Abstract
Empirical and theoretical studies implicate thalamocortical circuits in schizophrenia, supported by emerging resting-state functional connectivity studies (rs-fcMRI). Similar but attenuated alterations were found in bipolar disorder (BD). However, it remains unknown if segregated loops within thalamocortical systems show distinct rs-fcMRI alterations in schizophrenia. For instance, the mediodorsal (MD) nucleus, known to project to prefrontal networks, may be differently altered than the lateral geniculate nucleus (LGN), known to project to the occipital cortex. Also, it remains unknown if these circuits show different patterns of alterations in BD as a function of psychosis history, which may be associated with a more severe clinical course. We addressed these questions in 90 patients with chronic schizophrenia and 73 remitted BD patients (33 with psychosis history) matched to 146 healthy comparison subjects. We hypothesized that the MD vs LGN would show dissociations across diagnostic groups. We found that MD and LGN show more qualitative similarities than differences in their patterns of dysconnectivity in schizophrenia. In BD, patterns qualitatively diverged between thalamic nuclei although these effects were modest statistically. BD with psychosis history was associated with more severe dysconnectivity, particularly for the MD nucleus. Also, the MD nucleus showed connectivity reductions with the cerebellum in schizophrenia but not in BD. Results suggest dissociations for thalamic nuclei across diagnoses, albeit carefully controlling for medication is warranted in future studies. Collectively, these findings have implications for designing more precise neuroimaging-driven biomarkers that can identify common and divergent large-scale network perturbations across psychiatric diagnoses with shared symptoms.
Key words: schizophrenia, bipolar illness, connectivity, resting-state, thalamus, mediodorsal nucleus, cross-diagnostic comparisons
Introduction
Schizophrenia (SCZ) is a complex neurodevelopmental disorder hypothesized to affect distributed brain connectivity,1 with widespread disruptions in neuronal communication at the level of large-scale neural systems.2–6 Numerous theoretical models of this illness and empirical findings have implicated disruptions along thalamo-cortico-cerebellar circuits in SCZ.7,8 Recent resting-state functional connectivity studies (rs-fcMRI) have identified profound thalamocortical dysconnectivity in SCZ.9–11 These studies identified a particular pattern suggesting over-connectivity between the thalamus and sensory-motor regions, but under-connectivity between the thalamus and prefrontal-striatal-cerebellar circuits.9–11 Similar, but attenuated, effects were discovered in bipolar disorder (BD), known to share genetic risk and12 diagnostic and neural features with SCZ.13–15
While these emerging studies provide strong evidence for disruptions in thalamocortical information flow, it remains to be determined if distinct thalamocortical loops show qualitatively different rs-fcMRI alterations in SCZ; for instance, the mediodorsal (MD) nucleus known to project to prefrontal networks vs the lateral geniculate nucleus (LGN) known to project to the occipital cortex. We specifically considered the MD nucleus because of its dominant projections to prefrontal networks, which are of strong interest in SCZ.11 Conversely, the LGN represents a well-understood thalamic nucleus with a highly distinct connectivity profile than the MD nucleus, projecting primarily to the visual system, providing a test for specificity of MD effects. This comparison is important to determine if prefrontal-thalamic circuits show distinct spatial patterns of abnormalities or whether the patterns of dysconnectivity are largely similar to other nuclei, but differ in their magnitude. In other words, there could be important spatial differences in thalamic dysconnectivity patterns across nuclei, as well as magnitude of such disruptions. For instance, Woodward and colleagues10 identified dissociable patterns of dysconnectivity in SCZ between large cortical areas known to project to distinct thalamic subdivisions, providing important initial findings. However, it remains unknown if the whole-brain patterns of thalamocortical dysconnectivity between distinct thalamic subdivisions show qualitative dissociations in SCZ. Therefore, this study was designed to explicitly quantify similarities and differences in both magnitude and extent of thalamocortical dysconnectivity in SCZ across thalamic subdivisions known to project to different cortical territories. Finally, it remains unknown if different thalamic nuclei show unique patterns of dysconnectivity across diagnostic categories with shared clinical features, such as BD.
Prior studies identified a “graded” pattern of thalamocortical disruptions in BD, less pronounced than those identified in SCZ.9 However, it is important to further consider the complexity and heterogeneity of BD, especially with respect to co-occurrence of psychosis.16 Many clinical investigations divide patients based on the presence or absence of psychotic symptoms.13,17 Importantly, emerging connectivity findings suggest that co-occurrence of psychosis in BD may indeed be associated with a distinct clinical course and, perhaps, a more severe pattern of dysconnectivity in prefrontal networks.18 Yet, it remains unknown if identified thalamocortical alterations in SCZ can differentiate between BD patients with and without co-occurring psychosis. A corollary of this question is whether BD patients with psychosis history show thalamocortical dysconnectivity similar to SCZ.
Collectively, this work provides 3 key extensions from prior studies examining thalamic connectivity in SCZ. First, we examined if MD and LGN thalamic nuclei show dissociable dysconnectivity in SCZ when examined at the whole-brain level? This analysis provides further evidence for whole-brain thalamocortical dysconnectivity in SCZ across thalamic nuclei with known differences in projection patterns.19 Second, we examined if BD patients with and without psychosis history differ in their patterns of MD and LGN thalamic connectivity? Third, we tested if BD patients with psychosis history show a pattern of dysconnectivity more in line with SCZ results, but distinct from those identified in BD without psychosis? These two cross-diagnostic analyses provide evidence that thalamocortical dysconnectivity in SCZ may be a feature more closely associated with psychosis occurrence across clinical categories.
Materials and methods
Participants
We studied 3 groups of patients matched to their respective healthy comparison subjects (HCS), characterized across our prior studies9: (1) a group of patients diagnosed with SCZ (n = 90); (2) bipolar patients without psychosis history (BPW, n = 40); and (3) bipolar patients with psychosis history (BPP, n = 33). All participants were collected on the same scanner and carefully characterized in prior studies9 and used here to examine focused follow-up hypotheses centered on MD/LGN. All patients were recruited in the Hartford, CT area through outpatient clinics and community mental health services. HCS were recruited through community advertising and flyers. Signed informed consent approved by the Hartford Hospital and Yale University Institutional Review Board (IRB) was obtained from all participants. Detailed recruitment and diagnostic procedures were described previously.9,18 Of note, 90 demographically similar HCS were recruited in comparison with the SCZ group; 56 demographically similar HCS were recruited to match BD patients. All BD patients were remitted during the study and were divided into 2 demographically matched groups based on history of psychosis, using standardized procedures.13 Briefly, psychosis history of hallucinations and/or bizarre delusions within a mood episode was assessed with the lifetime psychosis module of the Structured Clinical Interview (SCID) for the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV),20 the Lifetime Dimensions of Psychosis Scale,21 and medical records review (when available). The absence of hallucinations and/or delusions during all affective episodes over an individual’s lifetime was evidence for nonpsychosis. Based on these criteria, 33 of the 73 individuals with BD met criteria for a lifetime history of mood-congruent psychotic symptoms. For the focused comparisons between BD groups and the SCZ sample, we selected a specific subset of 73 demographically similar SCZ patients (figure 5). While similar (table 1), SCZ patients differed from BD groups in gender proportion. However, gender, either when used as a covariate or as an independent variable, was not related to present effects.
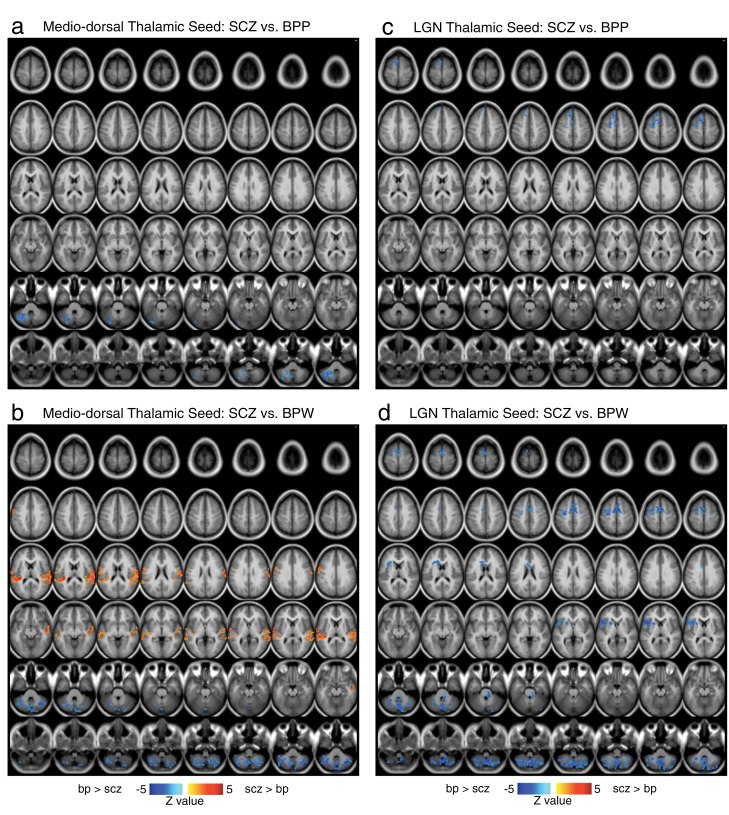
Fig. 5.
Differences in mediodorsal (MD) vs occipital-projecting (LGN) thalamic nuclei in schizophrenia (SCZ) vs bipolar disorder (BD) with and without psychosis history. Pairwise group comparisons between BPW and BPP groups relative to subgroup of matched SCZ subjects are presented for the MD seed (a and b) and the occipital-projecting LGN seed (c and d). Displayed results survived an independent type I error corrected analyses between each BD group and SCZ patients (see “Methods” section). These results generally indicate dissociable patterns for the MD vs LGN seeds and also different results for the BPP vs BPW groups relative to SCZ patients. Notably, SCZ exhibited reduced cerebellar connectivity with the MD seed relative to both BD groups, suggesting that cerebellar-thalamic dysconnectivity may be SCZ specific. Also, there seem to be more regional differences in BPW vs SCZ than BPP vs SCZ. The complete list of surviving foci is presented in table 5.
Table 1.
Schizophrenia Sample Demographics
| Characteristic | HCS (N = 90) | SCZ (N = 90) | Significance (SCZ vs CON) | |
|---|---|---|---|---|
| M (SD) | M (SD) | T Value/Chi-Square | P Values, 2 Tail | |
| Age (y) | 30.71 (11.99) | 32.93 (11.25) | 1.28 | .2 |
| Gender (% male) | 66.00 | 73.00 | 1.13 | .26 |
| Father’s education (y) | 14.37 (3.21) | 13.67 (3.47) | 1.42 | .16 |
| Mother’s education (y) | 13.99 (2.81) | 13.50 (2.92) | 1.15 | .25 |
| Participant’s education (y) | 15.24 (2.22) | 13.18 (2.21) | 6.26* | <.001 |
| Handedness (% right) | 84.21 | 80.00 | 0.85 | .4 |
| Signal to noise | 215.37 (45.25) | 206.81 (62.05) | 1.06 | .3 |
| % Frames flagged | 10.13 (7.89) | 17.63 (17.00) | 3.79* | <.001 |
| IQ estimate | 106.77 (8.92) | 97.78 (15.71) | 4.55* | <.001 |
| Medication (CPZ equivalents) | — | 229.00 (195.81) | — | — |
| PANSS positive symptoms | — | 15.80 (4.73) | — | — |
| PANSS negative symptoms | — | 14.34 (5.53) | — | — |
| PANSS general psychopathology | — | 30.48 (7.18) | — | — |
| PANSS total psychopathology | — | 60.51 (14.25) | — | — |
Note: PANSS, Positive and Negative Syndrome Scale; IQ, intelligence quotient; CPZ, chlorpromazine. Age, education levels, parental education, are expressed in years.
*Significant T statistic for the between-group t test.
All patients met either SCZ or BD diagnostic criteria as determined by experienced MA/PhD level clinician using SCID.22 Comorbid Axis I diagnosis of anxiety disorders and/or substance abuse (remitted for at least 6 months prior to this study) were allowed to increase representativeness of clinical samples. Eligible HCS had no current or lifetime mood or psychotic Axis I disorder as assessed by SCID-NP and no history of psychotic or mood disorders in first-degree relatives. Participant had no history of major medical or neurological condition. HCS had higher educational attainment relative to patients. However, educational differences are influenced by illness course23 and were thus not explicitly controlled. Also, as done previously,9,18 alcohol/drug use, anxiety, age, and illness duration did not alter findings (however, see “Limitations” section for a detailed discussion).
Current Symptoms and Medication
SCZ symptom severity was ascertained using the Positive and Negative Syndrome Scale.24 SCZ patients differed in medications profile for mood stabilizers, atypical antipsychotics, anxiolytics, and typical antipsychotics compared with BD patients (see “Limitations” section). Also, 9% of SCZ patients were unmedicated, whereas 18% of BPW and 15% of BPP groups were unmedicated. BD symptomatology was assessed using Young Mania Rating Scale (YMRS),25 21-item Hamilton Depression Scale (HAM-D),26 and the expanded version of the Brief Psychiatric Rating Scale.27 All BD participants were remitted >2 weeks prior to the participation in the study, determined by standardized cutoff values on YMRS and HAM-D scales (≥7). Bipolar subgroups (ie, BPP and BPW) were well matched for medications and current symptomatology (table 1).
Neuroimaging Data Acquisition
Scanning was performed at the Olin Neuropsychiatry Research Center on a Siemens-Allegra 3T scanner. Functional images sensitive to blood oxygenation level-dependent (BOLD) signal were collected with axial slices parallel to the anterior-posterior commissure (AC-PC) using a T2*-weighted gradient-echo, echo-planar sequence (TR/TE = 1500/27 ms, flip angle = 60°, field of view = 24 × 24cm, acquisition matrix = 64 × 64, voxel size = 3.43 × 3.43 × 4 mm), ensuring whole-brain coverage. Functional data collection lasted 5.25 minutes, resulting in 210 volumes (29 slices/volume, interslice gap = 1 mm). Subjects were instructed to remain awake in the scanner and keep their eyes open. A video camera was used to monitor subjects, ensuring they stayed awake. Subjects were excluded if they fell asleep or if their head movement exceeded 1 mm along any axis. Structural images were acquired using a T1-weighted, 3D magnetization-prepared rapid gradient-echo sequence (TR/TE/TI = 2200/4.13/766 ms, flip angle = 13°, voxel size [isotropic] = 0.8 mm, image size = 240 × 320 × 208 voxels), with axial slices parallel to the AC-PC line.
Neuroimaging Data Preprocessing and Analysis
All preprocessing followed our prior work and best practices in the clinical connectivity literature.2,28 Briefly, we performed (1) slice-time correction, debanding and normalization to whole-brain mode 1000, (2) removal of first 5 images from each run, (3) rigid body motion correction, (4) 12-parameter affine transform of the structural image to the Talairach coordinate system, and (5) coregistration of volumes to the structural image with 3 × 3 × 3 mm resampling. Furthermore, we employed the following rigorous quality assurance criteria for each participant, to ensure comparable BOLD quality: (1) signal-to-noise ratios (SNR) > 100. SNR was computed by obtaining the mean signal and SD for a given slice across the BOLD run, while excluding all non-brain voxels across all frames28; (2) no BOLD run with a single frame movement greater than 1 functional voxel as noted above; (3) all BOLDs were movement scrubbed29,30 and subjects with more than 50% frames flagged as potentially affected by movement artifacts were completely excluded from analyses. Specifically, first, frames in which sum of the displacement across all 6 rigid body movement correction parameters exceeded 0.5mm (assuming 50 mm cortical sphere radius) were identified. Second, root mean square (RMS) of differences in intensity between the current and preceding frame was computed across all voxels, divided by mean volume intensity and normalized to time series median. Frames in which normalized RMS > 1.6 were identified. We excluded frames flagged by either criterion. Moreover, 1 frame proceeding and 2 frames following the flagged frame were removed. Subject with >50% frames flagged were omitted from analyses.
After these criteria were implemented, there were no between-group differences in SNR or proportion of removed scrubbed frames for the BD and controls. There were, however, a higher proportion of frames scrubbed for the SCZ sample, suggesting more movement on average. Therefore, we ensured that the proportion of the removed frames did not significantly relate to reported effects involving the SCZ sample. We specifically used the proportion of scrubbed frames as a covariate across reported analyses. We also removed additional potentially spurious signal in resting-state data, as is standard practice.31 Briefly, all images were spatially smoothed using a 6 mm full-width-at-half-maximum Gaussian kernel and then underwent high (>0.009 Hz) and low (<0.08 Hz) pass temporal filtering, removal of nuisance signal from ventricles, deep white matter, as well as global mean signal, 6 rigid body motion correction parameters, and their first derivatives using in-house Matlab tools.32 All nuisance regressors (ie, ventricles, deep white matter and global mean signal) were defined via FreeSurfer software,33 based on individual subjects’ anatomically based segmentations (visually inspected for quality by a trained rater, A.A.).
Seed-based Functional Connectivity Analysis (rs-fcMRI) Based On Thalamic Anatomy
We focused on 2 specific thalamic seeds with known differences in their cortical projection patterns. We used a priori anatomically delineated thalamic subdivisions obtained using human cortical tractography.19,34 Specifically, we used the probabilistic thalamic atlas freely available in FSL, from which we derived thalamic seeds. As noted above, the key motivation behind the MD/LGN choice was based on findings by Woodward and colleagues,10 suggesting dissociable patterns of connectivity between these thalamic subdivisions. Moreover, the LGN provided a control nucleus for the MD, which is considered a central thalamic dysconnectivity locus in SCZ.
Subject-specific whole-brain thalamic maps were computed by extracting the average time series across all thalamic voxels for a given seed and computing a correlation with all other voxels. To obtain further specificity and avoid partial volume effects, for each subject, we defined a subset of thalamic voxels via FreeSurfer segmentation33 that explicitly overlapped with the FSL-defined seed. This way, the precise location of MD/LGN voxels was explicitly based on the overlap with individual-specific thalamic anatomy. Next, we computed a Fisher r-to-Z transform, yielding a connectivity map for each participant where each voxel’s value represents its thalamic connectivity. To examine between-group differences, all individual-subject maps were entered into appropriate second-level tests (either independent samples t test or 1-way ANOVA with 3 between-group levels [HCS, BPP, BPW]), which was computed within FSL’s Randomise tool with 10 000 permutations.35 All whole-brain type I error correction was accomplished via threshold-free-cluster-enhancement implemented in Randomise.36
We computed 2 follow-up analyses to further characterize the patterns of between-group differences for each of the seeds: (1) across both SCZ and BD samples, we computed a formal conjunction (ie, overlap), following whole-brain type I error correction between the MD and LGN-based results. This facilitated inspection of whether the 2 seeds produced similar or distinct regional dysconnectivity; (2) we computed post hoc pairwise between-group comparisons to characterize the 1-way ANOVA effect for the BD sample, as well as a directed comparison between each of the BD groups and SCZ (figures 4 and 5). These 2 follow-up analyses allowed us to better interpret the pattern of results across BPW and BPP relative to SCZ findings, informing whether the BPP sample is qualitatively closer to SCZ effects.
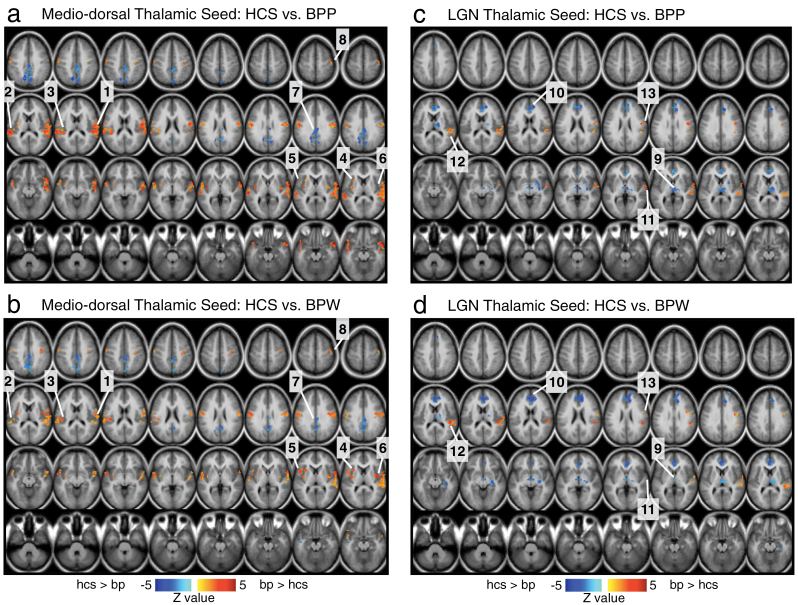
Fig. 4.
Differences in mediodorsal (MD) vs occipital-projecting (LGN) thalamic nuclei in bipolar disorder (BD) with and without psychosis history. To allow further inspection of BD results, pairwise group comparisons relative to healthy comparison subjects (HCS) are presented for the MD thalamic seed (a and b) and the occipital-projecting LGN thalamic seed (c and d). Results are shown within the regions surviving the 1-way ANOVA F test presented in figure 3. The main purpose here is to allow a qualitative inspection of the patterns of differences between the 2 bipolar groups and their matched HCS across the 2 thalamic seeds. In that sense, the figure is designed to complement figure 3 by highlighting precisely the same locations that survived the 1-way ANOVA in figure 3. As in figure 3, each of the surviving regions of interest (ROI) is marked with a number (ie, 1–8 for the MD seed and 9–13 for the LGN seed). These results generally indicate a qualitatively similar pattern for each of the seeds between the BPW and BPP groups. The key differences in magnitude of connectivity are (1) more pronounced over-connectivity along the sensory-motor areas and the MD thalamus specifically for the BPP group (ROIs 1–3); (2) more pronounced over-connectivity between the insula and MD thalamus specifically for the BPW group (ROIs 4–6); and (3) more pronounced under-connectivity between the medial prefrontal cortex and LGN thalamus specifically for the BPW group (ROI 10).
Findings were visualized using Caret 5.5 (http://brainvis.wustl.edu/wiki/index.php/Caret:Download) and NeuroLens (http://www.neurolens.org) software.
Results
MD and LGN Thalamic Connectivity in SCZ
We first investigated whether SCZ patients showed dissociable patterns of rs-fcMRI between MD and LGN thalamic nuclei that were defined anatomically based on their known projection patterns.19 Results indicated a pattern of dysconnectivity in SCZ that was spatially qualitatively similar across both seeds (see table 2 and figure 1). The general pattern indicated over-connectivity with sensory-motor regions, but under-connectivity with prefrontal-striatal and cerebellar clusters in SCZ vs HCS for both seeds. There were, however, several notable focal dissociations in patterns of dysconnectivity across the 2 thalamic nuclei (see figure 2 for the conjunction maps); for the LGN occipital-projecting seed SCZ patients exhibited over-connectivity with both primary visual cortex and anterior cingulate cortex; both patterns were not observed for the MD thalamic seed. Taken together, the SCZ-based findings suggest that the patterns of under-connectivity largely overlap between the MD and LGN thalamic seeds. In contrast, the over-connectivity shows notable differences across MD and LGN, perhaps, reflecting dissociable information loops between these thalamic nuclei.10 While the LGN and MD patterns were qualitatively similar, we formally tested whether the “severity” of dysconnectivity within the identified foci actually differed as a function of the thalamic seed, complementing our prior reports.9 To this end, we computed a follow-up Diagnosis (SCZ vs CON) × Seed (LGN vs MD) ANOVA for all identified regions (see table 2). All foci revealed a main effect of Diagnosis, suggesting a more severe pattern of dysconnectivity for SCZ vs CON for all the identified areas irrespective of the seed. However, a number of regions also revealed a Diagnosis × Seed interaction, suggesting that the pattern of dysconnectivity differed in severity for SCZ across the 2 nuclei, particularly for the MD seed. Therefore, while the maps across the 2 seeds were qualitatively similar in spatial extent, the interaction effect is in concert with our prior reports where we identified a generally more severe pattern for the MD nucleus.9
Table 2.
MD and LGN Results—SCZ Analysis
| X | Y | Z | Hemisphere | Anatomical Landmark | Mean T Value | P Value, 2 Tail | Cluster size (mm3) | Seed × Dx F Test (F/P Value) | Overlap |
|---|---|---|---|---|---|---|---|---|---|
| MD thalamic seed SCZ < HCS | |||||||||
| 0 | −59 | −35 | Midline | Cerebellum | −3.16 | .002 | 100575 | 5.36/.022* | Y |
| −18 | −100 | −18 | Left | Lingual gyrus (BA 18) | −2.76 | .006 | 918 | n.s. | Y |
| 1 | −99 | −15 | Midline | Lingual gyrus (BA 18) | −2.70 | .008 | 1080 | 7.67/.0061** | Y |
| −10 | −15 | 3 | Left | Mediodorsal thalamus | −3.25 | .001 | 9315 | 22.8/<.001*** | Y |
| 0 | 26 | 35 | Midline | Cingulate gyrus (BA 32) | −3.07 | .002 | 47682 | 12.4/<.001*** | Y |
| 21 | 6 | 1 | Right | Putamen | −2.80 | .006 | 1188 | n.s. | Y |
| −24 | 19 | 21 | Left | Caudate | −2.80 | .006 | 270 | 3.98/.048* | N |
| −10 | −32 | 19 | Left | Posterior cingulate | −2.93 | .004 | 378 | 3.18/.077trend | N |
| 6 | −17 | 31 | Right | Cingulate gyrus (BA 23) | −2.85 | .005 | 2889 | n.s. | Y |
| 8 | 58 | 35 | Right | Superior frontal gyrus (BA 9) | −2.67 | .008 | 378 | n.s. | Y |
| MD thalamic seed SCZ > HCS | |||||||||
| 7 | −73 | 14 | Right | Occipital cortex (BA 18) | 3.12 | .002 | 26136 | 7.22/.008** | Y |
| 1 | −23 | 29 | Midline | Cingulate gyrus (BA 23) | 3.35 | .001 | 120852 | 44.4/<.001*** | Y |
| −41 | −68 | 5 | Left | Middle occipital gyrus | 2.76 | .006 | 2457 | 7.67/.0062** | Y |
| −50 | −66 | 3 | Left | Middle temporal gyrus (BA 37) | 2.55 | .012 | 270 | n.s. | Y |
| 49 | −65 | 11 | Right | Middle temporal gyrus (BA 39) | 2.49 | .014 | 432 | n.s. | Y |
| −24 | −68 | 19 | Left | Precuneus (BA 31) | 2.71 | .007 | 405 | n.s. | Y |
| LGN thalamic seed SCZ < HCS | |||||||||
| 0 | −64 | −38 | Midline | Cerebellum | −3.17 | .002 | 79866 | n.s. | Y |
| 2 | −23 | −15 | Midline | Brainstem | −2.63 | .009 | 621 | n.s. | Y |
| 3 | −8 | 2 | Midline | Mediodorsal thalamus | −3.02 | 003 | 9018 | n.s. | Y |
| 36 | 33 | 35 | Right | Middle frontal gyrus (BA 9) | −3.18 | .002 | 13878 | n.s. | Y |
| −38 | 36 | 33 | Left | Middle frontal gyrus (BA 9) | −3.38 | .001 | 7884 | n.s. | Y |
| 2 | −13 | 27 | Midline | Cingulate gyrus (BA 23) | −2.92 | .004 | 14013 | 8.04/.005** | Y |
| 1 | 20 | 42 | Midline | Cingulate gyrus (BA 32) | −2.95 | .004 | 10800 | n.s. | Y |
| 4 | 58 | 33 | Midline | Superior frontal gyrus (BA 9) | −2.92 | .004 | 432 | n.s. | Y |
| LGN thalamic seed SCZ > HCS | |||||||||
| 22 | −44 | 18 | Right | Posterior parahippocampal gyrus | 3.01 | .003 | 53379 | n.s. | N |
| −1 | 24 | −4 | Midline | Anterior cingulate (BA 24/32) | 2.94 | .004 | 8424 | 8.89/.0033** | N |
| −37 | −78 | −12 | Left | Fusiform gyrus (BA 19) | 2.66 | .009 | 432 | n.s. | N |
| −53 | −27 | 4 | Left | Superior temporal gyrus (BA 22) | 2.97 | .003 | 7344 | n.s. | Y |
| 46 | −3 | −6 | Right | Insular cortex (BA 13) | 2.67 | .008 | 486 | n.s. | Y |
| −38 | −38 | 10 | Left | Superior temporal gyrus (BA 41) | 2.80 | .006 | 1890 | 6.83/.0098** | Y |
| −42 | −72 | 0 | Left | Middle occipital gyrus (BA 19) | 2.59 | .010 | 621 | n.s. | Y |
| −45 | −16 | 40 | Left | Post-central gyrus (BA 3) | 3.09 | .002 | 19251 | n.s. | Y |
| 12 | −16 | 74 | Right | Motor cortex | 2.75 | .007 | 891 | n.s. | Y |
Note: SCZ, schizophrenia patients; HCS, healthy comparison subjects; BA, Brodmann area. All statistics were calculated across all voxels for the identified cluster that survived the group t test presented in figure 1. Seed × Dx F test denotes an interaction between LGN vs MD seed and SCZ vs HCS groups; Dx, Diagnosis.
*Negative T value denotes a reduction for SCZ vs HCS.
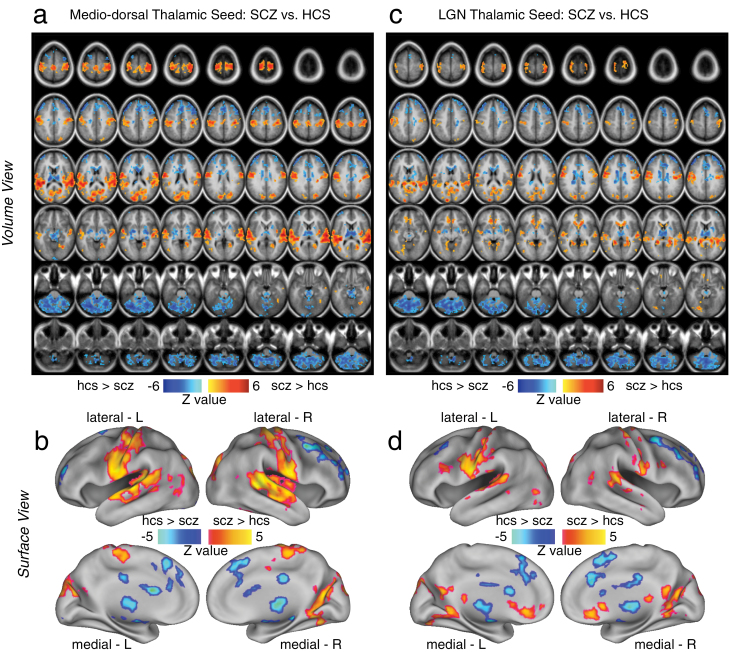
Fig. 1.
Differences in mediodorsal (MD) vs occipital-projecting thalamic nuclei in schizophrenia (SCZ). Threshold-free cluster enhancement36 whole-brain corrected volume and surface maps of group differences between SCZ patients and matched healthy comparison subjects (HCS). Results are shown for the MD prefrontal-projecting thalamic seed (panels a and b) and the lateral geniculate nucleus (LGN) occipital-projecting seed (panel b and c), both defined using the FSL atlas19 (see “Method” section for a detailed description of seed selection). Red foci mark regions where SCZ showed statistically higher connectivity than HCS, whereas blue foci show regions where SCZ show statistically lower connectivity than HCS for a given thalamic seed. For a complete list of regions and statistics see table 2. Note: Panels a and c show the results in a volume representation, whereas panels b and d show the same data mapped onto a surface representation.
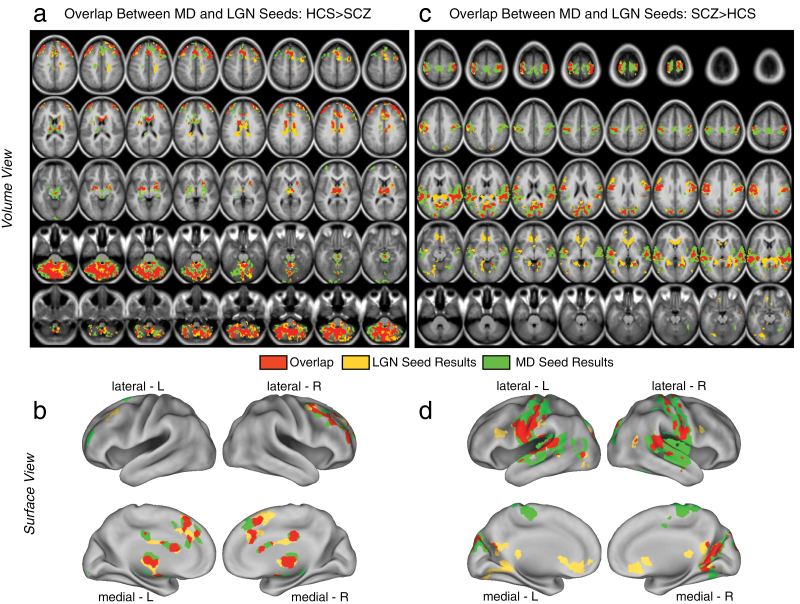
Fig. 2.
Formal overlap analysis between mediodorsal (MD) vs occipital-projecting thalamic nuclei group differences in schizophrenia (SCZ). (a and b) Regions exhibiting reductions for SCZ vs HCS are shown for the LGN seed-based results (yellow) vs MD seed-based results (green). The overlap is shown in red. (c and d) Regions exhibiting increases in connectivity for SCZ vs HCS are shown with the same color scheme as panel a. The overlap analysis (red) indicated similar patterns for MD and LGN seed-based results in areas showing reductions for SCZ vs HCS (panels a and b). In contrast, for areas showing increases in SCZ vs HCS, there were notable differences in between MD and LGN seeds (eg, LGN seed-based results indicated more prominent differences in both medial prefrontal clusters and parts of visual cortex, see table 2 for a complete list of overlapping foci). Note: Panels a and c show the results in a volume representation, whereas panels b and d show the same data mapped onto a surface representation.
MD and LGN Thalamic Connectivity in BD
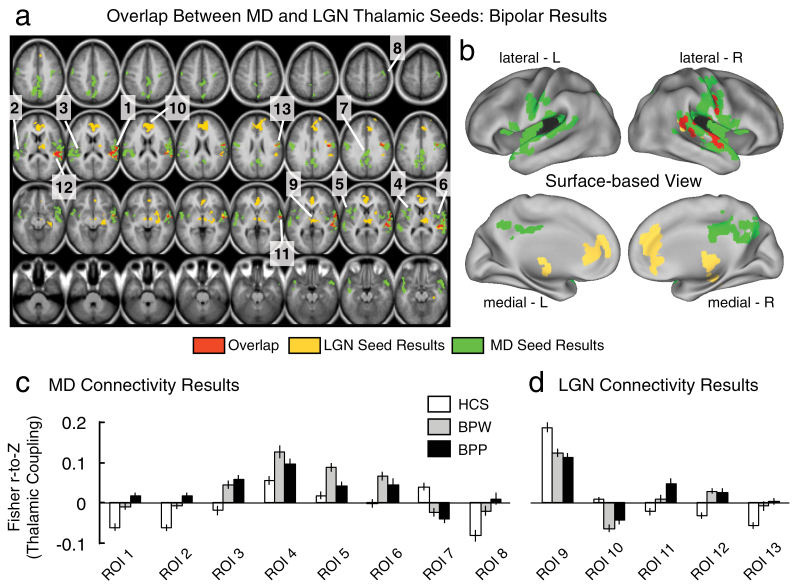
Next we examined whether thalamic connectivity patterns between MD and LGN seeds may also differ in BD. Furthermore, we specifically tested whether history of psychosis may be a key discriminating variable between BD patients. To test this possibility, we first computed a 1-way ANOVA at the whole-brain level with one between-group factor (HCS, BPW, and BPP) for each seed. Here we tested if MD or LGN seeds show unique patterns of dysconnectivity across bipolar groups. Complete findings are presented in figure 3, highlighting the ANOVA results for the MD thalamus (green foci) vs the LGN thalamic seed (yellow foci). As evident from the conjunction analysis (overlap shown in red foci), there were only a few places where the 2 seeds produced converging results (right lateral motor cortex and superior temporal gyrus around the auditory cortex). That is, the MD and LGN results largely diverged, showing dissociable patterns of between-group effects in BD. This pattern of findings was distinct from the SCZ results, whereby the MD/LGN patterns showed prominent spatial overlap. While the MD/LGN patterns differed in their spatial configuration, it remains possible that each of the results is uniquely driven by one of the BD groups (figure 3). In contrast to this hypothesis, a similar qualitative pattern of disturbances for BPW and BPP groups was generally observed (figure 4); the key difference was the magnitude of dysconnectivity across the 2 BD groups (see figures 3c and 3d for magnitudes of dysconnectivity). Another important BD result was the absence of cerebellar dysconnectivity across both MD and LGN analyses, which featured prominently in SCZ analyses (see figure 1). As for the SCZ effects, we also formally tested whether the “severity” of dysconnectivity within the identified foci actually differed as a function of the thalamic seed across the 2 bipolar subgroups. To this end, we computed a follow-up Diagnosis (BPP vs BPW) × Seed (LGN vs MD) ANOVA for all identified regions (see table 3). Three foci revealed a significant main effect of Diagnosis and a trend-level Diagnosis × Seed interaction, suggesting that the pattern of dysconnectivity differed in severity across the 2 nuclei for the BPP vs BPW group (see table 3). Interestingly, all three foci were centered on sensory-motor cortices (regions of interest [ROIs] 1, 2 and 11, see figure 3), for which BPP exhibited a more severe pattern of dysconnectivity. Collectively, these results suggest (1) that MD and LGN seeds show qualitative differences in dysconnectivity in bipolar illness, indicative of different disruptions; (2) That 3 foci centered on sensory-motor cortices exhibited a somewhat more severe pattern for the BPP group, particularly prominent for the MD seed.
Fig. 3.
Differences in mediodorsal (MD) vs occipital-projecting thalamic nuclei in bipolar disorder (BD) with and without psychosis history. (a and b) 1-way ANOVA results for the MD thalamic seed (green) and the occipital-projecting LGN thalamic seed (yellow) showing differences between bipolar groups (with and without psychosis history, BPP and BPW) and healthy comparison subjects (HCS). The formal overlap between the 2 seeds is shown in red. Each of the surviving regions of interest (ROI) is marked with a number, corresponding to the ROI label on the x-axis in panels c and d. (c) Magnitudes of connectivity between the MD seed and each of the identified foci across the 3 groups, to facilitate interpretation of the ANOVA effect. (d) Magnitudes of connectivity between the LGN seed and each of the identified foci across the 3 groups. The pattern shows that in only a few cases is the effect driven by psychosis history (ie, the BPP group). Also, as evident from the maps, the LGN and MD seeds revealed largely non-overlapping patterns of between-group differences (unlike patterns in SCZ). Error bars mark ±1 SEM. Complete maps of group pairwise comparisons (ie, BPW vs HCS and BPP vs HCS) are shown in figure 4; for a complete list of overlapping foci, see table 3. Note: Volume-based results are displayed in panel a, whereas panel b shows the same data mapped onto a surface representation.
Table 3.
MD and LGN Results—Bipolar Analyses
| X | Y | Z | Hemisphere | Anatomical Landmark | Mean T Value | P Value 2 Tail | Cluster Size (mm3) | Seed × Dx F Test (F/P Value) | Overlap | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPP vs HCS | BPW vs HCS | BPP vs BPW | BPP vs HCS | BPW vs HCS | BPP vs BPW | ||||||||
| MD thalamic seed F test results | |||||||||||||
| 53 | −15 | 9 | Right | Superior temporal gyrus (BA 22) | 2.79 | 1.99 | 0.96 | .006 | .049 | .340 | 19386 | 3.62/.06trend | Y |
| −52 | −18 | 10 | Left | Superior temporal gyrus (BA 41) | 2.84 | 2.01 | 0.95 | .006 | .047 | .345 | 11367 | 2.78/.1trend | N |
| −35 | −9 | 3 | Left | Insular cortex (BA 13) | 2.99 | 2.66 | 0.54 | .004 | .009 | .591 | 1134 | n.s. | N |
| −30 | 10 | 7 | Left | Anterior insular cortex (BA 13) | 1.84 | 3.13 | 1.11 | .069 | .002 | .271 | 459 | n.s. | N |
| −45 | 5 | 6 | Left | Insular cortex (BA 13) | 1.07 | 3.03 | 1.84 | .288 | .003 | .070 | 864 | 2.84/.1trend | N |
| 40 | −8 | 7 | Right | Insular cortex (BA 13) | 1.67 | 2.95 | 0.79 | .099 | .004 | .432 | 1053 | n.s. | N |
| 0 | −52 | 35 | Midline | Precuneus (BA 7) | −2.80 | −2.30 | 0.54 | .006 | .024 | .591 | 11232 | n.s. | N |
| 44 | −16 | 56 | Right | Precentral gytus (BA 4) | 2.77 | 2.33 | 0.64 | .007 | .022 | .524 | 594 | n.s. | N |
| LGN thalamic seed F test results | |||||||||||||
| 14 | −15 | 0 | Right | Thalamus | −2.44 | −2.21 | 0.28 | .017 | .030 | .780 | 5913 | n.s. | N |
| 3 | 37 | 15 | Midline | Anterior cingulate (BA 32) | −2.17 | −3.22 | 0.79 | .033 | .002 | .432 | 11070 | n.s. | N |
| 58 | −8 | 0 | Right | Superior temporal gyrus (BA 22) | 2.70 | 1.29 | 1.40 | .008 | .200 | .166 | 1485 | n.s. | Y |
| 52 | −33 | 14 | Right | Superior temporal gyrus (BA 41) | 2.35 | 2.67 | 0.12 | .021 | .009 | .905 | 4050 | n.s. | Y |
| 45 | −10 | 26 | Right | Precentral gyrus (BA 6) | 2.68 | 2.16 | 0.47 | .009 | .033 | .640 | 1107 | n.s. | Y |
Note: BPP, bipolar patients with psychosis history; BPW, bipolar patients without psychosis history; HCS, healthy control subjects; BA, Brodmann area. All statistics were calculated across all voxels for the identified cluster that survived the 1-way ANOVA F test presented in figures 3 and 4. Values marked in bold font denote the specific pairwise effect that is driving the 1-way ANOVA result. Seed × Dx F test denotes an interaction between LGN vs MD seed and BPW vs BPP groups; Dx, Diagnosis.
*Negative T value denotes a reduction for BPW or BPP vs HCS.
Comparing MD and LGN Thalamic Connectivity in BD vs SCZ
Based on the pattern of ANOVA results presented above, it may be possible that the BPP group shows a pattern of MD/LGN thalamic dysconnectivity that is more in line with SCZ. While the ANOVA results indicate notable between-group differences, it remains important to directly test this hypothesis by comparing each of the BD groups to SCZ patients directly (figure 5). To this end, we identified a subset of SCZ patients (N = 73) that were demographically similar to the BD patients (see “Methods” section). Direct post hoc pairwise comparisons revealed a pattern in line with a priori predictions—the BPP group showed fewer differences from the SCZ group apart from reduced cerebellum connectivity with MD thalamus in SCZ (figure 5a). Of note, there was no evidence surviving appropriate type I error correction that suggested differences in sensory-motor cortices between SCZ and BPP groups. In contrast, the SCZ group exhibited over-connectivity between MD thalamus and the sensory-motor cortices relative to BPW patients (figure 5c). Interestingly, the LGN seed was not associated with elevated sensory-motor coupling in SCZ relative to either of the BD groups (figures 5b and 5d). Collectively, these focused pairwise comparisons are consistent with the hypothesis that BPP patients may show a pattern of thalamocortical dysconnectivity that is more severe and closer to those observed in SCZ. Finally, these cross-diagnostic results underscore the importance of the thalamocerebellar dysconnectivity as a possible unique abnormality present in SCZ, but not as evident in BD.
Discussion
We investigated if thalamic subnuclei, with known differences in cortical projection pathways, exhibit dissociable disturbances in SCZ at the whole-brain level. We focused on the MD given its prefrontal projections, whereas LGN analyses established specificity of MD effects. Results revealed similar spatial patterns of whole-brain dysconnectivity in SCZ across nuclei, with some notable regional differences. Conversely, cross-diagnostic analyses focusing on BD revealed two novel effects: (1) while MD and LGN dysconnectivity qualitatively differed in BD these effects were modest quantitatively; (2) history of psychosis in BD was a key distinguishing variable suggesting that BPP patients exhibit thalamic dysconnectivity that is more SCZ-like.
MD and LGN Dysconnectivity Patterns in SCZ are Similar
The thalamus is segregated into distinct subnuclei that form parallel information processing loops with distinct cortical territories.37,38 This dissociation is most evident between MD and LGN, known to project to prefrontal vs occipital cortex. Mounting evidence implicates the thalamus as a key node in the disrupted information flow in SCZ.8,39–42 Importantly, distinct thalamic subdivisions (such as the MD and LGN) could show qualitatively dissociable patterns of dysconnectivity in SCZ due to unique cortical projection patterns. Establishing such finer-grained probes of thalamocortical disturbances in SCZ is important to delineate connectivity pathways that may be most sensitive to system-level disruptions caused by this illness. Voxel-wise pattern of thalamocortical dysconnectivity revealed similar patterns for the MD and LGN seeds in SCZ. The similarities were notable for cortical regions that showed reduced thalamic connectivity in SCZ (see figure 1, blue foci). Differences were more evident for those regions where SCZ showed thalamic over-connectivity: the LGN seed was associated with more pronounced dysconnectivity around primary visual regions and the anterior cingulate. The first observation is not surprising given that the LGN is the primary thalamic nucleus projecting to the occipital lobe. The anterior cingulate effect (see figure 2b, surface map) was more surprising. This effect was absent for the MD seed even when examining the maps at a lower threshold. It is important to consider, however, that resting-state functional connectivity captures both direct (monosynaptic) and more complex (polysynaptic) pathways that do not cleanly follow direct anatomical pathways.
These effects point to several future directions: First, it still remains to be established if the thalamocortical dysconnectivity in SCZ reflects changes in anatomical connectivity. Second, it will be important to test whether observed effects in any way relate to the heterogeneity of this dynamic neurodevelopmental illness. This could be tested in 2 ways: (1) examining if thalamocortical patterns alter as a function of illness progression (or appear even during early illness stages); (2) whether these patterns show heterogeneity across patients with specific symptom profiles. Third, no study has linked these system-level effects in chronic SCZ to observations following pharmacological manipulations (eg, the N-methyl-D-aspartate receptor antagonist effects).43–45 Such clinical-pharmacologic comparisons will be key to test mechanisms of specific neurotransmitter contributions to observed large-scale connectivity patterns in SCZ.43,44
MD and LGN Dysconnectivity Patterns in BD
We tested 2 novel follow-up cross-diagnostic questions: (1) are there qualitative differences between the MD and LGN seed results in BD? (2) Is history of psychosis in BD associated with a more severe pattern of thalamocortical dysconnectivity? Both questions were motivated by a number of observations suggesting that BD may share features with SCZ across genetic, neural system-level and behavioral levels of analyses.14,15,46 In particular, co-occurrence of psychosis in BD has been associated with a unique pattern of neuroimaging findings from those BD patients that follow a psychosis-free clinical curse using fully data-driven approaches.18 Delineating both shared vs distinct patterns of neural network abnormalities across the 2 conditions represents a vital effort as articulated by the National Institute of Mental Health Research Domain Criteria Initiative.47 The thalamus may represent a key node in this effort given its widespread connectivity with virtually all cortical territories. Moreover, prior investigations found that thalamic over/under connectivity identified in SCZ may be present in BD, just to a lesser extent.9 Yet, no study has examined whether this pattern is, perhaps, more severe for BD patients with co-occurring psychosis.
Our results revealed 3 findings with respect to BD: first, the MD/LGN seeds produced qualitatively distinct between-group connectivity patterns (although these differences were modest in magnitude). The patterns converged around the right motor cortex and superior temporal gyrus (see figure 3 and table 3). This suggests that, unlike in SCZ, there are some disparities in how the MD and LGN brain-wide connectivity patterns are affected in BD. Second, there was a conspicuous absence of any cerebellar effects in BD. That is, neither BPP nor BPW was associated with thalamocerebellar dysconnectivity—a prominent marker in SCZ and a key element of long-standing theoretical models of this disorder.8,39–42 Third, BPW and BPP patterns did not always qualitatively diverge, but rather the BPP group was associated with a somewhat more “severe” pattern of dysconnectivity than BPW (see below for further discussion). Relatedly, BPW showed localized alterations for the MD seed that were not present in BPP, specifically around bilateral anterior insular cortex (see figures 3b and 4, ROIs 4,5 and 6). These regional effects may reflect true underlying differences in specific circuit disturbances that could dissociate psychotic and nonpsychotic BD, perhaps, related to mood regulation vs psychosis.
Present BD findings also highlight a focus for future studies that aim to establish more robust biomarker-based classification via machine-learning tools.48 For instance, one possible application of these findings in subsequent independent investigations is to explicitly combine multiple seed-derived functional connectivity maps that have been associated with functional dissociations between diagnostic categories (as shown here). Consequently, such studies could form a “multi-modal” or “multi-variable” connectivity-based classification that is better able to separate patient subgroups (as opposed to focusing on any one circuit in particular). Lastly, the BD findings were identified in euthymic individuals (who were neither clinically depressed nor manic at the time of the scan). Therefore, the clinical state of the participants suggests that the observed markers may be related to a trait underlying system-level alterations, which persists even in the absence of frank mood symptoms at the time of the scan. This observation, however, leaves open the possibility that the BPP subgroup may indeed appear even more SCZ-like at the time of acute symptom exacerbation (ie, during psychotic episodes). Relatedly, present results may imply that worse dysfunction (ie, bipolar illness and co-occurring psychosis) is associated with worse patterns of thalamocortical dysconnectivity. Future studies in acutely symptomatic BPP patients are needed to test this possibility.49
BD With Psychosis History Shows Thalamocortical Dysconnectivity More Consistent With SCZ
A number of studies and theoretical models have hypothesized that BD with co-occurring psychosis may reflect a clinical condition closer neurobiologically to alterations found in SCZ. Indeed, prior work has shown that BD (without considering psychosis history) was associated with thalamocortical dysconnectivity similar to those found in SCZ.9 Here we tested for the first time whether BPP vs BPW show distinguishing features with respect to MD and LGN whole-brain connectivity patterns. We found, consistent with predictions, differences between the 2 BD groups (some of which are discussed above). We also quantitatively compared the 2 BD groups relative to SCZ patients matched for relevant demographic characteristics. These secondary analyses added 2 important insights: (1) for the MD seed there was a reduction in functional connectivity in BPW relative to SCZ (figure 5c) across bilateral sensory-motor regions, that is, we found over-connectivity in SCZ vs BPW but no such effect was evident for the SCZ vs BPP contrast (figure 5a). (2) There was clear evidence for reduced cerebellar-thalamic connectivity in SCZ relative to both BD groups. The cerebellar effect may be a uniquely distinguishing neural marker of SCZ directly related to the illness-specific pathophysiology. Future combined longitudinal/cross-sectional studies across diagnoses will be needed to directly test this possibility. Collectively, these cross-diagnostic comparisons are consistent with the hypothesis that BPP does exhibit a more severe pattern of SCZ-like thalamic dysconnectivity across certain areas, perhaps, due to common disturbances owing to shared genetic risk.12
Limitations
We noted that BD patients were euthymic. This is a key consideration because SCZ patients exhibited some active symptoms (table 1). Because we found similarities between BPP and SCZ, these effects imply that identified shared neural disturbances may relate to psychosis cross-diagnostically rather than to a state-like “psychosis” effect in SCZ specifically. Nevertheless, examining the impact of mood alterations on the observed effects will be important, independently of psychosis. Relatedly, BD and SCZ patients took different medication (see tables 1 and 4). Paradoxically, this raises confidence that the effects are indeed not entirely driven by medication in the SCZ group. Yet, it is impossible to fully ascertain whether lower (or different) level of medication in BD caused the “attenuated” patterns of dysconnectivity (an issue present across our prior cross-diagnostic studies).9 Although we did not find evidence that medication class or levels of chlorpromazine (CPZ) equivalents in SCZ explained effects, future studies that are able to study unmedicated chronic, first-episode patients or high-risk clinical groups need to establish the robustness of present findings independent of medication. Ultimately, to more fully inform the dichotomy between SCZ and BD with psychosis future studies will need to more carefully control for differences in medication. We noted that considering the clinical heterogeneity remains an important future direction. For instance, do these effects remain stable over time and present even during incipient stages of psychotic illness, or are they a marker of more chronic illness phases? Another limitation relates to prior history of substance use. While none of the participants met current substance abuse/dependence criteria at the time of the scan, prior substance/alcohol history was difficult to fully control and should be considered a limitation. Also, an often-neglected issue in rs-fcMRI studies is the inherently correlational conclusions that can be drawn. The observed alterations could reflect a cause or consequence of chronic SCZ and BD. Future pharmacological neuroimaging studies could provide a manipulation of specific neurotransmitter pathways that will help inform causal mechanisms.
Table 4.
Bipolar Sample Demographics
| Characteristic | BPP (N = 33) | BPW (N = 40) | HCS (N = 56) | Significance (HCS, BPP, BPW) | Significance (BPW vs BPP) | ||
|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | F Value/ Chi-Square | P Value, 2 Tail | T Value/ Chi-Square | P Value, 2 Tail | |
| Age (y) | 34.18 (10.9) | 30.20 (11.5) | 31.25 (10.3) | 1.29 | .28 | 1.51 | .14 |
| Gender (% female) | 21 (63%) | 32 (80%) | 32 (57%) | 5.52 | .06 | 2.43 | .12 |
| Education (y) | 13.94 (1.6) | 14.45 (2.1) | 15.11 (2.1) | 3.75 | .03 | 1.14 | .26 |
| Mother’s education (y) | 13.67 (3.0) | 14.26 (2.3) | 13.63 (2.6) | 0.77 | .47 | 0.96 | .34 |
| Father’s education (y) | 14.64 (3.4) | 15.00 (3.8) | 12.98 (3.9) | 3.97 | .02 | 0.43 | .67 |
| Mean parental education | 14.15 (2.9) | 14.63 (2.7) | 13.30 (2.9) | 2.45 | .09 | 0.67 | .50 |
| Clinical course | |||||||
| Age at diagnosis (y) | 18.27 (6.1) | 18.73 (6.9) | N/A | — | — | 0.29 | .77 |
| Duration of illness (y) | 15.91 (10.9) | 11.48 (9.1) | N/A | — | — | 1.89 | .06 |
| Current symptomatology | |||||||
| Depression (HAM-D) | 3.12 (3.1) | 4.33 (4.0) | 0.33 (0.7) | 25.80 | .0000 | 1.41 | .16 |
| Mania (YMRS) | 2.45 (3.3) | 2.88 (3.7) | 0.15 (0.4) | 14.55 | .0000 | 0.51 | .61 |
| Psychosis (BPRS) | 28.79 (4.4) | 27.90 (3.6) | 24.56 (1.0) | 23.56 | .0000 | 0.95 | .35 |
| Medications, n (%) | |||||||
| Mood stabilizer(s) | 18 (54%) | 19 (47%) | N/A | — | — | 0.36 | .55 |
| Antidepressant(s) | 11 (33%) | 20 (50%) | N/A | — | — | 2.06 | .15 |
| Atypical antipsychotic(s) | 15 (45%) | 10 (25%) | N/A | — | — | 3.36 | .07 |
| Anxiolytic/benzodiazepine(s) | 12 (36%) | 14 (35%) | N/A | — | — | 0.01 | .90 |
| Lithium | 8 (24%) | 5 (12%) | N/A | — | — | 1.70 | .19 |
| Unmedicated | 6 (18%) | 6 (15%) | N/A | — | — | 0.13 | .72 |
| Typical Antipsychotic(s) | 0 (0%) | 1 (2%) | N/A | — | — | 0.84 | .36 |
| Comorbid diagnoses, n (%) | |||||||
| Anxiety | 13 (39%) | 19 (47%) | N/A | — | — | 0.48 | .49 |
| Alcohol | 18 (54%) | 22 (55%) | N/A | — | — | 0.00 | .97 |
| Drug use history | 14 (42%) | 16 (40%) | N/A | — | — | 0.04 | .83 |
| Signal to noise | 217.71 (51.1) | 216.04 (54.0) | 215.45 (58.9) | 55.19 | .98 | 0.14 | .89 |
| % Frames flagged | 11.74 (9.5) | 10.82 (10.1) | 9.74 (10.4) | 0.42 | .66 | 0.43 | .67 |
Note: BPP, bipolar patients with psychosis history; BPW, bipolar patients without psychosis history; HCS, healthy control subjects; BPRS, Brief Psychiatric Rating Scale; HAM-D, Hamilton Depression rating scale; Hx, history; YMRS, Young Mania Rating Scale. Age, education levels, parental education, age at diagnosis and duration of illness are expressed in years. Of note, no pairwise BPP-BPW comparisons reached significance. As mentioned in the “Method” section, we selected a subset of SCZ (N = 73) patients that demographically matched BD patients for follow-up pairwise comparisons (shown in figure 5). If there were significant differences between clinical groups (eg, gender proportion and % frames scrubbed), we used these variables as covariates.
Table 5.
MD and LGN Results—Bipolar vs Schizophrenia Analyses
| X | Y | Z | Hemisphere | Anatomical Landmark | Mean T Value | P Value 2 Tail | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|---|---|
| BPP vs SCZ | BPW vs SCZ | BPP vs SCZ | BPW vs SCZ | ||||||
| MD thalamic seed SCZ vs BPW | |||||||||
| 57 | −27 | 9 | Right | Superior temporal gyrus (BA 42) | — | 2.86 | — | .005 | 14769 |
| −40 | −27 | −4 | Left | Temporal lobe (BA 22) | — | 2.54 | — | .012 | 891 |
| 41 | −30 | −6 | Right | Temporal lobe | — | 2.66 | — | .009 | 405 |
| −51 | −37 | 8 | Left | Middle temporal gyrus | — | 2.93 | — | .004 | 7209 |
| −58 | −9 | 18 | Left | Postcentral gyrus (BA 43) | — | 2.91 | — | .004 | 3240 |
| 39 | −27 | 11 | Right | Superior temporal gyrus (BA 13) | — | 2.67 | — | .009 | 648 |
| −33 | −61 | −43 | Left | Cerebellum | — | −2.81 | — | .006 | 7749 |
| 30 | −66 | −44 | Right | Cerebellum | — | −2.94 | — | .004 | 6561 |
| 0 | −76 | −38 | Midline | Cerebellum | — | −2.74 | — | .007 | 6453 |
| MD thalamic seed SCZ vs BPP | |||||||||
| −25 | −66 | −37 | Left | Cerebellum | −2.72 | - | .008 | - | 6831 |
| LGN thalamic seed SCZ vs BPW | |||||||||
| 1 | −66 | −45 | Midline | Cerebellum | — | −2.97 | — | .004 | 30834 |
| −31 | 11 | 8 | Left | Insula (BA 13) | — | −2.92 | — | .004 | 7128 |
| −1 | 0 | 53 | Midline | Medial frontal gyrus (BA 6) | — | −2.87 | — | .005 | 8397 |
| −30 | −7 | 51 | Left | Middle frontal gyrus (BA 6) | — | −2.85 | — | .005 | 3078 |
| LGN thalamic seed SCZ vs BPP | |||||||||
| −12 | 44 | 41 | Left | Superior frontal gyrus (BA 8) | −2.56 | — | .012 | — | 270 |
| −11 | 27 | 50 | Left | Superior frontal gyrus (BA 6) | −2.83 | — | .006 | — | 1620 |
| −13 | 2 | 55 | Left | Medial frontal gyrus (BA 6) | −2.83 | — | .006 | — | 4509 |
Note: BPP, bipolar patients with psychosis history; BPW, bipolar patients without psychosis history; SCZ, schizophrenia patients; BA, Brodmann area. All statistics were calculated across all voxels for the identified cluster that survived the group pairwise comparisons presented in figure 5.
*Negative T value denotes a reduction for SCZ vs BPW or BPP.
Conclusions
We provide 3 findings related to thalamocortical alterations in SCZ and BD: (1) we found qualitatively similar (rather than profoundly different) patterns of whole-brain MD/LGN thalamocortical dysconnectivity in SCZ at the whole-brain level (with some notable and possibly important exceptions); (2) BD was associated with more dissociable dysconnectivity across MD/LGN nuclei, albeit these qualitative differences were modest statistically; (3) BD patients with psychosis history exhibited dysconnectivity, in particular for the MD seed, that was more SCZ-like than that found for BD patients without psychosis history. These findings highlight a more finer-grained and complex pattern of thalamocortical information flow alterations across diagnostic categories with shared symptoms, which could help constrain and refine ongoing development for biomarker-driven diagnostic classification.50
Funding
National Institutes of Health grant (MH080912; PI: D.C.G.); National Institutes of Health grant (DP5OD012109-02; PI: A.A.); National Institutes of Health grants (MH43775, MH077945, MH074797 [PI: G.D.P.], R01-MH062349 to J.D.M.); National Institutes of Health Training Grant (T32GM 007205 to G.J.Y.); National Institutes of Health grant (MH096801; PI: M.W.C.); Fulbright Foundation (to A.S.); the Brain and Behavior Research Foundation Young Investigator Award (PI: A.A.).
Acknowledgments
We thank Anderson Winkler and Margaret Brumbaugh for help with initial organization of the clinical database. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939 [DOI] [PubMed] [Google Scholar]
- 2. Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salomon R, Bleich-Cohen M, Hahamy-Dubossarsky A, et al. Global functional connectivity deficits in schizophrenia depend on behavioral state. J Neurosci. 2011;31:12972–12981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anticevic A, Repovs G, Krystal JH, Barch DM. A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res. 2012;141:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42:27–33 [DOI] [PubMed] [Google Scholar]
- 9. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness [published online ahead of print]. Cereb Cortex 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2009;66:748–755 [DOI] [PubMed] [Google Scholar]
- 13. Glahn DC, Bearden CE, Barguil M, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–916 [DOI] [PubMed] [Google Scholar]
- 14. Khadka S, Meda SA, Stevens MC, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2011;2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodwin FK, Jamison KR. Manic Depressive Illness. New York, NY: Oxford University Press; 1990 [Google Scholar]
- 17. Pearlson GD, Wong DF, Tune LE, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995;52:471–477 [DOI] [PubMed] [Google Scholar]
- 18. Anticevic A, Brumbaugh MS, Winkler AM, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansen-Berg H, Behrens TE, Sillery E, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39 [DOI] [PubMed] [Google Scholar]
- 20. Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985 [Google Scholar]
- 22. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders. Washington, DC: American Psychiatric Press; 2001 [Google Scholar]
- 23. Glahn DC, Bearden CE, Bowden CL, Soares JC. Reduced educational attainment in bipolar disorder. J Affect Disord. 2006;92:309–312 [DOI] [PubMed] [Google Scholar]
- 24. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 25. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435 [DOI] [PubMed] [Google Scholar]
- 26. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296 [DOI] [PubMed] [Google Scholar]
- 27. Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters.” Int J Methods Psychiatr Res. 1993;3:221–244 [Google Scholar]
- 28. Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 2012;38:967–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355 [DOI] [PubMed] [Google Scholar]
- 34. Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757 [DOI] [PubMed] [Google Scholar]
- 35. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98 [DOI] [PubMed] [Google Scholar]
- 37. Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271 [DOI] [PubMed] [Google Scholar]
- 38. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381 [DOI] [PubMed] [Google Scholar]
- 39. Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andreasen NC, Arndt S, Swayze V, II, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298 [DOI] [PubMed] [Google Scholar]
- 42. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218 [DOI] [PubMed] [Google Scholar]
- 43. Anticevic A, Gancsos M, Murray JD, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109:16720–16725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Driesen NR, McCarthy G, Bhagwagar Z, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214 [DOI] [PubMed] [Google Scholar]
- 46. Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29:1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66:988–989 [DOI] [PubMed] [Google Scholar]
- 48. Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430 [DOI] [PubMed] [Google Scholar]
- 49. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]