Abstract
Significant amounts of arsenic have been found in the groundwater of many countries including Argentina, Bangladesh, Chile, China, India, Mexico, and the United States with an estimated 200 million people at risk of toxic exposure. Although chronic arsenic poisoning damages many organ systems, it usually first presents in the skin with manifestations including hyperpigmentation, hyperkeratoses, Bowen’s disease, squamous cell carcinoma, and basal cell carcinoma. Arsenic promotes oxidative stress by upregulating nicotinamide adenine dinucleotide phosphate oxidase, uncoupling nitric oxide synthase, and by depleting natural antioxidants such as nitric oxide and glutathione in addition to targeting other proteins responsible for the maintenance of redox homeostasis. It causes immune dysfunction and tissue inflammatory responses, which may involve activation of the unfolded protein response signaling pathway. In addition, the dysregulation of other molecular targets such as nuclear factor kappa B, Hippo signaling protein Yap, and the mineral dust-induced proto-oncogene may orchestrate the pathogenesis of arsenic-mediated health effects. The metalloid decreases expression of tumor suppressor molecules and increases expression of pro-inflammatory mitogen-activated protein kinase pathways leading to a tumor-promoting tissue microenvironment. Cooperation of upregulated signal transduction molecules with DNA damage may abrogate apoptosis, promote proliferation, and enhance cell survival. Genomic instability via direct DNA damage and weakening of several cellular DNA repair mechanisms could also be important cancer development mechanisms in arsenic-exposed populations. Thus, arsenic mediates its toxicity by generating oxidative stress, causing immune dysfunction, promoting genotoxicity, hampering DNA repair, and disrupting signal transduction, which may explain the complex disease manifestations seen in arsenicosis.
Keywords: Arsenic, Arsenicosis, Carcinogenesis, Pathogenesis, Oxidative stress
Introduction
Arsenic is a metalloid found abundantly within the earth’s crust complexed with the mineral, pyrite. Changes in soil pH, temperature, oxidation state, and solution composition readily dissociate arsenic from pyrite, leaving it free to enter the groundwater [1]. Though groundwater contamination is the most common source of arsenic toxicity to humans, occupational exposure may also cause arsenicosis [2,3]. Those employed in the production of agricultural products (e.g., pesticides and herbicides), wood preservatives, animal feed additives, electronics (e.g., semiconductors and transistors), glass, and pigment are at increased risk of exposure. Miners and metallic ore smelters may also be exposed to arsenic in the work-place [4–6]. Additionally, arsenic poses a threat to human health when ingested. Foods notorious for arsenic contamination include fish, cereal, algae [2], rice [7], and nutritional supplements (particularly those from endemic regions) [8].
Arsenic contamination is a serious and widespread global public health problem with a significant known groundwater burden in countries such as Argentina, Bangladesh, Chile, China, Ghana, India, Mexico, Taiwan, the United States, and Vietnam (Table 1). It is estimated that more than 200 million people are at risk of toxic arsenic exposure [7]. Between 20 and 45 million people living in Bangladesh alone are at risk of exposure to concentrations exceeding the country’s standard of 50 µg/L [9]. Similarly, approximately 15 million individuals living in China are exposed to concentrations exceeding its national standard of 10 µg/L. More than 10,000 people living in endemic and suspected endemic areas of China are currently suffering from arsenicosis [14]. On its 2011 substance priority risk list, the United States Agency for Toxic Substances and Disease Registry (ATSDR) ranked arsenic as the number one public health threat due to the prevalence of groundwater contamination and the substance’s toxic potential. The International Agency for Research on Cancer [4] classified inorganic arsenic compounds as class I human carcinogens, meaning sufficient evidence supports their carcinogenicity to humans [2,6]. The Joint Food and Agriculture Organization of the United Nations/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) re-evaluated the risks of arsenic exposure and found evidence of adverse effects in areas with drinking-water concentrations ranging between 50 and 100 µg/L. Currently, the WHO (2011) [6] recommends limiting the arsenic content of drinking water to 10 µg/L.
Table 1.
Arsenic concentrations and associated toxicities in countries worldwide.
| Country | Concentration in drinking water (µg/L) |
Arsenic-associated toxicities | Estimated number of exposed individuals (millions) |
|---|---|---|---|
| Argentina | <1 to 7550 | Bladder cancer, lung cancer | 2.0 |
| Bangladesh | <10 to >2700 | Various cancers, cardiovascular disease, infection [9] | 20–45 [9] |
| Chile | 600 to 800 | Bladder cancer, lung cancer, infection | 0.4 |
| China | <50 to 4400 | Skin cancer | 0.5–20 [10] |
| Ghana | <2 to 175 | Various cancers | <0.1 |
| India | <10 to >800 | Respiratory disease, immunotoxicity [11] | 1–43 [12] |
| Mexico | 5 to 43 | Impaired cognition, nutritional deficiencies | 0.4 |
| Taiwan | <1 to >3000 | Bladder cancer, lung cancer | >0.008 |
| United States | <1 to >3100 | Impaired cognition [13], increased urinary arsenic concentration | >3.0 |
| Vietnam | <0.1 to 810 | Increased arsenic concentration in hair | >3.0 |
Reproduced with permission from the un-copyrighted journal available for free reprinting, Environmental Health Perspectives [7].
Extensive epidemiological studies in countries such as Taiwan, Bangladesh, Chile, India, and Argentina have provided abundant information on the significant detrimental effects of chronic arsenic toxicity on public health [7]. Acute arsenic exposure, which is often accidental or due to suicide attempts, may cause nausea, vomiting, diarrhea, liver toxicity, and acute kidney injury [3–6]. Chronic arsenic exposure, the immensely more common problem, damages a broad range of organ systems in a time- and dose-dependent manner. Exposure to arsenic during pregnancy impacts human developmental processes resulting in lower birth weight, increased infant mortality, and neurological impairments in children. Impaired intellectual abilities, diminished motor function, and polyneuropathy are the most common neurological consequences of chronic arsenicosis [7,13,15,16]. Individuals suffering from arsenic toxicity are more susceptible to pulmonary diseases such as tuberculosis and bronchiectasis, conditions which also carry higher mortality rates in exposed populations [7,17]. In addition, evidence suggests that lung cancer is the most common cause of arsenic-related mortality. This well-known human carcinogen has also been implicated in cancers of the skin, bladder, liver, kidney, prostate, and uterus [3,4,18,19]. Arsenic-induced cardiotoxicity typically manifests as accelerated atherosclerosis [1] and increased coronary artery disease resulting in a consequentially increased risk of myocardial infarction [7,20–22]. Higher rates of diabetes and impaired glucose tolerance in arsenic-exposed individuals provide evidence of the metalloid’s impact on endocrine function [23]. Arsenic also has a significant impact on the immune response by dysregulating immune-related gene expression thus promoting susceptibility to infections and inappropriate inflammatory responses [7].
Though arsenic impacts diverse cellular processes in numerous organ systems, the symptoms of its toxicity are usually first manifest in the skin. Skin manifestations include hyperpigmentation, hyperkeratoses of the palms and soles [24,25], Bowen’s disease, squamous cell carcinoma, and basal cell carcinoma [3,8,26]. An association between arsenic exposure and Merkel cell carcinoma, a substantially less common disease, has also been identified [2]. The development of skin cancer in sun-protected areas is especially concerning for the existence of chronic arsenic exposure. These cutaneous manifestations serve as a surrogate marker for associated diseases such as hypertension, ischemic heart disease, atherosclerosis [1], peripheral vascular disease, diabetes mellitus, peripheral neuropathy [27], and Raynaud’s phenomenon [1,3,28]. This review unifies the body of evidence describing arsenic’s pathogenesis in light of novel mechanistic findings in the area.
Arsenic toxicity
The two oxidative states of arsenic are pentavalent arsenate (AsV) and the more toxic, trivalent arsenite (AsIII). In mammals, AsIII is absorbed through aquaporins 7 and 9, while phosphate transporters are believed to take up AsV [1]. Arsenic trioxide (ATO) is a compound used as a second-line chemotherapeutic agent in the treatment of some blood and bone marrow leukemias [29]. Similar to the metabolism of organic compounds, arsenic undergoes reduction, methylation, and glutathione conjugation in the liver to form more polar metabolites for excretion [30,31]. Historically, the methylation of inorganic arsenic (iAs) was believed to detoxify the metalloid [1] given that methylated metabolites such as monomethylarsinous acid (MMAsIII) and dimethylarsinous acid (DMAsIII) were found in the urine of exposed animals [1]. For the past decade, however, studies have demonstrated that methylated arsenites including MMAsIII and DMAsIII are more toxic than iAs [32]. In addition, MMAsIII is known to directly inhibit enzymes involved oxidative metabolism such as glutathione peroxidase, glutathione reductase, pyruvate dehydrogenase, and thioredoxin reductase, leading to an imbalance in oxidative cellular homeostasis [1,31,33].
Oxidative stress
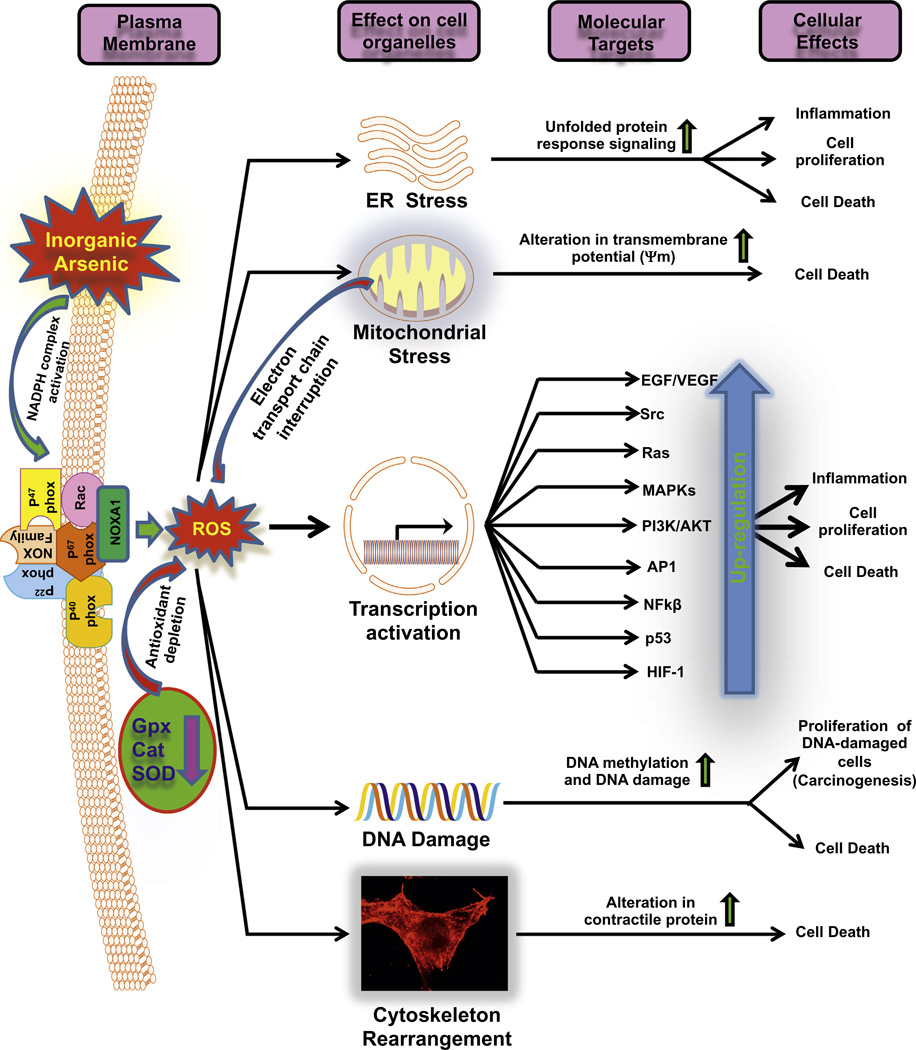
An overwhelming body of evidence has described arsenic’s ability to cause oxidative and nitrosative stress through the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [1,29,34,35]. Arsenic-induced oxidative stress has been measured directly by assaying ROS production and indirectly through the evaluation of reactive byproducts such as 8-hydroxydeoxyguanosine and lipid peroxides. Levels of antioxidant proteins such as glutathione, heme oxygenase-1, A170, and peroxiredoxin 1 have been used as predictors of oxidative stress [1,36]. Earlier, we were able to measure and quantify ROS production in real-time using the electron spin resonance spin trapping technique and confocal microscopy. Our findings implicated hydroxyl and hydrogen peroxide ROS in arsenic-induced genotoxicity using Chinese hamster ovary chromosomes and in epidermal keratinocytes. This confirmed the importance of ROS and the reduction of intracellular thiols, particularly glutathione, in the toxicity of arsenic [35]. In a subsequent investigation, we employed two complementary approaches to determine the cellular organelle responsible for ROS production in arsenic cytotoxicity. In the first approach, we found that cells without nuclei continued to demonstrate arsenic toxicity in a model fusing arsenic-treated cytoplasts with karyoplasts from controls. Our second approach, involving a model of arsenic-treated human–hamster hybrid cells compared to mitochondria-depleted controls, demonstrated the necessity for mitochondria in arsenic-mediated ROS production. These findings supported the conclusion that, despite the existence of other cellular organelles such as lysosomes, peroxisomes, endoplasmic reticulum, and membrane-bound NADPH oxidase, mitochondrial damage was necessary for arsenic-induced DNA damage to occur (Fig. 1; Liu et al., 2005 [34]). These findings were not wholly surprising since mitochondrial damage is known to release superoxide, leading to the formation of destructive RNS from the reaction between superoxide and nitric oxide (NO) [34,35].
Fig. 1.
Flow diagram showing arsenic-mediated enhancement in oxidative stress and consequent effects on various signaling pathways: Arsenic induces ROS by multiple mechanisms where various oxidant-generating enzymes are induced and antioxidants armory is dampened. These oxidants impair functions of various organelles leading to a complex toxic response which is manifested in the skin and various other organs. Altered signaling pathways following chronic exposure to arsenic generate an inflammatory and carcinogenic response.
Arsenic generates ROS by increasing the expression and activity of NAD(P)H oxidase (NADPHO), an enzyme that produces superoxide, through several mechanisms [1,37]. The protein p22phox, a critical subunit of the NADPHO complex responsible for electron transfer, is upregulated by arsenic exposure [38]. In fact, the inhibition of p22phox expression has been found to block arsenic-induced superoxide production and DNA damage in human vascular smooth muscle cells [39]. Arsenic also activates NADPHO by stimulating the translocation of Rac1, a low-molecular-weight guanosine triphosphate (GTP) binding protein responsible for NADPHO’s activation [1,40]. Additionally, the arsenic-induced activation of CDC42, a low-molecular-weight GTPase, also enhances NADPHO function [1].
Nitric oxide (NO), a small and ubiquitous molecule, is involved in regulating various cellular functions including cellular metabolism, growth, proliferation, and apoptosis [41]. NO plays a critical role in several endothelial cell vasoprotective functions such as vasodilation, reduced inflammation, and the inhibition of smooth muscle cell proliferation [42]. Systemic NO depletion has long been associated with chronic arsenic exposure [1]. Since NO depletion is considered the hallmark of endothelial dysfunction [42], it is postulated that diseases of the vessels such as atherosclerosis and peripheral vascular disease occur more frequently in arsenic-exposed individuals, which may stem from dysregulation of NO metabolism in endothelial cells. Many mechanisms explaining arsenic-induced NO depletion have been proposed [1,39]. The most commonly described mechanism is the consumption of NO by its reaction with excessive ROS generated by arsenic [43].
NO synthase (NOS) is the enzyme responsible for the formation of NO from L-arginine. Sumi et al. (2007) found that MMAsIII readily binds the thiol moiety of NOS, which inhibits its function and thus directly reduces the availability of NO [1]. There is also evidence to suggest that NOS is indirectly inhibited by arsenic. Tetrahydrobiopterin (BH4) is an important cofactor for the NOS enzyme complex. A study using a rabbit model with prolonged exposure to arsenic demonstrated a reduction in BH4 levels without a concomitant decrease in L-arginine concentrations. This imbalance was found to uncouple the enzyme, shifting its production from NO to superoxide instead [1,43]. Beyond this, endothelin-1 (ET1), a potent vasoconstrictor produced by endothelial cells, has also been implicated in arsenic toxicity [44]. ET1 has been found to increase superoxide levels by both stimulating NADPHO [45] and uncoupling NOS through the activation of ET1 receptors A and B [44]. Recently, Hossain et al. (2012a) [46] discovered arsenic’s connection to ET1. They found a dose-dependent relationship between arsenic exposure and plasma Big ET1 concentrations, a biological precursor to ET1, in a study of 304 chronically arsenic-exposed individuals in Bangladesh [46]. This study provided an example of arsenic’s ability to accelerate atherosclerosis through oxidative stress and to directly induce hypertension through ET1 production. Taken together, these findings demonstrate how arsenic induces oxidative stress through numerous mechanisms leading to the augmentation of ROS and the depletion of NO. The effects of arsenic on tissue oxidative stress and consequent modulation of various pathways are depicted in Fig. 1.
Immune dysfunction
Longstanding evidence suggests that arsenic plays a role in the disruption of both the innate and cell-mediated immune responses. Several studies found an association between lower respiratory tract infections and diarrhea in individuals with a history of chronic arsenic exposure [17,47,48]. Mouse models have demonstrated arsenic’s ability to reduce pulmonary antibacterial defenses mediated by innate immune cells. Other models have observed inhibited T cell function resulting in greater influenza virus infectivity in arsenic-exposed mice when compared to controls [49]. Impaired immune responses have been reported among patients treated with therapeutic ATO. Nouri et al. (2006) [50] found a greater incidence of herpes simplex and herpes zoster infections among multiple myeloma and colon cancer patients treated with ATO [50]. Recently, some evidence has emerged suggesting the mechanisms by which arsenic mediates disruption of the innate and adaptive immune responses. The unfolded protein response (UPR) is a ho-meostatic mechanism that is activated in response to endoplasmic reticulum (ER) stress, which has been implicated in carcinogenesis [51], immune dysfunction [49], as well as neurodegenerative diseases [52].
The UPR is activated in response to an excessive number of unfolded or misfolded proteins present in the ER lumen. Its activation involves three distinctive pathways that attempt to return the cell to homeostasis. Receptors in the ER lumen such as inositol-requiring enzyme-1 (IRE1), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6) act as sensors of unfolded proteins. IRE1 and PERK initiate signaling cascades that inhibit translation to decrease the ER protein burden. Stimulation of ATF6 signals for the transcription of additional chaperones, molecules responsible for protein folding. If stress continues unmitigated, additional pathways are activated that shift the cell toward apoptosis. Thus, moderate signaling through PERK is responsible for slowing protein translation while stronger activation leads to cell death [53]. Studies from our laboratory have shown that subchronic arsenic exposure activates all three signaling pathways of the UPR in the epidermis of SKH-1 mice. In these studies, we demonstrated arsenic’s ability to upregulate the IRE1, PERK, and ATF6 sensors of the UPR. These findings causally implicated ER stress in arsenic toxicity and provided one possible mechanism by which this metalloid altered cellular differentiation leading to cutaneous inflammation, which is known to occur at higher rates among individuals with chronic arsenic exposure. Treatment with antioxidants such as N-acetylcysteine (NAC) effectively blocked these effects in murine skin [24], providing evidence of the involvement of ROS in UPR activation.
In additional recent studies, we found that arsenic impacts several macrophage functions including bacterial engulfment, cytokine release, and bacterial digestion. To understand the mechanism by which this occurred, we identified two interrelated processes necessary for the alteration of macrophage function. ATO treatment disrupted murine macrophage function by simultaneously generating ROS and activating the UPR pathway through stimulation of upstream PERK. Inhibition of the UPR by the chemical chaperone, 4-phenylbutyric acid, with concomitant reduction in ROS production following NAC administration demonstrated the interdependence of these two mechanisms in this model system. These findings illustrate at least one important mechanism by which arsenic disrupts macrophage function [49].
Arsenic has also been found to exacerbate lipopolysaccharide-induced inflammation that is mediated by macrophages and monocytes. This occurs by the upregulation of p38 kinase activity through ROS stimulation of the Src kinase pathway. Src kinase is a non-receptor tyrosine kinase that has been implicated in the progression of many solid tumors. Arsenic exposure causes monocytic-lineage cells to mount an exaggerated and damaging immune response resulting in excessive production of inflammatory cytokines [54]. Pion et al. (2007) [55] have also described arsenic’s ability to harm the adaptive immune response. An in vitro study found that ATO exposure enhanced HIV’s ability to infect human dendritic cells by dampening post-viral-entry cellular defenses against the retrovirus [55]. Arsenic exposure of zebra fish embryos was associated with a 50-fold increase in viral load and a 17-fold increase in bacterial load of cells exposed to snakehead rhabdovirus and Edwardsiella tarda, respectively [56]. In human models, immunohistochemistry of Bowen’s disease lesions taken from chronically arsenic-exposed individuals demonstrated the presence of apoptotic CD4 + T lymphocytes. The same patients also had fewer peripheral CD4 + cells when compared to controls. Interestingly, Liao et al. (2009) [57] found the remaining CD4 + T cells to be resistant to arsenic-induced apoptosis. This resulted from decreased expression of tumor necrosis factor (TNF) receptor 1, a receptor responsible for binding the inflammatory and apoptotic cytokine, TNF [57]. In summary, arsenic damages innate and adaptive immunity through a variety of mechanisms, which ultimately reduce both immune cell function and number (Fig. 2).
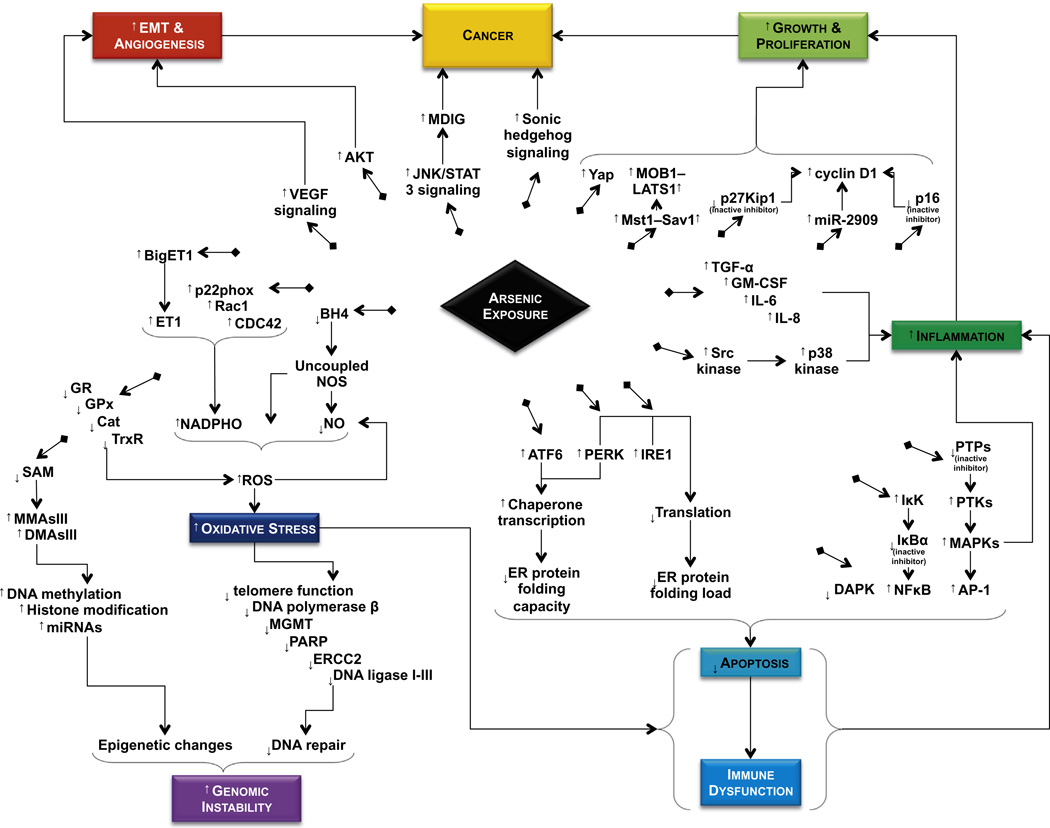
Fig. 2.
Summary of the effects of arsenic on various signal transduction pathways. Arsenic acts as a carcinogen by dysregulating multiple signal transduction pathways and proteins regulated by these pathways. Shown here are the effects of arsenic on the upregulation of ROS, NOS, VEGF, AKT, JNK/STAT, MDIG, Shh, Yap, Mst1-Sav1, cyclin D1, Src kinase, NFκB, AP-1, MAPKs, ATF6, PERK, IRE1, etc. and downregulation of p27Kip1, p16, DAPK, etc. Finally, it affects DNA methylation, DNA repair, and histone modification.
Genotoxicity
Arsenic is responsible for ubiquitous damage to mammalian tissues by disrupting chromosomes and damaging DNA strands. One study of 60 people residing in West Bengal, India found significantly increased incidence of chromosomal aberrations and errant sister chromatid exchanges in the DNA of arsenic-exposed individuals when compared to age-matched controls from the same socio-economic group [58]. Chronic arsenic exposure has also been found to cause epigenetic changes by modulating DNA methylation, histone maintenance, and mRNA expression [2,36]. The genotoxic effects of arsenic have been related to its ability to generate ROS and deplete methyl group donors such as S-adenosylmethionine (SAM). Tri- and pentavalent arsenic species receive methyl groups from SAM to form di- and monomethylated arsenic. Consequently, these arsenical compounds deplete the available pool of SAM and directly methylate DNA in an unregulated manner [2]. Oxidative damage, which is a reliable result of chronic arsenic exposure, can be predicted by neutrophilic DNA damage. It has been proposed as a measure of arsenicosis in exposed populations [59].
In addition to directly damaging DNA, arsenic also impacts several cellular DNA repair mechanisms. It inhibits nucleotide excision, base excision [3], and mismatch repair [60]. This is accomplished by its inhibition of DNA repair machinery such as I–III DNA polymerase β, O6-methyl-guanine-DNA methyltransferase, and poly ADP ribose polymerase [3] largely by taking the place of phosphate in ATP and by covalently bonding with sulfhydryl moieties of these enzymatic complexes [61]. Arsenic also disrupts proofreading during transcription through the inhibition of the Excision Repair Cross-Complementing rodent repair, complementation group 2 (ERCC2) protein [62]. Additionally, it promotes genomic instability by interfering with telomere function [63]. Investigations have also focused on the goal of reversing this damage. Roy et al. (2011) [64] found that curcumin, a naturally occurring compound frequently used in Indian food (curry preparations), enhanced the DNA repair capacity of arsenic-exposed individuals. Though these investigations are interesting, a translational component of this study in a clinical setting among patients chronically exposed to arsenic remains to be demonstrated. Altogether, the combination of direct genotoxicity and hampered DNA repair augment the mutagenicity of other cell stressors such as radiation and chemicals, thus demonstrating how arsenic acts synergistically with other carcinogens to enhance mutations and destabilize the genome. This aspect is highly relevant given the known variation in socioeconomic status of arsenic-exposed populations [65].
Disrupted signal transduction
Many of the deleterious effects caused by arsenic are mediated by aberrant activation of various signal transduction pathways. In general, the ability of arsenic to directly bind sulfhydryl (SH) moieties and/or conversion of SH to S–S by ROS generated in response to arsenic may result in many of its toxic effects. In fact, this interaction between arsenic and thiol groups may occur in over 200 known human proteins [66]. The most relevant pathways affected by arsenic may lead to the activation of oncogenes, the inhibition of tumor suppressors, and the upregulation of inflammatory pathways [1,27,67,68].
Recent evidence suggests that arsenic activates the canonical Hippo signaling pathway responsible for cell survival and proliferation [69]. This pathway has already been implicated in many malignancies including skin cancer [70]. Arsenic alters Hippo signaling through upregulation of several components of the pathway including STE20-like kinase 1/2 (Mst1), Salvador homolog 1 (Sav1), large tumor suppressor kinase 1/2 (LATS1), and Mps one binder kinase activator-like 1A (MOB1) [67].
Yes-associated protein (Yap), a known component of the Hippo signaling pathway, is a dual-function protein that serves distinctive roles depending upon its phosphorylation status. When phosphorylated, the protein regulates tight/adherens junctions of epithelium. When dephosphorylated, Yap acts as a transcription factor in the regulation of epithelial cell proliferation. One downstream target of dephosphorylated Yap is the GLI2 gene. GLI2 is a downstream effector of Sonic hedgehog (Shh) signaling, which has been implicated in arsenic-induced basal cell carcinoma [67,71]. Interestingly, arsenic was found to upregulate dephosphorylated Yap independent of Hippo activation in murine skin. The mechanism by which arsenic acts to upregulate this form of Yap has yet to be elucidated [67]. However, Li et al. (2013) [67] proposed that arsenic may induce Protein phosphatase 2A (PP2A), an enzyme known to regulate Yap function, and thus upregulate dephosphorylated Yap within cells (unpublished observations). Altogether, these findings demonstrate arsenic’s ability to promote skin cell proliferation and survival through alteration of both Hippo signaling and Hippo-independent Yap signaling pathways [67].
Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) is a transcription factor that has been implicated in chronic inflammation and various cancers [68]. It is retained in the cytoplasm by inhibitory κBα (IκBα) protein under normal conditions. However, constitutive activation of NFκB and migration to the nucleus lead to cell proliferation and growth [72]. When stimulated, IκBα kinase (IκK) phosphorylates IκBα, which is the targeted for ubiquitin-dependent proteasomal degradation allowing NFκB to translocate to the nucleus and transcribe genes responsible for cell survival. IκK has a thiol moiety that may be modified by AsIII leading to IκK activation. However, arsenic-mediated ROS have also been found to promote NFκB-mediated apoptosis [73–75]. These opposing findings, though perplexing, may point to the importance of the dose-dependent effects of arsenic. However, in-depth studies are required to clarify the situations that may lead to these diverse outcomes.
In this regard, a study of chronically exposed individuals proposed a different mechanism of arsenic-induced cell survival and proliferation. The authors found promoter hypermethylation and subsequent silencing of the pro-apoptotic death associated protein kinase (DAPK) and p16 genes in people with chronic arsenic exposure when compared to controls. This was associated with a higher risk of developing arsenic-induced skin lesions, ocular diseases, peripheral neuropathy, and respiratory diseases [27]. DAPK is serine/threonine protein kinase responsible for apoptosis via Fas ligand-TNF-α, tumor suppression, and detachment-induced cell death. p16 is a tumor suppressor gene that arrests the cell cycle at the growth 1 to synthesis (G1/S) transition by inhibition of the cyclin-D1/CDK4 complex [76]. In addition, arsenic exposure has been associated with decreased expression of cyclin-dependent kinase inhibitor 1B (p27Kip1), another inhibitor of the cyclin-D1/CDK4 complex [77]. Arsenic also promotes proliferation by altering the expression of cyclin-D1 itself. Sharma et al. found that arsenic upregulates cyclin-D1 through epigenetic modification by activating microRNA 2909 (miR-2909) [78].
Arsenic’s ability to disrupt signal transduction makes it an important carcinogen in many cancers in addition to those of the skin. Recent findings have demonstrated its ability to activate the mineral dust induced gene (MDIG), also known as myc-induced nuclear antigen 53 and nucleolar protein 52 by upregulating JNK and STAT3 pathways. MDIG is an oncogene that has been linked to lung [79], breast, colon, and gastric cancers, esophageal squamous cell, hepatocellular, and renal cell carcinomas, as well as some forms of lymphoma [80]. Evidence also suggests that this metalloid works through Akt (also known as protein kinase B) to promote epithelial to mesenchymal transition (EMT), invasion, and migration of arsenic-transformed bronchial epithelial cells [81]. Furthermore, arsenic has also been found to promote EMT and angiogenesis by increasing signaling through the β-catenin-vascular endothelial growth factor pathway [82].
Arsenic also acts by upregulating inflammatory pathways. Epidermal growth factor (EGF) binds the EGF receptor (EGFR) and ultimately activates mitogen-activated protein kinase (MAPK) pathways that play an important role in cellular stress response, inflammation, and growth. Notable MAPK pathways include the extracellular signal-regulated kinase1/2 (Erk1/2), p38 MAPK, and stress-activated protein kinase/Jun-N-terminal protein kinase (SAPK/ JNK) pathways. Erk1/2 is involved in growth and differentiation while p38 MAPK and SAPK/JNK are important in stress-induced inflammation and mitochondrial-dependent apoptosis. EGFR, an upstream effector of MAPK signaling, is normally inhibited by protein tyrosine phosphatases (PTPs) such as MAPK phosphatase 1. PTPs have a thiol group that may be subject to oxidation by arsenic via ROS. Oxidation of this moiety transiently inactivates PTPs, inhibitors of EGFR, thus enabling EGFR activation in the absence of EGF [1]. Exacerbating this effect, lower levels of the MAPK phosphatase 1 inhibitor have been found in arsenic treated cells when compared to controls [77].
Activation of the cutaneous p38 MAPK pathway increases inflammation as evidenced by alterations in helper T cell 1 (Th-1), Th-2, and Th-17 cytokines and their receptors [24,35]. Arsenic-induced MAPK signaling upregulates activated protein 1 (AP-1), a transcription factor which is known to regulate responses to cytokines, growth factors, stress, and infection, ultimately controlling apoptosis of the cell [1,3,83,84]. In addition, arsenic exposure increases keratinocyte expression of inflammatory cytokines such as TGF-α, GM-CSF, IL-6 and IL-8 [3]. Taken together, this large body of evidence suggests that arsenic orchestrates human disease by altering signal transduction, at least in part, through complex pathobiology involving the activation of oncogenes, inhibition of tumor suppressors, and upregulation of inflammatory pathways [1,27,67,68].
Skin effects of arsenic in murine models
Until recently, little was known of the clear mechanistic effects of arsenic toxicity in murine skin. Although arsenic is known to inhibit the cutaneous contact hypersensitivity response in mice [49], few studies have identified a relationship between chronic arsenic exposure and skin hyperkeratosis or cancer in the skin of mice. It was shown that the offspring of C3H mice exposed to arsenic compounds in utero failed to demonstrate increased incidence of skin tumors following treatment with topical applications of tumor-promoting phorbol ester [85]. Other studies in various animal models have not conclusively demonstrated that arsenic, alone, can induce tumors in murine skin [86–90]. Though arsenic’s ability to act alone as a skin carcinogen in mice has yet to be confirmed, an abundance of evidence demonstrates it acts as a co-carcinogen. Studies in Tg.AC skin tumor-prone mice showed greater incidence of skin papillomas when arsenic was combined with tumor-promoting phorbol ester versus controls [91]. However, the most well supported example of synergy has been shown between UV radiation and arsenic, a particularly relevant combination given the commonplace coexistence of these mutagens in human populations. Models using UVB-predominant exposure of mice with concomitant drinking water arsenic revealed shortened time to first skin tumor induction, increased total skin tumor number, larger tumor volume, and a greater percentage of invasive SCCs versus controls [89]. Recent in vitro studies combining arsenic and UV have supported this finding by demonstrating significantly greater oxidative damage in arsenic-treated cells when compared to the relevant controls [92]. Interestingly, this study and others found that arsenic treatment also modulated cellular stress responses by inhibiting physiologic UV-induced apoptosis [92,93]. This observed co-carcinogenicity was diminished by dietary antioxidants, once again implicating oxidative stress in arsenic’s pathogenesis [86]. These observations possess an important translational value because SCCs are observed in both murine and human studies. Although we previously described ROS-damaged DNA repair as one molecular mechanism to explain the synergism between arsenic and other carcinogens, recent studies have augmented this observation [67]. In a study of SKH-1 mice given arsenic-treated drinking water, we observed focal disruption in the basal layer of the epidermis as well as upregulated Yap signaling. Phosphorylated Yap, as mentioned previously, is involved in the maintenance epithelial tight/adherens junctions. These findings suggest inflammation and resultant oxidative damage stemming from an architectural disturbance as another possible pathway leading to arsenic-induced skin tumors [67].
Discussion
Arsenicosis is a serious and widespread global public health problem [6,65] with more than 200 million people at risk of toxic arsenic exposure from groundwater and food contamination [2,3,7]. Though the majority of data describing human disease resulting from arsenicosis has been collected from the developing world where its pathogenesis may involve additional factors such as malnutrition, mounting evidence suggests that arsenic poses a public health threat to certain populations in the United States as well. A recent case–control study in New Hampshire found positive relationship between urinary arsenic metabolites and SCC incidence in humans [94]. Another investigation observed that dietary arsenic from atypical sources such as dark meat fish (e.g., tuna steaks, mackerel, salmon, sardines, bluefish, and swordfish) correlated closely with toenail arsenic concentrations, a common marker for arsenic exposure [95]. Further investigation in a population-based case–control study by the same group supported the finding that demonstrated a positive relationship between the consumption of long chain fatty acids, typically found in fish oils, and toenail arsenic. Interestingly, an inverse relationship between other dietary lipids such as saturated fat and toenail arsenic were also observed. The authors proposed several explanations for these unexpected findings, however further study is needed to fully explain the human health implications observed [96]. As established previously, this well-known human carcinogen has been implicated in diseases of the lung, bladder, liver, kidney, prostate, and uterus. However, its effects on the skin typically occur first and often act as a precursor to other malignancy or disease [1–3,27,28].
In conclusion, these observations, combined with arsenic’s justified inclusion on the 2011 ATSDR substance priority risk list as the number one public health threat, signal the dire need to prioritize research investigations to understand the implications of arsenic exposure and to plan for attenuation of its predicted toxic manifestations in at-risk populations, worldwide. We have summarized in this review the body of evidence describing arsenic’s pathogenesis in the skin incorporating recently described novel mechanistic findings in the area.
Acknowledgement
MA’s original work described in this review was supported by National Institutes of Health grants R21 ES017494, R21 AR064595 and R01 CA138998.
Abbreviations
- AKT
RAC-alpha serine/threonine-protein kinase
- AP-1
activator protein-1
- ATF6
activating transcription factor 6
- BH4
tetrahydrobiopterin
- BigET1
big endothelin-1
- CDC42
cell division control protein 42 homolog
- DAPK
death associated protein kinase
- EMT
epithelial–mesenchymal transition
- ER
endoplasmic reticulum
- ERCC2
excision repair cross-complementing rodent repair complementation group 2
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IL
interleukin
- IRE1
inositol-requiring enzyme-1
- IκBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- IκK
IκB kinase
- LATS1
large tumor suppressor kinase 1/2
- MAPKs
mitogen-activated protein kinases
- MDIG
mineral dust-induced gene
- MGMT
O-6-methylguanine-DNA methyltransferase
- miR-2909
microRNA 2909
- miRNAs
microRNAs
- MOB1
Mps one binder kinase activator-like 1A
- Mst1
STE20-like kinase 1/2
- NADPHO
nicotinamide adenine dinucleotide phosphate oxidase
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- NOS
nitric oxide synthase
- p16
cyclin-dependent kinase inhibitor 2A
- p22phox
human neutrophil cytochrome βlight chain
- p27Kip1
cyclin-dependent kinase inhibitor 1B
- p38 kinase
p38 mitogen-activated protein kinase
- PARP
poly ADP ribose polymerase
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- PTKs
protein tyrosine kinases
- PTPs
protein tyrosine phosphatases
- Rac1
Ras-related C3 botulinum toxin substrate 1
- ROS
reactive oxygen species
- SAM
S-adenosyl methionine
- sav1
Salvador homolog 1
- Src kinase
proto-oncogene tyrosine-protein kinase Src
- TGF-α
transforming growth factor alpha
- VEGF
vascular endothelial growth factor
- Yap
Yes-associated protein
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.Kumagai Y, Sumi D. Arsenic: signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Annu. Rev. Pharmacol. Toxicol. 2007;47:243–262. doi: 10.1146/annurev.pharmtox.47.120505.105144. [DOI] [PubMed] [Google Scholar]
- 2.Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. J Toxicol. 2011;2011:431287. doi: 10.1155/2011/431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu HS, Liao WT, Chai CY. Arsenic carcinogenesis in the skin. J. Biomed. Sci. 2006;13(5):657–666. doi: 10.1007/s11373-006-9092-8. [Epub 2006 Jun 29] [DOI] [PubMed] [Google Scholar]
- 4.IARC. International Agency for Research on Cancer. Lyon, France: World Health Organization; 2012. A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts, Vol. 100C, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 5.Surdu S, Fitzgerald EF, Bloom MS, Boscoe FP, Carpenter DO, Haase RF, et al. Occupational exposure to arsenic and risk of nonmelanoma skin cancer in a multinational European study. Int. J. Cancer. 2013;133(9):2182–2191. doi: 10.1002/ijc.28216. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Background Document for Development of WHO Guidelines for Drinking-Water Quality. Geneva, Switzerland: World Health Organization; 2011. Arsenic in Drinking-Water. [Google Scholar]
- 7.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect. 2013;121(3):295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong SS, Tan KC, Goh CL. Cutaneous manifestations of chronic arsenicism: review of seventeen cases. J. Am. Acad. Dermatol. 1998;38(2 Pt 1):179–185. doi: 10.1016/s0190-9622(98)70596-1. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan SV, Johnston RB, Zheng Y. Arsenic in tube well water in Bangladesh: health and economic impacts and implications for arsenic mitigation. Bull. World Health Organ. 2012;90(11):839–846. doi: 10.2471/BLT.11.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, et al. Groundwater arsenic contamination throughout China. Science. 2013;341(6148):866–868. doi: 10.1126/science.1237484. [DOI] [PubMed] [Google Scholar]
- 11.Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environ. Health. 2013;12(1):73. doi: 10.1186/1476-069X-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas J, Sinha D, Mukherjee S, Roy S, Siddiqi M, Roy M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum. Exp. Toxicol. 2010;29(6):513–524. doi: 10.1177/0960327109359020. [Epub 2010 Jan 7] [DOI] [PubMed] [Google Scholar]
- 13.Wasserman GA, Liu X, Loiacono NJ, Kline J, Factor-Litvak P, van Geen A, et al. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ. Health. 2014;13(1):23. doi: 10.1186/1476-069X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu G, Sun D, Zheng Y. Health effects of exposure to natural arsenic in groundwater and coal in China: an overview of occurrence. Environ. Health Perspect. 2007;115(4):636–642. doi: 10.1289/ehp.9268. [Epub 2007 Jan 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, et al. Arsenic exposure and motor function among children in Bangladesh. Environ. Health Perspect. 2011;119(11):1665–1670. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syed EH, Poudel KC, Sakisaka K, Yasuoka J, Ahsan H, Jimba M. Quality of life and mental health status of arsenic-affected patients in a Bangladeshi population. J. Health Popul. Nutr. 2012;30(3):262–269. doi: 10.3329/jhpn.v30i3.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parvez F, Chen Y, Yunus M, Olopade C, Segers S, Slavkovich V, et al. Arsenic exposure and impaired lung function. Findings from a large population-based prospective cohort study. Am. J. Respir. Crit. Care Med. 2013;188(7):813–819. doi: 10.1164/rccm.201212-2282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 2008;116(2):158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Toxicology Program, Report on Carcinogens. [(accessed 14.04.28)];Research Triangle Park, NC:NTP. (12th ed.). 2011 < http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/arsenic.pdf>.
- 20.Chen Y, Wu F, Graziano JH, Parvez F, Liu M, Paul RR, et al. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am. J. Epidemiol. 2013;178(3):372–381. doi: 10.1093/aje/kwt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, Parvez F, et al. Interaction between arsenic exposure from drinking water and genetic susceptibility in carotid intima-media thickness in Bangladesh. Toxicol. Appl. Pharmacol. 2014;276(3):195–203. doi: 10.1016/j.taap.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ. Health Perspect. 2012;120(12):1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Xu J, Li F, Chaudhary SC, Weng Z, Wen J, et al. Unfolded protein response signaling and MAP kinase pathways underlie pathogenesis of arsenic-induced cutaneous inflammation. Cancer Prev. Res. (Phila.) 2011;4(12):2101–2109. doi: 10.1158/1940-6207.CAPR-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melkonian S, Argos M, Chen Y, Parvez F, Pierce B, Ahmed A, et al. Intakes of several nutrients are associated with incidence of arsenic-related keratotic skin lesions in Bangladesh. J. Nutr. 2012;142(12):2128–2134. doi: 10.3945/jn.112.165720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh SK, Bandyopadhyay D, Bandyopadhyay SK, Debbarma K. Cutaneous malignant and premalignant conditions caused by chronic arsenicosis from contaminated ground water consumption: a profile of patients from eastern India. Skinmed. 2013;11(4):211–216. [PubMed] [Google Scholar]
- 27.Banerjee N, Paul S, Sau TJ, Das JK, Bandyopadhyay A, Banerjee S, et al. Epigenetic modifications of DAPK and p16 genes contribute to arsenic-induced skin lesions and nondermatological health effects. Toxicol. Sci. 2013;135(2):300–308. doi: 10.1093/toxsci/kft163. [Epub 2013 Jul 20] [DOI] [PubMed] [Google Scholar]
- 28.Ahsan H, Steinmaus C. Invited commentary: use of arsenical skin lesions to predict risk of internal cancer: implications for prevention and future research. Am. J. Epidemiol. 2013;177(3):213–216. doi: 10.1093/aje/kws366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas-Schoemann A, Batteux F, Mongaret C, Nicco C, Chéreau C, Annereau M, et al. Arsenic trioxide exerts antitumor activity through regulatory T cell depletion mediated by oxidative stress in a murine model of colon cancer. J. Immunol. 2012;189(11):5171–5177. doi: 10.4049/jimmunol.1103094. [DOI] [PubMed] [Google Scholar]
- 30.Cullen WR. Chemical mechanism of arsenic biomethylation. Chem. Res. Toxicol. 2014;27(4):457–461. doi: 10.1021/tx400441h. [DOI] [PubMed] [Google Scholar]
- 31.Niedzwiecki MM, Hall MN, Liu X, Slavkovich V, Ilievski V, Levy D, et al. Interaction of plasma glutathione redox and folate deficiency on arsenic methylation capacity in Bangladeshi adults. Free Radic. Biol. Med. 2014 doi: 10.1016/j.freeradbiomed.2014.03.042. pii: S0891-5849(14)00160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brocato J, Costa M. Basic mechanics of DNA methylation and the unique landscape of the DNA methylome in metal-induced carcinogenesis. Crit. Rev. Toxicol. 2013;43(6):493–514. doi: 10.3109/10408444.2013.794769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, Slavkovich V, et al. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ. Health Perspect. 2013;121(9):1068–1074. doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu SX, Davidson MM, Tang X, Walker WF, Athar M, Ivanov V, et al. Mitochondrial damage mediates genotoxicity of arsenic in mammalian cells. Cancer Res. 2005;65(8):3236–3242. doi: 10.1158/0008-5472.CAN-05-0424. [DOI] [PubMed] [Google Scholar]
- 35.Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc. Natl Acad. Sci. U.S.A. 2001;98(4):1643–1648. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niedzwiecki MM, Hall MN, Liu X, Oka J, Harper KN, Slavkovich V, et al. A dose-response study of arsenic exposure and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Environ. Health Perspect. 2013;121(11–12):1306–1312. doi: 10.1289/ehp.1206421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol. Sci. 2011;123(2):305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271(38):23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 39.Lynn S, Gurr JR, Lai HT, Jan KY. NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ. Res. 2000;86(5):514–519. doi: 10.1161/01.res.86.5.514. [DOI] [PubMed] [Google Scholar]
- 40.Di-Poï N, Fauré J, Grizot S, Molnár G, Pick E, Dagher MC. Mechanism of NADPH oxidase activation by the Rac/Rho-GDI complex. Biochemistry. 2001;40(34):10014–10022. doi: 10.1021/bi010289c. [DOI] [PubMed] [Google Scholar]
- 41.Zhang YH, Casadei B. Sub-cellular targeting of constitutive NOS in health and disease. J. Mol. Cell. Cardiol. 2012;52(2):341–350. doi: 10.1016/j.yjmcc.2011.09.006. [Epub 2011 Sep 16] [DOI] [PubMed] [Google Scholar]
- 42.Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012;23(4):222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumagai Y. Fusion of field and laboratory studies on the investigation of arsenic. Yakugaku Zasshi. 2009;129(10):1177–1185. doi: 10.1248/yakushi.129.1177. [DOI] [PubMed] [Google Scholar]
- 44.Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J. Pharmacol. Exp. Ther. 2005;315(3):1058–1064. doi: 10.1124/jpet.105.091728. [Epub 2005 Sep 6] [DOI] [PubMed] [Google Scholar]
- 45.Li L, Watts SW, Banes AK, Galligan JJ, Fink GD, Chen AF. NADPH oxidase-derived superoxide augments endothelin-1-induced venoconstriction in mineralocorticoid hypertension. Hypertension. 2003;42(3):316–321. doi: 10.1161/01.HYP.0000084853.47326.F2. [DOI] [PubMed] [Google Scholar]
- 46.Hossain E, Islam K, Yeasmin F, Karim MR, Rahman M, Agarwal S, et al. Elevated levels of plasma Big endothelin-1 and its relation to hypertension and skin lesions in individuals exposed to arsenic. Toxicol. Appl. Pharmacol. 2012a;259(2):187–194. doi: 10.1016/j.taap.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Rahman A, Vahter M, Ekstrom EC, Persson LA. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ. Health Perspect. 2011;119:719–724. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AM, et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol. Lett. 2009;185:197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Srivastava RK, Li C, Chaudhary SC, Ballestas ME, Elmets CA, Robbins DJ, et al. Unfolded protein response (UPR) signaling regulates arsenic trioxide-mediated macrophage innate immune function disruption. Toxicol. Appl. Pharmacol. 2013;272(3):879–887. doi: 10.1016/j.taap.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nouri K, Ricotti CA, Jr, Bouzari N, Chen H, Ahn E, Bach A. The incidence of recurrent herpes simplex and herpes zoster infection during treatment with arsenic trioxide. J. Drugs Dermatol. 2006;5:182–185. [PubMed] [Google Scholar]
- 51.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013;5(206) doi: 10.1126/scitranslmed.3006767. 206ra138. [DOI] [PubMed] [Google Scholar]
- 53.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 54.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14(7):667–678. doi: 10.1634/theoncologist.2009-0009. PMC 3303596. PMID 19581523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pion M, Stalder R, Correa R, Mangeat B, Towers GJ, Piguet V. Identification of an arsenic-sensitive block to primate lentiviral infection of human dendritic cells. J. Virol. 2007;81:12086–12090. doi: 10.1128/JVI.00800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nayak AS, Lage CR, Kim CH. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio) Toxicol. Sci. 2007;98:118–124. doi: 10.1093/toxsci/kfm072. [DOI] [PubMed] [Google Scholar]
- 57.Liao WT, Yu CL, Lan CC, Lee CH, Chang CH, Chang LW, et al. Differential effects of arsenic on cutaneous and systemic immunity: focusing on CD4+ cell apoptosis in patients with arsenic-induced Bowen's disease. Carcinogenesis. 2009;30(6):1064–1072. doi: 10.1093/carcin/bgp095. [DOI] [PubMed] [Google Scholar]
- 58.Mahata J, Basu A, Ghoshal S, Sarkar JN, Roy AK, Poddar G, et al. Chromosomal aberrations and sister chromatid exchanges in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat. Res. 2003;534(1–2):133–143. doi: 10.1016/s1383-5718(02)00255-3. [DOI] [PubMed] [Google Scholar]
- 59.Pei Q, Ma N, Zhang J, Xu W, Li Y, Ma Z, et al. Oxidative DNA damage of peripheral blood polymorphonuclear leukocytes, selectively induced by chronic arsenic exposure, is associated with extent of arsenic-related skin lesions. Toxicol. Appl. Pharmacol. 2013;266(1):143–149. doi: 10.1016/j.taap.2012.10.031. [Epub 2012 Nov 8] [DOI] [PubMed] [Google Scholar]
- 60.Hossain MB, Vahter M, Concha G, Broberg K. Environmental arsenic exposure and DNA methylation of the tumor suppressor gene p16 and the DNA repair gene MLH1: effect of arsenic metabolism and genotype. Metallomics. 2012b;4(11):1167–1175. doi: 10.1039/c2mt20120h. [DOI] [PubMed] [Google Scholar]
- 61.Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity - a review. Hum. Exp. Toxicol. 2007;26(10):823–832. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- 62.Paul S, Banerjee N, Chatterjee A, Sau TJ, Das JK, Mishra PK, et al. Arsenic-induced promoter hypomethylation and over-expression of ERCC2 reduces DNA repair capacity in humans by non-disjunction of the ERCC2-Cdk7 complex. Metallomics. 2014 doi: 10.1039/c3mt00328k. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Bhattacharjee P, Banerjee M, Giri AK. Role of genomic instability in arsenic-induced carcinogenicity. A review. Environ. Int. 2013;53:29–40. doi: 10.1016/j.envint.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Roy M, Sinha D, Mukherjee S, Biswas J. Curcumin prevents DNA damage and enhances the repair potential in a chronically arsenic-exposed human population in West Bengal, India. Eur. J. Cancer Prev. 2011;20(2):123–131. doi: 10.1097/cej.0b013e328341017a. [DOI] [PubMed] [Google Scholar]
- 65.Argos M, Parvez F, Chen Y, Hussain AZ, Momotaj H, Howe GR, et al. Socioeconomic status and risk for arsenic-related skin lesions in Bangladesh. Am. J. Public Health. 2007;97(5):825–831. doi: 10.2105/AJPH.2005.078816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, et al. Arsenic: health effects, mechanisms of actions, and research issues. Environ. Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C, Srivastava RK, Elmets CA, Afaq F, Athar M. Arsenic-induced cutaneous hyperplastic lesions are associated with the dysregulation of Yap, a Hippo signaling-related protein. Biochem. Biophys. Res. Commun. 2013;438(4):607–612. doi: 10.1016/j.bbrc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;51:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 69.Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fei DL, Li H, Kozul CD, Black KE, Singh S, Gosse JA, et al. Activation of Hedgehog signaling by the environmental toxicant arsenic may contribute to the etiology of arsenic-induced tumors. Cancer Res. 2010;70(5):1981–1988. doi: 10.1158/0008-5472.CAN-09-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathas S, Lietz A, Janz M, Hinz M, Jundt F, et al. Inhibition of NF- kappaB essentially contributes to arsenic-induced apoptosis. Blood. 2003;102:1028–1034. doi: 10.1182/blood-2002-04-1154. [DOI] [PubMed] [Google Scholar]
- 73.Felix K, Manna SK, Wise K, Barr J, Ramesh GT. Low levels of arsenite activates nuclear factor-kappaB and activator protein-1 in immortalized mesencephalic cells. J. Biochem. Mol. Toxicol. 2005;19:67–77. doi: 10.1002/jbt.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP1 and NF-kappaB DNA binding activity and related gene expression. Toxicol. Lett. 2002;133:33–45. doi: 10.1016/s0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 75.Wijeweera JB, Gandolfi AJ, Parrish A, Lantz RC. Sodium arsenite enhances AP-1 and NFkappaB DNA binding and induces stress protein expression in precision-cut rat lung slices. Toxicol. Sci. 2001;61:283–294. doi: 10.1093/toxsci/61.2.283. [DOI] [PubMed] [Google Scholar]
- 76.Tyler LN, Ai L, Zuo C, Fan CY, Smoller BR. Analysis of promoter hypermethylation of death-associated protein kinase and p16 tumor suppressor genes in actinic keratoses and squamous cell carcinomas of the skin. Mod. Pathol. 2003;16:660–664. doi: 10.1097/01.MP.0000077516.90063.7D. [DOI] [PubMed] [Google Scholar]
- 77.Trouba KJ, Wauson EM, Vorce RL. Sodium arsenite-induced dysregulation of proteins involved in proliferative signaling. Toxicol. Appl. Pharmacol. 2000;164(2):161–170. doi: 10.1006/taap.1999.8873. [DOI] [PubMed] [Google Scholar]
- 78.Sharma M, Sharma S, Arora M, Kaul D. Regulation of cellular Cyclin D1 gene by arsenic is mediated through miR-2909. Gene. 2013;522(1):60–64. doi: 10.1016/j.gene.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 79.Sun J, Yu M, Lu Y, Thakur C, Chen B, Qiu P, et al. Carcinogenic metalloid arsenic induces expression of mdig oncogene through JNK and STAT3 activation. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.01.002. pii: S0304-3835(14)00016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu Y, Chang Q, Zhang Y, Beezhold K, Rojanasakul Y, Zhao H, et al. Lung cancer-associated JmjC domain protein mdig suppresses formation of tri-methyl lysine 9 of histone H3. Cell Cycle. 2009;8(13):2101–2109. doi: 10.4161/cc.8.13.8927. [DOI] [PubMed] [Google Scholar]
- 81.Wang Z, Yang J, Fisher T, Xiao H, Jiang Y, Yang C. Akt activation is responsible for enhanced migratory and invasive behavior of arsenic-transformed human bronchial epithelial cells. Environ. Health Perspect. 2012;120(1):92–97. doi: 10.1289/ehp.1104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Humphries B, Xiao H, Jiang Y, Yang C. Epithelial to mesenchymal transition in arsenic-transformed cells promotes angiogenesis through activating β-catenin-vascular endothelial growth factor pathway. Toxicol. Appl. Pharmacol. 2013;271(1):20–29. doi: 10.1016/j.taap.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ameyar M, Wisniewska M, Weitzman JB. A role for AP-1 in apoptosis: the case for and against. Biochimie. 2003;85(8):747–752. doi: 10.1016/j.biochi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Hossain E, Ota A, Takahashi M, Karnan S, Damdindorj L, Konishi Y, et al. Arsenic upregulates the expression of angiotensin II type I receptor in mouse aortic endothelial cells. Toxicol. Lett. 2013;220(1):70–75. doi: 10.1016/j.toxlet.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25(1):133–141. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- 86.Burns FJ, Uddin AN, Wu F, Nádas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: a dose-response study. Environ. Health Perspect. 2004;112:599–603. doi: 10.1289/ehp.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y, Megosh LC, Gilmour SK, Sawicki JA, O'Brien TG. K6/ODC trans-genic mice as a sensitive model for carcinogen identification. Toxicol. Lett. 2000;116:27–35. doi: 10.1016/s0378-4274(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 88.Morikawa T, Wanibuchi H, Morimura K, Ogawa M, Fukushima S. Promotion of skin carcinogenesis by dimethylarsinic acid in keratin (K6)/ ODC transgenic mice. Jpn J. Cancer Res. 2000;91:579–581. doi: 10.1111/j.1349-7006.2000.tb00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcino-gen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- 90.Uddin AN, Burns FJ, Rossman TG. Vitamin E and organoselenium pre- vent the cocarcinogenic activity of arsenite with solar UVR in mouse skin. Carcinogenesis. 2005;26:2179–2186. doi: 10.1093/carcin/bgi180. [DOI] [PubMed] [Google Scholar]
- 91.Tokar EJ, Benbrahim-Tallaa L, Ward JM, Lunn R, Sams 2nd RL, Waalkes MP. Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit. Rev. Toxicol. 2010;40(10):912–927. doi: 10.3109/10408444.2010.506641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun Y, Kojima C, Chignell C, Mason R, Waalkes MP. Arsenic transformation predisposes human skin keratinocytes to UV-induced DNA damage yet enhances their survival apparently by diminishing oxidant response. Toxicol. Appl. Pharmacol. 2011;255(3):242–250. doi: 10.1016/j.taap.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuo Z, Ouyang W, Li J, Costa M, Huang C. Cyclooxygenase-2 (COX-2) mediates arsenite inhibition of UVB-induced cellular apoptosis in mouse epidermal Cl41 cells. Curr. Cancer Drug Targets. 2012;12(6):607–616. doi: 10.2174/156800912801784802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilbert-Diamond D, Li Z, Perry AE, Spencer SK, Gandolfi AJ, Karagas MR. A population-based case-control study of urinary arsenic species and squamous cell carcinoma in New Hampshire, USA, Environ. Health Perspect. 2013;121(10):1154–1160. doi: 10.1289/ehp.1206178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cottingham KL, Karimi R, Gruber JF, Zens MS, Sayarath V, Folt CL, et al. Diet and toenail arsenic concentrations in a New Hampshire population with arsenic-containing water. Nutr. J. 2013;12:149. doi: 10.1186/1475-2891-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gruber JF, Karagas MR, Gilbert-Diamond D, Bagley PJ, Zens MS, Sayarath V, et al. Associations between toenail arsenic concentration and dietary factors in a New Hampshire population. Nutr. J. 2012;11:45. doi: 10.1186/1475-2891-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited reference
- 97.Kumar V, Abbas A, Aster J. Robbins Basic Pathology. Ninth ed. Elsevier Saunders; 2013. pp. 1–28. [Google Scholar]