Outcome Parameters
The primary outcome was defined as changes in weight gain at endpoint. As secondary outcomes, we extracted data on the following: clinically relevant weight change as defined in individual studies (eg, 7% or more weight loss), fasting glucose, hemoglobin A1c (HbA1c), fasting insulin, the homeostasis model of assessment of insulin resistance (HOMA-IR), total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. Intention-to-treat (ITT) datasets were used whenever available.
Data Analysis
Prior to the meta-analysis, risk of bias of the included studies were assessed using the Cochrane Risk of Bias Tool.7 When 2 or more studies were present per intervention, meta-analyses were performed using Review Manager, version 5.2.5 (The Cochrane Collaboration, http://ims.cochrane.org/revman). For continuous outcomes, mean differences were calculated using the inverse variance statistical method and random effects model to adjust for study heterogeneity. Unreported SD values were supplemented using procedures described in the supplementary appendix. Two-sided 95% CIs were used to assess significance, according to whether the CIs included the null value. For dichotomous outcomes, the Mantel-Haenszel statistical method and random effects model were used to calculate ORs. Furthermore, the number needed to treat (NNT) was calculated using equations described in the supplementary appendix.8 Study heterogeneity was quantified using the I 2 statistic9 with I 2 ≥50% indicating significant heterogeneity. The possibility of publication bias was assessed using funnel plots.10 Finally, for the primary outcome, we conducted subgroup and sensitivity analyses according to a priori defined study characteristics: (1) prevention vs treatment, (2) inpatients vs outpatients, (3) first-episode patients vs others, and (4) studies reporting SD values vs studies in which SD values were supplemented. Any additional, exploratory analyses that were performed were fully reported.
Results
Included Studies
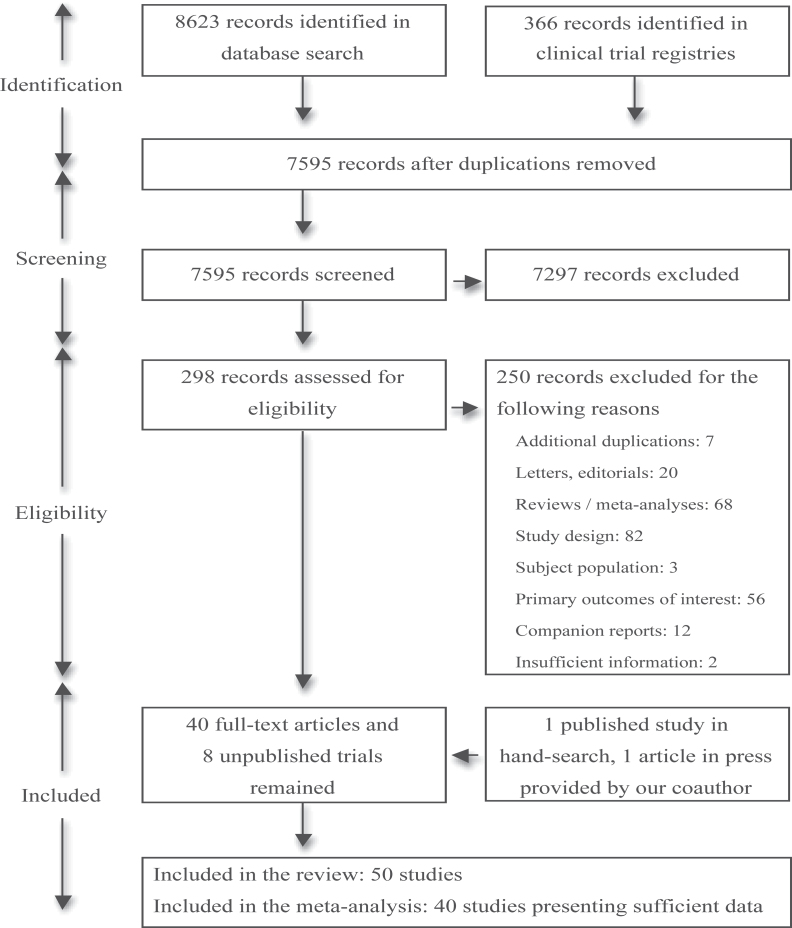
Fifty double-blind randomized placebo-controlled trials (41 published,4,5,11–49 8 unpublished [Eli Lilly and Company; University of North Carolina, Chapel Hill; University of Maryland; Mclean Hospital; Nathan Kline Institute for Psychiatric Research; Orexigen Therapeutics, Inc.; University of Massachusetts, Worcester; GW Pharmaceuticals Ltd—unpublished data], and 1 article in press,50 respectively) were included in the review (figure 1). The article in press was provided by one of our coauthors (W.W.F.). The total numbers of randomized subjects were N = 2298, 363, and 15 for published, unpublished, and in press studies, respectively.
Fig. 1.
Literature search results and study eligibility for meta-analysis.
Study characteristics are summarized in table 1, with reference to participants, interventions, comparisons, outcomes, and study design. Mean ± SD duration of randomized interventions was 12.2 ± 4.7 weeks (range: 4–24 weeks), and numbers of randomized subjects amounted to 54 ± 42 (range: 2–207). Studies were conducted in North America (n = 19) (Eli Lilly and Company; University of North Carolina, Chapel Hill; University of Maryland; Mclean Hospital; Nathan Kline Institute for Psychiatric Research; Orexigen Therapeutics, Inc.; University of Massachusetts, Worcester—unpublished data),12,13,15,16,18,21,22,32,40,44–46 Middle-East Asia (n = 10),4,5,19,20,25,35,37,41,42,48 East Asia (n = 6),26,27,29–31,47 South America (n = 6),23,24,28,33,38,43 Europe (n = 4) (GW Pharmaceuticals Ltd, unpublished data),17,39,50 and South Asia (n = 2),34,49 while one study was a multicontinental investigation.14 Recruitment locations were unreported in 2 studies.11,36 Regarding sources of funding, 18 studies (36%) received direct financial or material support from pharmaceutical companies (Eli Lilly and Company; University of North Carolina, Chapel Hill; Orexigen Therapeutics, Inc.; GW Pharmaceuticals Ltd—unpublished data),11,14,16,18,24,28,33,34,36,38,39,41,46,50 8 studies (16%) had unclear roles of funding,17,19,25,35,37,47–49 and 5 studies (10%) received investigator-initiated grants from pharmaceutical companies.12,13,21,22,45 The remaining studies did not have direct support from industry (University of Maryland; Mclean Hospital; Nathan Kline Institute for Psychiatric Research; University of Massachusetts, Worcester—unpublished data).4,5,15,20,23,26,27,29–32,40,42–44,46
Table 1.
Characteristics of Included DBRCTs
| Concomitant Drug/ Study (Year) | Duration (wk) | Study Population | Primary Antipsychotic | Intervention Groups (Daily Dose) | n, Randomized/Completeda | Defined Primary Outcome |
|---|---|---|---|---|---|---|
| Amantadine | ||||||
| Deberdt et al (2005) 11 | 8 | - Inpatients and outpatients, 55% with schizophrenia and related disorders, 45% with bipolar I disorder | OLZ | Amantadine 100–300 mg | 60/52 | Effects on body weight |
| - Previous weight gain ≥5% | Placebo | 65/59 | ||||
| Graham et al (2005) 12 | 12 | - Outpatients, 86% with schizophrenia and related disorders, 14% with bipolar disorder | OLZ | Amantadine up to 300 mg | 12/9 | Effects on BMI |
| - Previous weight gain ≥5 lb | Placebo | 9/9 | ||||
| Aripiprazole | ||||||
| Henderson et al (2009) 13 | 10 (4wk of each Tx with 2-wk interval) | - Outpatients with schizophrenia or schizoaffective disorder | OLZ | Aripiprazole 15 mg | 15/14 (crossover) | Effects on body weight, lipids, glucose metabolism, and psychopathology |
| - Previous BMI ≥30 or BMI ≥27 with other metabolic risk factors | Placebo | |||||
| Fleischhacker et al (2010) 14 | 16 | - Outpatients with schizophrenia | CLZ | Aripiprazole 5–15 mg | 108/97 | Effects on body weight |
| - Previous weight gain ≥2.5 kg | Placebo | 99/93 | ||||
| Fan et al (2013) 15 | 8 | - Outpatients with schizophrenia or schizoaffective disorder | CLZ | Aripiprazole 15 mg | 20/16 | Effects on glucose metabolism using the frequently sampled intravenous glucose tolerance test |
| Placebo | 18/14 | |||||
| Atomoxetine | ||||||
| Ball et al (2011) 16 | 24 | - Outpatients with schizophrenia or schizoaffective disorder | OLZ, CLZ, or CLZ + RIS | Atomoxetine 40–120 mg | 20/14 | Effects on body weight |
| - Previous weight gain of ≥7% | Placebo | 17/12 | ||||
| D-Fenfluramine | ||||||
| Goodall et al (1988) 17 | 12 | - Outpatients, 97% with schizophrenia or schizoaffective disorder (diagnostic criteria unspecified) | FPZ, FPX, or CPX | D-Fenfluramine 30 mg | 17/9 | Effects on body weight |
| - Receiving depot antipsychotics | Placebo | 16/7 | ||||
| - Previous BMI ≥27 | ||||||
| Dextroamphetamine | ||||||
| Modell et al (1965) 18 | 16 (8wk of Tx, no Tx 4wk before/after) | - Male inpatients with schizophrenia (diagnostic criteria unspecified) | Thioridazine, chlorpromazine, imipramine, or chlordiazepoxide | Dextroamphetamine 5 mg | 10/10 | Effects on body weight and appetite |
| Placebo | 10/10 | |||||
| Famotidine | ||||||
| Poyurovsky et al (2004) 19 | 6 | - Inpatients with first-episode schizophrenia or schizophreniform disorder | OLZ | Famotidine 40 mg | 7/7 | Effects on body weight |
| Placebo | 7/7 | |||||
| Fluoxetine | ||||||
| Poyurovsky et al (2002) 20 | 8 | - Inpatients with first-episode schizophrenia | OLZ | Fluoxetine 20 mg | 15/11 | Effects on body weight |
| Placebo | 15/13 | |||||
| Bustillo et al (2003) 21 | 16 | - Outpatients with schizophrenia or schizoaffective disorder | OLZ | Fluoxetine 20–60 mg | 15/11 | Effects on body weight |
| - Previous weight gain of ≥3% | Placebo | 15/9 | ||||
| Intranasal insulin | ||||||
| Li et al (2013) 22 | 8 | - Outpatients with schizophrenia or schizoaffective disorder | 54% OLZ, 46% other antipsychotics | Intranasal insulin 160 IU | 21/18 | Effects on body composition and lipid particle sizes using whole body dual-energy X-ray absorptiometry and nuclear magnetic resonance spectroscopy |
| Placebo | 24/21 | |||||
| Metformin | ||||||
| Baptista et al (2006) 23 | 14 | - Inpatients with schizophrenia or schizoaffective disorder | OLZ | Metformin 850–1700 mg | 20/19 | Effects on body weight |
| Placebo | 20/18 | |||||
| Baptista et al (2007) 24 | 12 | - Inpatients and outpatients, 95% with schizophrenia, 5% with bipolar disorder | OLZ | Metformin 850–2550 mg | 40/36 | Effects on body weight |
| Placebo | 40/36 | |||||
| Arman et al (2008) 25 | 12 | - Inpatients with schizophrenia or schizoaffective disorder | RIS | Metformin 1000 mg | 49 recruited, 16 completers in each group | Effects on body weight |
| - Age <20 | Placebo | |||||
| Wu et al (2008) 26 | 12 | - Outpatients with first-episode schizophrenia | CLZ, OLZ, RIS, or SLP | 2×2 design of: | 32/30 | Effects on body weight, BMI, waist circumference, glucose, insulin, and HOMA-IR |
| - Previous weight gain of >10% | - Lifestyle intervention [+]/[−] | 32/29 | ||||
| - Metformin 750mg/placebo | 32/30 | |||||
| 32/29 | ||||||
| Wu et al (2008) 27 | 12 | - Inpatients with first-episode schizophrenia | OLZ | Metformin 750 mg | 20/18 | Effects on body weight, BMI, waist circumference, waist-to-hip ratio, glucose, insulin, >7% gain in body weight, and HOMA-IR |
| Placebo | 20/19 | |||||
| Carrizo et al (2009) 28 | 14 | - Outpatients, 96% with schizophrenia or schizophreniform disorder | CLZ | Metformin 500–1000 mg | 31/24 | Effects on body weight |
| Placebo | 30/30 | |||||
| Wang et al (2012) 29 | 12 | - Outpatients with first-episode schizophrenia | CLZ, OLZ, RIS, or SLP | Metformin 1000 mg | 36/32 | Effects on body weight |
| - Previous weight gain of >7% | Placebo | 36/34 | ||||
| Wu et al (2012) 30 | 24 | - Female outpatients with first-episode schizophrenia | CLZ, OLZ, RIS, or SLP | Metformin 1000 mg | 42/39 | Effects on amenorrhea and body weight |
| - Experiencing amenorrhea with antipsychotic Tx | Placebo | 42/37 | ||||
| Chen et al (2013) 31 | 24 | - Inpatients/outpatients with schizophrenia or schizoaffective disorder | CLZ | Metformin 1500 mg | 28/28 | Effects on body weight and metabolic features |
| - Previous BMI ≥24 or having ≥1 defined metabolic abnormality | Placebo | 27/27 | ||||
| Jarskog et al (2013) 32 | 16 | - Outpatients with schizophrenia or schizoaffective disorder | Various antipsychotics including CLZ and OLZ | Metformin 1000–2000 mg | 75/58 | Effects on body weight |
| - Previous BMI ≥27 | Placebo | 73/58 | ||||
| Metformin-sibutramine combination | ||||||
| Baptista et al (2008) 33 | 12 | - Inpatients with schizophrenia | OLZ | Metformin 850–1700mg and sibutramine 10–20 mg | 15/13 | Effects on body weight, BMI, and waist circumference |
| Placebo | 15/15 | |||||
| Modafinil | ||||||
| Sudhakar et al (2008) 34 | 12 | - Inpatient/outpatient status undescribed, 57% with schizophrenia | CLZ, OLZ, or RIS | Modafinil 200 mg | 36/32 | Effects on body weight and daytime drowsiness |
| - On atypical antipsychotics for <2 wk | Placebo | 36/31 | ||||
| Nizatidine | ||||||
| Atmaca et al (2003) 35 | 8 | - Inpatients and outpatients with schizophrenia | OLZ | Nizatidine 300 mg | 18/17 | Effects on body weight and leptin |
| - Previous weight gain of >2.5 kg | Placebo | 17/17 | ||||
| Cavazzoni et al (2003) 36 | 16 | - Inpatients/outpatients with schizophrenia, schizoaffective disorder or schizophreniform disorder | OLZ | Nizatidine 300mg/600 mg | 57/35 | Effects on body weight |
| Placebo | 58/33 | |||||
| 60/37 | ||||||
| Atmaca et al (2004) 37 | 8 | - Inpatient/outpatient status undescribed | QTP | Nizatidine 300 mg | 14/13 | Effects on body weight and leptin |
| - Schizophrenia patients with “considerable weight gain” | Placebo | 14/12 | ||||
| Assunção et al (2006) 38 | 12 | - Outpatients with schizophrenia, schizoaffective disorder, or schizophreniform disorder | OLZ | Nizatidine 600 mg | 27 randomized to each group, 45 subjects completed | Effects on body weight |
| - Previous weight gain of ≥5% | Placebo | |||||
| Orlistat | ||||||
| Joffe et al (2008) 39 | 16 | - Inpatients/outpatients with “serious mental conditions” (diagnostic criteria unspecified) | CLZ or OLZ | Orlistat 360 mg | 35/28 | Effects on body weight |
| Placebo | 36/29 | |||||
| Phenylpropanolamine | ||||||
| Borovicka et al (2002) 40 | 12 | - Outpatients with treatment intolerant or resistant schizophrenia | CLZ | Phenylpropanolamine 75 mg | 8/6 | Effects on body weight |
| - Previous weight gain of >10% | Placebo | 8/6 | ||||
| Reboxetine | ||||||
| Poyurovsky et al (2003) 41 | 6 | - Inpatients with first-episode schizophrenia | OLZ | Reboxetine 4 mg | 13/10 | Effects on body weight |
| Placebo | 13/10 | |||||
| Poyurovsky et al (2007) 42 | 6 | - Inpatients with first-episode schizophrenia | OLZ | Reboxetine 4 mg | 31/22 | Effects on body weight |
| Placebo | 28/19 | |||||
| Reboxetine-betahistine combination | ||||||
| Poyurovsky et al (2013) 4 | 6 | - Inpatients with schizophrenia or schizophreniform disorder | OLZ | Reboxetine 4mg and Betahistine 48 mg | 29/22 | Effects on body weight |
| - Predominantly first-episode patients | Placebo | 14/10 | ||||
| Rosiglitazone | ||||||
| Baptista et al (2009) 43 | 12 | - Inpatients with schizophrenia | OLZ | Rosiglitazone 4–8 mg | 15/14 | Effects on body weight, waist circumference, HOMA-IR, HbA1c, and serum lipid levels |
| Placebo | 15/15 | |||||
| Henderson et al (2009) 44 | 8 | - Outpatients with schizophrenia or schizoaffective disorder | CLZ | Rosiglitazone 4 mg | 8/8 | Effects on HOMA-IR |
| - Previously showing insulin resistance or impaired glucose metabolism | Placebo | 10/10 | ||||
| Sibutramine | ||||||
| Henderson et al (2005) 45 | 12 | - Outpatients with schizophrenia or schizoaffective disorder | OLZ | Sibutramine 5–15 mg | 19/16 | Effects on body weight |
| - Previous BMI ≥30 or BMI ≥27 with other defined risk factors | Placebo | 18/15 | ||||
| Henderson et al (2007) 46 | 12 | - Outpatients with schizophrenia or schizoaffective disorder | CLZ | Sibutramine 5–15 mg | 11/10 | Effects on body weight |
| - Previous BMI ≥30 or BMI ≥27 with other defined risk factors | Placebo | 10/8 | ||||
| Biedermann (in press) 50 | 24 | - Outpatients with schizophrenia | Various antipsychotics including CLZ | Sibutramine 10 mg | 7/5 | Effects on body weight |
| - Previous weight gain of >7% or BMI >27 | Placebo | 8/5 | ||||
| Topiramate | ||||||
| Ko et al (2005) 47 | 12 | - Inpatients with schizophrenia | RIS, OLZ, QTP, or CLZ | Topiramate 200mg/100 mg | 66 recruited, 17, 16, and 20 completers in each group | Efficacy and tolerability as a weight-controlling agent |
| - Previous BMI ≥25 | Placebo | |||||
| Afshar et al (2009) 48 | 8 | - Outpatients with schizophrenia | CLZ | Topiramate 50–300 mg | 16 randomized to each group, numbers of dropouts are unclear | Efficacy and tolerability as an adjuvant to CLZ |
| - Poor clinical outcome despite Tx with several antipsychotics | Placebo | |||||
| Narula et al (2010) 49 | 12 | - Inpatients and outpatients with first-episode schizophrenia | OLZ | Topiramate 50–100 mg | 36/34 | Effects on body weight and biochemical/metabolic abnormalities |
| Placebo | 36/33 | |||||
| Zonisamide | ||||||
| Ghanizadeh et al (2013) 5 | 10 | - Outpatients, and inpatients close to discharge | Various antipsychotics including CLZ and OLZ | Zonisamide 50–100 mg | 21/19 | Effects on BMI and body weight |
| - Schizophrenia | Placebo | 20/20 | ||||
| Sibutramine | ||||||
| NCT00044187 (Eli Lilly and Company, unpublished data) | Unspecified | - Schizophrenia, schizophreniform disorder, schizoaffective disorder, and bipolar I disorder | OLZ | Sibutramine (dose unreported) | Estimated enrollment: 130 | Effects on body weight (no data) |
| Placebo | Study completed | |||||
| Amantadine | ||||||
| NCT00287352 (University of North Carolina, Chapel Hill, unpublished data) | 16 | - First-episode psychotic disorder, schizophreniform disorder, schizophrenia, schizoaffective disorder, and mood disorders with psychotic features | OLZ | Amantadine 300 mg | Enrollment: 40 | Effects on percentages of body fat, fat utilization, and other metabolic profiles (no data) |
| Placebo | Study completed | |||||
| Rimonabant | ||||||
| NCT00547118 (University of Maryland, unpublished data) | 16 | - Clinically stable inpatients and outpatients with schizophrenia or schizoaffective disorder | Second-generation antipsychotics (details undescribed) | Rimonabant 20 mg | 16 in total | Effects on body weight, metabolic parameters, cardiovascular disease risk, and food satiety (no data) |
| - Previous BMI ≥30 or BMI ≥27 with hyperlipidemia/hypertriglyceridemia | Placebo | Study terminated | ||||
| Naltrexone | ||||||
| NCT00567034 (Mclean Hospital, unpublished data) | 12 | - Schizophrenia or schizoaffective disorder | OLZ | Naltrexone 50 mg | Estimated enrollment: 52 | Effects on body weight and BMI (no data) |
| - Previous BMI ≥30 or BMI ≥27 with symptoms of the metabolic syndrome | Placebo | Recruitment status unknown | ||||
| Betahistine | ||||||
| NCT00709202 (Nathan Kline Institute for Psychiatric Research, unpublished data) | 12 | - Adolescents/young adults with schizophrenia and related psychotic disorders, bipolar disorder, autism | CLZ, OLZ, RIS or QTP | Betahistine 8–24 mg | Estimated enrollment: 40 | Effects on body weight and BMI (no data) |
| - Previous weight gain of >2% | Placebo | Recruitment status unknown | ||||
| Zonisamide SR | ||||||
| NCT00734435 (Orexigen Therapeutics, Inc., unpublished data) | 16 | - Outpatients with schizophrenia, schizoaffective disorder, or schizophreniform disorder | OLZ | Zonisamide SR 360 mg | Enrollment: 26 | Effects on body weight (no data) |
| Placebo | Study terminated | |||||
| Telmisartan | ||||||
| NCT00981526 (University of Massachusetts, Worcester, unpublished data) | 12 | - Outpatients with schizophrenia or schizoaffective disorder | CLZ or OLZ | Telmisartan 40–80 mg | Enrollment: 57 | Effects on insulin resistance and fasting triglycerides (no data) |
| Placebo | Study completed | |||||
| GWP42003:GWP42004 (40:1) | ||||||
| NCT01491490 (GW Pharmaceuticals Ltd., unpublished data) | 6 | - Schizophrenia, schizophreniform disorder, or acute psychosis with schizophrenia symptoms | OLZ | GWP42003:GWP42004 (40:1) | Enrollment: 2 | Effects on body weight |
| Placebo | Study terminated | |||||
Notes: BMI, body mass index (kg/m2); CLZ, clozapine; CPX, clopenthixol decanoate; DBRCT, double-blind randomized controlled trial; HOMA-IR, homeostasis assessment for insulin resistance model (fasting insulin [103 µIU/l] × fasting glucose [mmol/l]/22.5); FPX, flupenthixol decanoate; FPZ, fluphenazine decanoate; OLZ, olanzapine; QTP, quetiapine; RIS, risperidone; SLP, sulpiride; SR, sustained release; Tx, treatment.
aNumbers of randomized/completed subjects do not always match the numbers of subjects included in the analysis.
The effects of 20 and 8 unique interventions were investigated in published and unpublished trials, respectively. Medications used were the following: amantadine (total number of studies n = 2, total number of randomized subjects n = 146),11,12 aripiprazole (n = 3, n = 260),13–15 atomoxetine (n = 1, n = 37),16 D-fenfluramine (n = 1, n = 33),17 dextroamphetamine (n = 1, n = 20),18 famotidine (n = 1, n = 14),19 fluoxetine (n = 2, n = 60),20,21 intranasal insulin (n = 1, n = 45),22 metformin (n = 10, n = 757),23–32 metformin-sibutramine combination (n = 1, n = 30),33 modafinil (n = 1, n = 72),34 nizatidine (n = 4, n = 292),35–38 orlistat (n = 1, n = 71),39 phenylpropanolamine (n = 1, n = 16),40 reboxetine (n = 2, n = 85),41,42 reboxetine-betahistine combination (n = 1, n = 43),4 rosiglitazone (n = 2, n = 48),43,44 sibutramine (n = 3, n = 73),45,46,50 topiramate (n = 3, n = 170),47–49 and zonisamide (n = 1, n = 41).5 Unpublished studies used sibutramine (Eli Lilly and Company, unpublished data), amantadine (University of North Carolina, Chapel Hill, unpublished data), rimonabant (University of Maryland, unpublished data), naltrexone (Mclean Hospital, unpublished data), betahistine (Nathan Kline Institute for Psychiatric Research, unpublished data), zonisamide (Orexigen Therapeutics, Inc., unpublished data), telmisartan (University of Massachusetts, Worcester, unpublished data), and GWP42003:GWP42004 (40:1; GW Pharmaceuticals Ltd, unpublished data).
Thirty-seven studies (74%) used concomitant drugs as a treatment for preexisting metabolic adversities, while 13 studies (26%) initiated adjunctive medications simultaneously with antipsychotics in an effort to prevent such derangements. Forty-nine studies described the primary antipsychotic used; most studies included subjects using either clozapine or olanzapine (n = 45, 91.8%), while 4 studies (8.2%) investigated subjects who used neither of these drugs. Forty studies reported data on changes in body weight, while 21 and 16 studies reported additional outcomes related to glucose metabolism and lipids, respectively. Two published studies34,48 did not report sufficient data and thus were excluded from the meta-analysis. Moreover, none of the unpublished trials reported sufficient data to include in the meta-analysis: 7 studies (Eli Lilly and Company; University of North Carolina, Chapel Hill; University of Maryland; Mclean Hospital; Nathan Kline Institute for Psychiatric Research; Orexigen Therapeutics, Inc.; University of Massachusetts, Worcester—unpublished data) reported no data, and 1 study (GW Pharmaceuticals Ltd, unpublished data) reported data from only 2 subjects.
Risk of Bias
Risks of bias of the included studies are summarized in supplementary table 1. Although all studies were randomized trials, the methodology of random sequence generation and allocation concealment were often unreported, leading to “unclear risk” for selection bias in 40 studies (80%). Similarly, blinding of outcome assessors was often unspecified, resulting in “unclear risk” for detection bias in 34 studies (68%). In general, dropout cases were adequately explained, and data from ITT analyses were reported; only 7 studies (14%) had “high risk” for attrition bias for reasons including unbalanced dropouts between groups and reporting of completers analysis only. Six studies (12%) did not report full data on secondary outcomes and were judged to have “high risk” of selective reporting. For other bias, 3 reports (6%) did not specify the diagnostic criteria used and were judged to have “high risk” regarding study diagnoses. Taken together, only 6 studies (12%) showed a “low risk” for bias in all assessment criteria.
Meta-analyses
Effects on Body Weight.
Forty published studies representing 19 unique interventions reported data on changes in body weight (table 2). One study each, investigating nizatidine36 and topiramate,47 compared 2 different doses of active drugs with placebo; thus, placebo groups were included twice for these studies, in order to investigate a potential dose-effect relationship. Furthermore, Wu et al26 compared the effects of concomitant metformin to placebo in combination with or without lifestyle interventions, resulting in analyses of 2 sets of groups. Hence, the meta-analysis for effects on body weight consisted of 43 (ie, 40 + 3) comparisons between active drugs and placebo, and 76 (ie, 56 + 20)36,47 more subjects in the placebo groups than actually recruited.
Table 2.
Effects of Concomitant Drugs on Body Weight (kg), Results of 40 Studies, and Meta-analyses of 19 Interventions
| Concomitant Drug/Study | n, Analyzed | Mean ± SD Change at Endpoint (kg) | Mean Difference (95% CI) (kg) | I 2 (%) | ||
|---|---|---|---|---|---|---|
| Author (Year) | Active Drug | Placebo | Active Drug | Placebo | ||
| Amantadine (N = 2) | ||||||
| Deberdt et al (2005) 11 | 59 | 64 | −0.19±4.58 | 1.28±4.26 | −2.27 (−4.76, 0.23) | 40 |
| Graham et al (2005) 12 | 12 | 9 | −0.4±3.5 | 3.9±5.3 | ||
| Aripiprazole (N = 3) | ||||||
| Henderson et al (2009) 13 | 15 | 15 | −1.3±2.1 | 1.0±1.5 | −2.13 (−2.87, −1.39) | 0 |
| Fleischhacker et al (2010) 14 | 107 | 99 | −2.53±3.9 a | −0.38±3.9 a | ||
| Fan et al (2013) 15 | 16 | 14 | −1.5±2.3 | 0.3±2.3 | ||
| Atomoxetine (N = 1) | ||||||
| Ball et al (2011) 16 | 19 | 17 | −1.7±6.1b | −2.1±5.8b | — | — |
| D-Fenfluramine (N = 1) | ||||||
| Goodall et al (1988) 17 | 9 | 7 | −5.4±3.4 | −2.8±1.65 | — | — |
| Dextroamphetamine (N = 1) | ||||||
| Modell et al (1965) 18 | 10 | 10 | 0.9±4.3 | 0.1±2.3 | — | — |
| Famotidine (N = 1) | ||||||
| Poyurovsky et al (2004) 19 | 7 | 7 | 4.8±3.2 | 4.9±1.6 | — | — |
| Fluoxetine (N = 2) | ||||||
| Poyurovsky et al (2002) 20 | 15 | 15 | 6.1±5.3 | 5.9±4.6 | 0.75 (−1.76, 3.26) | 0 |
| Bustillo et al (2003) 21 | 15 | 15 | 3.0±5.3c | 1.7±4.6c | ||
| Intranasal Insulin (N = 1) | ||||||
| Li et al (2013) 22 | 18 | 21 | 0.8±1.9 | −2.8±9.6 | — | — |
| Metformin (N = 10) | ||||||
| Baptista et al (2006) 23 | 19 | 18 | 5.5±3.3d | 6.3±2.3d | −3.17 (−4.44, −1.90) | 88 |
| Baptista et al (2007) 24 | 36 | 36 | −1.40±3.2 | −0.18±2.8 | ||
| Arman et al (2008) 25 | 16 | 16 | 0.81±0.33 | 2.2±2.54 | ||
| Wu et al (2008) lifestyle [+] 26 | 32 | 32 | −4.7±3.2 e | −1.4±1.8 e | ||
| Wu et al (2008) lifestyle [−] 26 | 32 | 32 | −3.2±1.9 e | 3.1±1.9 e | ||
| Wu et al (2008) 27 | 18 | 19 | 1.9±2.72 | 6.87±4.23 | ||
| Carrizo et al (2009) 28 | 24 | 30 | −1.87±2.9 | 0.16±2.9 | ||
| Wang et al (2012) 29 | 32 | 34 | −3.3±3.9 c | 2.5±4.0 c | ||
| Wu et al (2012) 30 | 42 | 42 | −2.37±6.1 f | 2.15±6.1 f | ||
| Chen et al (2013) 31 | 28 | 27 | −3.2±3.1 | −0.2±2.1 | ||
| Jarskog et al (2013) 32 | 75 | 71 | −3.0±4.3 e | −1.0±4.2 e | ||
| Metformin-sibutramine combination (N = 1) | ||||||
| Baptista et al (2008) 33 | 13 | 15 | −2.8±3.2 | −1.4±2.6 | — | — |
| Nizatidine (N = 4) | ||||||
| Atmaca et al (2003) 35 | 17 | 17 | −4.5±2.2 | 2.3±0.9 | −2.03 (−4.53, 0.47) | 97 |
| Cavazzoni et al (2003) 36 ; 600 mg | 57 | 56 | 3.29±5.33 | 4.18±4.33 | ||
| Cavazzoni et al (2003) 36 ; 300 mg | 56 | 56 | 3.56±4.95 | 4.18±4.33 | ||
| Atmaca et al (2004) 37 | 14 | 14 | −1.0±0.6 | 1.2±1.2 | ||
| Assunção et al (2006) 38 | 27 | 27 | 1.1±0.6g | 0.7±1.2g | ||
| Orlistat (N = 1) | ||||||
| Joffe et al (2008) 39 | 31 | 32 | −1.25±4.33 | 0.44±3.73 | — | — |
| Phenylpropanolamine (N = 1) | ||||||
| Borovicka et al (2002) 40 | 8 | 8 | 1.36±21.8h | 1.36±16.6h | — | — |
| Reboxetine (N = 2) | ||||||
| Poyurovsky et al (2003) 41 | 10 | 10 | 2.45±2.72 | 5.45±3.09 | −1.90 (−3.07, −0.72) | 0 |
| Poyurovsky et al (2007) 42 | 31 | 28 | 3.31±2.73 | 4.91±2.45 | ||
| Reboxetine-betahistine combination (N = 1) | ||||||
| Poyurovsky et al (2013) 4 | 29 | 14 | 2.02±2.37 | 4.77±3.16 | — | — |
| Rosiglitazone (N = 2) | ||||||
| Baptista et al (2009) 43 | 14 | 15 | 3.2±4.5 | 2.2±2.3 | 0.26 (−1.83, 2.35) | 0 |
| Henderson et al (2009) 44 | 8 | 10 | −0.5±4.5i | 0.5±2.3i | ||
| Sibutramine (N = 3) | ||||||
| Henderson et al (2005) 45 | 19 | 18 | −3.8±1.1 | −0.8±0.7 | −2.86 (−4.72, −1.01) | 49 |
| Henderson et al (2007) 46 | 10 | 8 | −1.9±3.0b | −0.5±2.2b | ||
| Biedermann (in press) 50 | 5 | 6 | −6.1±6.7 | 1.9±3.5 | ||
| Topiramate (N = 2) | ||||||
| Ko et al (2005) 47 ; 200 mg | 17 | 20 | −5.45±13.1 j | −0.3±13.1 j | −5.20 (−9.55, −0.84) | 0 |
| Ko et al (2005) 47 ; 100 mg | 16 | 20 | −1.68±13.1j | −0.3±13.1j | ||
| Narula et al (2010) 49 | 33 | 34 | −1.27±13.1 f | 6.03±13.1 f | ||
| Zonisamide (N = 1) | ||||||
| Ghanizadeh et al (2013) 5 | 21 | 20 | −1.1±1.4 | 1.9±2.2 | — | — |
Notes: Statistically significant effects compared to placebo are shown in bold.
SD values were supplemented using the following methods:
aCalculated from 95% CIs of mean group difference.
bCalculated from SEs of within group difference.
cImputed from Poyurovsky et al (2002) 20 .
dAdditional data were provided by the authors.
eCalculated from 95% CIs of within group difference.
fCalculated from t values of between group difference.
gImputed from Atmaca et al (2004) 37 .
hCalculated from SEs measured in figures.
iImputed from Baptista et al (2009) 43 .
jImputed from Narula et al (2010) 49 .
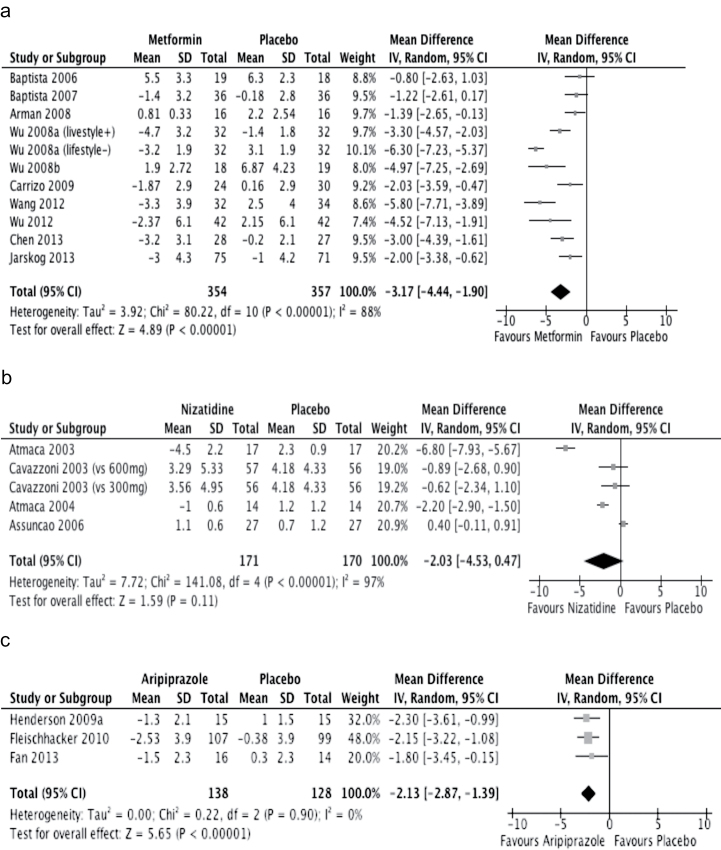
The results of the meta-analyses are displayed in table 2. Eight interventions (ie, aripiprazole, D-fenfluramine, metformin, reboxetine, reboxetine-betahistine combination, sibutramine, topiramate, and zonisamide) showed significant effects compared to placebo; of note, there was only one study each for D-fenfluramine,17 the reboxetine-betahistine combination,4 and zonisamide.5 Metformin was the most extensively studied drug, both regarding the number of studies and randomized subjects (n = 10, n = 757). This was followed by nizatidine (n = 4, n = 292) and aripiprazole (n = 3, n = 260).
Meta-analysis of 10 studies investigating the effects of metformin yielded a significant mean difference of −3.17 kg (95% CI: −4.44 to −1.90 kg) compared to placebo (figure 2a). However, the results were heterogeneous (I 2 = 88%), with a mixture of 3 negative23–25 and 7 positive studies.26–32 Most studies focused on adult patients who were, at least in part, treated with either clozapine or olanzapine; the exception was a negative trial in which the participants were adolescents receiving risperidone.25
Fig. 2.
Effects of metformin, nizatidine, and aripiprazole on body weight, mean difference (kg). (a) Metformin vs placebo. (b) Nizatidine vs placebo. (c) Aripiprazole vs placebo. IV, inverse variance. For each comparison, the small square represents the mean difference, and the horizontal line is the 95% CI. The diamonds represent the overall weighted mean differences. The width of the diamonds represents their 95% CI.
Four trials investigating the effects of nizatidine on body weight showed mixed results;35–38 the mean difference was not different from placebo (figure 2b). Again, the results were shown to be highly heterogeneous (I 2 = 97%).
Three studies reported on effects of add-on aripiprazole as a weight-controlling agent; the mean difference amounted to −2.13 kg (95% CI: −2.87 to −1.39 kg) compared to placebo (figure 2c). In a multicontinental investigation of 207 patients with schizophrenia who were receiving stable doses of clozapine, a significant weight loss was observed in those randomized to adjunctive aripiprazole.14 The remaining 2 studies were relatively small (n ≤ 30), with one study each showing positive13 and negative results.15
Among the remaining 16 interventions, sibutramine (n = 73) had 3 publications, while the following had 2 publications each: topiramate (n = 170), amantadine (n = 146), reboxetine (n = 85), fluoxetine (n = 60), and rosiglitazone (n = 48). Of these, pooled effects of topiramate, reboxetine, and sibutramine were superior to placebo.
Trials using the following medications were reported in one publication each: orlistat (n = 63), zonisamide (n = 41), intranasal insulin (n = 39), atomoxetine (n = 37), dextroamphetamine (n = 20), D-fenfluramine (n = 16), phenylpropanolamine (n = 16), and famotidine (n = 14). Of these, studies using zonisamide and D-fenfluramine showed positive results. Ghanizadeh et al5 reported modest but significant effects of zonisamide to decrease body weight in patients treated with various antipsychotics. Another trial performed by Li et al22 was the only study employing a nonoral form (intranasal insulin) of medication; however, no benefits were observed regarding body weight. Goodall et al17 were the only group to examine the effects of weight-modifying agents in subjects receiving depot antipsychotics; D-fenfluramine showed significant weight reduction compared to placebo.
Two studies investigated the synergetic effects of “polypill” to counteract weight gain. Baptista et al33 investigated the efficacy of a metformin-sibutramine combination on weight gain; weight reduction in the combination group was numerically higher but nonsignificant. Poyurovsky et al4 reported significant preventive effects of a reboxetine-betahistine combination on olanzapine-induced weight gain in comparison to placebo.
Subgroup and Sensitivity Analysis
In regard to body weight, the mean differences of trials performed in all a priori defined subgroups were different from placebo (supplementary figures 1–3). The mean difference of studies recruiting first-episode patients was especially large, amounting to −3.52 kg (95% CI: −4.93 to −2.11 kg) compared to placebo. To address whether first-episode patients would derive larger benefits in prevention trials, an exploratory subgroup analysis separating prevention studies into those recruiting first-episode patients vs others was performed; studies recruiting first-episode patients yielded a numerically larger mean difference of −2.41 kg (95% CI: −3.82 to −1.01 kg) (supplementary figure 4).
Subgroup analyses were also performed on the 10 metformin trials and 4 nizatidine trials to investigate reasons for heterogeneity (supplementary figures 5–6). An a priori defined subgroup analysis dividing metformin trials into those recruiting first-episode patients vs others decreased the heterogeneity in both subgroups to I 2 = 73% and 5%, respectively. In an additional, exploratory sensitivity analysis, a single comparison combining comprehensive lifestyle interventions with metformin26 was removed from the subgroup of first-episode trials; this decreased the heterogeneity to I 2 = 0%. Similarly, reasons for heterogeneity in the nizatidine trials were investigated; however, a priori defined subgroup analyses did not decrease the degree of heterogeneity. In an exploratory sensitivity analysis, the first published study35 which had an outlier effect size was excluded; however, heterogeneity still remained high at I 2 = 91%.
Finally, in an a priori defined sensitivity analysis, trials reporting SD values were analyzed separately from those in which unreported SD values were supplemented; mean differences remained significant in both groups (supplementary figure 7). Likewise, we examined the mean differences of metformin, nizatidine, aripiprazole, and sibutramine trials that reported SD values; the conclusions regarding their pooled effects remained largely the same, with exception to sibutramine which became nonsignificant (supplementary figure 8).
Publication Bias
Funnel plots of studies investigating the effects of concomitant metformin, nizatidine, and aripiprazole with respect to body weight are shown in supplementary figure 9. Asymmetry was observed among the metformin studies with smaller sample sizes, indicating a possibility of publication bias. Furthermore, all 8 unpublished trials reported insufficient data, suggesting a possibility of publication bias.
Clinically Relevant Weight Change
A limited number of studies presented data on percentages of clinically relevant weight change; 8 and 9 studies reported on weight loss and weight gain, respectively (supplementary tables 2–3). Cutoff points were defined as ≥7% change in body weight in most studies. All studies reporting on clinically relevant weight loss were treatment studies; aripiprazole (n = 1) and metformin (n = 4) showed significant effects compared to placebo, with a NNT of 9 and 3, respectively. In contrast, 7 of 9 studies reporting on clinically relevant weight gain were prevention studies. Metformin (n = 2), reboxetine (n = 2), and the reboxetine-betahistine combination (n = 1) were effective in preventing 7% or more weight gain, with a NNT of 10, 7, and 4, respectively.
Effects on Glucose Metabolism
Effects of concomitant medications on fasting glucose, HbA1c, fasting insulin, and HOMA-IR are displayed in supplementary tables 4–7. Metformin and topiramate significantly decreased fasting glucose levels, but the latter finding was supported by a single study.49 Pooling of a limited number of studies for aripiprazole13,15 and metformin24,28,32 resulted in a significant mean difference in HbA1c levels. In contrast, 9 and 8 metformin trials reported data on changes in fasting insulin and HOMA-IR, respectively; relatively consistent and robust effects were observed compared to placebo.
Effects on Blood Lipids
Changes in total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides are shown in supplementary tables 8–11. Pooling of 5 trials23,24,28,31,32 showed significant effects of metformin to improve triglycerides, while 3 aripiprazole trials13–15 resulted in significant mean differences for total cholesterol and LDL cholesterol. Two sibutramine studies45,46 also yielded a significant mean difference in total cholesterol. Only one study presented data in the context of clinical significance31: When applying the diagnostic criteria for metabolic syndrome, metformin was effective in reversing hypertriglyceridemia, but ineffective in enhancing HDL cholesterol.
Discussion
By pooling the effects of 40 studies representing 19 unique interventions with regard to body weight, we found concomitant metformin to be supported by the evidence, with a mean difference of −3.17 kg (95% CI: −4.44 to −1.90 kg) compared to placebo. Pooled effects for topiramate, sibutramine, aripiprazole, and reboxetine were also significant. Interventions were effective in all a priori defined subgroups, while first-episode patients may derive the most benefit. Patients receiving metformin were more likely to achieve clinically relevant weight loss in treatment trials, and less likely to experience clinically relevant weight gain in prevention trails, although the limited number of reports should be noted. The limited data with respect to glucose metabolism and lipids suggest that metformin and rosiglitazone improve insulin resistance, while aripiprazole, metformin, and sibutramine decrease cholesterol and triglyceride levels.
Obesity is prevalent among patients with schizophrenia,51–53 which can lead to various medical complications.54–57 Moreover, many antipsychotics precipitate weight gain,58–60 thereby increasing risks for metabolic complications. In this context, when the use of nonpharmacological interventions alone are insufficient, and switching antipsychotics to relatively weight-neutral agents is not feasible (eg, treatment-resistant patients receiving clozapine), pharmacological strategies to counteract these metabolic adversities need to be considered. In such instances, the use of adjunctive metformin appears to be the first choice, as positive effects on body weight, insulin resistance, and lipids have been consistently reported. A weight loss of −3.17 kg, albeit modest, may have clinically meaningful long-term benefits such as reduced risk of hypertension61 and diabetes62; however, such consequences will need replication in patients with schizophrenia.
The paucity of data regarding the genetic background, which affect response to medications that alleviate metabolic adversities, leaves an important question. For example, Fernández et al63 reported that specific genetic polymorphisms in the leptin promoter and leptin receptor genes were related to a blunted response to metformin in clozapine-treated patients. In a secondary analysis of the same patient group, they failed to find associations between peroxisome proliferator-activated receptor gamma 2 (PPAR-γ2) genotypes and the response to metformin.64 Future pharmacogenetic studies that control for sex and ethnicity in larger patient populations may identify subsets of patients who will benefit more from such pharmacological interventions.
Our findings align with the meta-analysis by Maayan et al2; we also found metformin, topiramate, reboxetine, and sibutramine to be effective in countering weight gain. Pooled effects on glucose metabolism and lipids were also similar with their report. In this updated report, we found effects of metformin to be supported by 4 additional studies (n = 359),29–32 thereby strengthening confidence regarding its efficacy. Differing from their report, we identified 3 studies13–15 investigating the effects of add-on aripiprazole, which resulted in modest but significant effects on weight gain, although such results should be interpreted in the context of risks and benefits of antipsychotic polypharmacy.65 Furthermore, our results align with the meta-analysis by Fiedorowicz et al.3 Similar to their conclusions, we found metformin and topiramate to be effective in countering weight gain, despite our meta-analysis having different numbers of studies included due to an updated search and different inclusion criteria. Moreover, while their report included a single study that examined the effectiveness of add-on aripiprazole to counter antipsychotic-induced weight gain,14 the present report identified 2 additional studies (n = 53),13,15 thereby enabling a meta-analysis. Differing from our report, Fiedorowicz et al meta-analyzed the effects of reboxetine41,42 with atomoxetine16 under the category of “norepinephrine reuptake inhibitors,” in which mean differences were not different from placebo. Finally, their review concluded that the use of sibutramine could not be recommended, taken that the drug has been withdrawn from markets worldwide66 due to evidence of increased risk of cardiovascular outcomes.67 Although our meta-analysis of 3 studies45,46,50 found sibutramine to be effective in regard to weight gain, and no serious adverse events were observed, we too caution its use in light of their potentially serious side effects.
Our results must be interpreted in light of various strengths and limitations. Although similar main conclusions were reached in previous reports,2,3 our main strength was that we followed the PRISMA statement to ensure transparent and complete reporting. Also, our search covered a wide range of metabolic adversities in published and unpublished studies. Addition, our focus on patients with schizophrenia will better guide evidence-based clinical decision making in this population. The present report also has some limitations. Firstly, the sample sizes and numbers of studies for most types of interventions were limited, and long-term effects beyond 24 weeks have not been investigated. Secondly, our report focused on the effects of concomitant drugs, and although interventions were generally well tolerated, a possibility of rare or long-term adverse effects of concomitant medications should be considered. Thirdly, a possibility of pharmacokinetic interactions remains and we did not review the specific mechanisms by which concomitant drugs counteract antipsychotic-induced metabolic adversities. Fourth, although blinding of outcome assessors may not be essential for trials investigating hard outcomes, only 10% of the studies reviewed had a “low risk” of bias in all assessment criteria, and sources of bias should be taken into account. Fifth, although funnel plots were used and unpublished trials were searched, a possibility of publication bias cannot be ruled out. Sixth, our search was designed to identify an extensive list of relevant studies; nevertheless, a possibility remains that we were not able to identify all relevant studies. Seventh, more than 90% of the included studies described either clozapine or olanzapine as primary antipsychotics; thus, it is unclear if our results are generalizable to patients receiving other types of antipsychotics. Eighth, our focus on pharmacological interventions to counter metabolic adversities is not intended to undermine the importance of nonpharmacological interventions; indeed, combining both may have synergetic and greater effects.26 Ninth, the application of our results to patients with other psychiatric disorders is cautioned. Finally, cost-effectiveness of concomitant drugs should also be taken into account. Moreover, the use of many agents included in this review entails off-label use.
We suggest that future studies should focus on long-term outcomes including potential consequences of metabolic adversities. Pharmacogenetic studies aiming to elucidate the individual responses to add-on medications are warranted. Finally, safety and tolerability of combination treatments should be given more attention. Notwithstanding these future tasks, the current literature suggests the use of metformin to counteract antipsychotic-induced weight gain and metabolic derangements in schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
None.
Supplementary Material
Acknowledgments
We thank Drs Man Wang and Trino Baptista for kindly providing additional data. No funding support was obtained for this report.
Some of the data were presented at the 28th meeting of the CINP World Congress of Neuropsychopharmacology on June 5, 2012 in Stockholm, Sweden; the 22nd meeting of the Japanese Society of Clinical Neuropsychopharmacology on October 19, 2012 in Utsunomiya, Japan; the 3rd Congress of the Asian College of Neuropsychopharmacology on September 13–14, 2013 in Beijing, China; and the 23rd meeting of the Japanese Society of Clinical Neuropsychopharmacology on October 24, 2013 in Okinawa, Japan.
Y.M. has received manuscript fees or speaker’s honoraria from Dainippon Sumitomo Pharma, Eli Lilly, and Wiley Japan within the past 3 years.
T.S. has received manuscript or speaker’s fees from Astellas, Dainippon Sumitomo Pharma, Eli Lilly, Elsevier Japan, Meiji Seika Pharma, Novartis Pharma, Otsuka Pharmaceutical, and Wiley Japan within the past 3 years.
A.N. has received speaker’s honoraria from Eli Lilly, Pfizer, Mochida Pharmaceutical, Yoshitomiyakuhin, Otsuka Pharmaceutical, Dainippon Sumitomo Pharma, and Mitsubishi Tanabe Pharma and textbook royalties from Igaku-shoin within the past 3 years.
K.Y. has received manuscript fees or speaker’s honoraria from Dainippon Sumitomo Pharma, Meiji Seika, and Wiley Japan within the past 3 years.
M.M. has received grants and/or speaker’s honoraria from Asahi Kasei Pharma, Astellas, Daiichi-Sankyo, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji Seika Pharma, Mochida Pharmaceutical, MSD, Novartis Pharma, Otsuka Pharmaceutical, Pfizer, Shionogi, Takeda, Mitsubishi Tanabe Pharma, and Yoshitomiyakuhin within the past 3 years.
W.W.F. has received research grants from Otsuka Pharmaceutical, Pfizer, Janssen Pharmaceutical, and Reckitt-Benckiser, as well as consulting honoraria from Lundbeck, Roche, Bristol-Myers Squibb, Otsuka Pharmaceutical, Janssen Pharmaceutical, Pfizer, MedAvante, and Merck. He has received speaker honoraria from Lundbeck, Janssen Pharmaceutical, Otsuka Pharmaceutical, and Roche, as well as holds stock from MedAvante.
H.U. has received grants from Pfizer, Astellas, Eisai, Otsuka Pharmaceutical, GlaxoSmithKline, Shionogi, Dainippon Sumitomo Pharma, Eli Lilly, Mochida Pharmaceutical, Meiji Seika Pharma, Janssen Pharmaceutical, and Yoshitomiyakuhin and speaker’s honoraria from Otsuka Pharmaceutical, Eli Lilly, Shionogi, GlaxoSmithKline, Yoshitomiyakuhin, Dainippon Sumitomo Pharma, Meiji Seika Pharma, Abbvie, and Janssen Pharmaceutical within the past 3 years.
References
- 1. Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev. 2007;1:CD005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35:1520–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiedorowicz JG, Miller DD, Bishop JR, Calarge CA, Ellingrod VL, Haynes WG. Systematic Review and Meta-analysis of Pharmacological Interventions for Weight Gain from Antipsychotics and Mood Stabilizers. Curr Psychiatry Rev. 2012;8:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poyurovsky M, Fuchs C, Pashinian A, Levi A, Weizman R, Weizman A. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology (Berl). 2013;226:615–622 [DOI] [PubMed] [Google Scholar]
- 5. Ghanizadeh A, Nikseresht MS, Sahraian A. The effect of zonisamide on antipsychotic-associated weight gain in patients with schizophrenia: a randomized, double-blind, placebo-controlled clinical trial. Schizophr Res. 2013;147:110–115 [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011 http://www.cochrane-handbook.org Accessed February 3, 2014.
- 8. Straus SE, Richardsong WS, Glasziou P, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM, Fourth Edition. Edinburgh: Churchill Livingstone; 2010 [Google Scholar]
- 9. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deberdt W, Winokur A, Cavazzoni PA, et al. Amantadine for weight gain associated with olanzapine treatment. Eur Neuropsychopharmacol. 2005;15:13–21 [DOI] [PubMed] [Google Scholar]
- 12. Graham KA, Gu H, Lieberman JA, Harp JB, Perkins DO. Double-blind, placebo-controlled investigation of amantadine for weight loss in subjects who gained weight with olanzapine. Am J Psychiatry. 2005;162:1744–1746 [DOI] [PubMed] [Google Scholar]
- 13. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;29:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleischhacker WW, Heikkinen ME, Olié JP, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010;13:1115–1125 [DOI] [PubMed] [Google Scholar]
- 15. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ball MP, Warren KR, Feldman S, McMahon RP, Kelly DL, Buchanan RW. Placebo-controlled trial of atomoxetine for weight reduction in people with schizophrenia treated with clozapine or olanzapine. Clin Schizophr Relat Psychoses. 2011;5:17–25 [PubMed] [Google Scholar]
- 17. Goodall E, Oxtoby C, Richards R, Watkinson G, Brown D, Silverstone T. A clinical trial of the efficacy and acceptability of D-fenfluramine in the treatment of neuroleptic-induced obesity. Br J Psychiatry. 1988;153:208–213 [DOI] [PubMed] [Google Scholar]
- 18. Modell W, Hussar AE. Failure of dextroamphetamine sulfate to influence eating and sleeping patterns in obese schizophrenic patients: clinical and pharmacological significance. JAMA. 1965;193:275–278 [DOI] [PubMed] [Google Scholar]
- 19. Poyurovsky M, Tal V, Maayan R, Gil-Ad I, Fuchs C, Weizman A. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a double-blind placebo-controlled pilot study. Eur Neuropsychopharmacol. 2004;14:332–336 [DOI] [PubMed] [Google Scholar]
- 20. Poyurovsky M, Pashinian A, Gil-Ad I, et al. Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. Am J Psychiatry. 2002;159:1058–1060 [DOI] [PubMed] [Google Scholar]
- 21. Bustillo JR, Lauriello J, Parker K, et al. Treatment of weight gain with fluoxetine in olanzapine-treated schizophrenic outpatients. Neuropsychopharmacology. 2003;28:527–529 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Li X, Liu E, et al. No effect of adjunctive, repeated dose intranasal insulin treatment on body metabolism in patients with schizophrenia. Schizophr Res. 2013;146:40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baptista T, Martínez J, Lacruz A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51:192–196 [DOI] [PubMed] [Google Scholar]
- 24. Baptista T, Rangel N, Fernández V, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophr Res. 2007;93:99–108 [DOI] [PubMed] [Google Scholar]
- 25. Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double-blind, placebo-controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Med J. 2008;29:1130–1134 [PubMed] [Google Scholar]
- 26. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185–193 [DOI] [PubMed] [Google Scholar]
- 27. Wu RR, Zhao JP, Guo XF, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165:352–358 [DOI] [PubMed] [Google Scholar]
- 28. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113:19–26 [DOI] [PubMed] [Google Scholar]
- 29. Wang M, Tong JH, Zhu G, Liang GM, Yan HF, Wang XZ. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138:54–57 [DOI] [PubMed] [Google Scholar]
- 30. Wu RR, Jin H, Gao K, et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2012;169:813–821 [DOI] [PubMed] [Google Scholar]
- 31. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74:e424–e430 [DOI] [PubMed] [Google Scholar]
- 32. Jarskog LF, Hamer RM, Catellier DJ, et al. ; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170:1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baptista T, Uzcátegui E, Rangel N, et al. Metformin plus sibutramine for olanzapine-associated weight gain and metabolic dysfunction in schizophrenia: a 12-week double-blind, placebo-controlled pilot study. Psychiatry Res. 2008;159:250–253 [DOI] [PubMed] [Google Scholar]
- 34. Sudhakar TP, Prasada Rao G, Lakshmi Prasuna P, John Vijay Sagar K. Study of effects of modafinil add-on therapy on excessive day time drowsiness and weight gain in patients on atypical antipsychotics. Indian J Psychol Med. 2008;30:24––31 [Google Scholar]
- 35. Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Nizatidine treatment and its relationship with leptin levels in patients with olanzapine-induced weight gain. Hum Psychopharmacol. 2003;18:457–461 [DOI] [PubMed] [Google Scholar]
- 36. Cavazzoni P, Tanaka Y, Roychowdhury SM, Breier A, Allison DB. Nizatidine for prevention of weight gain with olanzapine: a double-blind placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13:81–85 [DOI] [PubMed] [Google Scholar]
- 37. Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Kilic N. Nizatidine for the treatment of patients with quetiapine-induced weight gain. Hum Psychopharmacol. 2004;19:37–40 [DOI] [PubMed] [Google Scholar]
- 38. Assunção SS, Ruschel SI, Rosa Lde C, et al. Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine. Rev Bras Psiquiatr. 2006;28:270–276 [PubMed] [Google Scholar]
- 39. Joffe G, Takala P, Tchoukhine E, et al. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:706–711 [DOI] [PubMed] [Google Scholar]
- 40. Borovicka MC, Fuller MA, Konicki PE, White JC, Steele VM, Jaskiw GE. Phenylpropanolamine appears not to promote weight loss in patients with schizophrenia who have gained weight during clozapine treatment. J Clin Psychiatry. 2002;63:345–348 [DOI] [PubMed] [Google Scholar]
- 41. Poyurovsky M, Isaacs I, Fuchs C, et al. Attenuation of olanzapine-induced weight gain with reboxetine in patients with schizophrenia: a double-blind, placebo-controlled study. Am J Psychiatry. 2003;160:297–302 [DOI] [PubMed] [Google Scholar]
- 42. Poyurovsky M, Fuchs C, Pashinian A, et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double-blind placebo-controlled study. Psychopharmacology (Berl). 2007;192:441–448 [DOI] [PubMed] [Google Scholar]
- 43. Baptista T, Rangel N, El Fakih Y, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double-blind, placebo-controlled, 12-week trial. Pharmacopsychiatry. 2009;42:14–19 [DOI] [PubMed] [Google Scholar]
- 44. Henderson DC, Fan X, Sharma B, et al. A double-blind, placebo-controlled trial of rosiglitazone for clozapine-induced glucose metabolism impairment in patients with schizophrenia. Acta Psychiatr Scand. 2009;119:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Henderson DC, Copeland PM, Daley TB, et al. A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. Am J Psychiatry. 2005;162:954–962 [DOI] [PubMed] [Google Scholar]
- 46. Henderson DC, Fan X, Copeland PM, et al. A double-blind, placebo-controlled trial of sibutramine for clozapine-associated weight gain. Acta Psychiatr Scand. 2007;115:101–105 [DOI] [PubMed] [Google Scholar]
- 47. Ko YH, Joe SH, Jung IK, Kim SH. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28:169–175 [DOI] [PubMed] [Google Scholar]
- 48. Afshar H, Roohafza H, Mousavi G, et al. Topiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. J Psychopharmacol. 2009;23:157–162 [DOI] [PubMed] [Google Scholar]
- 49. Narula PK, Rehan HS, Unni KE, Gupta N. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophr Res. 2010;118:218–223 [DOI] [PubMed] [Google Scholar]
- 50. Biedermann F, Fleischhacker WW, Kemmler G, Ebenbichler CF, Lechleitner M, Hofer A. Sibutramine in the treatment of antipsychotic-induced weight gain: a pilot study in patients with schizophrenia [published online ahead of print December 01, 2013]. Int Clin Psychopharmacol. PMID: 24300751. [DOI] [PubMed] [Google Scholar]
- 51. Coodin S. Body mass index in persons with schizophrenia. Can J Psychiatry. 2001;46:549–555 [DOI] [PubMed] [Google Scholar]
- 52. Daumit GL, Clark JM, Steinwachs DM, Graham CM, Lehman A, Ford DE. Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness. J Nerv Ment Dis. 2003;191:799–805 [DOI] [PubMed] [Google Scholar]
- 53. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113:306–313 [DOI] [PubMed] [Google Scholar]
- 54. Bresee LC, Majumdar SR, Patten SB, Johnson JA. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: a population-based study. Schizophr Res. 2010;117:75–82 [DOI] [PubMed] [Google Scholar]
- 55. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. 2006;21:1133–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19–32 [DOI] [PubMed] [Google Scholar]
- 57. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69:514–519 [DOI] [PubMed] [Google Scholar]
- 58. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596––601 [DOI] [PubMed] [Google Scholar]
- 59. Lieberman JA, Stroup TS, McEvoy JP, et al. ; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223 [DOI] [PubMed] [Google Scholar]
- 60. Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21:517–535 [DOI] [PubMed] [Google Scholar]
- 61. Stevens VJ, Obarzanek E, Cook NR, et al. ; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11 [DOI] [PubMed] [Google Scholar]
- 62. Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 63. Fernández E, Carrizo E, Fernández V, et al. Polymorphisms of the LEP- and LEPR genes, metabolic profile after prolonged clozapine administration and response to the antidiabetic metformin. Schizophr Res. 2010;121:213–217 [DOI] [PubMed] [Google Scholar]
- 64. Fernández E, Carrizo E, Connell L, Baptista T. Pro12Ala polymorphism of the PPAR-γ2 gene, metabolic syndrome and response to metformin in clozapine-treated patients. Schizophr Res. 2012;137:262–263 [DOI] [PubMed] [Google Scholar]
- 65. Fleischhacker WW, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia [published online ahead of print May 02, 2012]. Int J Neuropsychopharmacol. 2012;1––11 PMID: 22717078. [DOI] [PubMed] [Google Scholar]
- 66. Sibutramine (Meridia) withdrawn. Med Lett Drugs Ther. 2010;52:88. [PubMed] [Google Scholar]
- 67. James WP, Caterson ID, Coutinho W, et al. ; SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.