Abstract
The FOCUS smartphone intervention was developed to provide automated real-time/real-place illness management support to individuals with schizophrenia. The system was specifically designed to be usable by people with psychotic disorders who may have cognitive impairment, psychotic symptoms, negative symptoms, and/or low reading levels. FOCUS offers users both prescheduled and on-demand resources to facilitate symptom management, mood regulation, medication adherence, social functioning, and improved sleep. In this study, 33 individuals with schizophrenia or schizoaffective disorder used FOCUS over a 1-month period in their own environments. Participants were able to learn how to use the intervention independently, and all but one participant completed the trial successfully and returned the smartphones intact. Completers used the system on 86.5% of days they had the device, an average of 5.2 times a day. Approximately 62% of use of the FOCUS intervention was initiated by the participants, and 38% of use was in response to automated prompts. Baseline levels of cognitive functioning, negative symptoms, persecutory ideation, and reading level were not related to participants’ use of the intervention. Approximately 90% of participants rated the intervention as highly acceptable and usable. Paired samples t tests found significant reductions in psychotic symptoms, depression, and general psychopathology, after 1 month of FOCUS use. This study demonstrated the feasibility, acceptability, and preliminary efficacy of the FOCUS intervention for schizophrenia and introduces a new treatment model which has promise for extending the reach of evidence-based care beyond the confines of a physical clinic using widely available technologies.
Key words: Mobile Health (mHealth), mobile interventions, depression, hallucinations, medication adherence, sleep, social functioning
Introduction
Schizophrenia is associated with high costs to individuals, their families, and society.1,2 Poorly managed, the illness can cause significant personal distress and impairment and is associated with increased risk for depression, anxiety, substance use, homelessness, victimization, hospitalization, and suicide.3–8
Over the last 2 decades several evidence-based psychosocial interventions have been developed to help individuals with schizophrenia better cope with symptoms, improve social functioning, maintain a healthier lifestyle, and engage in meaningful work, even in the context of a chronic mental health condition.9,10 However, these interventions are rarely available at clinical settings for a variety of reasons, including the lack of clinicians who are trained in these approaches, limited funding for psychosocial interventions, and poor utilization and ongoing engagement in treatments even when they are available.11–13
Mobile Health (mHealth) approaches that leverage mobile devices such as cellular phones and smartphones to support healthcare are promising for deployment of interventions that are unconstrained by the limitations of existing treatment settings.14,15 Mobile phones are carried on the person, typically turned on, and have near constant connectivity and access to multimedia resources. Thus, they can serve as conduits for interventions any time, and in almost any location.16 Furthermore, mobile phones are widely available, affordable, and are continuously dropping in cost; there are now over 6 billion mobile phones subscriptions worldwide, with the majority being used in low and middle income countries.17 In the United States, underserved populations now use “smartphones” (ie, mobile phones with computational capacities) as their primary method for accessing resources on the internet,18 and there is evidence that even homeless individuals with limited resources own and use mobile phones regularly.19,20 Mobile phone use among people with severe psychiatric disabilities is not dramatically different from that observed in the general population—a recent survey conducted among 1592 adults with serious mental illnesses found that 72% of respondents had mobile phones and used them for a range of functions including calling, texting, and email. Moreover, many individuals indicated that they would be interested in engaging in mobile interventions (eg, reminders, psychoeducation, contact with clinicians) via their mobile device.21
Early efforts to incorporate mobile phones into the clinical care of people with schizophrenia have produced mixed results. In one pilot study, participants received daily text message assessments sent to basic mobile phones from a remote server.22 If participants responded, the server engaged them with follow-up suggestions to improve their symptoms and functioning. Participants responded to 86% of assessments over 3 months, but clinical outcomes did not significantly change from baseline to posttrial. Some changes in self-reported distress from auditory hallucinations, social functioning, and medication adherence were recorded, but participants with lower cognitive functioning and more severe negative symptoms had greater difficulty negotiating the requirements of the protocol. The investigative team concluded that there was a need to develop interventions that capitalize on emerging smartphone technology that could enable more user-friendly and potent interventions.
In a second study, high risk for relapse outpatients and their family members received weekly text message requests to complete assessments of early warning signs of relapse on their mobile phones as part of a randomized controlled trial of an information technology–aided relapse prevention program.23 When participants reported problems, automated alerts were sent to their treating psychiatrists with a prompt to follow-up with a medication evaluation. Participants responded to 80% of weekly requests over a year, but practitioner adherence to the protocol was low and in many cases follow-up steps were not taken. When clinicians did respond accordingly, individuals in the intervention arm did significantly better than controls in terms of hospitalization, number of inpatient days, and costs. Taken together, these studies demonstrate that people with schizophrenia can successfully engage in mobile interventions, but that these should be user-centered in terms of the technology (ie, user-friendly, intuitive, engaging) and treatment model (ie, include self-management components that are independent of clinician engagement).
FOCUS: A User-Centered Smartphone System for Schizophrenia
Working together with patients and clinicians at community settings, our research team developed FOCUS, a smartphone system designed to support self-management of illness in individuals with schizophrenia. The system is grounded in 2 theoretical models: the cognitive model of psychosis24,25 and the stress-vulnerability model of schizophrenia.26,27 FOCUS aims to identify and dismantle dysfunctional beliefs that contribute to maintenance and distress associated with symptoms, and to interrupt the cyclical relationship between stress (eg, fatigue, interpersonal conflict, social isolation, poor medication adherence) and vulnerability that may lead to illness exacerbation.28 Through several iterative cycles of development and user feedback, we constructed a smartphone system that targets symptoms of psychosis, social functioning, mood problems, medication adherence, and sleep difficulties. We drew treatment content from an array of evidence-based interventions (ie, cognitive restructuring, behavioral tailoring, social skills training, illness management and recovery, anger management, behavioral activation, sleep hygiene), and adapted it so that it was suitable for delivery via smartphones. Content was distilled into brief interactive exchanges that are accompanied by illustrative images (ie, photographs, cartoons, touchscreen reminder buttons) that are displayed on the smartphone screen. To maximize usability, the system was developed in accordance with design principles for electronic resources for people with serious mental illness and cognitive impairment.29
FOCUS was initially tested with individuals with schizophrenia in laboratory conditions, and the system was adapted based on our observations of problems and user recommendations.30 Once we were satisfied that the system functioned well in controlled environments, we conducted a field trial in which 33 participants were provided with a smartphone installed with the FOCUS system, to use over 1 month in their own environments. We hypothesized that participants would find the system acceptable, usable, engaging, and helpful. We also hypothesized that participants’ cognitive functioning, negative symptoms, and levels of persecutory ideation would not impact their use of the system. To our knowledge, this is the first deployment of a smartphone intervention for schizophrenia.
Methods
Participants
The study was approved by the Committee for Protection of Human Subjects at Dartmouth. Thirty-three individuals with schizophrenia or schizoaffective disorder were recruited from community-based treatment programs in Chicago. Participants had a mean age of 45.9 (SD = 8.78). The sample was 61% male, 76% African American, 21% white, and 3% more than one race. Six percent identified as Latino. Participants reported an average of 12.7 years of education (SD = 2.32), but their average reading level was at the eighth grade level (assessed using the reading subsection in Wide Range Achievement Test—Fourth Edition, Wilkinson and Robertson31 [WRAT-4]). Sixty-one percent were living independently, 21% resided in a supervised living facility, and 18% were living with family members. The majority were unemployed (87.9%) and owned a mobile phone of some kind (87.5%). Of mobile phone users, 32% owned a smartphone. Participants reported an average of 6.40 (SD = 4.21) lifetime psychiatric hospitalizations. At baseline, the sample experienced moderate symptoms of schizophrenia (Positive and Negative Syndrome Scale, Kay et al32 [PANSS] total, M = 77.64, SD = 4.23; PANSS-positive score, M = 19.24, SD = 4.23; PANSS-negative score, M = 17.76, SD = 3.60), mild depressive symptoms (Beck Depression Inventory–Second Edition, Beck et al33 [BDI-2], M = 19.52, SD = 8.86), and subthreshold clinical insomnia (Insomnia Severity Index, Morin et al34 [ISI], M = 12.27, SD = 6.24). Participants’ beliefs that it was necessary for them to take medications were stronger than their medication-related concerns (Brief Medication Questionnaire, Svarstad et al35 [BMQ] necessity-concern differential, M = 5.15, SD = 5.69). On average, participants had moderate cognitive impairment (Brief Assessment of Cognition in Schizophrenia36 [BACS] t-score, M = 30.09, SD = 11.46).
Procedures
Participant Screening.
Clinical staff identified 95 individuals who were viable candidates for the study (ie, chart diagnosis, willingness to being contacted for research projects). Research staff contacted this group, and 58 expressed interest in participating in the study. After providing written informed consent, all potential participants were administered a structured diagnostic interview (Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition, First et al37) to verify diagnosis of schizophrenia or schizoaffective disorders. Potential participants then completed a battery of laboratory-based self-report and interview measures that included demographic information, measures of symptoms of schizophrenia (PANSS), symptoms of depression (BDI-2), and sleep difficulties (ISI). Potential participants were enrolled if they were 18 years of age or older, prescribed psychotropic medications, and had “mild” or higher severity scores on 2 of the following: hallucinatory behavior on the PANSS (P3 ≥ 3), passive/apathetic social avoidance or active social avoidance on the PANSS (N4 or G16 ≥ 3), BDI-2 total score ≥ 10, or ISI score ≥ 15. Candidates were excluded if they had hearing, vision, or motor impairments that made it impossible for them to use a smartphone (assessed on-site by study staff), if their reading level was below fourth grade, or if they were enrolled in another intervention study. Five individuals were found to be ineligible due to diagnosis, 6 were ineligible due to reading level, 13 were ineligible due to symptom severity criteria, and 1 was excluded due to enrollment in another study.
Pretrial.
Once enrolled, participants returned within a week and were administered the BACS and BMQ. This visit also included a shared decision-making session with research staff to establish the treatment targets each participant would work on (ie, receive daily prompts and content from the FOCUS system) over the course of the field trial. Every participant was assigned medication adherence as a treatment target because we wanted to have at least one element that was consistent across all participants, and this was identified as a high priority area by clinical staff. Two additional domains were chosen from the following options: social (encompasses interventions targeting persecutory ideation, anger management, and social skills training), mood problems (ie, depression and anxiety), auditory hallucinations, or sleep difficulties. The domain selection was informed by participants’ preference and data from the screening and baseline assessments; a study interviewer would identify measure scores that were particularly high and record concrete examples participants provided when asked about areas of greatest concern (eg, avoiding locations where voices were loudest, panic attacks, staying up at night ruminating). The interviewer would then reflect back to the participant their assessment of 2 “high priority” areas, and inquire whether they agree and would like to focus on these targets or if they preferred a different combination. Once they decided on the domains, the interviewer and participant would review their “typical day” and discuss when it would make most sense to address each domain (ie, the times they would get daily FOCUS prompts to engage).
Following treatment target selection, a 30-min training session was administered focusing on use of the smartphone provided and the different elements of the FOCUS system. Training on the use of the device included how to charge the phone, turning the phone on and off as well as locking the screen, how to use a touchscreen, how to make and receive calls, and how to save contact numbers. The FOCUS system was then demonstrated by a trained research assistant. The demonstration focused on in-the-moment use and selection of resources from the different on-demand options. Participants then had the opportunity to practice using FOCUS and ask questions as needed. Individuals were given a Motorola Droid 4 smartphone running the Android 4.1 operating system and charger and commenced their participation in the field trial only after they demonstrated proficiency in using the device and the FOCUS system in the laboratory. They were instructed to charge the phone at night and carry it with them wherever they go, but otherwise to go about their daily life as usual.
Field Trial.
Over the course of 1 month, the FOCUS system prompted participants to complete an assessment 3 times daily, on one of each of the 3 treatment targets between the hours of 9 am and 1 pm, 1 and 5 pm, and 5 and 9 pm (exact times within those ranges were determined randomly daily by the system). Participants were asked to respond to each system-generated assessment whenever possible. In response to the content of participant entries, the FOCUS system deployed tailored in-the-moment interventions. Participants were also instructed to use the host of on-demand FOCUS features as often as they liked, whenever they needed support. The FOCUS system automatically uploaded participant use data (ie, response to prompt rate, content of their responses to assessment, self-initiated use of resources) to a secure study server. Thus, so long as the smartphone was within reception, the research team could view user response data continuously. Study staff called each participant once per week to check-in and assist with any technical difficulties.
Posttrial.
After 1 month of use, participants returned the charger and smartphone and completed the PANSS, BDI-2, ISI, and BMQ, for a second time. Before debriefing, participants also completed a 26-item self-report acceptability/usability measure comprised of adapted items from the System Usability Scale,38 Post Study System Usability Questionnaire,39 Technology Assessment Model Measurement Scales,40 and Usefulness, Satisfaction, and Ease questionnaire.41 Participants were asked to rate their agreement with a series of statements about the intervention (see table 1 for all items).
Table 1.
Participant Acceptability/Usability Ratings
| Statement | Number of Participants Selecting Each Response | ||
|---|---|---|---|
| Disagree | Neutral | Agree | |
| I think that I would like to use FOCUS often | 1 (3.1%) | 7 (21.9%) | 24 (75%) |
| I found FOCUS to be very complicated | 26 (81.3%) | 2 (6.3%) | 4 (12.4%) |
| I thought FOCUS was easy to use | 3 (9.4%) | 1 (3.1%) | 28 (87.5%) |
| I think that I would need the support of a technical person to be able to use FOCUS | 24 (75%) | 2 (6.3%) | 6 (18.7%) |
| I found that the different parts of FOCUS work well together | 0 | 2 (6.3%) | 30 (93.7%) |
| I thought there was too much inconsistency in FOCUS | 24 (75%) | 3 (9.4%) | 5 (15.6%) |
| I would imagine that most people would learn to use FOCUS very quickly | 0 | 1 (3.1%) | 31 (96.9%) |
| I found FOCUS very awkward to use | 24 (75%) | 2 (6.3%) | 6 (18.7%) |
| I felt very confident using FOCUS | 2 (6.5%) | 1 (3.2%) | 28 (90.3%) |
| I needed to learn a lot of things before I could get going with FOCUS | 24 (75%) | 2 (6.3%) | 6 (18.7%) |
| Overall, I am satisfied with how easy it is to use FOCUS | 0 | 2 (6.3%) | 30 (93.7%) |
| I was able to complete the “modules” quickly in FOCUS | 1 (3.1%) | 5 (15.6%) | 26 (81.3%) |
| I felt comfortable using FOCUS | 1 (3.1%) | 2 (6.3%) | 29 (90.6%) |
| It was easy to learn to use FOCUS | 2 (6.9%) | 2 (6.9%) | 25 (86.2%) |
| Whenever I made a mistake using FOCUS, I could recover easily and quickly | 3 (9.4%) | 6 (18.8%) | 23 (81.8%) |
| It was easy to find the information I needed | 1 (3.1%) | 3 (9.4%) | 28 (87.5%) |
| The information provided for FOCUS was easy to understand | 1 (3.1%) | 4 (12.5%) | 27 (84.4%) |
| How things appeared on the screen was clear | 0 | 1 (3.1%) | 31 (96.9%) |
| If I have access to FOCUS, I will use it | 2 (6.3%) | 4 (12.5%) | 26 (81.3%) |
| I am satisfied with FOCUS | 1 (3.1%) | 2 (6.3%) | 29 (90.6%) |
| I would recommend FOCUS to a friend | 0 | 4 (12.5%) | 28 (87.5%) |
| FOCUS is fun to use | 1 (3.1%) | 4 (12.5%) | 27 (84.4%) |
| FOCUS works the way I want it to work | 2 (6.3%) | 7 (21.9%) | 23 (81.8%) |
| I feel I need to have FOCUS | 8 (25%) | 4 (12.5%) | 20 (62.5%) |
| FOCUS helped me manage my symptoms | 0 | 4 (12.5%) | 28 (87.5%) |
| FOCUS was interactive enough | 3 (9.4%) | 2 (6.3%) | 27 (84.3%) |
Participants were paid for the time they devoted to pre- and posttrial assessments. They received an unlimited data plan that enabled unrestricted calling, texting, and internet use on the smartphone during the trial. Daily engagement in the FOCUS intervention was not incentivized and participants were instructed to use the system as often as they wanted.
Description of the Mobile Intervention
The FOCUS system is comprised of 3 applications (apps) that are installed onto the smartphone, and a web-based dashboard. The first app prompts users to engage daily via auditory signals and visual notifications that appear on the screen. The second is the primary FOCUS app that uses interactive algorithms to generate brief assessments and interventions that the user progresses through using touchscreen buttons on the smartphone homescreen. The third is a Quick Tips app that allows users to access illness self-management resources and suggested coping strategies from a menu of options.
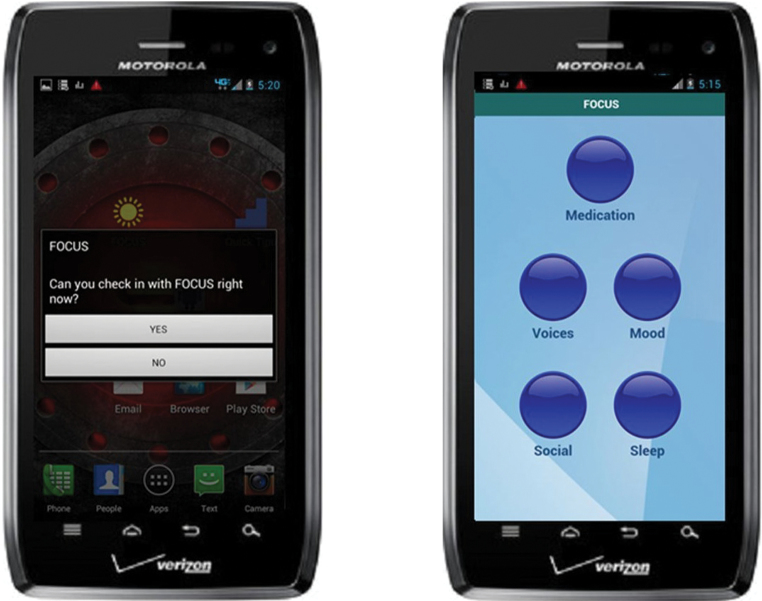
Once signaled by the prompting app (see figure 1), users can decide to engage or ignore the prompt. If they engage, the system will launch a brief assessment of their current status (eg, “How has your mood been today?”) with multiple choice touchscreen response options that appear below the question on the same screen. If the user endorses difficulties (eg, “Very bad. I’m very upset”) the system provides feedback (eg, “Looks like you could use some support. FOCUS is happy to help.”), followed by a more in-depth assessment (“Have you had any of these thoughts lately?”). Users’ responses determine the nature of the subsequent interventions they will receive (eg, see figure 2). Once they complete a sequence of screens, making selections as they progress and receiving interventions, the system signs off (“Thank you for using FOCUS. Have a nice day.”) until the next scheduled prompt.
Fig. 1.
FOCUS prompt and FOCUS home screen.
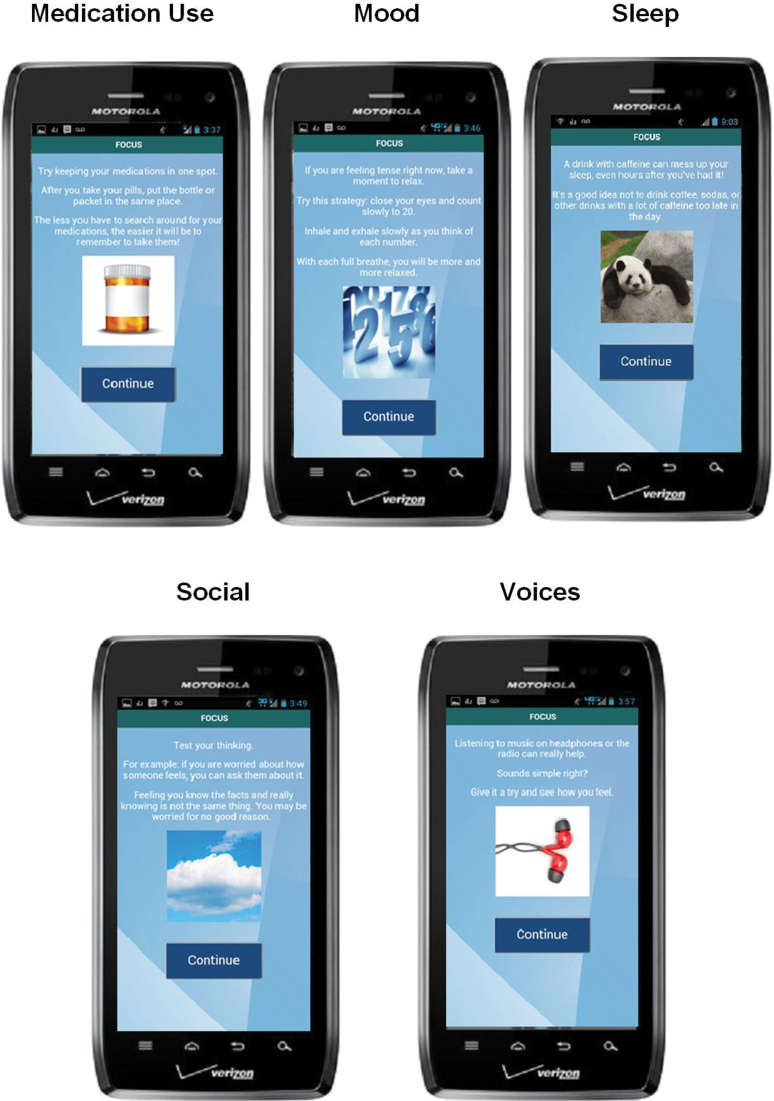
Fig. 2.
FOCUS intervention screen examples.
Users can access all intervention content “on-demand” whenever and wherever they choose, by going to the FOCUS homescreen and selecting any of the 5 treatment target icons (see figure 1) or by accessing Quick Tips for briefer noninteractive content. Each intervention sequence has multiple wording and image variations so that users do not encounter the exact same intervention every time, even if they make similar selections (see examples for FOCUS intervention screens for each target area in figure 2). When the smartphone has wireless connectivity, the FOCUS application transmits the use data to a secure study server. The data is then displayed as a continuously updated report on a secure web-based dashboard that the research team or authorized clinicians can access at any time.
Overview of Analyses
Descriptive statistics were derived from participants’ smartphone use data to characterize feasibility and acceptability. Pearson product-moment correlation coefficients were used to examine the association between baseline cognitive functioning (BACS), negative symptoms (PANSS-negative scale), persecutory ideation (suspiciousness item from the PANSS), and FOCUS system use (ie, days used, number of times used per day). Spearman’s rank correlation coefficients were used to examine the association between reading level (WRAT-4 grade estimate) and FOCUS system use. Paired samples t tests were used to test for differences between pretrial and posttrial clinical outcomes (PANSS, BDI-2, BMQ, ISI).
Results
Feasibility
One participant dropped out of the study after losing 2 study smartphones in the first week. The remaining 32 participants used the system successfully and returned the smartphone intact at the end of the trial. One participant did not use the smartphone for anything other than the FOCUS intervention. All other participants used a range of smartphone functions during the month, including calling (96.9%), texting (34.4%), email (15.2%), and accessing the internet (62.5%). System use data for 2 participants were lost due to technical problems during the automated data transfer to the study server. Therefore, we report on FOCUS system use data for 30 individuals.
On average, these participants used the FOCUS system on 86.5% of the days they had the smartphone (week 1 average: 6.7 days, week 4 average: 5.9 days). On days FOCUS was used, participants interacted with the system an average of 5.19 times (week 1 average: 6.4 times daily, week 4 average: 4.9 times daily). Participants initiated their interactions with FOCUS on 62.5% of the times it was used (ie, using on-demand interventions) and 37.5% of use was in response to prescheduled system prompts.
Baseline cognitive functioning (ie, BACS score), negative symptoms (PANSS-negative scale score), persecutory ideation (suspiciousness item from the PANSS), and reading level (WRAT-4 grade estimate) were not significantly associated with the percentage of days participants used FOCUS, or the number of times they used the system on those days (all Ps > .05). Overall, these results suggest the FOCUS smartphone intervention is feasible among people with schizophrenia.
Acceptability and Usability
Participant responses to the acceptability/usability measure are reported in table 1. Over 90% of participants thought the different components of the intervention worked well together, that content appeared on the screen clearly, and that people could learn to use FOCUS very quickly. They reported feeling very confident, comfortable, and satisfied using the intervention. Over 87% reported that it was easy to find the information they needed, that the intervention helped them manage their symptoms, and that they would recommend the system to a friend. A minority of participants reported some difficulties: approximately 12% found the intervention to be complicated, 18% thought they needed to learn more things before they could get started and found it awkward to use, and 6% thought they needed more technical support. Overall, these results suggest that the majority of participants found the FOCUS intervention acceptable and usable.
Preliminary Efficacy
Paired samples t tests indicated significant reductions in symptoms from pretrial to posttrial on the PANSS total (P = .002), PANSS positive (P < .001), PANSS general psychopathology (P < .001), and in depression on the BDI-2 (P = .003). Scores on the PANSS-negative subscale did not significantly change. There were also no significant changes in beliefs about medications (BMQ general and BMQ necessity-concern differential scores) or in sleep difficulties (ISI) (see table 2).
Table 2.
Pre- and Posttrial Clinical Measures (N = 32)
| Measure | Pretrial, mean (SD) | Posttrial, mean (SD) |
|---|---|---|
| Positive and Negative Syndrome Scale | ||
| Positive scale | 19.34 (4.26)** | 16.41 (4.06)** |
| Negative scale | 17.69 (3.64) | 18.22 (3.29) |
| General psychopathology scale | 40.88 (5.89)* | 36.75 (5.38)* |
| Total score | 77.59 (10.44)* | 71.47 (9.89)* |
| Beliefs About Medicines Questionnaire | ||
| Necessity-concern differential | 5.16 (5.78) | 3.53 (5.75) |
| General total | 21.28 (4.92) | 21.44 (4.58) |
| Insomnia Severity Index | 12.25 (6.34) | 11.56 (8.13) |
| Beck Depression Inventory-2 | 19.69 (8.94)* | 14.78 (10.28)* |
*P < .01, **P < .001 (paired samples t tests).
To examine whether there was an association between symptom change and the frequency with which participants used the intervention, we conducted Pearson correlations between changes in BDI-2 and PANSS scores, and the percentage of days participants used the system. We found a significant association between the change in participants’ BDI-2 scores and the percentage of days participants used FOCUS over the 1-month period (r = −.36, P < .05); the greater the reduction in depressive symptoms, the less often participants used the system. There was no significant association between PANSS scores and the percentage of days participants used the system.
Discussion
This study demonstrated that a smartphone intervention for illness management in individuals with schizophrenia is feasible and acceptable, and suggests the system may be clinically helpful. Participants reported high levels of satisfaction and an interest in continuing to use the smartphone system in the future. Acceptability of the smartphone intervention was high; participants used the system 86% of days they had the device. Additionally, individuals elected to use on-demand resources above and beyond the preprogrammed daily prompts; participant-initiated engagement accounted for over 60% of all intervention use. This finding is particularly important, given that participants were not given incentives to use the intervention during the trial and their access to other smartphone resources (ie, calling, texting, internet) was not dependent on their use of the FOCUS system. Participants’ use of the system was not hampered by their level of cognitive functioning, negative symptoms, persecutory ideation, or reading level.
Preliminary assessment of clinical efficacy suggested that the FOCUS intervention was helpful in reduction of positive symptoms of schizophrenia (PANSS-positive scale), general symptoms of psychopathology (PANSS general psychopathology scale), and depression (BDI-2) over the 1-month trial. There were no significant changes in sleep or beliefs about medications. Unlike the symptom-focused content, the smartphone system’s sleep interventions were designed to promote long-term lifestyle changes (ie, sleep hygiene) rather than in-the-moment coping strategies and might not provide immediate relief when deployed. For example, suggesting that an individual avoid naps during the day in order to facilitate a regular sleep/wake cycle may be less helpful in the moment when the person is struggling to fall asleep. It is also possible that positive sleep outcomes take longer than a month to emerge, and a longer trial would have produced stronger effects. At the beginning of the study most participants had stronger beliefs that medications were necessary for them to stay healthy than they had concerns about their use (BMQ necessity-concern differential), making it difficult to detect further increases in the importance of medication.
Overall, the clinical outcomes reported in this study are comparable or better than those produced by other psychosocial interventions9,10 and required a fraction of the cost. Even after accounting for the price of smartphone devices (and their possible replacement), data plans, and technical support staff needed to deploy FOCUS, it is much less resource intensive than services provided by a mental health professional at a clinic setting. Moreover, unlike scheduled face-to-face services, a mobile intervention is transportable and can be used in any location.
While the FOCUS system was used in the study as an adjunct to in-person services, one could also envision a future where evidence-based mHealth apps such as FOCUS are downloaded directly onto smartphones and used by individuals with little or no access to any mental health care. As familiarity with mobile technology increases, so may the range of potentially therapeutic options for using them to promote coping with psychiatric illnesses. For people who are now growing up with mobile technologies in hand (ie, “digital natives”) using the full range of smartphone capabilities will be intuitive (eg, uploading photos, using tools to adapt or generate their own content, connecting to social media). As a result, the level of sophistication and potential impact of mHealth interventions (ie, tailored, personalized, adaptive) will increase.
This study has several limitations. First, there was no control group, so it is not possible to determine whether the clinical improvements were related to use of the FOCUS system. Future research will need to evaluate FOCUS in more rigorously controlled studies and to examine whether symptom improvements persist over time. Second, the system was deployed for a relatively short amount of time. While it is encouraging to see some rapid therapeutic gains, future research will need to examine whether people find smartphone mHealth systems engaging and helpful over extended periods, or whether these are most suitable for time-limited care (eg, upon discharge from inpatient care, during symptom exacerbations). Third, the sample size was relatively small, impacting generalizability. It is also possible that a larger sample would be sufficiently powered to detect small changes in clinical outcomes that were not significant in the current study (eg, sleep ratings). However, whether these small improvements are clinically meaningful, is questionable. Finally, the clinical rater was not blinded to the study objectives and the nature of the intervention. This might have impacted their ratings on posttrial measures that require some clinical interpretation (ie, PANSS).
Clinical researchers have begun developing a number of other novel technologies to improve the accessibility and quality of care available to people with schizophrenia.42,43 Several of these approaches include web-based self-paced cognitive behavioral interventions for auditory hallucinations,44 online peer support and social therapy for first-episode psychosis,45 internet-based family intervention programs,46,47 computerized “relational agents” designed to enhance medication adherence and physical activity,48 and virtual reality paradigms for vocational rehabilitation49,50 and treatment of persecutory ideation.51 Many of these approaches can be adapted to smartphone and other mobile platforms that would allow patients to use them wherever and whenever they need them the most.
Conclusion
To our knowledge, this is the first study to demonstrate the feasibility, acceptability, and efficacy of a smartphone intervention for schizophrenia. Incorporating a user-centered approach in the intervention development process was essential to generating a system that can address many of the unique obstacles that people with schizophrenia face when attempting to engage in mHealth treatment. The integration of several adapted evidence-based psychosocial intervention strategies into a single mobile platform appeared promising. But the FOCUS system does not merely serve as a delivery system for existing interventions. Rather, it introduces a novel approach to clinical care for schizophrenia (ie, real-time, real-place, on-demand, self-navigated), ie, only made possible through recent advancements in mobile hardware, software, and telecommunication infrastructure. As smartphone and other mobile technologies continue to develop, they will create new and exciting opportunities for innovative mHealth systems that will enable continuous assessment and treatment. Leveraging both active (ie, self-report of symptoms and functioning) and passive (ie, sensors that capture behavior and physiology) patient data will undoubtedly increase the potency of treatments for people with schizophrenia in the years ahead.
Funding
National Institute of Mental Health (R34MH100195, Principal Investigator: D.B.-Z.); Dartmouth SYNERGY Center for Clinical and Translational Science.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Murray CJ, Lopez AD. The Global Burden of Disease. Geneva: World Health Organization; 1996 [Google Scholar]
- 2. Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull. 2004;30:279–293 [DOI] [PubMed] [Google Scholar]
- 3. Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162:370–376 [DOI] [PubMed] [Google Scholar]
- 5. Brekke JS, Prindle C, Bae SW, Long JD. Risks for individuals with schizophrenia who are living in the community. Psychiatr Serv. 2001;52:1358–1366 [DOI] [PubMed] [Google Scholar]
- 6. Sun SX, Liu GG, Christensen DB, Fu AZ. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23:2305–2312 [DOI] [PubMed] [Google Scholar]
- 7. Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dixon LB, Dickerson F, Bellack AS, et al. ; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36:48–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mueser KT, Deavers F, Penn DL, Cassisi JE. Psychosocial treatments for schizophrenia. Annu Rev Clin Psychol. 2013;9:465–497 [DOI] [PubMed] [Google Scholar]
- 11. Drake RE, Bond GR, Essock SM. Implementing evidence-based practices for people with schizophrenia. Schizophr Bull. 2009;35:704–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry K, Haddock G. The implementation of the NICE guidelines for schizophrenia: barriers to the implementation of psychological interventions and recommendations for the future. Psychol Psychother. 2008;81:419–436 [DOI] [PubMed] [Google Scholar]
- 13. Mojtabai R, Fochtmann L, Chang SW, Kotov R, Craig TJ, Bromet E. Unmet need for mental health care in schizophrenia: an overview of literature and new data from a first-admission study. Schizophr Bull. 2009;35:679–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ben-Zeev D. Mobile technologies in the study, assessment, and treatment of schizophrenia. Schizophr Bull. 2012;38:384–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luxton D, McCann R, Bush N, Mishkind M, Reger G. mHealth for mental health: integrating smartphone technology in behavioral healthcare. Prof Psychol Res Pr. 2011;42:505–512 [Google Scholar]
- 16. Proudfoot J. The future is in our hands: the role of mobile phones in the prevention and management of mental disorders. Aust N Z J Psychiatry. 2013;47:111–113 [DOI] [PubMed] [Google Scholar]
- 17. International Telecommunication Union. The World in 2011: ICT Facts and Figures. 2011. Retrieved from http://www.itu.int/ITU-D/ict/facts/2011/material/ICTFacts Figures2011.pdf [Google Scholar]
- 18. Smith A. Pew Internet and American Life Project: Americans and Their Cell Phones. Published 2011. Retrieved April 25, 2013 from http://pewinternet.org/Reports/2011/Cell-Phones.aspx [Google Scholar]
- 19. Eyrich-Garg KM. Mobile phone technology: a new paradigm for the prevention, treatment, and research of the non-sheltered “street” homeless? J Urban Health. 2010;87:365–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Post LA, Vaca FE, Doran KM, et al. New media use by patients who are homeless: the potential of mHealth to build connectivity. J Med Internet Res. 2013;15:e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ben-Zeev D, Davis KE, Kaiser S, Krzsos I, Drake RE. Mobile technologies among people with serious mental illness: opportunities for future services. Adm Policy Ment Health. 2013;40:340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Granholm E, Ben-Zeev D, Link PC, Bradshaw KR, Holden JL. Mobile Assessment and Treatment for Schizophrenia (MATS): a pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophr Bull. 2012;38:414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Španiel F, Hrdlička J, Novák T, et al. Effectiveness of the information technology-aided program of relapse prevention in schizophrenia (ITAREPS): a randomized, controlled, double-blind study. J Psychiatr Pract. 2012;18:269–280 [DOI] [PubMed] [Google Scholar]
- 24. Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195 [DOI] [PubMed] [Google Scholar]
- 25. Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. Br J Clin Psychol. 2002;41:331–347 [DOI] [PubMed] [Google Scholar]
- 26. Liberman RP, Mueser KT, Wallace CJ, Jacobs HE, Eckman T, Massel HK. Training skills in the psychiatrically disabled: learning coping and competence. Schizophr Bull. 1986;12:631–647 [DOI] [PubMed] [Google Scholar]
- 27. Zubin J, Spring B. Vulnerability–a new view of schizophrenia. J Abnorm Psychol. 1977;86:103–126 [DOI] [PubMed] [Google Scholar]
- 28. Mueser KT, Meyer PS, Penn DL, Clancy R, Clancy DM, Salyers MP. The Illness Management and Recovery program: rationale, development, and preliminary findings. Schizophr Bull. 2006;32(suppl 1):S32–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rotondi AJ, Sinkule J, Haas GL, et al. Designing websites for persons with cognitive deficits: design and usability of a psychoeducational intervention for persons with severe mental illness. Psychol Serv. 2007;4:202–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ben-Zeev D, Kaiser SM, Brenner CJ, Begale M, Duffecy J, Mohr DC. Development and usability testing of FOCUS: a smartphone system for self-management of schizophrenia. Psychiatr Rehabil J. 2013;36:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilkinson GS, Robertson GJ. Wide Range Achievement Test—Fourth Edition. Lutz, FL: Psychological Assessment Resources; 2006 [Google Scholar]
- 32. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 33. Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX: Psychology Corporation; 1996 [Google Scholar]
- 34. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37:113–124 [DOI] [PubMed] [Google Scholar]
- 36. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297 [DOI] [PubMed] [Google Scholar]
- 37. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSMI-IV Axis I Disorders-Patient Edition (SCID-I/P), Version 2.0. New York, NY: Biometrics Research, New York State Psychiatric Institute; 1995 [Google Scholar]
- 38. Brooke J. SUS-A quick and dirty usability scale. Usability Evaluation in Industry. 1996:189–194 [Google Scholar]
- 39. Lewis JR. Psychometric evaluation of the poststudy system usability questionnaire: the PSSUQ. In: Proceedings of the Human Factor Society Annual Meeting. 1992;36:1259 [Google Scholar]
- 40. Venkatech V, Davis FD. A theoretical extension of the technology acceptance model: four longitudinal field studies. Manage Sci. 2000;46:186–204 [Google Scholar]
- 41. Lund AM. Measuring usability with the USE questionnaire. Usability and User Experience. 2001;8:8 [Google Scholar]
- 42. Álvarez-Jiménez M, Gleeson JF, Bendall S, et al. Internet-based interventions for psychosis: a sneak-peek into the future. Psychiatr Clin North Am. 2012;35:735–747 [DOI] [PubMed] [Google Scholar]
- 43. Ben-Zeev D, Drake RE, Corrigan PW, Rotondi AJ, Nilsen W, Depp C. Using contemporary technologies in the assessment and treatment of serious mental illness. Am J Psychiatr Rehabil. 2012;15:357–376 [Google Scholar]
- 44. Gottlieb JD, Romeo KH, Penn DL, Mueser KT, Chiko BP. Web-based cognitive-behavioral therapy for auditory hallucinations in persons with psychosis: a pilot study. Schizophr Res. 2013;145:82–87 [DOI] [PubMed] [Google Scholar]
- 45. Alvarez-Jimenez M, Bendall S, Lederman R, et al. On the HORYZON: moderated online social therapy for long-term recovery in first episode psychosis. Schizophr Res. 2013;143:143–149 [DOI] [PubMed] [Google Scholar]
- 46. Glynn SM, Randolph ET, Garrick T, Lui A. A proof of concept trial of an online psychoeducational program for relatives of both veterans and civilians living with schizophrenia. Psychiatr Rehabil J. 2010;33:278–287 [DOI] [PubMed] [Google Scholar]
- 47. Rotondi AJ, Anderson CM, Haas GL, et al. Web-based psychoeducational intervention for persons with schizophrenia and their supporters: one-year outcomes. Psychiatr Serv. 2010;61:1099–1105 [DOI] [PubMed] [Google Scholar]
- 48. Bickmore TW, Puskar K, Schlenk EA, Pfeifer LM, Sereika SM. Maintaining reality: relational agents for antipsychotic medication adherence. Interact Comput. 2010;22:276–288 [Google Scholar]
- 49. Bell MD, Weinstein A. Simulated job interview skill training for people with psychiatric disability: feasibility and tolerability of virtual reality training. Schizophr Bull. 2011;37(suppl 2):S91–S97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsang MMY, Man DWK. A virtual reality-based vocational training system (VRVTS) for people with schizophrenia in vocational rehabilitation. Schizophr Res. 2013;144:51–62 [DOI] [PubMed] [Google Scholar]
- 51. Freeman D. Studying and treating schizophrenia using virtual reality: a new paradigm. Schizophr Bull. 2008;34:605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]