Abstract
Background
The development of intensity-modulated radiotherapy (IMRT) has revolutionized the management of nasopharyngeal carcinoma (NPC). The purpose of this study was to evaluate the prognostic value and classification of TNM stage system for retropharyngeal lymph node (RLN) metastasis in NPC in the IMRT era.
Material and Methods
We retrospectively reviewed data from 749 patients with biopsy-proven, non-metastatic NPC. All patients received IMRT as the primary treatment. Chemotherapy was administered to 86.2% (424/492) of the patients with stage III or IV disease.
Results
The incidence of RLN metastasis was 64.2% (481/749). Significant differences were observed in the 5-year disease-free survival (DFS; 70.6% vs. 85.4%, P<0.001) and distant metastasis-free survival (DMFS; 79.2% vs. 90.1%, P<0.001) rates of patients with and without RLN metastasis. In multivariate analysis, RLN metastasis was an independent prognostic factor for disease failure and distant failure (P = 0.005 and P = 0.026, respectively), but not for locoregional recurrence. Necrotic RLN metastases have a negative effect on disease failure, distant failure and locoregional recurrence in NPC with RLN metastasis (P = 0.003, P = 0.018 and P = 0.005, respectively). Survival curves demonstrated a significant difference in DFS between patients with N0 disease and N1 disease with only RLN metastasis (P = 0.020), and marginally statistically significant differences in DMFS and DFS between N1 disease with only RLN metastasis and other N1 disease (P = 0.058 and P = 0.091, respectively). In N1 disease, no significant differences in DFS were observed between unilateral and bilateral RLN metastasis (P = 0.994).
Conclusions
In the IMRT era, RLN metastasis remains an independent prognostic factor for DFS and DMFS in NPC. It is still reasonable for RLN metastasis to be classified in the N1 disease, regardless of laterality. However, there is a need to investigate the feasibility of classifying RLN metastasis as N1a disease in future by a larger cohort study.
Introduction
Although nasopharyngeal carcinoma (NPC) is rare in most regions of the world, it is endemic in certain regions, especially Southeast Asia. The incidence of NPC is approximately 30–80 per 100,000 per year in Southern China [1]. The nasopharynx contains a well-developed network of lymph nodes, and the retropharyngeal lymph nodes (RLN) are regarded as one of the key lymph nodes in NPC [2]. Due to the limitations of CT imaging, RLN metastases are isodense and contiguous with the primary tumor, and can be difficult to identify as a separate mass on CT scans; therefore, the true incidence of RLN metastasis was previously underestimated [3]–[5]. However, due the superior soft tissue contrast and multiplanar capacity of MRI, the incidence of RLN metastasis in NPC is currently approximately 70% [5], [6], and RLN metastasis has received increasing attention in recent years.
The recent seventh edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) staging system for NPC incorporated RLN metastasis into the tumor-node-metastasis (TNM) classification, and classified it as N1 disease regardless of laterality [7], [8]. However, this revision was based on patients treated with a two-dimensional conventional radiotherapy technique. Intensity-modulated radiotherapy (IMRT) offers an improved tumor target conformity, allows safer dose escalations and yields superior results in NPC compared to 2D-CRT, primarily by achieving a higher local tumor control rate [9]. IMRT has gradually replaced two-dimensional conventional radiotherapy as the primary radiotherapy modality for the treatment of NPC; however, the introduction of new therapeutic technologies may require a revaluation of the prognostic value and appropriate classification of TNM staging system for RLN metastasis in NPC.
In this study, we analyzed the outcomes of patients with NPC staged by MRI prior to treatment who subsequently received IMRT and the current standard systemic treatments, to investigate whether it is still reasonable to classify RLN metastasis in NPC as N1 disease in the IMRT era.
Methods and Materials
Patient characteristics
Approval for retrospective analysis of the patient data was obtained from the ethics committee of Sun Yat-sen University Cancer Center. Written consent was waived, while oral consent from the patients was obtained via telephone and documented by telephone recording. All 749 patients with newly diagnosed, biopsy-proven, non-metastatic NPC who were treated at Sun Yat-Sen University Cancer Center using IMRT between January 2003 and December 2007 were retrospectively reviewed. The clinicopathologic characteristics of the patients are shown in Table 1.
Table 1. The clinicpathological characters of 749 patients in this study.
| Characteristic | N | Column (%) |
| Age | ||
| >50 | 553 | 73.8 |
| ≤50 | 196 | 26.2 |
| Gender | ||
| Male | 580 | 77.4 |
| Female | 169 | 22.6 |
| Histologic type | ||
| WHO II/III | 744 | 99.3 |
| WHO I | 5 | 0.7 |
| T category | ||
| T1 | 177 | 23.6 |
| T2 | 140 | 18.7 |
| T3 | 264 | 35.2 |
| T4 | 168 | 22.4 |
| N category | ||
| N0 | 184 | 24.6 |
| N1 | 409 | 54.6 |
| N2 | 106 | 14.2 |
| N3 | 50 | 6.7 |
| Stage | ||
| I | 78 | 10.4 |
| II | 179 | 23.9 |
| III | 282 | 37.7 |
| Iva-b | 210 | 28.0 |
| Chemotherapy | ||
| No | 214 | 28.6 |
| Concurrent | 233 | 33.5 |
| Concurrent + Induction | 246 | 32.8 |
| Concurrent + Adjuvant | 46 | 6.1 |
| PET-CT | ||
| Yes | 162 | 21.6 |
| No | 587 | 78.4 |
All patients completed a pre-treatment evaluation including complete patient history, physical examination, hematology and biochemistry profiles, neck and nasopharyngeal MRI, chest radiography, abdominal sonography and a single photon emission computed tomography (SPECT) whole body bone scan. 18-F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)-CT was performed on 162/749 patients (21.6%). All patients were retrospectively re-staged according to the 7th edition of UICC/AJCC staging system. The distribution of disease stages was: stage I, 10.4%; stage II, 23.9%; stage III, 37.7% and stage IVa–b, 28.0% (Table 1).
MRI techniques and criteria for retropharyngeal lymph node and other cervical lymph node metastasis
All patients underwent MRI scans using a 1.5-T system (Signa, General Electric, CV/i; General Electric Healthcare, Chalfont St. Giles, United Kingdom). The area from the suprasellar cistern to the inferior margin of the sternal end of clavicle was examined with a head and neck combined coil. T1-weighted fast spin-echo images in the axial, coronal and sagittal planes (repetition time of 500–600 ms and echo time of 10–20 ms), and T2-weighted fast spin-echo MR images in the axial plane (repetition time of 4000–6000 ms and echo time of 95–110 ms) were obtained before injection of contrast material. After intravenous injection of Gd-DTPA at a dose of 0.1 mmol/kg body weight, spin-echo T1-weighted axial and sagittal sequences, and spin-echo T1-weighted fat-suppressed coronal sequences were performed sequentially using parameters similar to those used before injection of contrast. The section thickness for the axial plane was 5 mm with a 1 mm interslice gap, and 6 mm with a 1 mm interslice gap for the coronal and sagittal planes.
Two radiologists specializing in head and neck cancers evaluated all of the scans independently. Any disagreements were resolved by consensus. The diagnostic MRI criteria for metastatic lymphadenopathy included: 1) lateral RLN with a minimal axial diameter of ≥5 mm and any node seen in the median retropharyngeal group, or lymph nodes with a minimal axial diameter ≥11 mm in the diagastric region or ≥10 mm for all other cervical nodes except the retropharyngeal group; 2) lymph nodes of any size with central necrosis or a contrast-enhanced rim; and 3) nodal grouping: the presence of three or more contiguous and confluent lymph nodes, each of which should have a minimal axial diameter of 8–10 mm [10]–[12]. The criteria for extranodal neoplastic spread (ENS) in RLN was the presence of indistinct nodal margins, irregular nodal capsular enhancement or infiltration into the adjacent fat or muscle [13].
Treatment
All patients received IMRT as the primary treatment. The patients were immobilized in supine position by a thermoplastic mask. After administration of intravenous contrast material, 3-mm CT slices, depicting the area of the head until 2 cm below the sterno-clavicular joint, were acquired. The primary tumor and the upper-neck area above the caudal edge of the cricoid cartilage were treated by IMRT. Target volumes were in agreement with the International Commission on Radiation Units and Measurements Reports 50 and 62. The contoured images were transferred to an integrated IMRT planning and delivery system (Peacock, Corvus 3.0, NOMOS Corporation, Sewickley, Pa). The prescribed radiation dose was defined as follows: a total dose of 68 Gy in 30 fractions at 2.27 Gy per fraction to the planning target volume (PTV) of the primary gross tumor volume (GTV-P), 60 to 64 Gy to the PTV of nodal gross tumor volume (GTV-N), 60 Gy to the PTV of CTV-1 (i.e., high-risk regions), and 54 Gy to the PTV of CTV-2 (i.e., low-risk regions) and CTV-N (i.e., neck nodal regions). The treatment was delivered by a dynamic, multileaf, intensitymodulating collimator (called MIMiC). For the lower neck, an anterior cervical field was used. All patients were treated with one fraction daily over 5 days per week.
Chemotherapy was administered to 86.2% (424/492) of the patients with stage III or IV disease. The chemotherapy regimens included concurrent chemotherapy alone, concurrent chemotherapy combined with induction chemotherapy and/or adjuvant chemotherapy in conjunction with a platinum-based therapeutic clinical trial. Reasons for deviation from institutional guidelines included patients' refusal, age (≥70 years), organ severe dysfunction (diabetes, cardiac dysfunction, renal insufficiency, liver insufficiency, et al) that would suggest intolerance to chemotherapy. When possible, salvage treatments such as intracavitary brachytherapy, surgery and chemotherapy were provided in the event of documented relapse or persistent disease.
Follow-up
Median follow-up was 81 months (range, 3–127 months). Each patient was assessed weekly during treatment for treatment response and toxicity, and every 2–3 months during the first 2 years and every 3–6 months during years 3–5 after radiotherapy. Endoscopy, CT or MRI scans of the head and neck were performed every 3 months during the first year and annually during years 2–5. Patients with residual or recurrent local disease underwent biopsy to confirm malignancy. Additional tests were ordered when indicated to evaluate for local or distant failure.
Statistical analysis
All analyses were performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). Actuarial rates were estimated by the Kaplan-Meier method; survival curves were compared using the log-rank test [14]. The following endpoints (measured from the start of treatment to the first defining event) were estimated: locoregional relapse-free survival (LRRFS), local relapse-free survival (LRFS), and nodal relapse-free survival (NRFS), DMFS, disease-free survival (DFS) and overall survival (OS).
Multivariate analyses with the Cox proportional hazards model were used to test for independent significance by backward elimination of insignificant explanatory variables [15]. The Cox proportional hazards model was used to calculate hazard ratios (HRs). Two-tailed P values <0.05 were considered statistically significant.
Results
Incidence of RLN metastasis
In this study, no patient had a metastatic median RLN, and the incidence of lateral RLN metastasis in the current study was 64.2% (481/749 patients). Thirty-two percent (154/481) of patients with RLN metastasis had no evidence of cervical lymph node (CLN) metastasis, and 79.6% (327/411) patients with CLN metastasis had evidence of RLN involvement.
Of 481 the patients with RLN metastasis, 63.2% (304/481) had unilateral RLN involvement, whereas 36.8% (177 of 481) had bilateral involvement. The mean minimal and maximal axial diameters of the RLN metastases were 9.61±4.31 mm (range, 5–28 mm) and 12.66±5.61 mm (range, 5–36 mm). The incidence of RLN necrosis was 13.3% (64/481) and the incidence of ENS was 21.8% (105/481).
Prognostic value of RLN metastasis
There were 56/749 (7.5%) patients developed recurrence, including 34 patients (4.5%) with isolated local recurrences, 15 patients (2.0%) with isolated regional nodal recurrences, and 7 patients (0.9%) with both local and regional nodal recurrence. In addition, there were 129 (17.2%) patients developed distant metastases and 149 (19.9%) died. The 5-year survival rates were: LRRFS, 92.9%; DMFS, 83.1%; DFS, 75.9% and OS, 83.9%.
Significant differences were observed in the 5-year DFS (70.6% vs. 85.4%, P<0.001), DMFS (79.2% vs. 90.1%, P<0.001) and LRRFS (90.5% vs. 97.0%, P = 0.010) rates of patients with and without RLN metastasis (Figure 1). Multivariate analysis was performed to adjust for various prognostic factors; the following known important prognostic variables were included in the Cox proportional hazards model: age (≤50 vs.>50 years), gender, T-classification, chemotherapy (yes vs. no), bilateral CLN metastasis (yes vs. no), dimension of CLN metastases (≤6 vs.>6 cm) and the location of CLN metastasis (with supraclavicular lymph nodes metastasis vs. without supraclavicular lymph nodes metastasis). Dimension of CLN metastases (≤6 vs.>6 cm) was measured based on maximal diameter by palpation. RLN metastasis was an independent prognostic factor for disease failure and distant failure (HR = 1.663, 95% CI: 1.169–2.365, P = 0.005, and HR = 1.682, 95% CI: 1.065–2.655, P = 0.026, respectively), but not for locoregional recurrence (Table 2).
Figure 1. Survival curves for nasopharyngeal carcinoma (NPC) patients with and without retropharyngeal lymph node (RLN) metastasis.
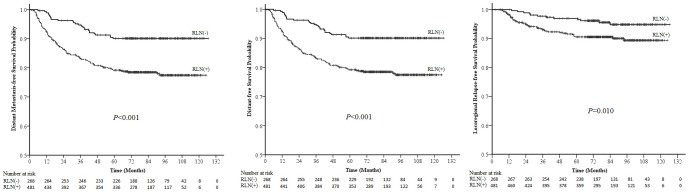
RLN (-): NPC patients without RLN metastasis; RLN (+): NPC patients with RLN metastasis.
Table 2. Summary of multivariate analysis of prognostic factors in 749 patients with nasopharyngeal carcinoma.
| Endpoint | Variable | B | P * | HR | 95% CI for HR |
| Distant failure | Retropharyngeal lymph node (yes vs. no) | 0.520 | 0.026 | 1.682 | 1.065–2.655 |
| Age (≤50 vs. 50 years) | 0.406 | 0.040 | 1.501 | 1.019–2.211 | |
| T-classification | 0.451 | <0.001 | 1.570 | 1.307–1.887 | |
| CLN dimension (≤6 vs. > 6 cm) | 1.268 | <0.001 | 3.555 | 1.965–6.434 | |
| CLN location (with SCLN vs. without SCLN) | 0.896 | 0.003 | 2.450 | 1.351–4.443 | |
| Bilateral CLN (yes vs. no) | 0.511 | 0.013 | 1.668 | 1.114–2.497 | |
| Disease failure | Retropharyngeal lymph node (yes vs. no) | 0.509 | 0.005 | 1.663 | 1.169–2.365 |
| Age (≤50 vs. 50 years) | 0.656 | <0.001 | 1.927 | 1.427–2.602 | |
| T classification | 0.435 | <0.001 | 1.544 | 1.335–1.787 | |
| CLN dimension (≤6 vs. >6 cm) | 1.068 | <0.001 | 2.908 | 1.691–5.001 | |
| CLN location (with SCLN vs. without SCLN) | 0.818 | 0.002 | 2.266 | 1.342–3.826 | |
| Bilateral CLN (yes vs. no) | 0.362 | 0.040 | 1.436 | 1.016–2.029 | |
| Locoregional recurrence | Retropharyngeal lymph node (yes vs. no) | NS | |||
| T-classification | 0.438 | 0.001 | 1.550 | 1.183–2.031 | |
| Bilateral CLN (yes vs. no) | 0.681 | 0.022 | 1.975 | 1.105–3.530 | |
Abbreviations: CI = confidence interval; HR = hazard ratio; CLN = cervical lymph nodes; NS = not significant; SCLN = Supraclavicular lymph node.
*P values were calculated using an adjusted Cox proportional-hazards model. The following known important prognostic variables were included in the Cox proportional hazards model: age (≤50 vs. >50 years), gender, T-classification, chemotherapy (yes vs. no), bilateral CLN metastasis (yes vs. no), dimension of CLN metastases (≤6 vs. >6 cm), CLN location (with SCLN vs. without SCLN) and RLN metastasis (yes vs. no).
All of the MRI-determined nodal variables were analyzed in the 481 patients with RLN metastasis using univariate analyses and multivariate analyses. The RLN variables were categorized as follows: minimal axial diameters (<10 vs. ≥10 mm MID), necrosis (no vs. yes), laterality (unilateral vs. bilateral) and ENS (no vs. yes). Univariate analysis revealed that necrosis had significant prognostic value for DMFS, DFS and LRRFS (P<0.001, P<0.001 and P<0.001; Table 3). After adjusting for various prognostic factors including age, sex, T-classification, N-classification and chemotherapy, necrosis remained significant for disease failure, distant failure and locoregional recurrence (HR = 1.795, 95%CI: 1.214–2.654, P = 0.003; HR = 1.752, 95%CI:1.100–2.790, P = 0.018 and HR = 2.614, 95%CI: 1.339–5.103, P = 0.005; Table 4).
Table 3. Five-year survival rates for 481 nasopharyngeal carcinoma patients with retropharyngeal lymph nodes metastasis according to the characteristics of retropharyngeal lymph node metastasis.
| Size (minimal axial diameters) | Necrosis | Laterality | Extranodal neoplastic spread | |||||||||
| <10 mm | ≥10 mm | P * | No | Yes | P * | Unilateral | Bilateral | P * | No | Yes | P * | |
| DMFS | 86.5 | 71.9 | <0.001 | 81.6 | 62.8 | <0.001 | 82.5 | 73.6 | 0.032 | 81.6 | 70.3 | 0.001 |
| DFS | 78.3 | 62.9 | <0.001 | 73.3 | 53.1 | <0.001 | 73.6 | 65.5 | 0.054 | 74.1 | 58.1 | 0.002 |
| LRRFS | 93.5 | 87.3 | 0.017 | 92.0 | 80.0 | <0.001 | 91.5 | 88.9 | 0.310 | 92.0 | 85.2 | 0.019 |
Abbreviations: DFS = disease-free survival; DMFS = Distant metastasis-free survival; LRRFS = Locoregional relapse-free survival.
*P values were calculated by the unadjusted log-rank test.
Table 4. Summary of multivariate analysis of prognostic factors in 481 nasopharyngeal carcinoma patients with retropharyngeal lymph node metastasis (RLN) metastasis.
| Endpoint | Variable | B | P * | HR | 95% CI for HR |
| Disease failure | Age (≤50 vs. 50) | 0.659 | <0.001 | 1.933 | 1.374–2.721 |
| T-classification | 0.330 | <0.001 | 1.391 | 1.167–1.658 | |
| N-classification | 0.349 | <0.001 | 1.417 | 1.203–1.670 | |
| Necrosis | 0.585 | 0.003 | 1.795 | 1.214–2.654 | |
| MID (<10 vs. ≥10 mm) | 0.329 | 0.063 | 1.389 | 0.983–1.964 | |
| Distant failure | Age (≤50 vs. 50 years) | 0.412 | 0.064 | 1.510 | 0.976–2.335 |
| T-classification | 0.318 | 0.004 | 1.374 | 1.107–1.706 | |
| N-classification | 0.413 | <0.001 | 1.511 | 1.249–1.828 | |
| Necrosis | 0.561 | 0.018 | 1.752 | 1.100–2.790 | |
| MID (<10 vs. ≥10 mm) | 0.490 | 0.026 | 1.632 | 1.059–2.515 | |
| Locoregional recurrence | Necrosis | 0.961 | 0.005 | 2.614 | 1.339–5.103 |
| MID (<10 vs. ≥10 mm) | 0.570 | 0.083 | 1.767 | 0.929–3.364 |
Abbreviations: CI = confidence interval, MID = minimal axial diameters.
*P values were calculated using an adjusted Cox proportional-hazards model. The following known important prognostic variables were included in the Cox proportional hazards model: minimal axial diameters of RLN (<10 vs. ≥10 mm MID), necrosis of RLN (no vs. yes), laterality of RLN (unilateral vs. bilateral) and extra nodal neoplastic spread of RLN (no vs. yes), age (≤50 vs. 50), sex, T-classification, N-classification and chemotherapy (no vs. yes).
Survival according to N classification
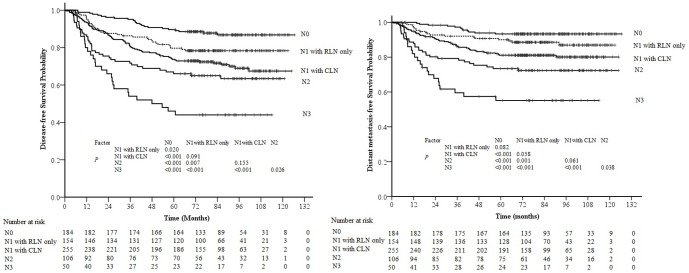
According to the seventh edition of AJCC staging system, RLN is included as a criterion for N1 disease, and 154 (20.6%) N0 patients would be upgraded to N1 disease (N1 with RLN only). All 749 patients were divided into five groups: N0 disease, N1 disease with retropharyngeal lymph node metastasis and without CLN metastasis (N1 with RLN only), N1 disease with CLN metastasis (N1 with CLN), N2 disease, and N3 disease. The survival curves demonstrated a significant difference in DFS between patients with N0 disease and N1 with RLN only (P = 0.020). The differences in DMFS and DFS between N1 with RLN only and N1 with CLN were marginally statistically significant (P = 0.058 and P = 0.091, respectively; Fig. 2).
Figure 2. Survival curves for patients with nasopharyngeal carcinoma (NPC) stratified by the N classification of the 7th edition of the UICC/AJCC staging system for NPC.
N1 + RLN only: N1 disease with retropharyngeal lymph node metastasis and without cervical lymph node metastasis; N1 + CLN: N1 disease with cervical lymph node metastasis.
In the N1 disease group, no significant differences were observed in the DFS, MDFS, LRFS or NRFS rates of patients with unilateral and bilateral RLN metastasis (P = 0.994, P = 0.752, P = 0.398 and P = 0.08 respectively).
Discussion
The TNM staging system is crucial for predicting prognosis, guiding treatment strategy for different risk groups, and facilitating the exchange of information between oncology centers [8], [16]. The TNM staging system is continually being modified to account for new developments in diagnostic and therapeutic techniques. There is little controversy that IMRT is the treatment of choice for NPC, as dosimetric studies have demonstrated the clear advantages of IMRT in terms of improving the dose conformity for complex tumor targets and better protecting the adjacent organs at risk [17], [18]. This is the first study to demonstrate that it is reasonable for RLN metastasis in NPC to be classified as N1 disease in the IMRT era.
Prognostic value of RLN metastasis
Several studies have reported that neck lymph node involvement in NPC spreads in an orderly manner down the neck [19], [20]. RLN metastasis is very common in NPC, as the RLNs are the first echelon lymph node [21]. When treated with two-dimensional conventional radiotherapy, patients with NPC and RLN metastasis had a poor prognosis [7]. In this study of patients treated with IMRT, RLN metastasis remained an independent prognostic factor for DFS and DMFS, even after adjustment for various prognostic factors. It is possible that conventional two-dimensional and three-dimensional conformal radiotherapy and IMRT do not have a significantly different effect on DMFS in patients with RLN metastasis. A number of studies have confirmed that IMRT has improved local control, but not distant control, in NPC [9], [17], [22]. In this study, the DMFS rate was only 83.1%, indicating that distant failure remains a challenge in patients with RLN metastasis. Therefore, the inclusion of RLN metastasis in the UICC/AJCC staging system would be useful to guide treatment planning, and additional therapeutic improvements are required to achieve a favorable outcome in patients with RLN metastasis.
Central necrosis is considered to be a late event in the biological evolution of tumor metastases within lymph nodes [23]. It has been confirmed that central necrosis primarily occurs in lymph nodes approximately 20.0 mm or larger, and it appears that central necrosis characteristically occurs after massive tumor infiltration [24]. There are few reports about the prognostic value of necrosis in RLN metastases in NPC. In this study, we found that necrosis of RLN metastases had a negative effect on survival in NPC. Tumor necrosis is believed to represent the endpoint of severe, chronic hypoxia in tissues distal to functional blood vessels. Tumor hypoxia may be one factor accounting for the poor prognosis of patients with necrotic RLN metastases [25]. Although IMRT offers improved tumor target coverage, the lack of oxygen in areas of hypoxia not only makes the tissues less susceptible to radiotherapy, but also induces the transcription of a variety of genes which promote tumor progression and increase tumor aggressiveness compared to non-hypoxic tumors [26], [27], which may explain why necrosis had significant prognostic value for all endpoints (DFS, DMFS and LRRFS). We propose that RLN necrosis should be adopted as a factor to enhance individualized NPC patient prognostication and clinical decision making, especially as it is simple to assess and could easily be incorporated into routine histopathological examinations.
Classification for RLN metastasis
Due to the limited diagnostic capabilities prior to the era of MRI imaging, consistent guidelines for the designation of RLN metastasis could not be identified in previous TNM staging systems; RLN metastasis was only incorporated into the TNM classification of the most recent 7th edition of the UICC/AJCC staging system for NPC. Evidence from two retrospective studies indicated that patients with RLN alone, regardless of laterality, have a similar risk of distant metastasis (DM) as patients with N1 disease [7], [28]. However, all of the patients in one of these studies [7] underwent conventional radiotherapy, and in the other study [28], only 12.7% of the patients underwent IMRT and there were no stratification analyses according to the radiation technique. IMRT achieves a significantly higher survival rate in NPC than CRT [9]; therefore, it was necessary to reevaluate whether it is still reasonable for RLN metastasis to be classified as N1 disease in the IMRT era.
This study, in which all patients received IMRT as the primary treatment, demonstrates that it is still reasonable for RLN metastasis in NPC to be classified as N1 disease, regardless of laterality. There are a number of reasons for this suggestion: Firstly, it is well recognized that the RLNs are the first echelon node in NPC. In most cases, and unlike CT, MRI can discriminate the RLNs from the primary tumor, so RLN metastasis should be classified in the N-classification, not the T-classification. Secondly, the survival curves in this study demonstrated a significant difference in DFS between patients with N0 disease and N1 disease with RLN only; therefore, it is still reasonable for RLN metastasis to be classified as N1 disease in patients treated with IMRT. Lastly, no significant differences were observed in the DFS, MDFS and LRFS rates of patients with unilateral and bilateral RLN metastasis, so RLN laterality does not need to be considered in future revisions of the staging system.
However, it was also observed that the differences in DMFS and DFS between N1 with RLN only and N1 with CLN were marginally statistically significant, and the survival curve could be separated, so there is a need to investigate the feasibility of classifying RLN metastasis as N1a disease in future by a larger cohort study.
It should be emphasized that this study was retrospective, and these results should be confirmed in a prospective study in the future. However, the current analysis of a large number of patients who received a systemic staging workup and were treated at single institution offers valuable information for evaluating appropriate classification of TNM stage for RLN metastasis in the current MRI/IMRT era. More importantly, all patients were treated with IMRT, in compliance with the requirements for studies aimed at formulating improvements in staging systems [16].
In conclusion, in the IMRT era, RLN metastasis remains an independent prognostic factor in NPC, and necrotic RLN metastases have a negative effect on survival in NPC. It is still reasonable for RLN metastasis to be classified in the N1 disease, regardless of laterality. However, there is a need to investigate the feasibility of classifying RLN metastasis as N1a disease in future by a larger cohort study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data files are available from the figshare database (DOI:10.6084/m9.figshare.1120584).
Funding Statement
This work was supported by grants from the China Scholarship Council (No. 201308440052, the National Natural Science Foundation of China (No. 81101695 and No. 81071836), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2010), and the Innovation Team Development Plan of the Ministry of Education (IRT1297). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J (1997) Cancer incidence in five continents. IARC, Lyon France 143: 814–815. [Google Scholar]
- 2. Sham JST, Choy D, Wei WI (1990) Nasopharyngeal carcinoma: orderly neck node spread. Int J Radiat Oncol Biol Phys 19: 929–933. [DOI] [PubMed] [Google Scholar]
- 3. Ng SH, Chang TC, Ko SF, Yen PS, Wan YL, et al. (1997) Nasopharyngeal carcinoma: MRI and CT assessment. Neuroradiology 39: 741–746. [DOI] [PubMed] [Google Scholar]
- 4. Olmi P, Fallai C, Colagrande S, Giannardi G (1995) Staging and follow-up of nasopharyngeal carcinoma: magnetic resonance imaging versus computerized tomography. Int J Radiat Oncol Biol Phys 32: 795–800. [DOI] [PubMed] [Google Scholar]
- 5. Liao XB, Mao YP, Liu LZ, Tang LL, Sun Y, et al. (2008) How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography? Int J RadiatOncolBiol Phys 72: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 6. Ng SH, Chang JT, Chan SC, Ko SF, Wang HM, et al. (2004) Nodal metastases of nasopharyngeal carcinoma: patterns of disease on MRI and FDG-PET. Eur J Nucl Med Mol Imaging 31: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 7. Ma J, Liu L, Tang L, Zong J, LinA, et al (2007) Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res 13: 1445–152. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Fritz AG, Byrd DR, Greene FL, Compton CC, et al. (2010) AJCC cancer staging manual. Springer, Berlin Heidelberg New York, 41–56.
- 9. Lai SZ, Li WF, Chen L, Luo W, Chen YY, et al. (2011) How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 80: 661–668. [DOI] [PubMed] [Google Scholar]
- 10. Van den Brekel MW, Castelijns JA, Snow GB (1990) Cervical lymph node metastasis: Assessment of radiologic criteria. Radiology 177: 379–384. [DOI] [PubMed] [Google Scholar]
- 11. Lam WW, Chan YL, Leung SF, Metreweli C (1997) Retropharyngeal lymphadenopathy in nasopharyngeal carcinoma. Head Neck 19: 176–181. [DOI] [PubMed] [Google Scholar]
- 12. King AD, Tse GM, Ahuja AT, Yuen EH, Vlantis AC, et al. (2004) Necrosis in metastatic neck nodes: Diagnostic accuracy of CT, MR imaging, and US. Radiology 230: 720–726. [DOI] [PubMed] [Google Scholar]
- 13. King AD, Tse GM, Yuen EH, To EW, Vlantis AC, et al. (2004) Comparison of CT and MR imaging for the detection of extranodal neoplastic spread in metastatic neck nodes. Eur J Radiol 52: 264–70. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan EL, Meier P (1958) Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 53: 457–481. [Google Scholar]
- 15. Cox DR (1972) Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological) 34: 187–220. [Google Scholar]
- 16. Groome PA, Schulze K, Boysen M, Hall SF, Mackillop WJ (2001) A comparison of published head and neck stage groupings in carcinomas of the oral cavity. Head Neck 23: 613–624. [DOI] [PubMed] [Google Scholar]
- 17. Ng WT, Lee MC, Hung WM, Choi CW, Lee KC, et al. (2011) Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 79: 420–428. [DOI] [PubMed] [Google Scholar]
- 18. Xia P, Fu KK, Wong GW, Akazawa C, Verhey LJ (2000) Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 48: 329–337. [DOI] [PubMed] [Google Scholar]
- 19. Sham JST, Choy D, Wei WI (1990) Nasopharyngeal carcinoma: orderly neck node spread. Int J Radiat Oncol Biol Phys 19: 929–933. [DOI] [PubMed] [Google Scholar]
- 20. Tang L, Mao Y, Liu L, Liang SB, Chen Y, et al. (2009) The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer 115: 680–688. [DOI] [PubMed] [Google Scholar]
- 21. King AD, Ahuja AT, Leung SF, Lam WW, Teo P, et al. (2000) Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck 22: 275–281. [DOI] [PubMed] [Google Scholar]
- 22. Kong F, Ying H, Huang S, Du C, Zhou J, et al. (2014) Preliminary results of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: a retrospective study of 364 patients. Eur Arch Otorhinolaryngol DOI 10.1007/s00405-014-2900-2. In press [DOI] [PubMed] [Google Scholar]
- 23. Som P (1987) Lymph nodes of the neck. Radiology 165: 593–600. [DOI] [PubMed] [Google Scholar]
- 24. Don DM, Anzai Y, Lufkin RB, Fu YS, Calcaterra TC (1995) Evaluation of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Laryngoscope 105: 669–674. [DOI] [PubMed] [Google Scholar]
- 25. Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, et al. (2006) Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 24: 2098–2104. [DOI] [PubMed] [Google Scholar]
- 26. Toustrup K, Sorensen BS, Lassen P, Wiuf C, Alsner J, et al. (2012) Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol 102: 122–129. [DOI] [PubMed] [Google Scholar]
- 27. Toustrup K, Sorensen BS, Nordsmark M, Busk M, Wiuf C, et al. (2011) Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 71: 5923–5931. [DOI] [PubMed] [Google Scholar]
- 28. Tang L, Li L, Mao Y, Liu LZ, Liang SB, et al. (2008) Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma detected by magnetic resonance imaging: prognostic value and staging categories. Cancer 113: 347–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data files are available from the figshare database (DOI:10.6084/m9.figshare.1120584).