Abstract
Injectable biodegradable hydrogels, which can be delivered in a minimally invasive manner and formed in situ, have found a number of applications in pharmaceutical and biomedical applications, such as drug delivery and tissue engineering. We have recently developed an in situ crosslinkable citric acid-based biodegradable poly (ethylene glycol) maleate citrate (PEGMC)/hydroxyapatite (HA) composite, which shows promise for use in bone tissue engineering. In this study, the mechanical properties of the PEGMC/HA composites were studied in dynamic linear rheology experiments. Critical parameters such as monomer ratio, crosslinker, initiator, and HA concentrations were varied to reveal their effect on the extent of crosslinking as they control the mechanical properties of the resultant gels. The rheological studies, for the first time, allowed us investigating the physical interactions between HA and citric acid-based PEGMC. Understanding the viscoelastic properties of the injectable gel composites is crucial in formulating suitable injectable PEGMC/HA scaffolds for bone tissue engineering, and should also promote the other biomedical applications based on citric acid-based biodegradable polymers.
1 Introduction
Inorganic bioceramics such as hydroxyapatite (HA), tricalcium phosphate (TCP), and biphasic calcium phosphates (BCP) are commonly used fillers, which have been used to confer osteoconductivity and strength in bone tissue engineering grafts.[1, 2] These inorganic materials have been incorporated into crosslinkable biodegradable polymers for cell and drug delivery or directly used as bone fillers.1-4 It is imperative that the role of inorganic fillers on the crosslinking mechanism should be understood before a suitable injectable bone scaffold is developed. As the gelation kinetics and stiffness of the gel determine the overall performance of the injectable materials, the mechanical behavior of various hydrogels (e.g. poly(propylene fumarate),5 polymethylmethacrylate,2 and hydroxypropylmethyl cellulose6) filled with HA have been widely characterized and studied. While polymer compositional factors on gelation kinetics have been reported in different composite systems,7-9 the rheological properties of injectable polymer/HA composites have not yet been studied.
We have recently developed a family of citric acid-based biodegradable elastomers (CABEs) for various biomedical applications.10-17 For these materials, citric acid is a robust multifunctional monomer, which provides valuable pendant functionality to give CABEs unique advantages over existing biomaterials. Citric acid is not only used to participate in the ester bond network crosslink formation in CABEs, but also enhances hemocompatibility, balances the hydrophilicity of the polymer network, provides hydrogen bonding, and additional binding sites for bioconjugation to confer further functionality such as optical properties.18, 19 In particular for bone tissue engineering, CABEs such as poly(diol citrates) have shown uniqueness over other injectable polymers as the rich −COOH from the citrate units of the CABEs prompt calcium chelation to facilitate polymer/HA interaction resulting in enhanced mechanical properties and mineralization. To expand the uses of CABEs in injectable tissue engineering, we have recently developed a water-soluble injectable polymeric biomaterial, poly (ethylene glycol maleate citrate) (PEGMC) which demonstrated excellent injectability, in situ crosslinkability, degradability, and biocompatibility both in vitro and in vivo.20 PEGMC is an unsaturated linear polyester oligomer composed of citric acid, polyethylene glycol (Mw = 200 Da), and maleic anhydride, which are crosslinked through the vinyl groups of maleate units in the PEGMC polymer backbones.
An important feature of a suitable injectable hydrogel/HA composite system is that it should be easily injected and rapidly crosslinked in a time frame that allows for mixing of the gel with HA prior to injection. In order to develop such injectable systems, a thorough rheological study is important. For example, the moduli can be measured over time to determine the time-dependant viscoelastic properties of hydrogel/HA composite. Since chemical crosslinking occurs as a function of time, it severely hinders the measurement of the frequency dependent moduli during the gelation. Thus, multi-wave rheology experiments can be an alternative approach to expedite the measurement of storage and loss moduli by applying multiple frequencies simultaneously in oscillatory experiments. The scope of this study is to observe the effect of HA fillers, particularly at large loading compositions (~50 wt.-%, close to the percentage of inorganic components in natural bone), on the viscoelastic properties of hydrogel composites. In addition, we examine the effect of the concentration of vinyl functionalities (i.e. monomer ratio) on the rheological properties of a chemically crosslinked PEGMC hydrogel system.
2 Experimental
Synthesis of PEGMC Pre-Polymers
HA [Mw: 502.32, assay >90% (as Ca3(PO4)2); particle size: >75 μm (0.5%), 45–75 μm (1.4%), < 45 μm (98.1%)] was purchased from Fluka (St. Louis, MO, USA). All other chemicals were purchased from Sigma Aldrich (St. Louis, MO), except where mentioned otherwise. All chemicals were used as received.
PEGMC is an oligomeric pre-polymer synthesized by the polycondensation of polyethylene glycol (Mw = 200 Da) (PEG), citric acid (CA), and vinyl group containing maleic anhydride (MA).20 The resulting PEGMC pre-polymer was purified by dialyzing in deionized (DI) water with a 500 Da molecular weight cut off membrane for 2 days. Finally, the PEGMC pre-polymer was freeze-dried for additional 2 days. To understand the effect of the different molar concentration of monomers on the mechanical behaviors of the polymers, a series of pre-polymers was synthesized with the ratios of MA/CA varied as 0.8/0.2, 0.6/0.4 and 0.4/0.6 while keeping the overall diol to the acid ratio as 1:1. The corresponding PEGMC pre-polymers are denoted as PEGMC(0.8), PEGMC(0.6), and PEGMC(0.4), respectively.
Preparation of PEGMC/HA Composites
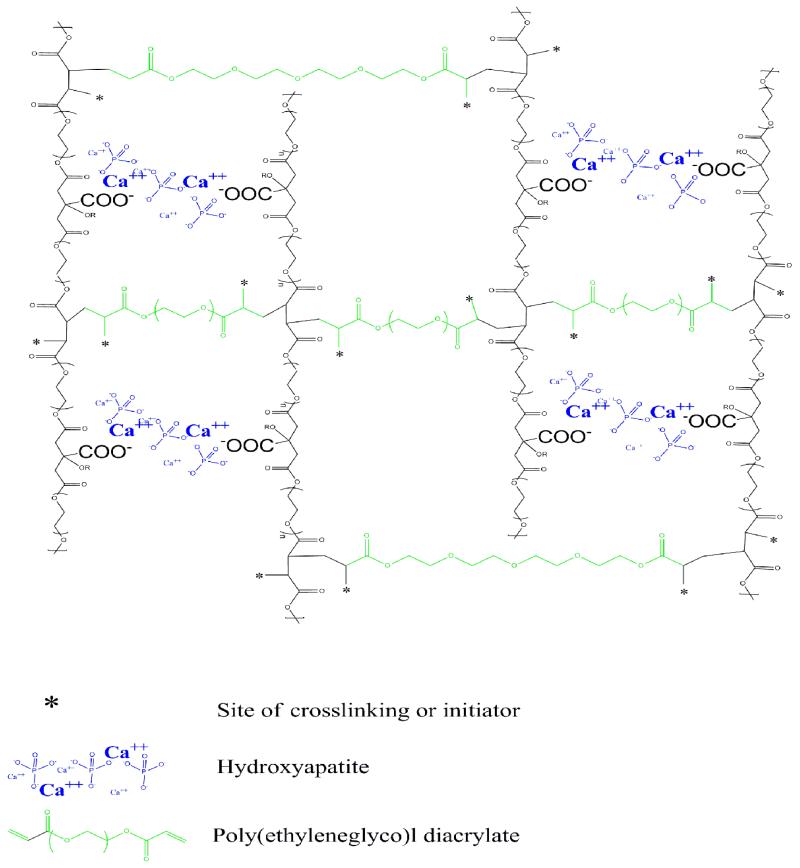
Oligomeric PEGMC pre-polymers were crosslinked using poly(ethylene diacrylate) (PEGDA) as a crosslinker and redox initiator after mixing with HA particles (Figure 1). 0.3 g of PEGMC pre-polymer was dissolved in 1 ml of DI water followed by mixing 0.15 g of PEGDA (Mw = 700 Da) and 0.3 g of HA, which was further neutralized by 0.3-0.5 g of sodium bicarbonate. 25 mM of ammonium persulfate (APS) and N, N, N’, N’-Tetramethyl-1-,2-diaminomethane (TEMED) were then added to the above composites to initiate crosslinking reaction, and form crosslinked PEGMC. The weight percentages of HA to composite (PEGMC/HA) varied as 0%, 30%, 50%, and 70%. The weight by volume percentages of crosslinker (PEGDA) to the injectable form varied as 3%, 6%, and 15%. The molar concentrations of initiators were 25 mM, 50 mM, and 75 mM.
Figure 1.
Schematic representation of the chemical structure of PEGMC/HA composites.
Hydrogel morphology and porosity measurement: The crosslinked PEGMC hydrogels and PEGMC/HA composites were soaked in water for 24 hr to achieve equilibrium swelling. The swollen hydrogels were cryosectioned to obtain 20 μm thick sections. The sections were placed on glass slides and then dried at room temperature. The morphology of the hydrogel composite at a fully swollen state was observed under a Leica DMLP microscope (Leica Microsystems Inc., Bannockburn, IL) fitted with a Nikon E500 camera (Nikon Corp., Japan). Image J analysis software was used to determine porosity. Briefly, six micrographs randomly obtained from hydrated samples were sectioned and converted from analogical to digital image (8-bit conversion). The porosity was calculated via the ratios of void areas over section areas. More than 10 sections were analyzed and averaged to obtain mean and standard deviation of porosity and pore diameters. Statistical analysis was performed using one way-ANOVA with post hoc NeumaneKeuls testing. A p-value of 0.05 or less was considered statistically significant.
Rheological measurements
Rheology experiments were conducted during the gelation process at 37 °C using a TA Instruments’ strain controlled ARES-G2 rheometer with 25 mm parallel plates and the gap was kept at 1.9 mm for all the samples. PEGMC hydrogels and their composites were prepared as described above. The mixtures were mixed well before adding TEMED solution and transferred immediately to the parallel plates of the rheometer after adding TEMED and stirring for 3 seconds.
Chemically crosslinked polymers undergo a liquid to solid transition in which the sol- and gel-like characteristics can be observed before and after gel-point. First, we conduct frequency sweep experiments and also multi-wave tests to verify that Winter-Chambon (WC) criteria is valid for our samples and consequently the gel-point of PEGMC was determined by monitoring the cross-over of viscous and elastic moduli in rheology measurements. Characteristic behavior of a hydrogel can be defined as the solid-like response (G’ > G”) at short time scales (high frequency) and liquid-like response (G’ < G”) at long times (low frequency). As the chemical crosslinking occurs as a function of time, it is difficult to measure the frequency dependent moduli during the gelation. Thus, multiwave rheology experiments can expedite the measurement of storage and loss moduli by applying multiple frequencies simultaneously in oscillatory experiments with time to evaluate the gel point. In this method, an oscillatory small strain γ(t) was imposed to the sample:
| (1) |
where m is the number of the superimposed harmonics, and γi and ωi denote the amplitude and the frequency of the i-th harmonic, respectively. The frequencies of the harmonics were ωn=nωf with the fundamental frequency ωf. In our studies, ωf=10 rad/s (n =1, 5, 10) and γi = 2%. Data collection started immediately after the sample was loaded and continued for 1000 s. The cross-over point of G’ and G” is correlated with the gel point which is based on the fact that WC criteria is valid for our chemically crosslinked gels.
Frequency sweep experiments from 0.1 to 100 rad/s were conducted on samples with varying polymer compositions and filler amounts after gelation. The strain amplitude in frequency sweep measurements was 10% to remain within the linear region of viscoelasticity. Viscoelastic properties were characterized by the complex shear modulus
| (2) |
and complex viscosity
| (3) |
3 Results and Discussion
3.1 PEGMC/HA composite formation, morphology observation, and porosity analyses
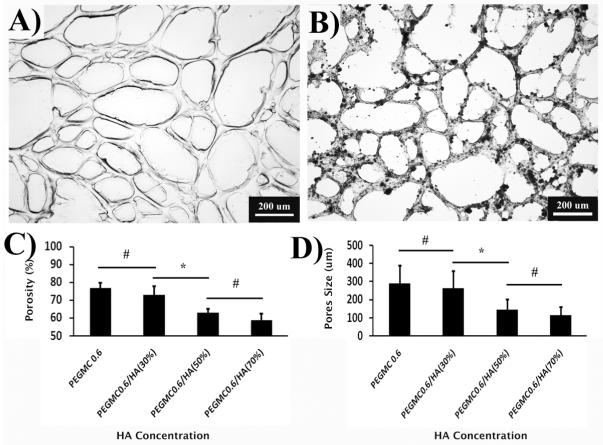
PEGMC was synthesized by a convenient polycondensation reaction, which resulted in a water-soluble polyester oligomer pre-polymer with double bonds in polymer backbone and pendant carboxyl and hydroxyl functionalities.20 PEGMC was able to be homogeneously mixed with HA particles and further crosslinked into a hydrogel composite through radical polymerization where PEGDA was used as the crosslinker (Figure 1). The PEGMC/HA composite was easily injectable through a 27-gauge needle prior to crosslinking (Figure 2A), and was rapidly transformed into a mechanically stable solid hydrogel composite within 5 to 15 min (Figure 2B). At a fully swollen state, PEGMC hydrogels were highly porous (Figure 3A). The HA particles were uniformly dispersed within the PEGMC matrix while still maintaining the highly porous structure as observed in Figure 3B. The porosity analyses indicated that pure PEGMC hydrogels exhibited a porosity of 76.88± 3.06% with average pores diameter 289.93 ± 98.75 μm, but did not show significiently difference from those of the hydrogel composites with 30% of HA particles. However, increasing HA content to 50% and 70% significantly decreased both porosity and pore diameters. Figure 3B also clearly showed that HA particles are well distributed throughout the polmer matrix.
Figure 2.
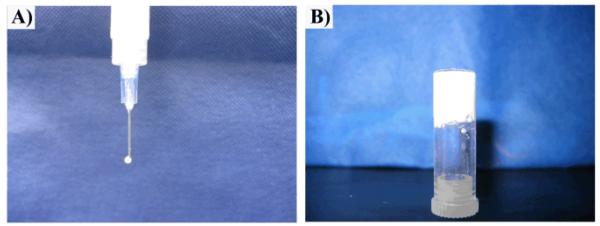
Photograph of PEGMC0.4/HA (50%) composites injected through 27-gauge needle before (A) and after (B) 5-min crosslinking.
Figure 3.
Photomicrographs of the PEGMC (0.6)/HA hydrogels at swollen state. (A) Pure PEGMC (0.6) hydrogel, (B) PEGMC (0.6)/HA (30%), (C) Porosity of PEGMC (0.6) and PEGMC (0.6)/HA (30%, 50%, and 70%), and D) Pore sizes of PEGMC (0.6) and PEGMC (0.6)/HA (30%, 50%, and 70%). Black spots in the image B represent HA “*” and “#” represent p value <0.05 and >0.05 respectively.
3.2 Effects of maleic anhydride/citric acid ratios
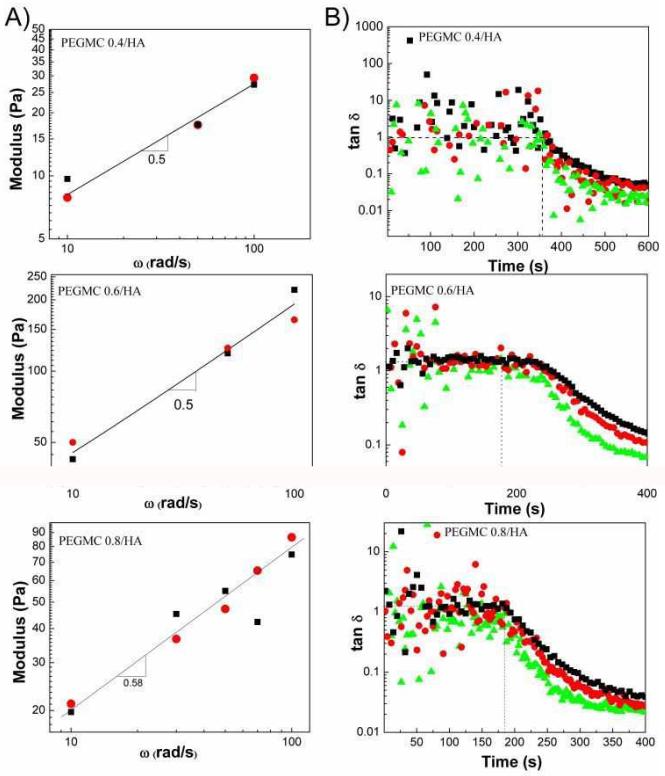
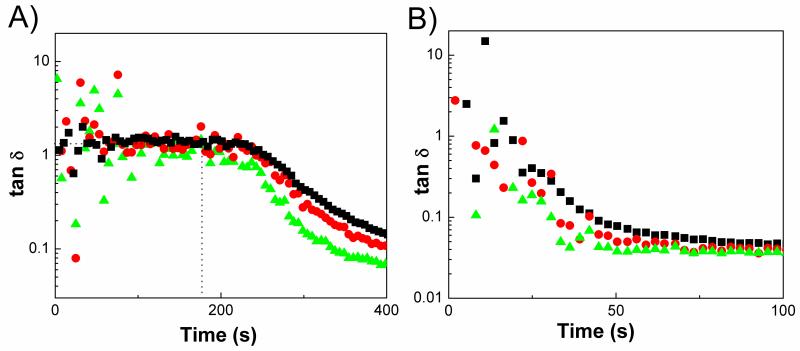
It has been shown that the intersection points of loss tangent (tanδ = G”/G’) at different frequencies correspond to the gel point of physically crosslinked systems.21-23 This behavior indicates that the modulus scaling with different frequencies perform in the same manner as G’, G”~ ωn, where n is the relaxation exponent. As the samples become more elastic, loss tangent decreases monotonically. In other words, G’, which represents the stiffness of the material, increases at a faster rate than G” as sample goes through liquid-solid transition. Crossover of G’ and G” coincides with the gel point only in certain cases, where the relaxation exponent (n) is 0.5 and tan δ is 1. We have determined the relaxation exponent, n, which was calculated from the slopes of the lines in G’ and G”-frequency data. The slopes were found to be 0.5 and 0.58 for samples prepared from different monomer ratios (Figure 4A). PEGMC/HA composites prepared from PEGMC with different molar ratios of maleic anhydride and citric acid (PEGMC (0.4), PEGMC (0.6), and PEGMC (0.8)), crosslinked by 15 (w/v)% PEGDA, 50 wt% of HA/composites ratio and initiated by 25 mM of APS and TEMED were tested in multi-wave tests. Figure 4B showed the multi-wave time sweep results of PEGMC/HA composites of varying maleic acid concentration where tanδ is independent of frequency (tanδ equals to 1) until gelation is complete. Tan δ lines for different frequencies coincide at the gel-point. This clearly shows that Winter-Chambon criteria applies to our chemically-crosslinked hydrogels in the presence of fillers. We do not present the multi-wave test data for the pure hydrogels because of the scattered moduli data during the gelation as the samples were very liquid-like even in the measurements conducted at 100rad/s.
Figure 4.
(A Change of elastic and loss moduli with frequency at gel time at 37°C for PEGMC (0.4), (0.6), (0.8) with HA fillers (G’: circle; G”: square). (B) Loss tangent versus time for PEGMC composites at different frequencies: 10 rad/s (triangle), 50 rad/s (circle), 100 rad/s (square). (Crosslinker: 15 (w/v)%, initiator =25 mM, HA/composites = 50 wt%.)
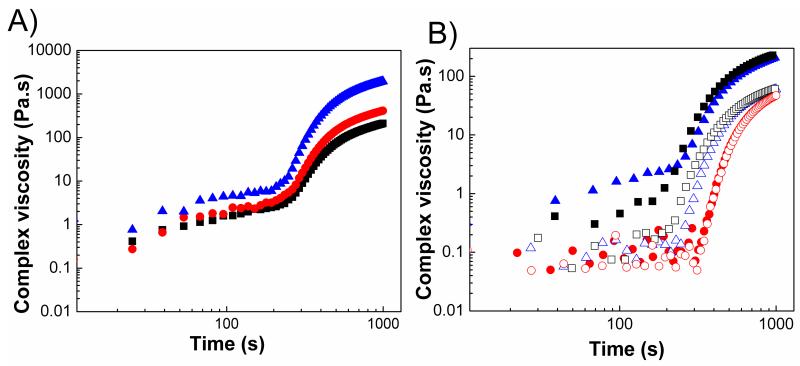
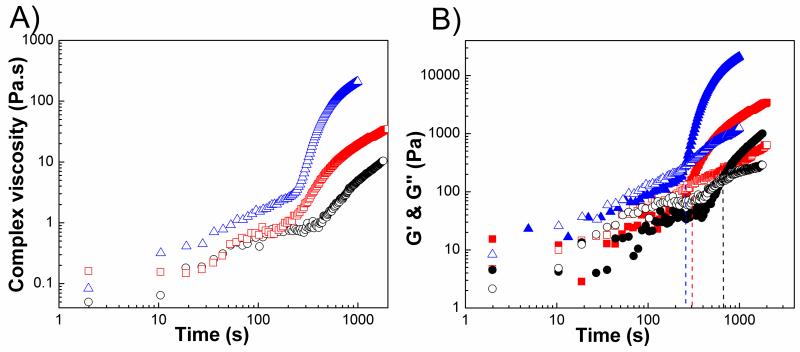
Figure 5A showed that complex viscosity diverged at the sol-gel transition. Viscosity diverges in lower frequencies because gels behave like solids at short time scales. We also compared the complex viscosity of the same gel with and without fillers to reveal the effect of fillers on complex viscosity. Figure 5B shows that in the case of PEGMC(0.6)/HA and PEGMC (0.8)/HA, viscosity slightly increased with HA. However in the case of PEGMC(0.4)/HA, viscosity did not change with the fillers upon the gel formation, which means that injection of this composite will be easier due to its lower viscosity (50 Pa.s).
Figure 5.
Complex viscosity as a function of time (A) PPEGMC(0.6)/HA at different frequencies (10 rad/s: triangle, 50 rad/s: circle, 100 rad/s: square; (B) PPEGMC(0.4) (circle), PPEGMC(0.6) (triangle), and PPEGMC(0.8) (square) at 100 rad/s. Closed symbols represent the composites and open symbols represent pure gels. (Crosslinker: 15 (w/v)%, initiator =25 mM, HA/composites = 50 wt%.)
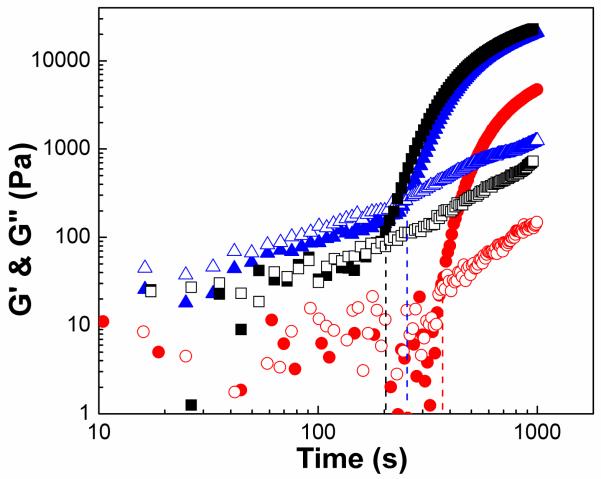
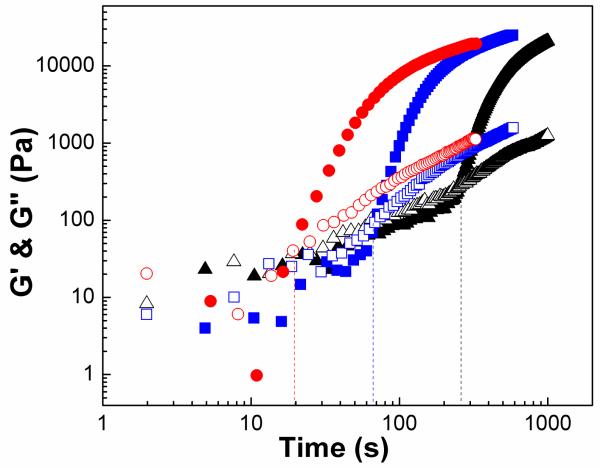
To characterize viscoelastic properties of gel composites, we performed time sweep oscillatory experiments to monitor G’ and G” during the crosslinking of PEGMC(0.4)/HA, PEGMC(0.6)/HA, and PEGMC(0.8)/HA (Figure 6). Initially, G” was larger than G’, as the viscous properties of the liquid state dominated. As the reaction proceeds, the composite precursor gained elastic properties resulting in a gel-like state of the hydrogel composite. Although, both of the moduli increased, the slope of G’ with respect to time was higher than that of G”. This difference in the rates leads a point of cross-over of G’ and G”, which represents the gelation time. As illustrated in Figure 6, PPEGMC(0.8)/HA showed the shortest gelation time around 212 s followed by PEGMC(0.6)/HA (265 s) and PEGMC(0.4)/HA (374 s). These results suggest that gelation times are mainly controlled through the monomer composition PEGMC(0.8)/HA, which contained the highest molar concentration of maleic anhydride (vinyl group), showed the shortest gelation time and could easily react with the terminal alkene crosslinker (PEGDA). All the samples were dry when they reach to 1000s in Figure 6. It is also worth noting that the G’ and G” of PEGMC(0.6)/HA and PEGMC(0.8)/HA composites at 1000 seconds did not differ significantly. The proximity of the moduli data of PEGMC (0.6) and (0.8) suggests that the vinyl groups located in the middle of polymer chains of PEGMC(0.6) and PEGMC(0.8) may not have reacted.
Figure 6.
Evolution of viscoelastic properties of PEGMC/HA hydrogel composites during gelation: Loss and storage moduli as a function of time at 100 rad/s for composites PEGMC(0.4)/HA (circle), PPEGMC(0.6)/HA (triangle), PPEGMC(0.8)/HA(square). (Crosslinker: 15 (w/v)%, initiator = 25 mM, HA/composites = 50 wt%, frequency = 100 rad/s. Filled symbols represent G’ and open symbols are for G”.) The crossovers of G’ and G” are at 212s, 265s, 374s.
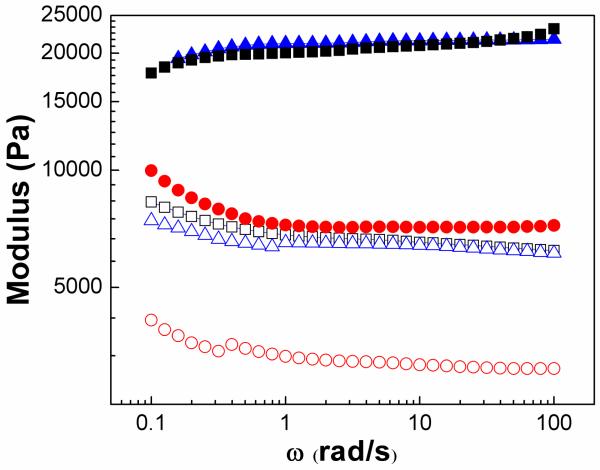
Frequency sweep oscillatory experiments were conducted on dried (fully incubated at 37°C for 1000s and then transferred onto plates of rheometer) hydrogel composites at 37°C. Samples behaved like stronger solids with the elastic modulus reaching up to 8 kPa for PEGMC(0.4)/HA, 20kPa for PEGMC(0.6)/HA, and 20 kPa for PEGMC(0.8)/HA (Figure 7).
Figure 7.
Storage moduli for PPEGMC (0.4) (circle), PPEGMC (0.6) (triangle), PPEGMC(0.8) (square) versus frequency at 37°C of the fully dried composites. Applied strain is 10%. Filled symbols represent G’ for composites and open symbols are for gel. (Crosslinker: 15 (w/v)%, initiator =25 mM, HA/composites = 50 wt%).
3.3 Effects of crosslinker concentration
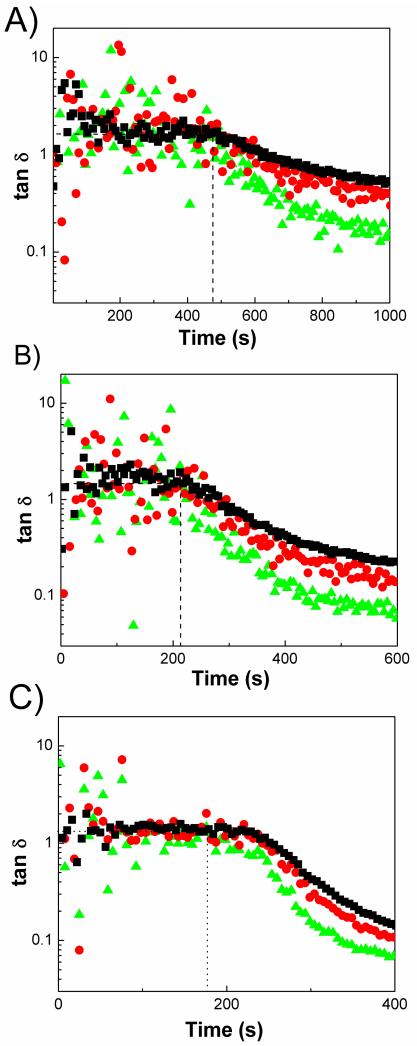
Another parameter that influences the crosslinking kinetics of PEGMC/HA composites is the concentration of the crosslinker. To understand the influence of the crosslinker concentration on the complex viscosity and loss tangent, we formulated PEGMC(0.6)/HA initiated with 25 mM of initiator with various concentration of PEGDA (3, 6, and 15 %(w/v)) with a constant sample thickness. Multiwave-tests conducted at 10, 50 and 100 rad/s on gels showed that tan δ was 1 (Figure 8) and the intersection point where tan δ becomes frequency independent indicates roughly the gelation time, which increases from 200 s to 500 s as crosslinker amount decreases. Figure 9A shows a typical diversion of complex viscosity at the sol-gel transition. The viscosity of composites with the highest crosslinker concentration (15% (w/v)) has resulted an increase in viscosity by a factor of 20. As seen in Figure 9B, an increase in crosslinker concentration significantly influenced the gelation time of the composites. PEGMC(0.6)/HA composite crosslinked with 15% (w/v) of crosslinker showed a rapid gelation time of 256 seconds whereas the gelation point was delayed to 302 seconds and 668 seconds in the cases of PEGMC(0.6)/HA composites crosslinked with 6% (w/v) and 3% (w/v) of crosslinker, respectively. It is desirable to have an injectable system with a reasonably short gelation time, but one should take into consideration that it should not hinder the process of implantation. Another careful observation in Figure 9B reveals that there was a significant difference in the loss moduli of the composites at 1000 seconds of crosslinking. PEGMC (0.6)/HA composites crosslinked with 3% (w/v) of crosslinker showed a loss moduli of 1000 Pa which increased by 3 fold and 20 fold when crosslinker was increased to 6% (w/v) and 15% (w/v). This data clearly indicated that the hydrogel composite with higher crosslinker concentrations could achieve higher mechanical stability within short period of time. Thus, lower concentrations of the crosslinker may be more favorable in the case of cell delivery to the targeted site, whereas use of higher crosslinker concentrations may be more applicable when quick mechanical stability of depot is desired as in the case of acellular cement fillers in the defected bone cavity.
Figure 8.
Loss tangent versus time for PEGMC0.6/HA crosslinked by using (A) 3% (B) 6% (C) 15% at different frequencies: 10 rad/s (triangle), 50 rad/s (circle), 100 rad/s (square). (HA/composites = 50 wt%, initiator =25 mM)
Figure 9.
Effect of crosslinker concentrations on viscoelastic properties of PEGMC(0.6)/HA hydrogel composite. (A) Complex viscosity versus time at 100 rad/s for varying crosslinker amounts: 3% (circle), 6% (square), 15% (triangle). (B) Loss and storage moduli as a function of time at 100 rad/s for composites prepared using different crosslinker concentrations. (Initiator concentrations: 25 mM, HA/composites = 50 wt% . Filled symbols represent G’ and open symbols are for G”. The crossovers of G’ and G” are at 256s, 302s and 668s.)
3.4 Effects of initator concentration
We further extended our investigation to the influence of initiator concentration on the crosslinking kinetics of the composites. We formulated PEGMC(0.6)/HA crosslinked with 15% (w/v) of crosslinker initiated with 25, 50, and 75 mM of initiator (APS and TEMED). Figure 10 shows that gelation occurred significantly faster with increasing initiator concentrations. For example, composites initiated with 25 mM of initiator showed a gelation point at 256 seconds, which was significantly shortened to 66 seconds and 20 seconds when the initiator concentration was increased to 50 mM and 75 mM, respectively. There was no noticeable effect on the final moduli of the hydrogels prepared with different initiator concentrations at 1000 seconds. Loss tangent-time data from multiwave experiments has shown that a clear gelation point can be obtained for 25 mM initiator. However, decay of tan δ is observed for 75mM as the gelation was very quick (Figure 11). Although, an increase in initiator concentration accelerated the gelation time, the toxicity of initiating system is also a concern of injectables for in vivo applications. This study could help in designing formulations to balance the gelation time and biocompatibility of PEGMC injectable composites.
Figure 10.
Loss and storage moduli evolution over time of PEGMC(0.6)/HA hydrogel composite prepared with different initiator concentrations: 25 mM (triangle), 50 mM (square), 75 mM (circle). (Crosslinker: 15 (w/v)%, HA/composites = 50 wt%, frequency: 100 rad/s. Filled symbols represent G’ and open symbols are for G”). The crossovers of G’ and G” are at 20s, 66s and 256s.
Figure 11.
Loss tangent versus time of PEGMC(0.6)/HA hydrogel composite prepared with different initiator concentrations: (A) 25 mM (B) 75mM. (Crosslinker: 15 (w/v)%, HA/composites = 50 wt%.) Different symbols present frequencies of: 10 rad/s (triangle), 50 rad/s (circle), 100 rad/s (square).
3.5 Effects of HA concentration
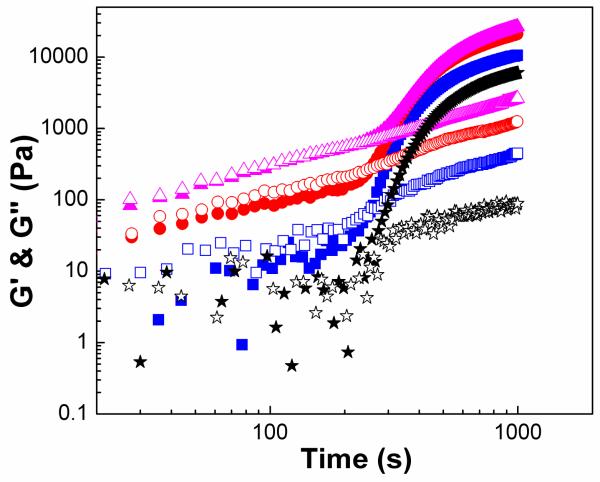
We have demonstrated that PEGMC/HA composites exhibit highly porous structures in the presence of HA fillers, which is important for cellular infiltration and proliferation. HA particles were well distributed within PEGMC network. The complex viscosity of the composites increased with the addition of HA particles with an exception in the case of PEGMC(0.4)/HA, which did not change the complex viscosity after the addition of HA particles. Next, we investigated if there is any influence of HA particles addition on the gelation time of the PEGMC/HA composites. Hydrogel composites were formulated with 30%, 50% and 70% HA/composite weight ratios. Interestingly, with 70% loading G’ was not lower than G”. This result suggests that fillers may potentially suppress the chemical crosslinking. The moduli of the composites increased by a factor of 10 with 50-70% loading when compared to the moduli of unfilled gel (Figure 12). These results indicate that the well-dispersed HA particles may facilitate drawing PEGMC together by ionic interaction, which resulted in almost the same crossover-points for all samples in Figure 12 and a slight increment in mechanical strength.
Figure 12.
Loss and storage moduli evolution over time for PEGMC (0.6)/HA composites with 0 (star), 30% (square), 50% (circle) and 70% (triangle) ratio of HA/composite. (Crosslinker: 15 (w/v)%, initiator = 25 mM, frequency = 100 rad/s.) Filled symbols represent G’ and open symbols are for G”.
3.6 Interactions of PEGMC and HA
Biodegradable polymers like poly(propylene fumarate), poly(lactide ethylene oxide-fumarate) have been mixed with carbon nanotubes, hydroxyapatite, and calcium phosphate cement (CPC) to reinforce the gels.5, 24, 25 However, the rheological behaviors of such composite systems were rarely studied.5, 26, 27
A recent solid-state nuclear magnetic resonance (NMR) study has shown that the surfaces of the apatite crystals in bone are strongly bound with citrate molecules.28 This study demonstrated that citrate is a strongly bound, integral part of the nanocomposites in bone. However, such natural existence of citrate in bone and the interactions of citrate with apatite were not fully considered in scaffold designs for bone tissue engineering. We have previously developed a novel citric acid-based biodegradable polymer composite with HA particles, poly(octamethylene citrate)/HA (POC/HA).17 POC/HA induced rapid mineralization, demonstrated excellent osteoconductivity, and elicited minimal inflammatory response in vivo in a rabbit knee model, which were presumably ascribed to the key interactions of HA and citrate-containing POC. However, no data was proposed to support such interactions in these composites. The current PEGMC/HA is an injectable form of POC/HA. To understand the interactions of PEGMC and HA, we systematically varied the PEGMC compositions (monomer ratios, crosslinker, HA and initiator concentrations) for our rheology experiments. We found that HA fillers do not influence the gelation kinetics of chemically crosslinked gels but they improve the elastic moduli through ionic interactions between HA and the polymer. PEGMC carries rich pendant −COOH from the citrate units located on the polymer backbone which can chelate with the calcium of HA. We conclude that when fillers interact strongly with the polymer, they are better dispersed and hence they do not change the gelation kinetics. On the contrary, fillers that interact weakly with the polymer can block the reactive sites and thereby retard the crosslinking kinetics as previously reported for gels with colloidal silica fillers.29, 30 Our results supported the homogenous distribution of HA particles within PEGMC matrix where HA particles act as bridges to facilitate the radical polymerization (crosslinking reaction) of PEGMC and crosslinkers. This feature makes PEGMC different from any other existing injectable biodegradable polymers and brings multiple benefits to PEGMC as mentioned previously.
4 Conclusion
We have systematically studied the viscoelastic properties of newly developed citric acid-based injectable biodegradable PEGMC/HA composites. As expected, increasing initiator and crosslinker concentrations could accelerate the gelation kinetics. It was an interesting finding that the addition of HA to PEGMC did not improve the stiffness of the composite hydrogel at a large extent. We have concluded that the moduli of the composites were mainly controlled by the PEGMC matrix. Rheology measurements on PEGMC/HA allowed us to assess the effect of interactions between HA and PEGMC on the mechanical properties for the first time, which greatly improved our understanding on the citric acid-based biodegradable polymer/HA composites for orthopedic applications.
Acknowledgements
DG, PN, RT and JY acknowledge support in part by a NIH award R21EB009795 from the National Institute of Biomedical Imaging and Bioengineering(NIBIB), a National Science Foundation (NSF) CAREER award (0954109), and a high impact/high risk award (RP110412) from Cancer Prevention & Research Institute of Texas (CPRIT). YJ and PA acknowledge support from the University of Missouri Research Board Grant (CB000383-RB) and Stevens Institute of Technology.
Notes and references
- 1.Habraken WJ, Wolke JG, Jansen JA. Adv. Drug Deliv. Rev. 2007;59:234–248. doi: 10.1016/j.addr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Moursi AM, Winnard AV, Winnard PL, Lannutti JJ, Seghi RR. Biomaterials. 2002;23:133–144. doi: 10.1016/s0142-9612(01)00088-6. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa T, Ohgushi H, Tamai S. J. Biomed. Mater. Res. 1996;32:481–492. doi: 10.1002/(SICI)1097-4636(199611)32:3<481::AID-JBM23>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Khanna R, Katti KS, Katti DR. Acta. Biomater. 2011;7:1173–1183. doi: 10.1016/j.actbio.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Lee KW, Wang S, Yaszemski MJ, Lu L. Biomaterials. 2008;29:2839–2848. doi: 10.1016/j.biomaterials.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss P, Bohic S, Lapkowski M, Daculsi G. J. Biomed. Mater. Res. 1998;41:167–170. doi: 10.1002/(sici)1097-4636(199807)41:1<167::aid-jbm20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Moura MJ, Figueiredo MM, Gil MH. Biomacromolecules. 2007;8:3823–3829. doi: 10.1021/bm700762w. [DOI] [PubMed] [Google Scholar]
- 8.Weng L, Chen X, Chen W. Biomacromolecules. 2007;8:1109–1115. doi: 10.1021/bm0610065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh K, Shu XZ, Mou R, Lombardi J, Prestwich GD, Rafailovich MH, Clark RA. Biomacromolecules. 2005;6:2857–2865. doi: 10.1021/bm050361c. [DOI] [PubMed] [Google Scholar]
- 10.Tran RT, Thevenot P, Gyawali D, Chiao JC, Tang LP, Yang J. Soft Matter. 2010;6:2449–2461. doi: 10.1039/C001605E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Biomaterials. 2006;27:1889–1898. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 12.Dey J, Xu H, Shen JH, Thevenot P, Gondi SR, Nguyen KT, Sumerlin BS, Tang LP, Yang J. Biomaterials. 2008;29:4637–4649. doi: 10.1016/j.biomaterials.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, Kapadia M, Carroll TJ, Ameer GA. Adv. Mater. 2006;18:1493–1498. [Google Scholar]
- 14.Gyawali D, Tran RT, Guleserian KJ, Tang L, Yang J. J. Biomater. Sci. Polym. Ed. 2010;21:1761–1782. doi: 10.1163/092050609X12567178204169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran RT, Thevenot P, Zhang Y, Gyawali D, Tang L, Yang J. Materials. 2010;3:1375–1389. doi: 10.3390/ma3021375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Webb AR, Ameer GA. Adv. Mater. 2004;16:511–516. [Google Scholar]
- 17.Qiu H, Yang J, Kodali P, Koh J, Ameer GA. Biomaterials. 2006;27:5845–5854. doi: 10.1016/j.biomaterials.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA, Tang L. Proc Natl Acad Sci U S A. 2009;106:10086–10091. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran RT, Zhang Y, Gyawali D, Yang J. Recent Pat. Biomed. Eng. 2009;2:216–227. [Google Scholar]
- 20.Gyawali D, Nair P, Zhang Y, Tran RT, Zhang C, Samchukov M, Makarov M, Kim HK, Yang J. Biomaterials. 2010;31:9092–9105. doi: 10.1016/j.biomaterials.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winter HH, Chambon F. J. Rheol. 1986;30:367–382. [Google Scholar]
- 22.Winter HH, Mours M. Adv. Polym. Sci. 1997;134:164–234. [Google Scholar]
- 23.Chambon F, Winter HH. J. Rheol. 1987;31:683–697. [Google Scholar]
- 24.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Biomacromolecules. 2006;7:2237–2242. doi: 10.1021/bm060391v. [DOI] [PubMed] [Google Scholar]
- 25.Chang CH, Liao TC, Hsu YM, Fang HW, Chen CC, Lin FH. Biomaterials. 2010;31:4048–4055. doi: 10.1016/j.biomaterials.2010.01.124. [DOI] [PubMed] [Google Scholar]
- 26.Peter SJ, Kim P, Yasko AW, Yaszemski MJ, Mikos AG. J. Biomed. Mater. Res. 1999;44:314–321. doi: 10.1002/(sici)1097-4636(19990305)44:3<314::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Peter SJ, Lu L, Kim DJ, Mikos AG. Biomaterials. 2000;21:1207–1213. doi: 10.1016/s0142-9612(99)00254-9. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y-Y, Rawal A, Schmidt-Rohr K. Proc. Natl. Acad. Sci. U. S. A. 2010;107:22425–22429. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiou BS, Raghavan SR, Khan SA. Macromolecules. 2001;34:4526–4533. [Google Scholar]
- 30.Raghavan SR, Chen LA, McDowell C, Khan SA, Hwang R, White S. Polymer. 1996;37:5869–5875. [Google Scholar]