Abstract
Background and Aims: Schizophrenia leads to significant personal costs matched by high economic costs. Cognitive function is a strong predictor of disabilities in schizophrenia, which underpin these costs. This study of cognitive remediation therapy (CRT), which has been shown to improve cognition and reduce disability in schizophrenia, aims to investigate associations between improvements in cognition and cost changes. Methods: Eighty-five participants with schizophrenia were randomized to receive CRT or treatment as usual and were assessed at baseline, posttherapy, and 6 month follow-up. Four structural equation models investigated associations between changes in cognitive function and costs of care. Results: All 4 models provided a good fit. Improvement in 3 individual cognitive variables did not predict total cost changes (model 1). But improvement in a single latent cognition factor was associated with a reduction in depression, which in turn was associated with reduced subsequent total costs (model 2). No significant associations with constituent daycare and special accommodation cost changes were apparent with 3 individual cognitive change variables (model 3). But improvement in a single latent cognitive change variable was associated with subsequent reductions in both daycare and special accommodation costs (model 4). Conclusion: This study exemplifies a method of using cost changes to investigate the effects and mechanisms of CRT and suggests that executive function change may be an important target if we are to reduce disability and resultant health and social care costs.
Key words: cognition, functioning, executive function, working memory, cognitive predictors
Introduction
A diagnosis of schizophrenia is frequently characterized by recurrent episodes of psychosis and chronic and pervasive disability, affecting employment, social relationships, and independent living.1 These problems not only detrimentally impact recovery and quality of life but also are associated with high economic costs, resulting directly from medication, psychiatric admissions, and outpatient mental healthcare and indirectly from the high levels of support for the concomitant disability.2,3 An economic costs report by the Schizophrenia Commission estimated the total societal cost in England to be £11.8 billion per year.4
One of the best predictors of the disabilities that drive high costs in schizophrenia is cognitive function, which is pervasively and chronically impaired.5 Overall neuropsychological performance, as well as specific executive function, working and long-term memory and processing speed scores, are consistently strongly associated with employment,6–8 functional outcome9; social functioning,10 dependence on psychiatric care,11 and quality of life.12 Cognitive impairment is associated with medical treatment nonadherence13 and poor medical decision-making capacity,14 which may also negatively influence costs.15 Although the relationship between cognition and functioning is not necessarily direct,16,17 we might extrapolate that cognitive function will be related to costs.
This has been investigated in only 2 studies. In a study of older psychiatric patients, overall cognitive impairment was longitudinally associated with higher mental healthcare costs.18 Using an adult schizophrenia sample, our own group investigated associations between health and social care costs and type and severity of cognitive impairment using structural equation models (SEMs).19 Although no significant relationships were found between 3 independent cognitive components and costs, models with covarying cognitive components and with a single global cognitive construct both fit the data and showed a significant relationship between poor cognition and high costs.
If this association between costs and cognitive function is causal, improvements in cognitive function may lead to reduced costs. Cognitive remediation therapy (CRT) is a psychological therapy that aims to improve cognitive functioning in people with schizophrenia. There is good evidence of moderate effect sizes for durable cognitive benefits and functional improvements following CRT.20 In a randomized controlled trial (RCT) of CRT compared with treatment as usual (TAU), for which the baseline data were investigated for the cost analyses by Patel et al,19 there was a nonsignificant cost advantage for the therapy group posttreatment.21 On the other hand, in a smaller RCT, there were increased costs in day care only for the CRT group, suggesting greater uptake of day care services.22
CRT studies investigating mechanisms of change have shown that improvements in functional outcome are predicted by improvements in cognition, particularly in executive function and memory.16,17,23–25 No study has investigated cognitive change predictors of cost change.
In this study, we extended the model building in Patel et al19 to investigate associations between changes in cognitive function and costs over time following CRT. Data were from the same RCT reported in Patel et al19 and Wykes et al.21 Previously, structural equation modelling investigated 2 models that showed a significant cross-sectional relationship between cognition and cost at baseline, using (a) 2 covarying cognitive components and (b) a single latent cognitive construct.19 However, here we use change in these variables over time rather than the baseline values. We include change in positive symptoms, depression, social withdrawal, and antisocial behavior scores as possible predictors of costs. We hypothesized that improvements in cognition at posttreatment would be associated with reduced subsequent total costs and that, as with the previous study, a model using a single latent cognitive component may provide the best fit. To elucidate the relationships further, we also looked at constituent costs. We hypothesized that improved cognition would be associated with increased community day-based service costs, as people engage more frequently with services, but that special accommodation costs (ie, staffed residential care, including long-stay psychiatric rehabilitation wards) be reduced because this is likely to be underpinned by the social functioning disability that may be reduced following an improvement in cognitive function.
Method
Design
This was a single blind RCT comparing CRT with TAU. CRT was associated with significant durable improvements in memory and a significant improvement in cognitive flexibility posttreatment but not at follow-up.21 The trial registration number is ISRCTN44277627.
Participants
Participants were 85 people (1) with a DSM-IV diagnosis of schizophrenia, (2) aged at least 17 years, (3) at least 1 problem behavior on the social behavior schedule (SBS),26 and (4) cognitive inefficiency: (a) “poor” score on the Rivermead Behavioural Memory test,27 (b) <16th percentile on the Wisconsin Card Sorting test (WCST),28 and/or (c) “poor” score on the Hayling Sentence Completion test.29 They were recruited from local community mental health teams in the South London and Maudsley NHS Trust in a structured geographical rotation.
Procedure
All participants gave informed, written consent and were assessed at baseline (“time 1”), posttherapy (“time 2”) and 6 months posttherapy (“time 3”). Assessments were carried out by a graduate psychologist who was blind to group allocation although there were occasional accidental disclosures. Symptoms were rated by a blind independent psychiatrist. Social behavior data were collected from clinician or relative informants who were independent but not blind.
Measures
The following subset of measures was used. Demographic and clinical history information was collected from participants and clinical records. Premorbid intelligence quotient (IQ) was assessed using the National Adult Reading test.30
Cognition.
These are categorized using the 3 cognitive domains represented in the SEMs that were initially based on the component structure suggested by a previous exploratory principal components study.16 An additional variable was included in the “response inhibition” factor to increase its robustness.
Cognitive Shifting
Trail-making test31—time taken for part B.
Behavioural Assessment for the Dysexecutive Syndrome (BADS)32: Zoo map test—total raw score.
BADS: Key Search—total raw score.
Spatial Response Inhibition (SRI)33—median time taken for incompatible response condition minus simple reaction time.
Stroop test34—Color-word task, number correct in 120 seconds.
Verbal Working Memory
Wechsler Adult Intelligence scale, Third Edition (WAIS-III-UK)35: Letter-Number Sequencing—total raw score.
Controlled Oral Word Association test36—total correct.
BADS: Six Elements—total raw score.
WAIS-III-UK: Digit Span—total raw score.
Response Inhibition
WCST—perseverative errors.
WCST—categories completed.
Hayling Sentence Completion test—converted error score.
Symptoms.
Positive and Negative Syndrome Scale (PANSS)37—total positive symptoms and depression items.
Social Functioning.
SBS—antisocial behavior and social withdrawal subscores.
Costs. Health, social care, and criminal justice system resource use was assessed using the Client Service Receipt Inventory,38 completed retrospectively at the 3 time points, based on participant self-report and/or information from healthcare staff and case records. Costs at time 1 and time 3 were based on the previous 6-month period, and at time 2 on the previous 3-month period, so were multiplied by 2 for consistency. Unit costs were combined with individual-level resource volumes to obtain a cost-per-participant. Unit costs for health and social care services were based on UK national estimates, adjusted to reflect higher London costs.39 Medication costs were based on prices reported by the Joint Formulary Committee.40 Specialist education services costs were estimated from Chartered Institute of Public Finance and Accountancy statistics.41 Social security benefit rates were obtained from the UK Department for Work and Pensions.42 Estimates for police contact costs were made using Finn et al.43 Unit costs were standardized to 2000/2001 prices. All costs were reported as mean values in pounds sterling (£). Three cost variables were used: (1) total costs, (2) special accommodation (ie, staffed residential care, including long-stay psychiatric rehabilitation wards) costs, and (c) community day-based service costs.
Therapy
An individual pencil-and-paper CRT program targeting attention, working and long-term memory, and executive functioning used a series of simple repetitive tasks that gradually increase in difficulty and that can be individually tailored.44 The tasks are taught using scaffolding and errorless learning to help people develop the use of strategies to facilitate a more organized and systematic approach. Explicit attempts are made to transfer these strategies to everyday living skills, which are identified through personal therapy goals. The aim was to deliver therapy on at least 3 days a week until participants had received 40 sessions. Therapists were graduate psychologists supervised by a clinical psychologist, and therapist fidelity was checked using the therapists’ session records and direct observation.
Statistical Analysis
Thirteen out of eighty-five cases had some missing data. Missing values for all but the cost variables were imputed for 11 cases (final n = 85). Imputation was carried out using the regression method imputation function in SPSS, based on all cognition, social behavior, and symptomatology variables included in the models. Seven cases had missing cost data. This was not imputed because there was no reasonable basis on which to do so. All analyses were carried out in Mplus that uses a maximum likelihood method, so that the models could be fitted even when there are some missing values on the outcomes. The 7 cases with missing cost data were similar to those included in terms of age, gender, time since first contact with psychiatric services, predicted premorbid IQ, PANSS total score, and present accommodation. The missing participants, however, had significantly more years in education (mean years in education 13 vs 11) and lower SBS scores (mean of 6 vs 13). Such a profile would suggest that the excluded cases may have had lower costs than those included in the SEMs although their accommodation use was similar.
Where necessary, cognitive variables were multiplied by −1 to ensure that a high score indicated good performance.
For the cognition variables, symptom, and functioning variables, change scores were calculated from time 1 to time 2. For costs, 2 change scores were calculated: from time 1 to time 2 and from time 1 to time 3.
Using change scores for (1) cognition, (2) social function, (3) symptoms, and (4) costs, a series of SEMs were fitted. Initially, we fitted 2 models based on the models identified in Patel et al.19 A second pair of models was subsequently fitted to investigate the associations between cognitive change and constituent costs. In each case, if the initial full model was not a good fit, the symptom and social functioning change variables were subsequently excluded. In each model, all pathways were estimated.
Model 1—Three Cognitive Variables, Total Costs.
Initially, 3 cognition factors, representing change in verbal working memory, change in cognitive shifting, and change in response inhibition, were intended for inclusion in the first SEM. However, change in the individual variables was not in a uniform direction within factors, and an exploratory factor analysis of the cognitive change scores suggested a 5-factor model and therefore the 3-factor model was excluded. The alternative model fitted had 3 individual cognitive variables in place of each factor: change in Digit Span (verbal working memory), change in the Trail-Making test (cognitive shifting), and change in Hayling Task (response inhibition), selected because they each had the highest loadings on the relevant cognitive change factor in the initial model. In addition, change in the functioning and symptom variables were included. Finally, changes in total costs (time 1 to time 2 or time 3) were included and allowed to be dependent on both the cognition factors and treatment group.
Model 2—Single Latent Cognitive Factor, Total Costs.
A single cognition factor directly based on change scores was estimated. This factor was allowed to be dependent on treatment group. Change in functioning and symptoms were also included. The same 2 measures of change in health care costs were included as in model 1.
Model 3—Three Cognitive Variables, Special Accommodation, and Day Care Costs.
This model was the same as model 1 but included change in special accommodation costs and change in daycare costs (time 1 to time 2 and time 1 to time 3) instead of total costs.
Model 4—Single Latent Cognitive Factor, Special Accommodation and Day Care Costs.
This model was the same as model 2 but included change in special accommodation costs and change in day care costs (time 1 to time 2 and time 1 to time 3) instead of total costs.
Results
Participant Characteristics (n = 85)
Seventy-three percent were men, and there was a mean age of 36 years. Eighty-four percent were married. Forty-five percent were white, 16% black Caribbean, 5% black African, 8% black other, 11% Indian, and 16% described as “other” (n = 84). This ethnic diversity is characteristic of the population of people with a schizophrenia diagnosis in inner London.45 The mean number of years in education was 12 (range 2–17 years) and mean estimated full-scale IQ was 93 (69–127). Fifty-two percent had been in contact with psychiatric services for >10 years. Twenty-seven percent participants were living alone or with a spouse, 22% with a parent or other relative, 20% in supervised accommodation, and 31% were hospital inpatients. Ten percent people were currently in paid, voluntary, or sheltered employment and 12% had never been employed (n = 84). Ninety-six percent participants were receiving state social security benefits (n = 84).
Supplementary table 1 shows baseline scores for the cognition, social function, and symptom variables. Use of health and social care services and societal costs in the previous 6 months from baseline are shown in supplementary table 2. Fifty-four percent participants were currently living in a hospital or other specialized accommodation, such as a 24 hour–staffed residential setting. The use of hospital outpatient services was dominated by 3 participants who each had a mean of 55 day hospital attendances over the 6-month period. Only 1 person had any criminal justice contacts (1 night in a police cell). Seventy-five percent used atypical antipsychotic medication and only person reported no prescribed medications. Overall, the mean total cost was £15 113.91 (SD £10 929.23) for the 6-month period prior to the baseline assessment. There were no participants with zero costs.
Supplementary table 3 shows summaries of each of the variables used in the SEMs. Some of these variables have been rescaled by dividing by a constant, because the latent factors can be most easily estimated when the variance of the individual indicators is on a similar scale.
Model 1—Three Cognitive Variables, Total Costs.
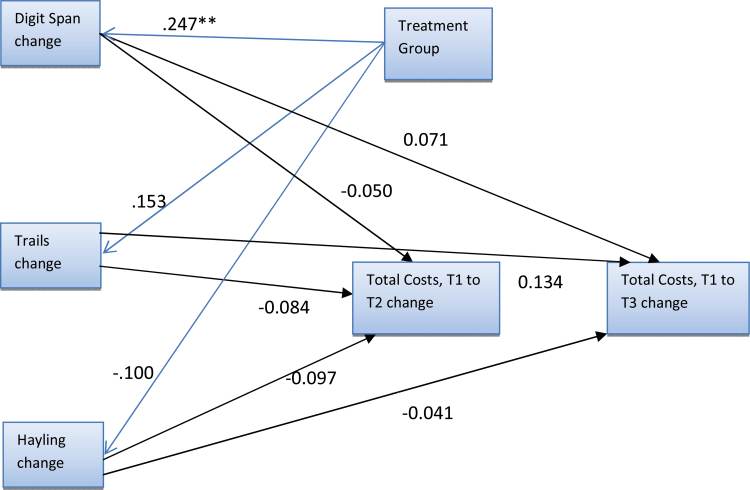
The goodness-of-fit statistics of the initial full model indicated poor fit and so symptom and social functioning change variables were excluded. This subsequent model was a good fit (table 1). There were no significant associations between cognitive change and total cost change. Treatment group significantly predicted Digit Span change (figure 1).
Table 1.
Goodness-of-Fit Statistics for Structural Equation Models
| χ2 | Df | P | |
|---|---|---|---|
| Model 1 | 2.697 | 3 | .4407 |
| Model 2 | 159.968 | 136 | .0784 |
| Model 3 | 2.697 | 3 | .4407 |
| Model 4 | 122.749 | 108 | .01572 |
Fig. 1.
Model with 3 cognitive variables with standardized parameter estimates. All pathways were estimated but small parameter estimates are not shown. **P < .05.
Model 2—Single Latent Cognitive Factor, Total Costs Variable.
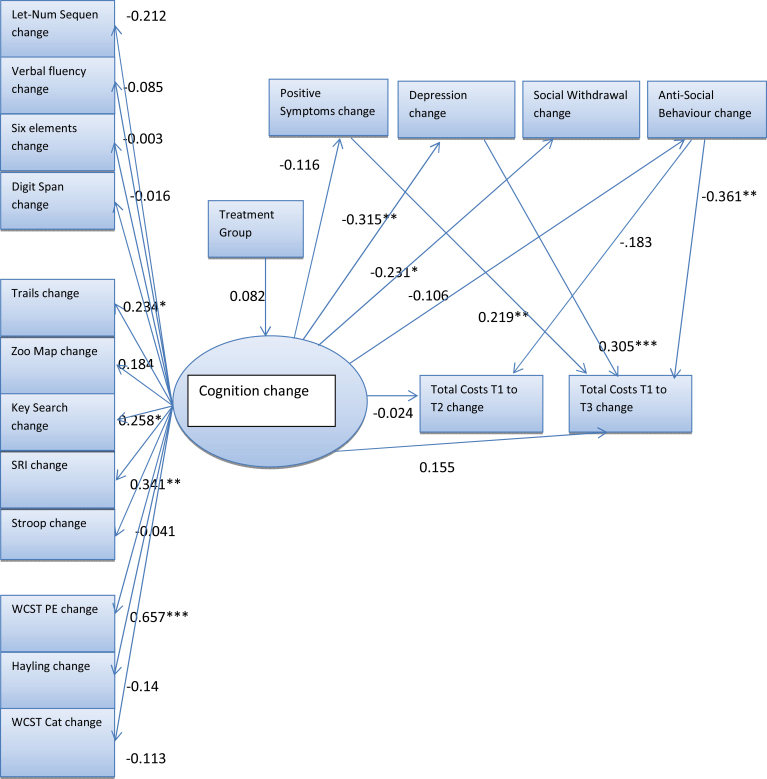
There was reasonable model fit. Some cognitive change scores had negative loadings on the latent cognition factor, so increased cognitive factor change scores cannot strictly be interpreted as an improvement in cognition. However, all significant loadings on the latent cognitive factor were in a positive direction. Cognitive improvement was significantly associated with a reduction in depression, which in turn led to significantly decreased costs at time 3. Reduced antisocial behavior and increased positive symptoms were also significantly associated with increased costs at time 3. Neither of the functioning nor symptom changes was significantly associated with cognitive changes (figure 2).
Fig. 2.
Single latent cognition factor model with standardized parameter estimates. Although all pathways were estimated in the model, some small parameter estimates are not shown. *P < .1; **P < .05, ***P < .01.
Model 3—Three Cognitive Variables, Special Accommodation, and Day Care Costs.
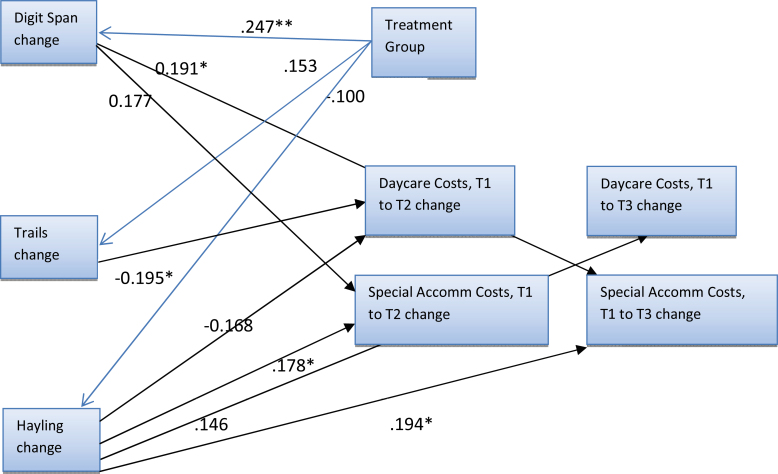
Treatment group predicted change in Digit Span. No other associations were significant (figure 3).
Fig. 3.
Model with 3 cognitive variables and 2 cost variables, with standardized parameter estimates. Although all pathways were estimated, small parameter estimates <0.1 are not shown. *P < .1; **P < .05, ***P < .01.
Model 4—Single Latent Cognitive Factor, Special Accommodation, and Day Care Costs.
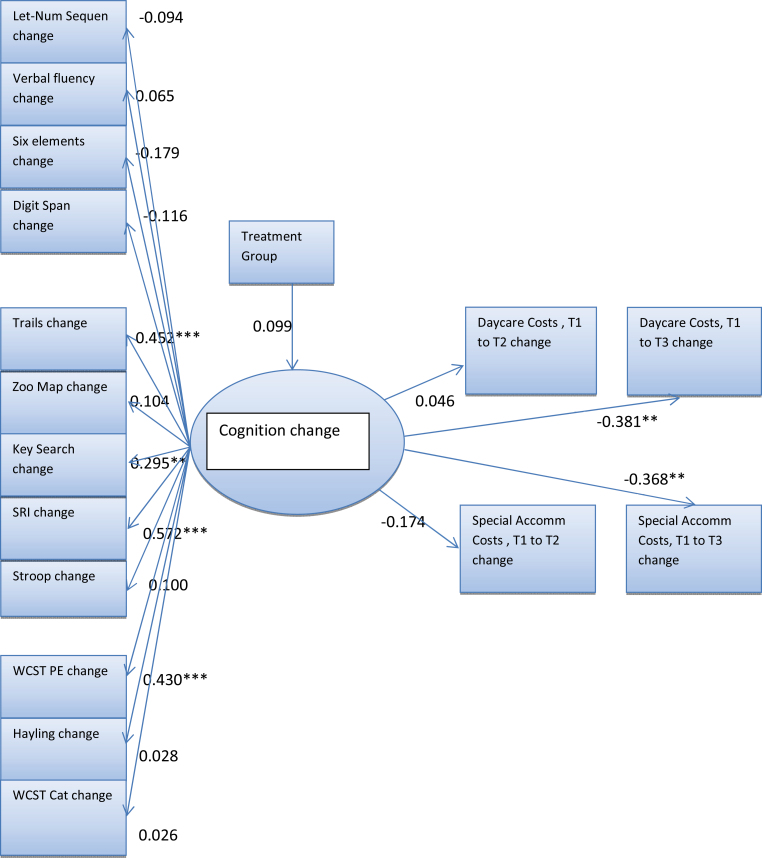
This model showed significant associations between improvements in cognition and reductions in both day care and special accommodation costs at time 3 (figure 4).
Fig. 4.
Single latent cognition factor model with standardized parameter estimates. Although all pathways were estimated in the model, some small parameter estimates are not shown. *P < .1; **P < .05, ***P < .01.
Discussion
Does Change in Cognition Predict Total Cost Change?
Two SEMs investigated the association between cognitive change and cost change. Cognitive change was represented either as (1) 3 individual working memory and executive function change variables or as (2) a single latent global cognitive change factor. Within the first model, the only significant association was between treatment group and digit span change, which is consistent with previous findings that CRT had a positive effect on verbal working memory.21 In the second model, there was no direct relationship between cognitive and total cost changes, but improved cognition predicted reduced depression, which in turn was associated with a reduction in subsequent costs. These associations may reflect an indirect relationship between cognition and subsequent cost change. The link between reduced depression and lower costs makes conceptual sense because better mood is likely to be associated with behavioral activation and an increased capacity to work (leading to a reduction in the reliance on state benefits) and to carry out activities of daily living independently (leading to reduced need for residential or community support). The cognition-depression link can be accounted for by changes in depression-related state-dependent cognitive impairments.46 Alternatively, it may be mediated by a third factor, such as increased self-esteem. This is consistent with findings from Rose et al,47 which showed that if participants noticed improvements in their cognition, then self-esteem improved. Presumably, perceptions of improvements were more likely if cognition had actually improved. Whatever the mechanism, depression may make a useful intervention target if a goal is to reduce costs, and this may better be targeted directly through medication and CBT rather than indirectly through CRT.
Increased costs were also significantly associated with an increase in positive symptoms and a decrease in antisocial behavior in model 2. The former may reflect increased psychiatric acute admissions as a result of worsening mental state, but the latter finding is more difficult to interpret. Because costs were calculated in terms of service use rather than allocation, it maybe that with a decrease in antisocial behavior, patients were able and willing to engage to a greater extent in health care services.
Does Change in Cognition Predict Change in Day Care or Special Accommodation Costs?
Both SEMs were a good fit. The first, with 3 individual cognitive variables, showed no significant associations between cognitive and cost changes. The second, with 1 latent cognition factor, showed that improvements in cognition were associated with significant reductions in subsequent day care and special accommodation (ie, staffed residential care, including long-stay psychiatric rehabilitation wards) costs. The residential care finding was consistent with our hypothesis that improved cognition would lead to a reduction in disability and a consequential reduced requirement for specialized accommodation. The reduced day care costs finding was not consistent with our hypothesis, nor findings from Wykes et al.22 Although our study related to improved cognition specifically, rather than receiving CRT more generally, it seems unlikely that the differences in results could be attributed to the nonspecific effects of CRT because Wykes et al included a therapist-matched control therapy. An alternative explanation may be the difference in sample size. Wykes and coworkers’ study included only 35 people and so would have been more susceptible to relatively few people making substantial changes.
Change in the single latent cognition factor was consistently a better predictor of change in costs than the 3 individual cognitive variables. Although we cannot draw conclusions relating to causation from a correlational study, this may suggest that the quantity rather than type of cognitive improvement is important in promoting cost change. Alternatively, it is of note that, although the single latent cognition change factor was estimated using all 12 cognitive change scores, change scores for only 4 (Trails, Key Search, Spatial Response Inhibition, and WCST perseverative errors) had significant loadings on this factor. Therefore, this cognitive change factor appears to reflect certain aspects of executive function change and not change in working memory or even global cognition. The finding that improved executive functioning is predictive of improved functioning is becoming increasingly consistent in the literature.16,23,25,48 This is supportive of models that suggest that the transfer of new cognitive skills to everyday living skills relies on top-down, executive thinking processes, such as metacognition,49 rather than the development of bottom-up basic processing skills that then cascade forward to more complex thinking.50
Although this study is limited by its relatively modest sample size and the presence of some missing data (which were estimated to avoid further exclusion of participants), we were able to estimate models of good fit and to identify significant associations between cognitive change and costs. Due to limitations of numbers, we were not able always to include the symptom and function variables that might have elucidated relationships between cognition and functioning. However, this approach serves as an exemplar of what may be possible in terms of investigating the relationships between cognitive change and cost outcomes.
Two characteristics of costs may need to be considered in investigating these relationships. First, changes in service use, such as special accommodation, may be uncommon and rely on large functioning changes to occur. Small functional changes may not affect cost and a longer time span may be required for significant change to occur. Our trial took place over a relatively short time frame and with no additional adjunctive rehabilitation opportunities to accompany the CRT. It is notable that cognitive changes were specifically associated with subsequent cost change, rather than costs occurring during the same period as the therapy. Thus, while it is encouraging that despite these constraints significant associations were apparent, studies taking place over longer time periods and with concomitant rehabilitation may be more successful in elucidating associations between cognitive and cost changes.
A second characteristic of measures of cost is that the variance is very wide and may be skewed by costs for a few participants. For example, inpatient costs, even for a brief admission can disproportionately swamp more moderate costs of regular appointments or medication. To avoid this problem, in our second pair of models, we excluded inpatient costs and focused only on day care and special accommodation. On the other hand, while many costs may only affect a few participants, the fact that a wide range of functional difficulties may be reflected in costs for different uses of resources may make costs a useful way to account for functional change, which may manifest itself in enormously varied ways between individuals.
In summary, this study exemplifies a method of investigating some of the effects and mechanisms of treatment using a rarely studied outcome—cost. It generated a number of hypotheses, particularly that executive change may be important if we are to reduce disability and resultant costs to society and healthcare systems, and that depression may play an important and frequently overlooked role in accounting for links between cognitive and functional changes.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Grant from the Department of Health, UK (RFG 757).
Supplementary Material
Acknowledgments
Til Wykes, Clare Reeder, and Victoria Harris would like to acknowledge the support of the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London. Til Wykes would also like to acknowledge the support of her NIHR Senior Investigator award. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679 [DOI] [PubMed] [Google Scholar]
- 2. Gustavsson A, Svensson M, Jacobi F, et al. ; CDBE2010Study Group. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779 [DOI] [PubMed] [Google Scholar]
- 3. Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. 2012;11:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rethink. Effective Interventions for Schizophrenia – The Economic Case. London, UK: Rethink; 2012 [Google Scholar]
- 5. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445 [DOI] [PubMed] [Google Scholar]
- 6. Tsang HW, Leung AY, Chung RC, Bell M, Cheung WM. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiar. 2010;44:495–504 [DOI] [PubMed] [Google Scholar]
- 7. Bell M, Bryson G, Greig T, Corcoran C, Wexler BE. Neurocognitive enhancement therapy with work therapy: effects on neuropsychological test performance. Arch Gen Psychiatry. 2001;58:763–768 [DOI] [PubMed] [Google Scholar]
- 8. McGurk SR, Mueser KT, DeRosa TJ, Wolfe R. Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophr Bull. 2009;35:319–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peña J, Segarra R, Ojeda N, García J, Eguiluz JI, Gutiérrez M. Do the same factors predict outcome in schizophrenia and non-schizophrenia syndromes after first-episode psychosis? A two-year follow-up study. J Psychiatr Res. 2012;46:774–781 [DOI] [PubMed] [Google Scholar]
- 10. Hoe M, Nakagami E, Green MF, Brekke JS. The causal relationships between neurocognition, social cognition and functional outcome over time in schizophrenia: a latent difference score approach. Psychol Med. 2012;42:2287–2299 [DOI] [PubMed] [Google Scholar]
- 11. Wykes T. Predicting symptomatic and behavioural outcomes of community care. Br J Psychiatry. 1994;165:486–492 [DOI] [PubMed] [Google Scholar]
- 12. Lin CH, Huang CL, Chang YC, et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146:231–237 [DOI] [PubMed] [Google Scholar]
- 13. Jeste SD, Patterson TL, Palmer BW, Dolder CR, Goldman S, Jeste DV. Cognitive predictors of medication adherence among middle-aged and older outpatients with schizophrenia. Schizophr Res. 2003;63:49–58 [DOI] [PubMed] [Google Scholar]
- 14. Okonkwo OC, Griffith HR, Copeland JN, et al. Medical decision-making capacity in mild cognitive impairment: a 3-year longitudinal study. Neurology. 2008;71:1474–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bambauer KZ, Soumerai SB, Adams AS, Zhang F, Ross-Degnan D. Provider and patient characteristics associated with antidepressant nonadherence: the impact of provider specialty. J Clin Psychiatry. 2007;68:867–873 [DOI] [PubMed] [Google Scholar]
- 16. Reeder C, Newton E, Frangou S, Wykes T. Which executive skills should we target to affect social functioning and symptom change? A study of a cognitive remediation therapy program. Schizophr Bull. 2004;30:87–100 [DOI] [PubMed] [Google Scholar]
- 17. Reeder C, Smedley N, Butt K, Bogner D, Wykes T. Cognitive predictors of social functioning improvements following cognitive remediation for schizophrenia. Schizophr Bull. 2006;32(suppl 1):S123–S131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mackin RS, Delucchi KL, Bennett RW, Areán PA. The effect of cognitive impairment on mental healthcare costs for individuals with severe psychiatric illness. Am J Geriatr Psychiatry. 2011;19:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel A, Everitt B, Knapp M, et al. Schizophrenia patients with cognitive deficits: factors associated with costs. Schizophr Bull. 2006;32:776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485 [DOI] [PubMed] [Google Scholar]
- 21. Wykes T, Reeder C, Landau S, et al. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br J Psychiatry. 2007;190:421–427 [DOI] [PubMed] [Google Scholar]
- 22. Wykes T, Reeder C, Williams C, Corner J, Rice C, Everitt B. Are the effects of cognitive remediation therapy (CRT) durable? Results from an exploratory trial in schizophrenia. Schizophr Res. 2003;61:163–174 [DOI] [PubMed] [Google Scholar]
- 23. Eack SM, Greenwald DP, Hogarty SS, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60:1468–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spaulding WD, Reed D, Sullivan M, Richardson C, Weiler M. Effects of cognitive treatment in psychiatric rehabilitation. Schizophr Bull. 1999;25:657–676 [DOI] [PubMed] [Google Scholar]
- 25. Wykes T, Reeder C, Huddy V, et al. Developing models of how cognitive improvements change functioning: mediation, moderation and moderated mediation. Schizophr Res. 2012;138:88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wykes T, Sturt E. Social behaviour schedule [SBS]. In: Wade DT, ed. Measurement in Neurological Rehabilitation. New York, NY: Oxford University Press; 1992:283–284 [Google Scholar]
- 27. Wilson B, Cockburn J, Baddeley A. Rivermead Behavioural Memory Test. Bury St. Edmonds, UK: Thames Valley Test Company; 1985 [Google Scholar]
- 28. Heaton R, Chelune G, Talley J, Kay G, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources, Inc; 1993 [Google Scholar]
- 29. Burgess PW, Shallice T. The Hayling and Brixton Tests. Bury St. Edmonds, UK: Thames Valley Test Company/Pearson Assessment; 1997 [Google Scholar]
- 30. Nelson H, Willison J. National Adult ReadingTest (NART): Test Manual. 2nd ed. London, UK: Nfer Nelson; 1991 [Google Scholar]
- 31. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276 [Google Scholar]
- 32. Wilson B, Alderman N, Burgess P, et al. Behavioural Assessment of the Dysexecutive Syndrome BADS. Bury St. Edmonds, UK: Thames Valley Test Company; 1996 [Google Scholar]
- 33. Wykes T, Sturt E, Katz R. The prediction of rehabilitative success after three years. The use of social, symptom and cognitive variables. Br J Psychiatry. 1990;157:865–870 [DOI] [PubMed] [Google Scholar]
- 34. Trenerry M, Crosson B, DeBoe J, Leber W. The Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; 1989 [Google Scholar]
- 35. Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997 [Google Scholar]
- 36. Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1994 [Google Scholar]
- 37. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 38. Beecham JK, Knapp M. Costing psychiatric interventions. In: Thornicroft G, Brewin C, Wing JK, eds. Measuring Mental Health Needs. London: Gaskell; 1992:163–183 [Google Scholar]
- 39. Netten A, Rees T, Harrison G. Unit Costs of Health and Social Care. Canterbury, Kent: University of Kent at Canterbury, PSSRU; 2001. OECD. Purchasing Power Parities. Comparative Price Levels. http://www.oecd.org/dataoecd/61/54/18598754. pdf Accessed October 14, 2004 [Google Scholar]
- 40. Joint Formulary Committee. British National Formulary. 44th ed. London, UK: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2002. http://www.bnf.org/bnf/ Accessed February 7, 2003 [Google Scholar]
- 41. CIPFA. Education Statistics 1999–2000 Actuals. London, UK: Chartered Institute of Public Finance and Accountancy; 2000 [Google Scholar]
- 42. Department for Work and Pensions 2003. Benefits and services http://webarchive.nationalarchives.gov.uk/+/http://www.dwp.gov.uk/lifeevent/benefits Accessed March 17, 2014.
- 43. Finn W, Hyslop J, Truman C. Mental Health Multiple Needs and the Police. London, UK: Revolving Doors Agency; 2000 [Google Scholar]
- 44. Delahunty A, Reeder C, Wykes T, Morice R, Newton E. Revised Cognitive Remediation Therapy Manual. London, UK: Institute of Psychiatry; 2002 [Google Scholar]
- 45. Coid JW, Kirkbride JB, Barker D, et al. Raised incidence rates of all psychoses among migrant groups: findings from the East London first episode psychosis study. Arch Gen Psychiatry. 2008;65:1250–1258 [DOI] [PubMed] [Google Scholar]
- 46. Liu SK, Chiu CH, Chang CJ, Hwang TJ, Hwu HG, Chen WJ. Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry. 2002;159:975–982 [DOI] [PubMed] [Google Scholar]
- 47. Rose D, Wykes T, Farrier D, Doran AM, Sporle T, Bogner D. What do clients think of cognitive remediation therapy? A consumer-led investigation of satisfaction and side effects. Am J Psychiatr Rehabil. 2008;11:181–204 [Google Scholar]
- 48. Wykes T, Reeder C, Corner J, Williams C, Everitt B. The effects of neurocognitive remediation on executive processing in patients with schizophrenia. Schizophr Bull. 1999;25:291–307 [DOI] [PubMed] [Google Scholar]
- 49. Wykes T, Reeder C. Cognitive Remediation Therapy for Schizophrenia: Theory and Practice. London, UK: Brunner Routledge; 2005 [Google Scholar]
- 50. Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.