Abstract
Background
The Trachinotus ovatus (Teleostei, Carangidae) is an economically important marine fish species in the world. However, the lack of genomic information regarding this species limits our understanding of the genetics and biological mechanisms in Trachinotus ovatus. In this study, high throughput transcriptome sequencing was used to obtain comprehensive genomic information in Trachinotus ovatus.
Principal Findings
Transcriptome sequencing was performed by using Illumina paired-end sequencing technology. The 98,534,862 high quality reads were yielded, and were de novo assembled into 156,094 unigenes with an average sequence length of 1179 bp. Transcriptome annotation revealed that 75,586 and 67,923 unigenes were functionally annotated in the NCBI non-redundant database and Swiss-Prot protein database, respectively. Functional analysis demonstrated that 67,923 unigenes were grouped into 25 Cluster of Orthologous Groups (COG) functional categories, 37,976 unigenes were clustered into 61 Gene Ontology (GO) terms, and 38,172 unigenes were assigned to 275 different Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Based on the transcriptome dataset, a large number of unigenes associated with reproduction, growth and immunity were identified. Furthermore, a total number of 38,794 simple sequence repeats (SSRs) were discovered and 16 polymorphic loci were characterized in Trachinotus ovatus.
Conclusion/Significance
The present study is the first transcriptome analysis of a fish species belonging to the genus Trachinotus and provides a valuable genomic resource for novel gene discovery, gene expression and regulation studies, and the identification of genetic markers in Trachinotus ovatus and the other fish of the genus Trachinotus.
Introduction
There are 20 species of carangid fish that belong to the genus Trachinotus. They are distributing all over the world and possess very desirable biological characteristics such as fast adaptation to controlled conditions, rapid growth, and so on. Therefore, fish species belonging to the genus Trachinotus have long been considered as promising candidates for mariculture [1]. According to the Food and Agriculture Organization of the United Nations (FAO) fishery statistics, the global aquaculture production of the carangid fish had exceeded 113,000 tons in 2012, with a value of approximately 458 million US dollars [2].
Trachinotus ovatus (Linnaeus 1758), the most popular cultured species of the genus Trachinotus, mainly inhabits in tropical and subtropical waters of the eastern Atlantic Ocean, and is also found in the Mediterranean and along the African coast, including offshore islands [3]. Due to its fast growth rate, pleasing flavor and increased market demand, Trachinotus ovatus is recognized as one of the most economically important marine fish species. With the wide use of large-scale marine cage culture in the Asia-Pacific region, including China and Southeast Asian countries, production of this species has increased in momentum in recent years.
However, with the expansion of large-scale culture and the neglect of fishery management and conservation, the genetic diversity of Trachinotus ovatus appears to be declining. Several biological changes including early age of sexual maturation, low growth rate, and increasing disease susceptibility have been found in cultured Trachinotus ovatus [4]. Since 2001, frequent disease occurrence has been reported in cultured Trachinotus ovatus each year in the southern coast of China, resulting in great economic losses [5], [6].
In spite of its significant economic importance, there is very little existing basic research on Trachinotus ovatus. Although some DNA markers (amplified fragment length polymorphism and microsatellite markers) have been developed [7], [8], the genetic diversity and population structure of Trachinotus ovatus cannot be effectively assessed with the existing genetic information. With the exception of a few studies on growth performance and feeding in confined environments [9]–[11], there is currently only limited information on other important traits such as reproduction, growth and immunity in Trachinotus ovatus. Moreover, owing to the lack of genomic data, molecular-genetic association studies remain unexplored in this species. Therefore, all areas of research, particularly the basic research on the genetics and on the gene networks involved in the regulation of reproduction, growth and immune response should be strengthened to support the sustained development of the Trachinotus ovatus industry.
Despite the rapid development of high-throughput sequencing technologies, genome sequencing is still expensive and takes long time. Transcriptome sequencing is a good choice for quickly and cost-effectively obtaining large-scale genetic information and functional genes in non-model species that do not have reference genomic information. Transcriptome analysis has now been widely applied in the fields of ecology, genetics, and molecular biology, playing valuable roles in gene discovery, gene expression and regulation, developing molecular markers and so on [12]–[16].
In the present study, using the Illumina paired-end sequencing technology, transcriptome analysis on various tissues of Trachinotus ovatus was performed. The genes involved in biological processes associated with reproduction, growth, and immunity were analyzed, and a set of microsatellite markers was developed. Our study provides abundant genomic information for future research into the genetics and molecular biology of Trachinotus ovatus.
Materials and Methods
Sample Preparation and RNA Extraction
Ten two-year-old Trachinotus ovatus with body weight of approximately 1000 ± 100 g were obtained from the Daya Bay Aquaculture Center, Guangdong, China. Fish were anesthetized and sacrificed by decapitation. Tissue samples were collected immediately and snap frozen in liquid nitrogen. All animal experiments were conducted in accordance with the guidelines and approval of the respective Animal Research and Ethics Committees of Sun Yat-Sen University.
Total RNA was isolated from different tissues of female and male Trachinotus ovatus (brain, pituitary, liver, gill, heart kidney, kidney, spleen, testes, and ovaries) using Trizol Reagent (Invitrogen, USA) according to the manufacturer's instructions. The concentration of total RNA was estimated by measuring the absorbance at 260 nm using a 2100 Bioanalyzer (Agilent Technologies, USA), and the RNA integrity was checked by ethidium bromide staining of 28S and 18S ribosomal bands on a 1% agarose gel. Equal volumes of RNA from each tissue were pooled and used for cDNA synthesis.
cDNA Library Construction and Sequencing
The cDNA library construction and sequencing was carried out as described by Li [17]. Briefly, Poly (A) mRNA was isolated from 20 ug of total RNA using oligo-dT beads (Qiagen, Germany), and was broken into short fragments (200 nt) in fragmentation buffer. The short fragments were used to synthesize first-strand cDNA by using random hexamer-primed reverse transcription, and then the second-strand cDNA was synthesized using RNase H and DNA polymerase I. The double-stranded cDNAs were purified using the QIAquick PCR extraction kit (Qiagen, Germany). After washing with EB buffer, the purified products were used for end reparation poly (A) addition and ligated to sequencing adapters. Following agarose gel electrophoresis, the adaptor-ligated fragments (200 bp ± 25 bp) were further extracted on the agarose gel and enriched by PCR to construct the final cDNA library, which was sequenced on the Illumina HiSeq 2000 sequencing platform using single-end paired-end technology in Beijing Genomics Institute-Shenzhen, Shenzhen, China. The original data process to sequences, base-calling and quality value calculation were achieved by the Illumina Genome Analyzer Pipeline (version 1.6), and 100 bp paired-end reads were obtained.
Illumina Reads Processing and Assembly
A Perl program was written to select clean reads. Low-quality reads that were more than 50% bases with quality lower than 20 in one sequence, ambiguous reads containing more than 5% unknown bases, and reads containing adaptor sequences were removed. Then the clean reads were assembled using Trinity to construct unique consensus sequences [18].
Functional Annotation and Classification
Using the BLASTx tool which was developed to evaluate the similarities between two sequences [19], the filtered transcripts were search against the National Center for Biotech-nology Information (NCBI) non-redundant protein (Nr) database (http://www.ncbi.nlm.nih.gov/), the Swiss-Prot protein database (http://www.expasy.ch/sprot) [20], the Cluster of Orthologous Groups (COG) (http://www.ncbi.nlm.nih.gov/COG/) [21], and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database [22] with an E-value cut off of 1e-5. Protein sequences from the databases which had the highest similarity scores were used as the functional annotation for the related unigene. Blast2GO was applied to get the annotation results of unigenes in Gene Ontology (GO) database (http://www.geneontology.org/) [23]. The WEGO software (http://wego.genomics.org.cn/cgibin/wego/index.pl) [24], a statistics tool, was then used to classify the annotation results of unigenes in GO database. Meanwhile, the unigenes were also aligned to the KEGG database to annotate the signal pathways.
Simple Sequence Repeat (SSR) Detection and Primer Design
Potential SSR markers were detected among the 156,094 unigenes using the MIcroSAtellite identification tool (MISA) (http://pgrc.ipk-gatersleben.de/misa/) [25]. We searched for SSRs with motifs ranging from di- to hexa-nucleotides in size. Given difficulties in distinguishing genuine mono-nucleotide repeats from polyadenylation products and some mono-nucleotide repeats that were generated by base mismatches or sequencing errors, mono-nucleotide repeats were discarded in this study. According to the MISA results, primer pairs flanking each SSR locus were designed using the Primer3 program (http://www.broadinstitute.org/genome_software/other/primer3.html) [26].
Survey of SSR Polymorphism
Fin samples were randomly collected from 30 six-month old Trachinotus ovatus individuals, which were the progeny of one broodstock population in the Daya Bay Aquaculture Center, Guangdong, China. All the samples were placed in absolute ethanol and kept frozen at −20°C until DNA extraction. Polymorphism was tested in the 30 Trachinotus ovatus individuals. Genomic DNA was extracted from the fin samples using the salting-out procedure [27]. PCR was performed on a Veriti Thermal Cycler in a total volume of 20 uL containing 0.4 uM of each primer, 10× PCR buffer (Genstar, China), and 100 ng DNA. Cycling conditions consisted of initial denaturation at 94°C for 5 min, 35cycles of 45 s at 94°C,40 s at the annealing temperature, 40 s at 72°C, and a final cycle of 5 min at 72°C. Allele sizes were estimated according to the pBR322 DNA/MspI marker (TianGen, China) after PCR products were separated on 8% denaturing polyacrylamide gel. The expected and observed heterozygosities together with an analysis of Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium were calculated using GENEPOP 4.0 [28]. Null allele frequencies were calculated with MICRO-CHECKER2.2.3 [29]. The significant values for all multiple tests were corrected by the sequential Bonferroni's procedure [30]. Polymorphism information content (PIC) was calculated using the PIC-CALC 0.6 software.
Results and Discussion
Sequencing, Assembly and Evaluation
Using the Illumina sequencing platform, a total of 99,714,868 raw reads were produced. After strict quality control and data filtration, 98,534,862 cleaned reads were harvested, with an average length of 100 bp (Table 1). All cleaned reads generated in this study have been deposited in the NCBI Sequence Read Archive (SRA) database (accession number: SRA161953).
Table 1. Statistics of the assembled transcripts and unigenes.
| Total number | Total Nucleotides base pair (bp) | Average length(bp) | N50 | |
| Raw Reads | 99,714,868 | 9,971,486,800 | 100 | – |
| Clean Reads | 98,534,862 | 9,853,486,200 | 100 | – |
| Unigenes | 156,094 | 184,107,954 | 1179 | 2404 |
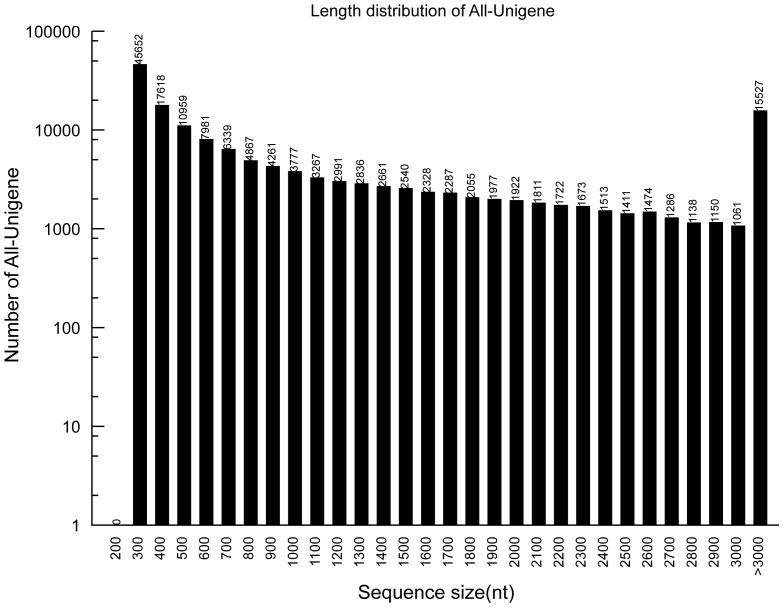
Using the Trinity assembly program, 156,094 unigenes were generated by de novo assembly, with an average length of 1179 bp and an N50 of 2404 bp (Table 1). The mean length of the unigenes in this study was longer than those assembled in other teleost fish such as Misgurnus anguillicaudatus (387 bp) [31], Oncorhynchus mykiss (662 bp) [32], Scophthalmus maximus (671 bp) [33] using de novo transcriptome assembly or 454 sequencing. Furthermore, the number of assembled unigenes and their length distribution were also analyzed. About 53,930 unigenes were >1,000 bp in length (Figure 1).
Figure 1. Length distribution of all unigenes of Trachinotus ovatus.
In order to assess the extent of coverage of the assembled unigenes and of evaluating the effects of coverage depth on unigene assembly, the ratio of the assembled unigene length to Danio rerio ortholog length against coverage depth was calculated. As shown in Figure 2A, the coding regions of most of Danio rerio orthologs could be effectively covered by the assembled Trachinotus ovatus unigenes. There were 10,219 unigenes with the ratio within 0.8 to 1.2, providing abundant long transcripts for gene function studies in Trachinotus ovatus. Additionally, it was observed that coverage depth was not directly proportional to coverage of the coding regions, although increased coverage depth could improve the extent of transcript coverage to a certain degree. The total percentage of Danio rerio ortholog coding sequences that were covered by all Trachinotus ovatus unigenes was also plotted. As shown in Figure 2B, 1,679 Danio rerio orthologs could be covered by Trachinotus ovatus unigenes with a percentage more than 80%. In addition, there were about 2,337 Danio rerio orthologs with coverage varying from 50% to 80%, and 2,725 orthologs with coverage below 20%. These data indicate that the assembled unigenes in this current study are of high quality of the present assembly.
Figure 2. Comparison of Trachinotus ovatus unigenes to Danio rerio orthologous coding sequences.
(A) The ratio of Trachinotus ovatus unigene length to Danio rerio ortholog length was plotted against Trachinotus ovatus unigene coverage depth. (B) The total percentage of Danio rerio ortholog coding sequence covered by all Trachinotus ovatus unigenes.
Annotation of Unigenes
Using the BLASTx algorithm (E-value <10−5), all assembled unigenes was searched against the databases of NCBI Nr, Swiss-Prot, COG, and KEGG. The annotation results were demonstrated through the Venn diagram (Figure 3). Of the 156,094 unigenes, 75,586 and 67,923 had homologous sequences in the Nr and Swiss-Prot protein databases, while 27,798 and 38,127 unigenes could be classified by COG and KEGG databases, respectively. In total, 15,145 unigenes could be simultaneously annotated by all four databases.
Figure 3. Venn diagram of annotation results against Nr, SwissProt, COG and KEGG databases.
The number in each color block indicates the number of unigenes that is annotated by single or multiple databases.
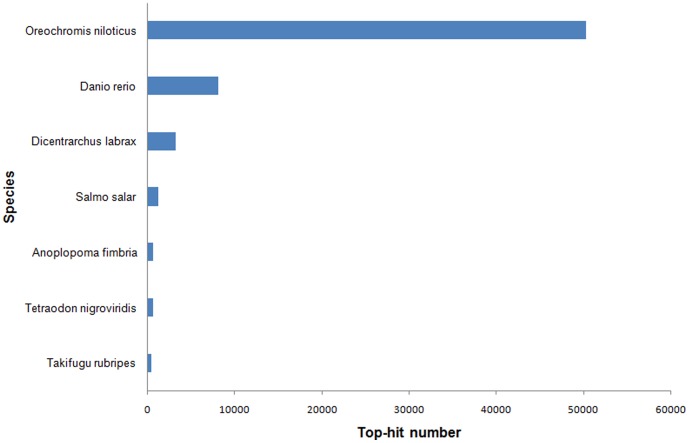
A BLASTx top-hit species distribution showed that 50,324 unigenes exhibited similarity to the sequences of Oreochromis niloticus, 8,173 to the sequences of Danio rerio, 3,322 to the sequence of Dicentrarchus labrax, and 1,342 to the sequence of Salmo salar (Figure 4). These results reveal that the gene content is phylogenetic conserved between Trachinotus ovatus and Oreochromis niloticus, and indicate that Trachinotus ovatus is more closely related to Oreochromis niloticus, both of which belong to the order Perciformes.
Figure 4. Top-hit species distribution for sequences from Trachinotus ovatus submitted BLASTX against the NCBI-Nr database.
Only fish species were present in this study.
COG, GO and KEGG Classification
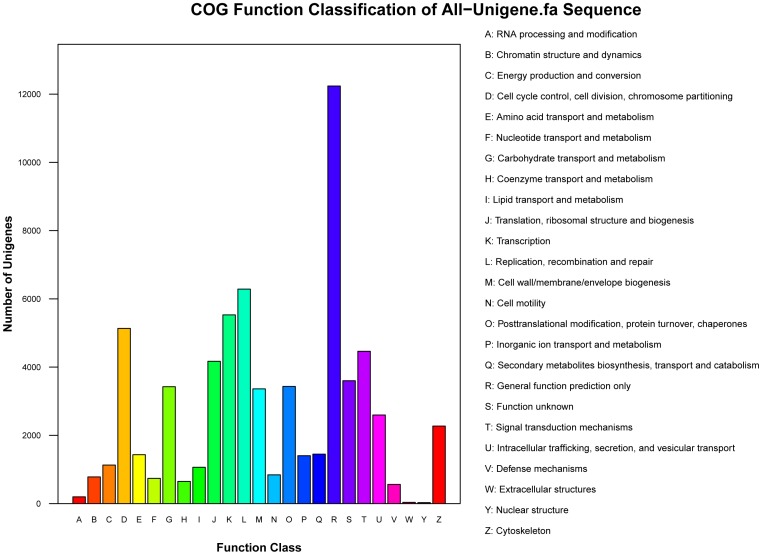
For functional prediction and classifications, all unigenes were aligned to the COG database. Together, 67,923 unigenes were grouped into 25 COG classifications (Figure 5). The largest cluster was the general function prediction only (18.31%), followed by replication, recombination and repair (9.41%), transcription (8.28%), cell cycle control, cell division and chromosome partitioning (7.68%), signal transduction mechanisms (6.68%), translation, ribosomal structure and biogenesis (6.24%), function unknown (5.39%), posttranslational modification, protein turnover, and chaperones (5.14%) and carbohydrate transport and metabolism (5.13%) (Table S1).
Figure 5. Clusters of Orthologous Groups (COG) functional classification of the Trachinotus ovatus transcriptome.
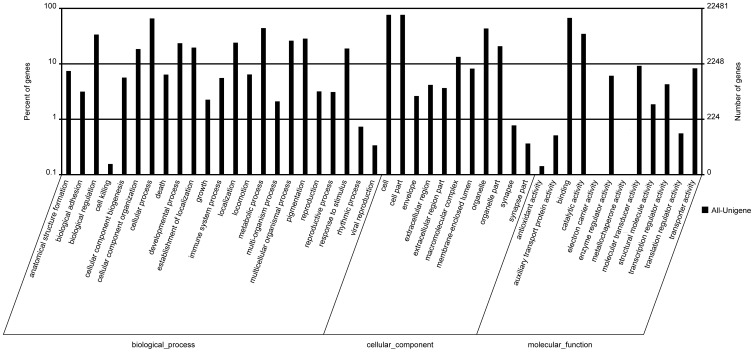
Gene Ontology is a standardized system for gene functional classification, containing three domains which are classified by biological process, cellular components and molecular functions of gene products [34]. Gene ontology analysis of our dataset showed that a total of 22,481 unigenes were annotated. Of these, 17,838 were grouped into cellular component, 18,064 were grouped into molecular function, and 18,823 were grouped into biological process (Figure 6). In the category of biological process, a large proportion of unigenes were related to cellular progress and metabolic progress. Within the category of cellular component, unigenes related to cell and cell part represented the largest clusters. Under the category of molecular function, a high percentage of unigenes was involved in binding and catalytic activity.
Figure 6. Gene Ontology (GO) analysis and functional classification of the Trachinotus ovatus transcriptome.
For further identification of the biological pathways in Trachinotus ovatus, we mapped the assembled sequences to the reference of typical pathways in the KEGG database. The 38,172 unigenes were matched to 275 different KEGG pathways (Table S2). Among these sequences, 6,639 were classified into metabolism groups, mostly involving in purine metabolism (776), glycerophospholipid metabolism (409), pyrimidine metabolism (358), and glycerolipid metabolism (341). The second largest cluster is the genetic information processing (5,364), involving degradation (1,146), repair (247), transcription and replication (172) and so on. There were 14,981, 9,849, and 1,339 sequences that were classified into cellular processing, human disease and environment information, respectively.
Identification of Sequences Related to Reproduction
Fish reproduction is the precondition for large-scale commercial culture and breeding. The artificial reproductive manipulation in Trachinotus ovatus has been established. However, the regulatory mechanisms behind reproductive development in this species are poorly understood and there is a lack of effective techniques for reproductive manipulation, leading to the abuse of hormone in current culture practices. Reproductive activities in fish are mainly controlled by gonadotropin-releasing hormone (GnRH)-gonadotropic hormone (GtH) axis [35]. GnRH agonists and exogenous GtH preparations have been widely used to induce ovulation and spermiation in cultured fish [36]. Therefore, understanding the regulation of GnRH and GtH is highly important for understanding reproductive activities in Trachinotus ovatus. In the transcriptome data, the transcripts for three subtypes of GnRH and different GtH subunits, including the complete coding DNA sequence of follicle-stimulating hormone subunit beta, luteinizing hormone subunit beta were indentified (Text S1). Moreover, the transcripts for kisspeptin, tachykinin3, aromatase, estrogen receptors, and androgen receptors were also found (Text S1). Kisspeptin and tachykinin3 are considered to be the key regulators of GnRH secretion in vertebrates [37]. Aromatase, estrogen receptors, and androgen receptors are critical factors in gonad responding to the actions of GnRH and GtH [38]. These sequences provide valuable information for elucidating the regulatory mechanisms of reproductive axis in Trachinotus ovatus. Further studies will likely focus on the role of the GnRH-GtH axis in the regulation of reproductive activities and on establishing highly effective techniques for the induction of gamete maturation, ovulation, and spermiation in Trachinotus ovatus.
In addition, Trachinotus ovatus is a unique species without any obvious phenotypic characteristics that can be used to distinguish the males and females. Difficulty in identifying the sexes has greatly hampered the artificial breeding program. In order to isolate sex-specific molecular markers, identification of genes related to sex determination and differentiation is necessary. Through the GO analysis, there were about 714 unigenes related to the reproductive regulation (Table S3), including forkhead box L2 that and doublesex and mab-3 related transcription factor 1 (DMRT1), which are key genes in the control of sex determination and differentiation in vertebrates [39]. The Realtime PCR analysis revealed that the expression of DMRT1 was much higher in the testes than in the ovaries (Figure S1), which is consistent with the findings in other fish species [40]–[42]. Studies have found that overexpression of DMRT1 could induce a female-to-male sex-reversal in Nile tilapia [43]. A linkage map in zebrafish has revealed the sex determination locus harboring DMRT1 gene [44]. Therefore, studying the roles of DMRT1 and other sex related genes in sex determination and differentiation will be useful in finding sex-specific markers that can help solve the problem of sex identification in Trachinotus ovatus.
Identification of Sequences Related to Growth and Metabolism
Improving the growth rate of cultivated fish and selecting fish with desired traits including rapid growth are major objectives in aquaculture. Elucidation of the regulatory mechanisms behind growth control will help achieve these goals. Similar to other vertebrates, fish growth is also controlled by growth hormone/insulin-like growth factor I (GH/IGFI) axis [45]. In this study, the genes coding for GH, IGFs and their receptors were identified in the transcriptome of Trachinotus ovatus (Text S2). Meanwhile, the signal transducers associated with GH/GHR signaling pathway and IGF/IGFR signaling pathway were also found (Text S2, Table S4). Furthermore, we also discovered genes that regulate GH and IGFs, such as the growth hormone releasing hormone (GHRH), pituitary adenylate cyclase-activating polypeptide (PACAP), somatostatins, insulin-like growth factor-binding proteins (IGFBP) (Text S2). GHRH and PACAP are the main regulators that promote GH release, while somatostatins play an inhibitory role [46]. IGFBP act as the carrier proteins for IGF [47]. With these sequences, a full view of the endocrine regulatory network of growth axis in Trachinotus ovatus can be constructed. Nevertheless, further molecular biology and biochemical studies are required to confirm the roles that these gene products play in Trachinotus ovatus growth.
Growth is a highly complex process that involves the regulation of appetite, muscle growth, weight gain and, protein and lipid metabolism. We found a few genes related to appetite and muscle development, including neuropeptide Y (NPY), pro-opiomelanocortin (POMC), leptin and myostatin (Text S2). NPY and POMC exert opposite roles in stimulating or inhibiting feeding, respectively [48]. Leptin has been recognized to regulate energy intake and energy expenditure [49]. Myostatins act as negative regulators of muscle growth [50]. Polymorphisms in myostatin genes have been identified in several fish species and are associated with growth traits [51]–[52]. These may be developed to growth-related markers for the molecular marker-assisted breeding.
In addition, signaling pathways involving carbohydrate, protein, and lipid metabolism were found in the KEGG pathway analysis (Table S2). Studies of these metabolic signaling pathways in combination with previous studies on growth performance and digestion [9]–[11] will expand our understanding of growth control in Trachinotus ovatus and will help optimize the artificial feeding for its aquaculture.
Identification of Sequences Related to Immunity
Aquaculture practices have been found to reduce genetic variability in hatchery-reared stocks, which may result in loss of disease resistance [53]–[54]. In recent years, frequent outbreaks of infectious diseases in fish farming have become a great threat to the Trachinotus ovatus industry [5]–[6]. However, we have little knowledge on the immune system of this species. In order to establish efficient defenses against pathogenic infections, the first step is to understand the immune system of Trachinotus ovatus and to identify genes and pathways involved in its immune response. GO classification revealed that 4,249 and 1,250 unigenes belonged to two subcategories: response to stimulus and immune system process respectively (Table S5, Table S6). KEGG pathways analysis revealed 29 immune-related pathways, including the toll-like receptor (TLR) signaling pathway, antigen processing and presentation, intestinal immune network for Immunoglobulin A (IgA) production, natural killer cell mediated cytotoxicity and so on (Table S7). These signaling pathways provide comprehensive information for the understanding of the Trachinotus ovatus immune system.
The toll-like receptor family is an important group of pattern-recognition receptors which are expressed on antigen-presenting cells, participating in innate immune responses and the subsequent promotion of adaptive immune responses [55]. We identified 10 different TLR transcripts in our transcriptome dataset. Moreover, we also found the genes belong to the TLR signaling pathway, such as myeloid differentiation factor 88 (Text S3). The major histocompatibility complex (MHC) are important molecules that help the immune system recognize foreign substances by binding peptide fragments derived from pathogens and presenting them to T cells [56]. Transcripts encoding MHC class I and II were also identified in the transcriptome dataset (Text S3). MHC genes have been considered as candidate markers associated with disease resistance as well, as it were found to be highly polymorphic in teleosts [57]–[59]. Further analysis of TLR and MHC genes will provide insights into Trachinotus ovatus immune defense. Additionally, some transcripts coding for cytokines were discovered in this study, including 18 different interleukins (Text S3). Cytokines play important roles in mediating immune responses in vertebrates. Many recombinant cytokines have been approved for clinical use [60], and these small soluble proteins also have the potential to be used as immunostimulants in aquaculture. However, their primary structures are not conserved among species, and their cDNA sequences are not easily cloned in non-model fish species by traditional homology cloning strategies. These sequence information will facilitate functional studies of cytokines in Trachinotus ovatus.
SSR Discovery: Distribution and Frequencies
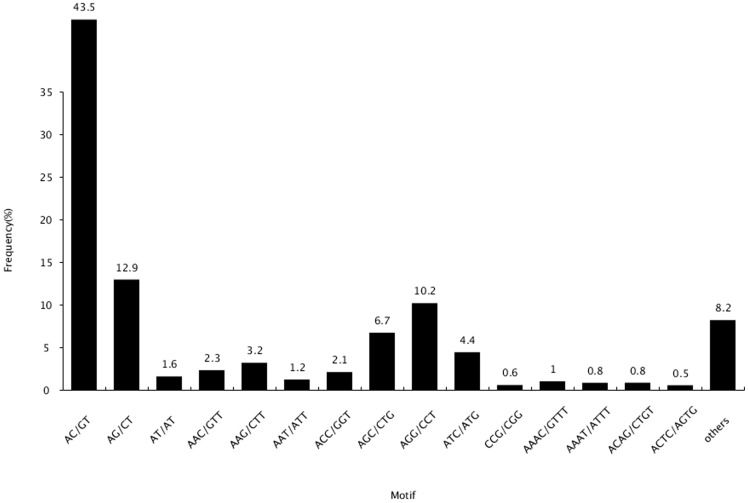
Simple sequence repeats, also known as microsatellites, are tandem repeats of 1–6 nucleotides found in all prokaryotic and eukaryotic genomes [61]. They have been widely used in genetic linkage map construction, population genetic studies, molecular marker-assisted breeding and so on [62]. As there is no genome sequence information, transcriptomes are a useful alternative for SSR markers discovery of in Trachinotus ovatus. In our study, all of assembled sequences were applied to exploit potential SSRs using MISA software [25]. In total, 38,794 SSRs were recognized in 30,295 sequences (19.41%), and 6,616 sequences (4.24%) contained more than one SSR. There were 2,118 SSRs present in compound formation. Di-nucleotide repeats were the most abundant SSR motif (58.07%), followed by tri- (31.19%), tetra- (7.60%), penta- (1.88%) and hexa- (1.27%) nucleotide repeats (Table 2). A summary on the frequency of SSRs with different numbers of tandem repeats were presented in Figure 7. SSRs with six tandem repeats occurred at the highest frequency (26.21%), followed by those with five tandem repeats (19.27%), seven tandem repeats (16.30%), and nine tandem repeats (11.90%). Among the di-nucleotide repeats, the AT/GT motif accounted for the majority of SSRs (43.5%), followed by the AG/CT di-nucleotide repeat motif (12.9%), and the AT/TA di-nucleotide repeat motif (1.6%). The most frequent motifs in the tri-nucleotide repeats were ACG/CTG (10.2%).
Table 2. Distribution of identified SSRs using the MISA software.
| Motif | Repeat numbers | Total | % | |||||||||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ≥15 | |||
| Di- | 0 | 0 | 6560 | 4079 | 3480 | 4610 | 3121 | 655 | 23 | 0 | 0 | 1 | 22529 | 58.07 |
| Tri- | 0 | 6299 | 3434 | 2235 | 116 | 0 | 5 | 1 | 1 | 1 | 2 | 4 | 12098 | 31.19 |
| Tetra- | 1701 | 1095 | 144 | 3 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2948 | 7.60 |
| Penta- | 656 | 56 | 7 | 2 | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 728 | 1.88 |
| Hexa- | 391 | 27 | 22 | 5 | 4 | 5 | 5 | 3 | 8 | 0 | 5 | 16 | 491 | 1.27 |
| Total | 2748 | 7477 | 10167 | 6324 | 3605 | 4618 | 3132 | 659 | 33 | 1 | 7 | 23 | 38794 | 100.00 |
| % | 7.08 | 19.27 | 26.21 | 16.30 | 9.29 | 11.90 | 8.07 | 1.70 | 0.09 | 0 | 0.02 | 0.06 | 100.00 | - |
Figure 7. Frequency of classified repeat types of SSRs.
Polymorphism Test of SSR Markers
To detect the genetic polymorphism of these microsatellite markers in the transcriptome, 331 pairs of SSR primers were designed for validation. In our study, 290 primer pairs were successfully amplified using Trachinotus ovatus genomic DNA (File S8). A large number of these microsatellite-containing transcripts could be annotated, most of which were associated with the metabolic pathways and immune system. Using the 290 primer pairs, 30 Trachinotus ovatus fish were analyzed, and 16 highly polymorphic microsatellite loci were found (Table 3). The rate of polymorphic microsatellites isolated in this study was low, perhaps because the tested individuals came from the same fish farm. More polymorphic microsatellites may be developed if more geographically distant populations were examined. Of the 16 polymorphic markers, the number of alleles per locus ranged from 2 to 14, with an average of 5.8. The observed and expected heterozygosities ranged from 0.152 to 0.663 and from 0.337 to 0.848, with an average of 0.318 and 0.682, respectively. The PIC values of 13 microsatellite loci were higher than 0.5, while that of the other 3 loci were between 0.29 and 0.46. Amongst the 16 SSR markers developed, 10 were located in unigenes annotated with known functions. The mammalian counterparts of these unigenes are involved in the regulation of various important cellular processes. For example, CCAAT/enhancer-binding protein beta 2 is an important transcription factor that controls the expression of genes involved in immune responses [63]. Retinoic acid receptor responder protein 3 is thought to act as a tumor suppressor or growth regulator [64]. Fam40b is essential for the differentiation of murine embryonic stem cells [65]. The potential links between these expressed sequence tag-simple sequence repeats (EST-SSRs) and some interesting phenotypes should be explored in further study.
Table 3. Characteristics of 16 polymorphic microsatellite loci.
| Unigene ID | SSRs | No. of alleles | Ho | He | PIC | BLASTX, sequence description |
| 0102325 | (TG)9 | 8 | 0.165 | 0.835 | 0.780 | similar to KIAA1486 protein |
| 0102780 | (CA)9 | 3 | 0.663 | 0.337 | 0.292 | No |
| 0041000 | (CTG)20 | 7 | 0.352 | 0.648 | 0.576 | CCAAT/enhancer-binding protein beta 2 |
| 0096447 | (GT)9 | 5 | 0.292 | 0.708 | 0.650 | transforming protein RhoA-like |
| 0141384 | (AC)9 | 14 | 0.152 | 0.848 | 0.807 | No |
| 0095395 | (TG)10 | 4 | 0.261 | 0.740 | 0.674 | No |
| 0120853 | (CA)9 | 5 | 0.349 | 0.651 | 0.581 | No |
| 0118815 | (CAGCTC)12 | 8 | 0.210 | 0.790 | 0.744 | similar to expressed protein |
| 0128444 | (TG)10 | 4 | 0.281 | 0.719 | 0.655 | No |
| 0019285 | (CA)9 | 5 | 0.257 | 0.743 | 0.682 | E3 ubiquitin-protein ligase RNF220-like |
| 0090156 | (CAGCTC)12 | 7 | 0.226 | 0.774 | 0.728 | serine/threonine protein kinase |
| 0110112 | (TG)9 | 2 | 0.492 | 0.508 | 0.375 | No |
| 0141617 | (CA)10 | 4 | 0.352 | 0.648 | 0.570 | retinoic acid receptor responder protein 3-like |
| 0106655 | (TG)9 | 4 | 0.457 | 0.543 | 0.460 | cell adhesion molecule 4-like |
| 0091717 | (GT)9 | 4 | 0.335 | 0.666 | 0.589 | protein FAM40B-like |
| 0108204 | (AC)10 | 10 | 0.246 | 0.754 | 0.716 | golgin subfamily B member 1-like |
Ho, observed heterozygosity; HE, expected heterozygosity; PHWE, Hardy-Weinberg probability; PIC, polymorphic information content.
However, although the remaining 6 polymorphic microsatellite loci were came from transcribed regions, their sequences showed no similarity to any known protein when BLAST searches were performed on nucleotide and protein database. A possible explanation is that these microsatellite-containing sequences belong to microsatellite-containing transcripts. Another possible explanation may be that some sequences may be derived from poorly conserved untranslated regions. EST-SSRs have been recognized to be conserved and have cross-species transferability [66]. Therefore, these transcriptome-derived microsatellite markers are of high value, and can be employed for Trachinotus ovatus population genetic studies and linkage map construction in the future.
Conclusion
For the first time, the whole transcriptome was studied in a fish species belonging to the genus Trachinotus. A large number of genes related to reproduction, growth and metabolism, and immune response were identified in the transcriptome dataset, providing abundant genomic data for future studies on the molecular mechanisms behind important physiological processes in Trachinotus ovatus. At the same time, many microsatellites were discovered and 16 polymorphic microsatellite loci were developed in this study, which are useful markers for genetic studies and marker-assisted selection in Trachinotus ovatus.
Supporting Information
Relative mRNA amounts of DMRT1 in adult gonads of Trachinotus ovatus .
(DOCX)
The functional classification of the COG classes for the transcriptome of Trachinotus ovatus.
(XLSX)
KEEG pathway analysis for the transcriptome of Trachinotus ovatus.
(XLSX)
GO terms associated with reproduction.
(XLSX)
IGF/IGFR signaling pathways.
(XLSX)
GO terms associated with response to stimulus.
(XLSX)
GO terms associated with immune system process.
(XLSX)
KEEG pathway analysis for immune response.
(XLSX)
Information of 290 SSRs primer pairs derived from unigenes.
(XLSX)
Putative sequences related to reproduction in the transcriptome of Trachinotus ovatus .
(TXT)
Putative sequences related to growth and metabolism in the transcriptome of Trachinotus ovatus .
(TXT)
Putative sequences related to immunity in the transcriptome of Trachinotus ovatus .
(TXT)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Figures, tables and some data are within the paper and its Supporting Information files, and all sequences in this study have been deposited in NCBI SAR database (accession number: SRA161953).
Funding Statement
This work was supported by the National 863 Program of China (No. 2012AA10A407) and Special Fund for Agro-scientific Research in the Public Interest (201403008). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jory DE, Iversen ES, Lewis RH (1985) Culture of fishes of the genus Trachinotus (Carangidae) in the western Atlantic: prospects and problems. J World Aquacult Soc 16(1–4): 87–94. [Google Scholar]
- 2.FAO website, Global Aquaculture Production (online query). Available: http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en. Accessed 2014 May 9.
- 3.Froese R, Pauly D (2014) Fishbase, a Global Information System on Fishes: http://www.fishbase.org. World Wide Web electronic publication. Accessed 2014 April.
- 4. Ou YJ, Ji L, Li JE, Fan CY, Wang G (2013) Correlation analysis of major morphometric traits and body weight of selective group at different month ages of Trachinotus ovatus . Journal of Fisheries of China 37(7): 961–969 (in Chinese).. [Google Scholar]
- 5. Zhou YC, Zhu CH, Zhang B, Su YQ (2001) Isolation and prevention of the pathogen causing large scale death on Trachinotus ovatus . Mar Sci 25 (4): 40–44 (in Chinese).. [Google Scholar]
- 6. Wang R, Feng J, Su Y, Ye L, Wang J (2013) Studies on the isolation of Photobacterium damselae subsp. piscicida from diseased golden pompano (Trachinotus ovatus Linnaeus) and antibacterial agents sensitivity. Vet Microbiol 162(2–4): 957–963. [DOI] [PubMed] [Google Scholar]
- 7. Peng M, Chen XH, Jiang WM, Chen XL, Yang CL, et al. (2011) Optimization of AFLP Fingerprinting and Screening of Primer Pairs in Genetic Diversity Analysis of Trachinotus ovatus . Southwest China Journal of Agricultural Sciences 24 (3): 1152–1155 (in Chinese).. [Google Scholar]
- 8. Sun LY, Zhang DC, Jiang SG, Guo HY, Zhu CY (2011) Isolation and characterization of 21 polymorphic microstatellites in golden pompano Trachinotus ovatus . Conser Genet Res 5(4): 1107–1109. [Google Scholar]
- 9. Zhang Q, Yu HR, Tong T, Tong WP, Dong LF, et al. (2014) Dietary supplementation of Bacillus subtilis and fructooligosaccharide enhance the growth, non-specific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against Vibrio vulnificus . Fish Shellfish Immun 38(1): 7–14. [DOI] [PubMed] [Google Scholar]
- 10.Ma ZH, Guo HY, Zheng PL, Wang L, Jiang SG, et al. (2014) Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem (in press). [DOI] [PubMed]
- 11. Lin SM, Mao SH, Guan Y, Lin X, Luo L (2012) Dietary administration of chitooligosaccharides to enhance growth, innate immune response and disease resistance of Trachinotus ovatus . Fish Shellfish Immun 32(5): 909–13. [DOI] [PubMed] [Google Scholar]
- 12. Garg R, Patel RK, Tyagi AK, Jain M (2011) De novo assembly of chickpea transcriptome using short reads for gene discovery and marker identification. DNA research 18(1): 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blanca J, Cañizares J, Roig C, Ziarsolo P, Nuez F, et al. (2011) Transcriptome characterization and high throughput SSRs and SNPs discovery in Cucurbita pepo (Cucurbitaceae). BMC genomics 12(1): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji PF, Liu GM, Xu J, Wang XM, Li JT, et al. (2012) Characterization of common carp transcriptome: sequencing, de novo assembly, annotation and comparative genomics. PloS one 7(4): e35152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma KY, Qiu GF, Feng JB, Li JL (2012) Transcriptome analysis of the oriental river prawn, Macrobrachium nipponense using 454 pyrosequencing for discovery of genes and markers. PloS one 7(6): e39727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao ZX, Luo W, Liu H, Zeng C, Liu XL, et al. (2012) Transcriptome analysis and SSR/SNP markers information of the blunt snout bream (Megalobrama amblycephala). PloS one 7(8): e42637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li P, Deng WQ, Li TH, Song B, Shen YH (2013) Illumina-based de novo transcriptome sequencing and analysis of Amanita exitialis basidiocarps . Gene 532(1): 63–71. [DOI] [PubMed] [Google Scholar]
- 18. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29(7): 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3): 403–410. [DOI] [PubMed] [Google Scholar]
- 20. Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, et al. (2003) The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res 31(1): 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, et al. (2003) The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, et al. (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36: D480–D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21(18): 3674–3676. [DOI] [PubMed] [Google Scholar]
- 24. Ye J, Fang L, Zheng Hk, Zhang Y, Chen J, et al. (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34 suppl 2: W293–W297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thiel T, Michalek W, Varshney RK, Graner A (2003) Exploiting EST databases for the development of cDNA derived microsatellite markers in barley (Hordeum vulgareL.). Theor Appl Genet 106(3): 411–422. [DOI] [PubMed] [Google Scholar]
- 26. Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386. [DOI] [PubMed] [Google Scholar]
- 27. Howe JR, Klimstra DS, Cordon-Cardo C (1997) DNA extraction from paraffin-embedded tissues using a salting-out procedure: A reliable method for PCR amplification of archival material. Histol Histopathol 12(3): 595–601. [PubMed] [Google Scholar]
- 28. Raymond M, Rousset F (1995) GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86 (3): 248–249. [Google Scholar]
- 29. Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4(3): 535–538. [Google Scholar]
- 30. Rice WR (1989) Analyzing tables of statistical tests. Evolution 43(1): 223–225. [DOI] [PubMed] [Google Scholar]
- 31. Long Y, Li Q, Zhou BL, Song GL, Li T, et al. (2013) De novo assembly of mud loach (Misgurnus anguillicaudatus) skin transcriptome to identify putative genes involved in immunity and epidermal mucus secretion. PLoS One 8(2): e56998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salem M, Rexroad CE, Wang J, Thorgaard GH, Yao J (2010) Characterization of the rainbow trout transcriptome using Sanger and 454-pyrosequencing approaches. BMC Genomics 11: 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pereiro P, Balseiro P, Romero A, Dios S, Forn-Cuni G, et al. (2012) High-throughput sequence analysis of turbot (Scophthalmus maximus) transcriptome using 454-pyrosequencing for the discovery of antiviral immune genes. PloS one 7(5): e35369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, et al. (2000) Gene Ontology: tool for the unification of biology. Nat Genet 25(1): 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ (2010) Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol 165(3): 412–437. [DOI] [PubMed] [Google Scholar]
- 36. Mylonas CC, Fostier A, Zanuy S (2010) Broodstock management and hormonal manipulations of fish reproduction. Gen Comp Endocrinol 165(3): 516–534. [DOI] [PubMed] [Google Scholar]
- 37. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ (2010) Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol 34(3): 211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaron Z, Levavi-Sivan B (2011) Endocrine Regulation of Fish Reproduction. In: Farrell A.P., (ed.), Encyclopedia of Fish Physiology: From Genome to Environment, volume 2, pp. 1500–1508. San Diego: Academic Press. [Google Scholar]
- 39. Baroiller JF, D'Cotta H, Saillant E (2009) Environmental effects on fish sex determination and differentiation. Sex Dev 3(2–3): 118–135. [DOI] [PubMed] [Google Scholar]
- 40. Guo YQ, Cheng HH, Huang X, Gao S, Yu HS, et al. (2006) Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem Biophys Res Commun 330(3): 950–957. [DOI] [PubMed] [Google Scholar]
- 41. Veith AM, Schäfer M, Klüver N, Schmidt C, Schultheis C, et al. (2006) Tissue-specific expression of dmrt genes in embryos and adults of the platyfish Xiphophorus maculatus . Zebrafish 3(3): 325–337. [DOI] [PubMed] [Google Scholar]
- 42. Ijiri S, Kaneko H, Kobayashi T, Wang DS, Sakai F, et al. (2008) Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus . Biol Reprod 78(2): 333–341. [DOI] [PubMed] [Google Scholar]
- 43. Wang DS, Zhou LY, Kobayashi T, Matsuda M, Shibata Y, et al. (2008) Doublesex- and Mab-3-related transcription factor-1 repression of aromatase transcription, a possible mechanism favoring the male pathway in tilapia. Endocrinology 151(3): 1331–1340. [DOI] [PubMed] [Google Scholar]
- 44. Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, et al. (2011) An SNP-Based Linkage Map for Zebrafish Reveals Sex Determination Loci. G3 (Bethesda) 1(1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rousseau K, Dufour S (2007) Comparative aspects of GH and metabolic regulation in lower vertebrates. Neuroendocrinology 86(3): 165–174. [DOI] [PubMed] [Google Scholar]
- 46. Li WS, Lin HR (2010) The endocrine regulation network of growth hormone synthesis and secretion in fish: emphasis on the signal integration in somatotropes. Sci China Life Sci 53(4): 462–470. [DOI] [PubMed] [Google Scholar]
- 47. Duan CM, Xu QJ (2005) Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol 142(1–2): 44–52. [DOI] [PubMed] [Google Scholar]
- 48. Valassi E, Scacchi M, Cavagnini F (2008) Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18(2): 158–168. [DOI] [PubMed] [Google Scholar]
- 49. Morton GJ, Schwartz MW (2011) Leptin and the central nervous system control of glucose metabolism. Physiol Rev 91(2): 389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gabillard JC, Biga PR, Rescan PY, Seiliez I (2013) Revisiting the paradigm of myostatin in vertebrates: insights from fishes. Gen Comp Endocrinol 194: 45–54. [DOI] [PubMed] [Google Scholar]
- 51. Nadjar-Boger E, Funkenstein B (2011) Myostatin-2 gene structure and polymorphism of the promoter and first intron in the marine fish Sparus aurata: evidence for DNA duplications and/or translocations. BMC Genet 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun YH, Yu XM, Tong JG (2012) Polymorphisms in Myostatin Gene and Associations with Growth Traits in the Common Carp (Cyprinus carpio L.). Int J Mol Sci 13(11): 14956–14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lind CE, Evans BS, Knauer J, Taylor JU, Jerry DR (2009) Decreased genetic diversity and a reduced effective population size in cultured silver-lipped pearl oysters (Pinctada maxima). Aquaculture 286 (1–2): 12–19. [Google Scholar]
- 54. Meng ZN, Yang S, Fan B, Wang L, Lin HR (2012) Genetic variation and balancing selection at MHC class II exon 2 in cultured stocks and wild populations of orange-spotted grouper (Epinephelus coioides). Genet Mol Res 11(4): 3869–3881. [DOI] [PubMed] [Google Scholar]
- 55. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4): 783–801. [DOI] [PubMed] [Google Scholar]
- 56. Neefjes J, Jongsma ML, Paul P, Bakke O (2011) Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 11(12): 823–836. [DOI] [PubMed] [Google Scholar]
- 57. Langefors A, Lohm J, Grahn M, Andersen O, von ST (2001) Association between major histocompatibility complex class IIB alleles and resistance to Aeromonas salmonicida in Atlantic salmon. Proc Biol Sci 268(1466): 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang YX, Chen SL, Liu YG, Sha ZX, Liu ZJ (2006) Major histocompatibility complex class IIB allele polymorphism and its association with resistance/susceptibility to Vibrio anguillarum in Japanese flounder (Paralichthys olivaceus). Mar Biotechnol (NY) 8(6): 600–610. [DOI] [PubMed] [Google Scholar]
- 59. Xu TJ, Chen SL, Ji XS, Sha ZX (2009) Molecular cloning, genomic structure, polymorphism and expression analysis of major histocompatibility complex class IIA and IIB genes of half-smooth tongue sole (Cynoglossus semilaevis). Fish Shellfish Immun 27(2): 192–201. [DOI] [PubMed] [Google Scholar]
- 60. Sirko A, Vanek T, Gora-Sochacka A, Redkiewicz P (2011) Recombinant cytokines from plants. Int J Mol Sci 12(6): 3536–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zane L, Bargelloni L, Patarnello T (2002) Strategies for microsatellite isolation: a review. Mol Ecol 11(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 62. Selkoe KA, Toonen R J (2006) Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett 9(5): 615–629. [DOI] [PubMed] [Google Scholar]
- 63. Campion CG, Labrie M, Grosset AA, St-Pierre Y (2014) The CCAAT/Enhancer-Binding Protein Beta-2 Isoform (CEBPβ-2) Upregulates Galectin-7 Expression in Human Breast Cancer Cells. PloS one 9(5): e95087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. DiSepio D, Ghosn C, Eckert RL, Deucher A, Robinson N, et al. (1998) Identification and characterization of a retinoid-induced class II tumor suppressor/growth regulatory gene. Proc Natl Acad Sci U S A 95(25): 14811–14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wagh V, Doss MX, Sabour D, Niemann R, Meganathan K, et al. (2014) Fam40b is required for lineage commitment of murine embryonic stem cells. Cell Death Dis 5: e1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xie F, Sun G, Stiller JW, Zhang B (2011) Genome-wide functional analysis of the cotton transcriptome by creating an integrated EST database. PLoS One 6(11): e26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative mRNA amounts of DMRT1 in adult gonads of Trachinotus ovatus .
(DOCX)
The functional classification of the COG classes for the transcriptome of Trachinotus ovatus.
(XLSX)
KEEG pathway analysis for the transcriptome of Trachinotus ovatus.
(XLSX)
GO terms associated with reproduction.
(XLSX)
IGF/IGFR signaling pathways.
(XLSX)
GO terms associated with response to stimulus.
(XLSX)
GO terms associated with immune system process.
(XLSX)
KEEG pathway analysis for immune response.
(XLSX)
Information of 290 SSRs primer pairs derived from unigenes.
(XLSX)
Putative sequences related to reproduction in the transcriptome of Trachinotus ovatus .
(TXT)
Putative sequences related to growth and metabolism in the transcriptome of Trachinotus ovatus .
(TXT)
Putative sequences related to immunity in the transcriptome of Trachinotus ovatus .
(TXT)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Figures, tables and some data are within the paper and its Supporting Information files, and all sequences in this study have been deposited in NCBI SAR database (accession number: SRA161953).