Abstract
Schizophrenia is a highly complex and severe neuropsychiatric disorder with an unknown etiopathology. Evidence for a dysregulated immune system in both the risk for and progression of schizophrenia has recently been overwhelming. Importantly, chronic low-grade inflammation both in the periphery and central nervous system has been shown to contribute predominantly to the pathogenesis of schizophrenia in a subset of individuals. Inflammation in the central nervous system is mediated by a range of proinflammatory cytokines, resident immune cells such as microglia, and brain infiltrating peripheral immunocompetent cells, such as T lymphocytes. Recently, Th17 cells, a subset of T helper cells have emerged as crucial players in mucosal defense against infections. It is linked to atopic, inflammatory, and autoimmune disorders. The risk factors/mechanisms leading to low-grade inflammation in schizophrenia are diverse and include infectious agents, stress, trauma, environmental toxins, genetic vulnerability, physical inactivity, obesity, poor diet, and sleep disruption. Herein, we propose that fetal programming of cellular immune components driven by intrauterine adversity can lead to the generation of long-lasting effector/memory Th17 cells. Th17 cells can disrupt the blood-brain barrier, infiltrate the central nervous system, and, along with other cytokines and microglia, lead to neuroprogression through neuroinflammation in schizophrenia.
Key words: Th17 cells, IL-17 cytokine, inflammation, neuroprogression, schizophrenia, pathogenesis, etiology, fetal
Introduction
Schizophrenia has long been considered as a disorder involving the immune system. The macrophage-T lymphocyte theory of schizophrenia, proposed in 19921 became one of the strong propositions supporting autoimmune and/or immunoinflammatory origin of schizophrenia. This was further supported by multiple lines of evidence showing altered functions of immune cells and components.2–4 Importantly, altered T-cell number and function has consistently been demonstrated in schizophrenia.5 In acute schizophrenia, higher CD3+ and CD4+ T-cell numbers were observed.6 A recent study demonstrated an activated T-cell network in schizophrenia.7 Altered distributions of T-cell subsets in cerebrospinal fluid (CSF)8 and higher densities of T lymphocytes were also reported in the hippocampus of patients with schizophrenia,9 providing indirect evidence of blood-brain barrier (BBB) impairment and T-cell infiltration. Animal studies have elucidated that peripheral T-cell disruption can lead to cognitive and behavioral impairment, further implying the relevance of T cells in brain and behavior.10 Interestingly, reduced number and diminished functions of T cells in schizophrenia have also been reported by few studies. Acute paranoid schizophrenia was found to be accompanied by a reduced T-cell defenses.11 In vitro studies have demonstrated significantly reduced proliferative responses of T cells to stimulation in schizophrenia.12 Such responses were found to be functionally related to cell cycle machinery, intracellular signaling, oxidative stress, and metabolism of T cells.12 In addition, recent pathway analyses of data from genome-wide association studies suggest that genes related to immune functions, involved in antigen processing and cell adhesion molecules relevant to T cells, are significantly associated with schizophrenia.13 Furthermore, epigenetic studies also indicated that several genes involved in the activation of T cells were differentially methylated in schizophrenia.14 Taken together, these data strongly suggest a significant role of T cells in the pathophysiology of schizophrenia.
The T cells are a crucial component of the adaptive immune response. Upon activation, naive CD4+ T cells differentiate into a variety of effector Th subsets, each with its unique cytokine profile and functions. The Th subsets were initially classified as Th1 and Th2 cells based on their ability to produce different patterns of cytokines and perform different effector functions.15,16 Recently, a new subset of Th cells, termed as Th17 cells have been identified, which play critical roles in infection, autoimmunity, and inflammation, particularly in mucosal surfaces of the lungs, skin, and gut.17,18 Th17 cells secrete high amounts of IL-17A, IL-17F, IL-21, IL-22, and GM-CSF. Its master regulator is the rorc gene, which encodes the gene for Retinoic-acid Receptor-related Orphan Receptor gamma t, RORγt.19 Th17 cells display a great deal of context-dependent plasticity, either in clearing specific pathogens or in inducing autoimmune tissue inflammations.20 In addition to Th17 cells, CD4+CD25+FOXP3 regulatory T (Treg) cells have also been recently described as classical Treg cells, essential in maintaining peripheral tolerance, suppressing activation of the immune system, preventing autoimmune diseases, and limiting chronic inflammation.21 Emerging findings suggest an interplay between IL-17 producing Th17 cells and CD4+CD25+FOXP3 regulatory T cells in controlling inflammatory and autoimmune disorders.22 In this article, we propose that maternal immune activation (MIA) induced by intrauterine infection and other factors can be accompanied by the preferential generation of Th17 cells that subsequently might lead to priming the process of neuroprogression in schizophrenia through neuroinflammation.
Role of Th17 Cells in Neuro-immune cross talk
Emerging research indicates that Th17 cells can infiltrate the central nervous system (CNS) through the capillary and postcapillary venules of the BBB by disrupting tight junctions of BBB via the direct effects of IL-17A and IL-22 on endothelial cells.23 In fact, Th17 cell and human brain endothelial cell interactions are becoming increasingly important aspects of transmigration and CNS inflammation.24 Th17 cells may reach the CNS from blood to the CSF at the choroid plexus.25 This is supported by studies that Th17 cells constitutively express the chemokine receptor CCR6, whose ligand, CCL20, is also constitutively expressed by epithelial cells of choroid plexus.26 It is noteworthy that a cytokine, IL-23, is specially known for its ability to generate, expand, and stabilize autoreactive Th17 cells. A study has demonstrated that IL-23 drives encephalo-tropism of Th17 cells by promoting BBB disruption.27 Such brain-homing capability of Th17 cells might contribute to the neuroinflammation, implicated in the pathogenesis of many neurological disorders.
In parallel, given that Th17 cells play a predominant role in mucosal cell integrity and defense, the hypothesis that altered gut permeability may play a role in schizophrenia takes on another dimension.28 This may be putatively related to impairment of the gut’s ability to block uptake of exogenous psychotomimetic compounds or, akin to what is described in depression, may be related to commensal bacterial translocation and the development of autoimmunity.29 A surrogate marker of bacterial translocation, soluble CD14, was shown to be elevated in schizophrenia.30 Although these hypotheses remain tentative, and require further investigation, emerging research suggests that immunomodulatory properties of gut microbiota extend to the brain. Some specific intestinal microbial species known to induce Th17 cells can initiate the inflammatory cascade in the CNS. Germ-free mice colonized with segmented filamentous bacteria were found to have increased Th17 cells in both the colon and small intestine, as well as within the spinal cord and developed experimental autoimmune encephalomyelitis (EAE).31
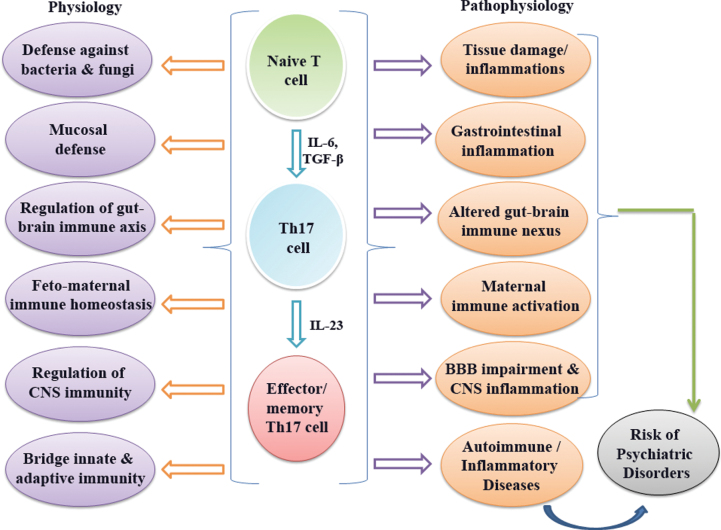
It is being increasingly recognized that Th17 cells play a pivotal role in the pathogenesis of autoimmune encephalomyelitis and they have emerged as a new player of neuroinflammation in multiple sclerosis.32–34 Th17 cells are found in focal lesions in EAE and intimately interact with CNS resident cells, such as astrocytes, microglia, and neurons.35 Th17 cells activate microglia in the CNS and lead to local production of IL-1β, tumor necrosis factor-α, and IL-6, which strongly implies its key role in neuroinflammation. This is accomplished by generating reactive oxygen species in mitochondria for antimicrobial purposes,36 resulting in oxidative stress,37 which is increasingly considered a potential biomarker in the etiopathophysiology and clinical course of schizophrenia.38 Oligodendrocyte lineage cells are found to be sensitive to IL-17-mediated toxicity.39 Interestingly, in animal model of Alzheimer’s disease, Th17 cell-mediated neuroinflammation is found to be involved in neurodegeneration.40 Taken together, it has now been established both in animal and human that Th17 cells can initiate CNS autoimmunity by supporting Th17-cell pathogenecity.41 Roles of Th17 cells in physiological and pathophysiological conditions are summarized in figure 1.
Fig. 1.
Summary of functional roles of Th17 cells in physiology and pathophysiology.
Hypothesis
On the basis of various direct and indirect data, a significant role of Th17 cells in the pathogenesis of schizophrenia is proposed. Higher percentages of Th17 cells were reported in recent onset, first-episode and drug-naive schizophrenia subjects.7,42 In addition, levels of IL-17, a signature cytokine of Th17 pathway, were elevated in a drug naive, first-episode schizophrenia cohort.42 Importantly, the proportion of Th17 cells and plasma levels of IL-17 were positively correlated with the overall severity of clinical symptoms based on Positive and Negative Symptoms Scale (PANSS) scores.42 Contrary to this, decreased levels of IL-17 are also reported in schizophrenia.43 Low levels of IL-17 were noted in first-episode and medication-free schizophrenia patients.44 Decreased levels of IL-17 were also reported in chronic- and antipsychotic-medicated people with schizophrenia, where pathway analyses demonstrated that cytokines representing IL-17 pathway were positively correlated with PANSS scores,45 indicating the potential role of IL-17 pathway in the etiology of schizophrenia.
The discrepancies in the above findings could be attributed to a number of parameters such as inadequate number of subjects studied, disease status (recent onset/chronic), types of cytokine evaluated, effects of age, sex, race, body mass index, smoking habits and psychotropic medications. Differentiation and maintenance of IL-17-producing Th17 cells are dependent on various transcription factors (RORγt, STAT3) and cytokines, such as IL-1, IL-1β, transforming growth factor-β (TGF-β), IL-23, and IL-6. Certain cytokines such as Interferon-γ (IFN-γ), IL-4, and IL-27 produced by Th1, Th2, and antigen-presenting cells, respectively, are known to suppress Th17 cells. Interestingly, in 1 study,45 slightly lower levels of IL-1β, IL-6, and IL-17 were found, and in another study,44 along with IL-6 and TGF-β, higher levels of Th17-suppressing cytokines such as IFN-γ, IL-4, and IL-27 were reported. Although TGF-β exerts both the immune-suppressive and proinflammatory functions, in inflammatory conditions, TGF-β promotes Th17 pathway. In addition to this, IL-23 is a potent initiator of Th17 pathway and IL-23/IL-17 axis acts as a critical mediator of autoimmune inflammatory diseases. A limitation of the above studies is that assessment of potential interactions between the Th17-activator (TGF-β/IL-1β/IL-6/IL-23), Th17-attenuator (IFN-γ/IL-2/ IL-4/ IL-27), and Th17-effector cytokines (IL-17A, IL-17F, IL-21, IL-22) in schizophrenia was not carried out. Therefore, the precise role of Th17 pathway in schizophrenia is yet to be empirically discerned.
Growing evidence suggests hyperactive TGF-β pathway in schizophrenia.46–48 In addition, cytokines such as IL-1β and IL-6, initiators of the Th17 pathway, are consistently implicated in the pathogenesis of schizophrenia.49,50 Moreover, in contrast to Th1 and Th2 immunity, Th17 cells demonstrate high-grade plasticity, and such plasticity allows Th17 cells a functional adaptation to various physiological situations during immune responses. Th17 cells display enhanced antitumor immunity and play important roles in transplant rejection. Th17 cells are more effective in host defense against microbes, especially bacteria and some fungi.51 Th17 cells regulate innate immune responses and participate in bacterial clearance during CNS infection.52 In addition, Th17 cells play an important role in gastrointestinal tract function. Th17 cells bridge innate and adaptive immunity and mount robust antimicrobial inflammatory responses. Although the functional plasticity of Th17 cells provides protection against microbes, they also mediate pathological inflammation. In a recent study, Th17 plasticity in autoimmune arthritis was shown to be driven by the inflammatory environment.53
As infections strongly increase the risk for schizophrenia, a role of Th17 cells is predicted. Th17 pathway is linked to the pathogenesis of several autoimmune and inflammatory disorders.54 Th17 cells are involved in the differential regulation of CNS autoimmunity. Inflammation in the brain parenchyma occurs only when Th17 cells outnumber Th1 cell.55 Interestingly, a 30-year population-based register study has shown that a prior autoimmune disease and history of hospitalization with infection increased the risk schizophrenia by 29% and 60%, respectively.56 The association of autoimmune diseases and schizophrenia is mediated by inflammation, brain-reactive antibodies, shared genetic factors, and common etiologic components like infections.57 Increased prevalence of multiple autoantibodies including antibodies to neuronal surface antigens is found in schizophrenia.58 Some of the antigens, such as N-methyl-d-aspartate receptor (NMDAR), a central component of synaptic plasticity, learning and memory act as a target of autoimmune reaction.59 Antibodies to NMDAR has been identified in schizophrenia.60,61 Antibodies that target proteins involved in synaptic function can cause limbic autoimmune encephalitis in some cases and this has also been linked to systemic autoimmune diseases.62 Limbic encephalitis is known to be one of the best-appreciated causes of rapid behavioral dysfunction and is increasingly being implicated with schizophrenia.63,64
Mild encephalitis, characterized by low-level neuroinflammation is associated with a variety of psychopathological symptoms. Mild encephalitis hypothesis of schizophrenia has received wide appreciation in recent years.65,66 The mild encephalitis hypothesis is thought to be triggered by infections, autoimmunity, toxicity, or trauma. Multiple epidemiological studies have demonstrated association of prenatal infection, prenatal stress, famine, environmental toxins, etc. with schizophrenia risk. All these factors are known to affect developmental immune system, crucial phases of neurodevelopment, and brain structures and functions.67,68 There is a growing recognition that MIA during pregnancy also shapes the immunological phenotypes of offspring.69 Offspring of immunostimulated pregnant mice were found to preferentially develop Th17 cells.70,71 These and various other findings based on altered immune components lend further support toward the autoimmune/inflammatory hypothesis of schizophrenia.72
Emerging research indicates that the gut microbiota have the capacity to modulate brain immunity and functions.73 However, an altered gut microbiome can lead to the development of autoimmune CNS disorders by fostering the production of brain-reactive autoantibodies74 and behavioral and physiological abnormalities in neurodevelopmental disorders.75 Th17 cells play important role in intestinal immune homeostasis; however, intestinal dysbiosis can lead to gastrointestinal inflammation through activation of Th17 cells.18 Notably, gastrointestinal inflammation is found to be associated with immune activation and the pathogenesis of schizophrenia.76 Considering the role of Th17 cells in gastrointestinal inflammation and the relevance of gastrointestinal inflammation in neurodevelopmental disorders, a significant role of Th17 pathway in schizophrenia seems imperative.
Schizophrenia has been proposed to be associated with neuroinflammation, as supported by multiple postmortem brain studies showing upregulated expression of inflammatory gene signatures.50,77 Prenatal infection can lead to developmental neuroinflammation and might cause microglial activation with ensuing neuroinflammation in the adult CNS.78,79 Given that microbial infections are an established risk factor for schizophrenia, and the role of the Th17 system particularly in mucosal defense, this is an intriguing nexus. Such findings provide evidence supporting an association of neuroinflammation with schizophrenia and establish an immune context for the mild encephalitis hypothesis of schizophrenia. Considering the importance of Th17 cells in the initiation of encephalitis and association of encephalitis with schizophrenia, an encephalitogenic function of Th17 cells can be envisaged in schizophrenia. In addition, Human endogenous retroviruses are shown to be associated with proinflammatory and neurotoxic cascades in schizophrenia.80 Interestingly, human endogenous retrovirus triggers Th17 cytokine response in various immune-mediated disorders.81 This further highlights the potential implications of Th17 cells in schizophrenia.
Other relevant findings implicating Th17 pathway in neuropsychiatric disorders has come from studies showing higher level of IL-17 in bipolar disorder and a correlation between increased serum level of IL-17A with the severity of autism.82,83 Furthermore, increased Th17 cells are found to be associated with depression both in human and animals.84,85 The shared inflammatory hypothesis of schizophrenia, bipolar disorder, autism, and immunoinflammation-induced priming of schizophrenia for increased expression of depression86–88 provide further support toward Th17-mediated pathogenesis of schizophrenia.
Proposals
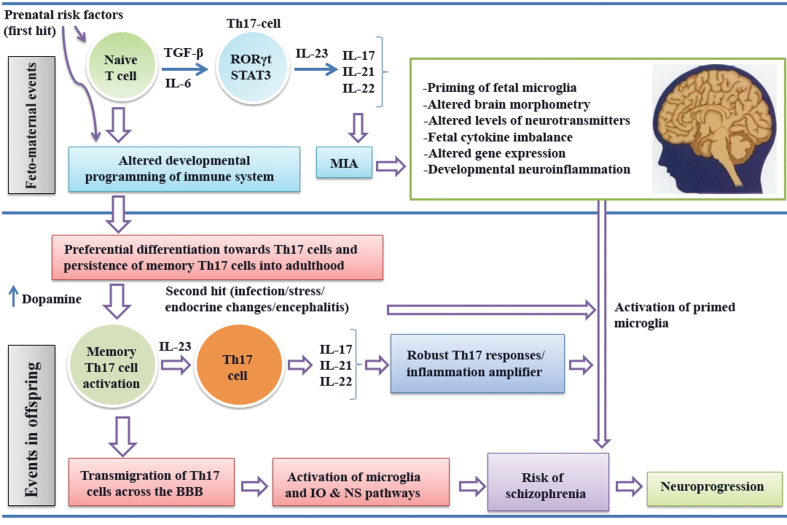
Prenatal infection and MIA strongly predisposes offspring to schizophrenia risk. It is hypothesized that prenatal infection might selectively lead to the development of Th17 cells. Prenatal infection affects fetal brain development and enhances the risk of schizophrenia in offspring through the production IL-6.89 Interestingly, IL-6 induces differentiation of Th17 cells from both naive and memory CD4+ T cells, and higher levels of IL-6 in schizophrenia is an established finding.49 This may be linked to polymorphisms of the IL-6 rs1800795*C allele, which is associated with higher IL-6 blood levels in schizophrenia.90 Prenatal infection activates toll-like receptor and amplifies the proinflammatory response by producing various inflammatory cytokines. This has been hypothesized to be one of the important mechanisms leading to inflammation and neuroprogressive changes in schizophrenia.91 Activation of TLRs thus could potentiate Th17-cell differentiation, further implying a significant role of prenatal infection in Th17-cell homeostasis. Human Th17 cells remain in the body as a long-lived proliferating effector memory T cell with unique and functional genetic characteristic.92,93 Activation of Th17 cells by a second hit (such as stress, infection, endocrine changes, etc.) and dysregulation of Th17 signaling may drive to chronic inflammation. The activated Th17 cells might infiltrate brain by disrupting the BBB and also lead to the activation of microglial cells. The release of Th17 effector cytokines and proinflammatory molecules from activated microglia can induce immunoinflammatory, oxidative, and nitrosative stress pathways that subsequently may enhance the risk of schizophrenia and neuroprogression. The effector cytokines of Th17 responses may provide a link between inflammation and neurodegeneration in schizophrenia.
Dopamine is known to interact directly with dopaminergic receptors on normal human T cells94 and is involved in activation of T cells through upregulation of the expression of T-cell adhesion molecules. This helps T cells in trafficking and extravasation across blood vessels and tissue barriers including the CNS. Increased expression of dopamine receptors in T cells and a significantly higher expression of dopamine receptor 3 mRNA in T cells are reported in schizophrenia.95,96 It has been recently demonstrated that stimulation of dopamine receptor D5 expressed on dendritic cells can potentiate Th17-mediated immunity.97 In addition, antagonizing the dopamine D1-like receptor was found to inhibit Th17-cell differentiation.98 Recently, dopamine was found to upregulate Th17 phenotype in individuals with generalized anxiety disorder.99 The dopamine hypothesis is one of the most widely accepted constructs in schizophrenia. Considering the role of hyperdopaminergia in schizophrenia, it is proposed that Th17 responses could be significantly amplified in schizophrenia by dopamine. Similarly, the glutamate theory of schizophrenia is of relevance to Th17 immunity.100,101 Knockout mice missing the metabotropic glutamate receptor-4 (mGluR4) showed increased sensitivity to experimental models of autoimmune encephalomyelitis mediated principally by increased IL-17-producing Th17 cells. Selective mGluR4 enhancers reduced sensitivity to autoimmune encephalomyelitis, which appears to be mediated by regulatory T (Treg) cells.102 A summary of the proposed hypothesis has been depicted in figure 2.
Fig. 2.
Hypothetical model summarizing aspects of Th17 pathway–mediated immunopathogenesis of schizophrenia (IO&NS, Immuno-inflammatory, oxidative & nitrosative stress).
Translational Implications and Evaluation of the Hypothesis
Pathogenetic paradigms invoking prenatal infection and maternal immune activation offer novel translational applications with potential to prevent this devastating disorder.103 A growing body of evidence suggest IL-17 pathway as a major therapeutic target in autoimmune and inflammatory diseases.104,105 A recent study has demonstrated that inhibition of STAT3 blocks Th17 development and inhibits experimental autoimmune uveitis.106 Therapeutic targeting of STAT pathways is thus gaining importance in CNS diseases.107 In addition to this, cytokines such as IL27p28/IL12p40 inhibit CNS diseases and/or CNS autoimmunity by antagonizing Th17 responses.108,109
The proposed hypothesis can be evaluated by examining the proportion of Th17 cells in human subjects with schizophrenia, well-characterized, first-episode, drug-naive, and ethnically homogeneous subsets of patients and controls matched for gender, age, parental socioeconomic conditions, and other confounding factors that potentially influence Th17-mediated immunity. In addition, determination of the peripheral and brain levels of various cytokines of Th17 pathway and their potential interactions with other Th1 and Th2 cytokines and other immune mediators including chemokines will lead to a better understanding of the significance of Th17 pathway in schizophrenia. The effects of Th17 activation and its derived cytokines on brain morphometry and cognitive functions in schizophrenia can also be tested by structural neuroimaging and neuropsychological testing. The temporal relationship between the symptom course of schizophrenia and changes in the blood Th17 products can be used as useful indicators for validating the hypothesis. Dopamine receptors expressed on immune cells modulate Th17-mediated inflammation.110 This is supported by studies showing the attenuation of Th17-mediated immune response by a dopamine D1-like receptor antagonist.111 Therefore, studies emphasizing the effects of antipsychotic drugs on Th17 pathway and the relationships between antipsychotic drugs modulating specific positive and negative symptoms with the changing proportion of Th17 cells will help to establish a mechanistic link between Th17 pathway and the etiopathogenesis of schizophrenia. Further, improved understanding of the influence of Th17 cells on the chronicity of schizophrenia patients especially with respect to negative symptoms and with underlying neurodegenerative changes might help us to determine the impact of Th17 cells on the fundamental aspects of this disorder.
The proposed hypothesis can also be tested by developing a murine model. The impact of prenatal infection on the development of Th17 cells and subsequent inflammation could be tested by subjecting mice to bacterial lipopolysaccharide (LPS) and/or viral polyinosinic: polycytidylic acid (poly I:C) components, followed by estimation of different proinflammatory cytokines and oxidative/nitrosative stress markers. Further, the effects of Th17 effector cytokines on fetal brain development could be examined by applying various imaging procedures and by studying the expression profile of the markers involved in regulation of apoptotic and necrotic pathways in fetal brain tissues. The potential impact of dopamine on Th17 responses can be tested in vitro with the help of dopamine agonists and antagonists. The effect of prenatal inflammation on cognitive and behavioral attributes of the mice could be analyzed by testing their behavior using established paradigms for prepulse inhibition, latent inhibition, and other relevant social interaction measures.
The long-term effects of prenatal immune events on neurochemistry, neuromorphometry, and behavioral attributes could be monitored in the mice challenged with LPS/poly I:C. The attributes of long-lived Th17 cells on brain and behavior can be examined at different time intervals at postnatal life and may be correlated with changes of neurotransmitters, brain volume, and learning and memory. Furthermore, the detailed functional significance of Th17 pathway in brain can also be derived from knockout mice lacking important components of Th17 pathway.
Conclusion
Functional plasticity of Th17 cells in immunity and disease is quite well established. Recent understanding of the capacity of Th17 cells to infiltrate brain and induce neuroinflammation has generated renewed interest in delineating the immunopathogenesis of CNS disorders. Peripheral immune components and disruption of BBB are increasingly linked to psychosis. Although multiple lines of evidence link schizophrenia to chronic low-grade inflammation, the contribution of systemic immune changes to neuroinflammation is yet to be fully discerned. The proposed hypothesis could fill the gap and might establish a causal link between prenatal infection, a persistent systemic immune perturbations, and neuroinflammation in schizophrenia and offer novel treatment targets.
Funding
National Health and Medical Research Council Senior Principal Research Fellowship (1059660 to M.B.).
Acknowledgements
M.B. has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier and Woolworths, has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay and Wyeth, and served as a consultant to Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck Merck and Servier. M.D. has no conflicts of interest in relation to the subject of this study
References
- 1. Smith RS. A comprehensive macrophage-T-lymphocyte theory of schizophrenia. Med Hypotheses. 1992;39:248–257 [DOI] [PubMed] [Google Scholar]
- 2. Smith RS, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses. 1995;45:135–141 [DOI] [PubMed] [Google Scholar]
- 3. Müller N, Riedel M, Ackenheil M, Schwarz MJ. The role of immune function in schizophrenia: an overview. Eur Arch Psychiatry Clin Neurosci. 1999;249(suppl 4):62–68 [DOI] [PubMed] [Google Scholar]
- 4. Müller N, Riedel M, Ackenheil M, Schwarz MJ. Cellular and humoral immune system in schizophrenia: a conceptual re-evaluation. World J Biol Psychiatry. 2000;1:173–179 [DOI] [PubMed] [Google Scholar]
- 5. Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73:993–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sperner-Unterweger B, Whitworth A, Kemmler G, et al. T-cell subsets in schizophrenia: a comparison between drug-naive first episode patients and chronic schizophrenic patients. Schizophr Res. 1999;38:61–70 [DOI] [PubMed] [Google Scholar]
- 7. Drexhage RC, Hoogenboezem TA, Cohen D, et al. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;14:746–755 [DOI] [PubMed] [Google Scholar]
- 8. Nikkilä H, Müller K, Ahokas A, Miettinen K, Andersson LC, Rimón R. Abnormal distributions of T-lymphocyte subsets in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res. 1995;14:215–221 [DOI] [PubMed] [Google Scholar]
- 9. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26:1273–1279 [DOI] [PubMed] [Google Scholar]
- 10. Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steiner J, Jacobs R, Panteli B, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260:509–518 [DOI] [PubMed] [Google Scholar]
- 12. Craddock RM, Lockstone HE, Rider DA, et al. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2:e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aberg KA, Liu Y, Bukszár J, et al. A comprehensive family-based replication study of schizophrenia genes. JAMA Psychiatry. 2013;70:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, Chen J, Ehrlich S, et al. Methylation Patterns in Whole Blood Correlate With Symptoms in Schizophrenia Patients [epub ahead of print]. Schizophr Bull. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357 [PubMed] [Google Scholar]
- 16. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173 [DOI] [PubMed] [Google Scholar]
- 17. Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133 [DOI] [PubMed] [Google Scholar]
- 20. Muranski P, Borman ZA, Kerkar SP, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352 [DOI] [PubMed] [Google Scholar]
- 22. Mai J, Wang H, Yang XF. Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front Biosci (Landmark Ed). 2010;15:986–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haqqani AS, Stanimirovic DB. Intercellular interactomics of human brain endothelial cells and th17 lymphocytes: a novel strategy for identifying therapeutic targets of CNS inflammation. Cardiovasc Psychiatry Neurol. 2011;2011:175364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523 [DOI] [PubMed] [Google Scholar]
- 26. Sallusto F, Impellizzieri D, Basso C, et al. T-cell trafficking in the central nervous system. Immunol Rev. 2012;248:216–227 [DOI] [PubMed] [Google Scholar]
- 27. Gyülvészi G, Haak S, Becher B. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur J Immunol. 2009;39:1864–1869 [DOI] [PubMed] [Google Scholar]
- 28. Wei J, Hemmings GP. Gene, gut and schizophrenia: the meeting point for the gene-environment interaction in developing schizophrenia. Med Hypotheses. 2005;64:547–552 [DOI] [PubMed] [Google Scholar]
- 29. Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141:55–62 [DOI] [PubMed] [Google Scholar]
- 30. Severance EG, Gressitt KL, Stallings CR, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4615–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aranami T, Yamamura T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS). Allergol Int. 2008;57:115–120 [DOI] [PubMed] [Google Scholar]
- 33. Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74:1–13 [DOI] [PubMed] [Google Scholar]
- 35. Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651 [DOI] [PubMed] [Google Scholar]
- 36. Zhi L, Ustyugova IV, Chen X, Zhang Q, Wu MX. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J Immunol. 2012;189:1639–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huppert J, Closhen D, Croxford A, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–1034 [DOI] [PubMed] [Google Scholar]
- 38. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kang Z, Wang C, Zepp J, et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci. 2013;16:1401–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang J, Ke KF, Liu Z, Qiu YH, Peng YP. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of aβ1-42-induced Alzheimer’s disease model rats. PLoS One. 2013;8:e75786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huber M, Heink S, Pagenstecher A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ding M, Song X, Zhao J, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51C:78–82 [DOI] [PubMed] [Google Scholar]
- 43. Schwarz E, Guest PC, Rahmoune H, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry. 2012;17:494–502 [DOI] [PubMed] [Google Scholar]
- 44. Borovcanin M, Jovanovic I, Radosavljevic G, et al. Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J Psychiatr Res. 2012;46:1421–1426 [DOI] [PubMed] [Google Scholar]
- 45. Dimitrov DH, Lee S, Yantis J, et al. Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: potential role for IL-17 pathway. Schizophr Res. 2013;151:29–35 [DOI] [PubMed] [Google Scholar]
- 46. Kalkman HO. Altered growth factor signaling pathways as the basis of aberrant stem cell maturation in schizophrenia. Pharmacol Ther. 2009;121:115–122 [DOI] [PubMed] [Google Scholar]
- 47. Jia P, Wang L, Meltzer HY, Zhao Z. Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr Res. 2010;122:38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frydecka D, Misiak B, Beszlej JA, et al. Genetic variants in transforming growth factor-β gene (TGFB1) affect susceptibility to schizophrenia. Mol Biol Rep. 2013;40:5607–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214 [DOI] [PubMed] [Google Scholar]
- 51. Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holley MM, Kielian T. Th1 and Th17 cells regulate innate immune responses and bacterial clearance during central nervous system infection. J Immunol. 2012;188:1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nistala K, Adams S, Cambrook H, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–14756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med. 2011;6:487–495 [DOI] [PubMed] [Google Scholar]
- 55. Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310 [DOI] [PubMed] [Google Scholar]
- 57. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol Psychiatry. 2014;75:300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ezeoke A, Mellor A, Buckley P, Miller B. A systematic, quantitative review of blood autoantibodies in schizophrenia. Schizophr Res. 2013;150:245–251 [DOI] [PubMed] [Google Scholar]
- 59. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pollak TA, McCormack R, Peakman M, Nicholson TR, David AS. Prevalence of anti-N-methyl-d-aspartate (NMDA) antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychol Med. In press. [DOI] [PubMed] [Google Scholar]
- 61. Steiner J, Walter M, Glanz W, et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278 [DOI] [PubMed] [Google Scholar]
- 62. Kayser MS, Dalmau J. The emerging link between autoimmune disorders and neuropsychiatric disease. J Neuropsychiatry Clin Neurosci. 2011;23:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lennox BR, Coles AJ, Vincent A. Antibody-mediated encephalitis: a treatable cause of schizophrenia. Br J Psychiatry. 2012;200:92–94 [DOI] [PubMed] [Google Scholar]
- 64. Moscato EH, Jain A, Peng X, Hughes EG, Dalmau J, Balice-Gordon RJ. Mechanisms underlying autoimmune synaptic encephalitis leading to disorders of memory, behavior and cognition: insights from molecular, cellular and synaptic studies. Eur J Neurosci. 2010;32:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bechter K. Updating the mild encephalitis hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:71–91 [DOI] [PubMed] [Google Scholar]
- 66. Muller N, Bechter K. The mild encephalitis concept for psychiatric disorders revisited in the light of current psychoneuroimmunological findings. Neurol Psychiatry Brain Res. 2013:19:87–101 [Google Scholar]
- 67. Meyer U, Weiner I, McAlonan GM, Feldon J. The neuropathological contribution of prenatal inflammation to schizophrenia. Expert Rev Neurother. 2011;11:29–32 [DOI] [PubMed] [Google Scholar]
- 68. Altamura AC, Pozzoli S, Fiorentini A, Dell’osso B. Neurodevelopment and inflammatory patterns in schizophrenia in relation to pathophysiology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:63–70 [DOI] [PubMed] [Google Scholar]
- 69. Mandal M, Donnelly R, Elkabes S, et al. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun. 2013;33:33–45 [DOI] [PubMed] [Google Scholar]
- 70. Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Preferential development of Th17 cells in offspring of immunostimulated pregnant mice. J Reprod Immunol. 2010;87:97–100 [DOI] [PubMed] [Google Scholar]
- 71. Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun. 2011;25:863–871 [DOI] [PubMed] [Google Scholar]
- 72. Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry. 2014;75:324–331 [DOI] [PubMed] [Google Scholar]
- 73. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712 [DOI] [PubMed] [Google Scholar]
- 74. Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. 2013;25:488–795 [DOI] [PubMed] [Google Scholar]
- 75. Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Severance EG, Alaedini A, Yang S, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hwang Y, Kim J, Shin JY, et al. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry. 2013;3:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34 [DOI] [PubMed] [Google Scholar]
- 79. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115–121 [DOI] [PubMed] [Google Scholar]
- 80. Perron H, Hamdani N, Faucard R, et al. Molecular characteristics of Human Endogenous Retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry. 2012;2:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ariza ME, Williams MV. A human endogenous retrovirus K dUTPase triggers a TH1, TH17 cytokine response: does it have a role in psoriasis? J Invest Dermatol. 2011;131:2419–2427 [DOI] [PubMed] [Google Scholar]
- 82. Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. 2012;9:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. do Prado CH, Rizzo LB, Wieck A, et al. Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology. 2013;38:667–676 [DOI] [PubMed] [Google Scholar]
- 84. Chen Y, Jiang T, Chen P, et al. Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Res. 2011;188:224–230 [DOI] [PubMed] [Google Scholar]
- 85. Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hope S, Melle I, Aukrust P, et al. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. 2009;11:726–734 [DOI] [PubMed] [Google Scholar]
- 87. Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26R–33R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Anderson G, Maes M, Berk M. Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:101–114 [DOI] [PubMed] [Google Scholar]
- 89. Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zakharyan R, Petrek M, Arakelyan A, Mrazek F, Atshemyan S, Boyajyan A. Interleukin-6 promoter polymorphism and plasma levels in patients with schizophrenia. Tissue Antigens. 2012;80:136–142 [DOI] [PubMed] [Google Scholar]
- 91. Venkatasubramanian G, Debnath M. The TRIPS (Toll-like receptors in immuno-inflammatory pathogenesis) Hypothesis: a novel postulate to understand schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:301–311 [DOI] [PubMed] [Google Scholar]
- 92. Kryczek I, Zhao E, Liu Y, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function. Eur J Immunol. 2001;31:3504–3512 [DOI] [PubMed] [Google Scholar]
- 95. Brito-Melo GE, Nicolato R, de Oliveira AC, et al. Increase in dopaminergic, but not serotoninergic, receptors in T-cells as a marker for schizophrenia severity. J Psychiatr Res. 2012;46:738–742 [DOI] [PubMed] [Google Scholar]
- 96. Boneberg EM, von Seydlitz E, Pröpster K, Watzl H, Rockstroh B, Illges H. D3 dopamine receptor mRNA is elevated in T cells of schizophrenic patients whereas D4 dopamine receptor mRNA is reduced in CD4+ -T cells. J Neuroimmunol. 2006;173:180–187 [DOI] [PubMed] [Google Scholar]
- 97. Prado C, Contreras F, González H, et al. Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J Immunol. 2012;188:3062–3070 [DOI] [PubMed] [Google Scholar]
- 98. Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 2008;373:286–291 [DOI] [PubMed] [Google Scholar]
- 99. Ferreira TB, Kasahara TM, Barros PO, et al. Dopamine up-regulates Th17 phenotype from individuals with generalized anxiety disorder. J Neuroimmunol. 2011;238:58–66 [DOI] [PubMed] [Google Scholar]
- 100. Berk M, Plein H, Csizmadia T. Supersensitive platelet glutamate receptors as a possible peripheral marker in schizophrenia. Int Clin Psychopharmacol. 1999;14:119–122 [DOI] [PubMed] [Google Scholar]
- 101. Berk M, Plein H, Belsham B. The specificity of platelet glutamate receptor supersensitivity in psychotic disorders. Life Sci. 2000;66:2427–2432 [DOI] [PubMed] [Google Scholar]
- 102. Fallarino F, Volpi C, Fazio F, et al. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat Med. 2010;16:897–902 [DOI] [PubMed] [Google Scholar]
- 103. Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hu Y, Shen F, Crellin NK, Ouyang W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann N Y Acad Sci. 2011;1217:60–76 [DOI] [PubMed] [Google Scholar]
- 105. Toussirot E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets. 2012;11:159–168 [DOI] [PubMed] [Google Scholar]
- 106. Yu CR, Lee YS, Mahdi RM, Surendran N, Egwuagu CE. Therapeutic targeting of STAT3 (signal transducers and activators of transcription 3) pathway inhibits experimental autoimmune uveitis. PLoS One. 2012;7:e29742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Egwuagu CE, Larkin Iii J. Therapeutic targeting of STAT pathways in CNS autoimmune diseases. JAKSTAT. 2013;2:e24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem. 2012;287:36012–36021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chong WP, Horai R, Mattapallil MJ, et al. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. J Autoimmun. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Prado C, Contreras F, González H, et al. Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J Immunol. 2012;188:3062–3070 [DOI] [PubMed] [Google Scholar]
- 111. Nakagome K, Imamura M, Okada H, et al. Dopamine D1-like receptor antagonist attenuates Th17-mediated immune response and ovalbumin antigen-induced neutrophilic airway inflammation. J Immunol. 2011;186:5975–5982 [DOI] [PubMed] [Google Scholar]