Abstract
Background and Aims
Endothelial small- and intermediate-conductance KCa channels, SK3 and IK1, are key mediators in the endothelium-derived hyperpolarization and relaxation of vascular smooth muscle and also in the modulation of endothelial Ca2+ signaling and nitric oxide (NO) release. Obesity is associated with endothelial dysfunction and impaired relaxation, although how obesity influences endothelial SK3/IK1 function is unclear. Therefore we assessed whether the role of these channels in the coronary circulation is altered in obese animals.
Methods and Results
In coronary arteries mounted in microvascular myographs, selective blockade of SK3/IK1 channels unmasked an increased contribution of these channels to the ACh- and to the exogenous NO- induced relaxations in arteries of Obese Zucker Rats (OZR) compared to Lean Zucker Rats (LZR). Relaxant responses induced by the SK3/IK1 channel activator NS309 were enhanced in OZR and NO- endothelium-dependent in LZR, whereas an additional endothelium-independent relaxant component was found in OZR. Fura2-AM fluorescence revealed a larger ACh-induced intracellular Ca2+ mobilization in the endothelium of coronary arteries from OZR, which was inhibited by blockade of SK3/IK1 channels in both LZR and OZR. Western blot analysis showed an increased expression of SK3/IK1 channels in coronary arteries of OZR and immunohistochemistry suggested that it takes place predominantly in the endothelial layer.
Conclusions
Obesity may induce activation of adaptive vascular mechanisms to preserve the dilator function in coronary arteries. Increased function and expression of SK3/IK1 channels by influencing endothelial Ca2+ dynamics might contribute to the unaltered endothelium-dependent coronary relaxation in the early stages of obesity.
Introduction
Endothelial calcium-activated K (KCa) channels, including small conductance (SK3 or KCa2.3) and intermediate conductance (IK1 or KCa3.1) isoforms, are important effectors modulating arterial tone, since their opening is a starting point in the so-called non-chemical endothelial-derived hyperpolarization (EDH). This response causes vascular smooth muscle (VSM) relaxations resistant to nitric oxide (NO) synthases (NOS) and cyclooxygenases inhibitors [1], [2] and implies electrotonical coupling between endothelial and VSM cells which leads to VSM hyperpolarization and relaxation [1], [3]. The EDH-mediated response is initiated with the increase in endothelial intracellular Ca2+ concentration ([Ca2+]i which activates SK3 and IK1 channels and causes endothelial cell hyperpolarization [2], [4]. Besides the role of SK3 and IK1 channels in the non-chemical EDH response, it is now well established that activation of these channels increases the driving force for Ca2+ entry into endothelial cells [5]–[7]. This implies that SK3/IK1 channel-mediated hyperpolarization of endothelial cell itself can modulate activation of endothelial NOS and thus NO release and relaxation [8]–[11].
Obesity is associated with cardiovascular and metabolic disorders such as insulin resistance, impaired glucose tolerance, hypertension, and dyslipidemia, jointly referred to as metabolic syndrome. Each of these disorders is an independent predictor of cardiovascular events thus, obese patients have increased prevalence of cardiovascular co-morbidities e.g. type 2 diabetes, hyperlipidemia, hypertension, heart disease, and stroke as well as inflammation [12]. Obesity is associated with endothelial dysfunction and impaired relaxation [12], [13] which has mainly been ascribed to the diminished bioavailability of endothelium-derived NO as a consequence of increased reactive oxygen species formation and of the abnormal profile of proinflammatory cytokines release from the inflamed adipose tissue [14]–[17]. In contrast, EDH-mediated relaxation appears to persist or even compensate for the loss of NO-mediated relaxation under obesity conditions [18]–[21].
Concerning coronary endothelial function in obesity, clinical and experimental studies have shown preserved basal coronary blood flow [22] and unaltered, attenuated, or even augmented vasodilator responses to endothelial agonists in coronary arterioles from humans [23] and experimental models of obesity [24]–[26]. This has led to the suggestion that coronary arteries initially adapt to match the higher metabolic demand in obesity by preserving their vasodilator function and they are somehow “resistant” to the early endothelial dysfunction that occurs in other vascular beds [27].
Endothelium-dependent relaxation in large coronary arteries seems to be mainly due to NO release [28] while the contribution of EDH-mediated responses is of larger importance in small coronary arteries [29]. Regarding the role of KCa channels in the endothelium-dependent relaxations of coronary arteries in obesity both impaired [30] and preserved function [31] have been reported for the large conductance KCa (BKCa) channels, while the function of endothelial SK3/IK1 channels remains largely unexplored. In this regard, we have previously described preserved endothelial relaxations mediated by NO in coronary arteries of an animal model of genetic obesity and insulin resistance [26], [32]. In order to better understand the mechanisms underlying this preserved coronary endothelial response, the present study was designed to assess whether SK3 and IK1 channels contribute to the endothelial NO-mediated relaxation in coronary arteries, and if so, to determine whether these channels may be involved in the signaling pathway for this preservation.
Methods
1.1. Ethics Statement
This study was conducted in compliance with the European Directive for the Protection of Animals Used for Scientific Purposes (2010/63/EU). All animal care and experimental protocols were approved by ethical committee of Complutense University of Madrid (Spain).
1.2. Animal model
Male Obese Zucker Rats (fa/fa, OZR) and their counterpart, Lean Zucker Rats (fa/-, LZR) were provided from Charles River Laboratories (Barcelona, Spain). Animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and euthanized by decapitation and exsanguination. The depth of anesthesia was evaluated by pinching the animal's paw with forceps and all efforts were made to minimize suffering. The heart was quickly removed and placed in cold (4°C) physiological saline solution (PSS) of the following composition (mM): 119 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, 1.5 CaCl2, 24.9 NaHCO3, 0.027 EDTA, and 11 glucose; bubbled with a mixture of 95% O2 and 5% CO2, resulting in a pH 7.4.
1.3. Isolation and mounting of small coronary arteries
Intramyocardial second order branches of the left anterior descending coronary artery were carefully dissected as previously described [32], [33]. Arterial segments from OZR and LZR were mounted in microvascular myographs (DMT, Denmark) and equilibrated at 37°C. For each individual artery, the internal circumference L100 corresponding to a transmural pressure of 100 mmHg for a relaxed vessel in situ was calculated. The arteries were set to an internal circumference L1 equal to 0.9 times L100, at which tension development is maximal [32].
1.4. Experimental procedures for the functional assays
At the beginning of each experiment, coronary arteries were challenged twice with 124 mM K+ (KPSS) equivalent to PSS except that NaCl was exchanged for KCl on an equimolar basis. Endothelium-dependent relaxations were evaluated in serotonin (5-HT)-precontracted coronary arteries from LZR and OZR by constructing concentration-dependent response curves to acetylcholine (ACh, 10 nM-30 µM) in the absence and presence of the NOS inhibitor L-NG nitro arginine (L-NOARG, 100 µM), the selective SK3 channel blocker apamin (0.5 µM), the selective IK1 channel blocker 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM34), (0.4 µM), and a combination of apamin plus TRAM34. Since ACh-induced relaxations in coronary arteries of both LZR and OZR are mostly mediated by NO [26], the effect of SK3 and IK1 channel blockers was also assessed on the relaxations to the NO donor S-nitroso-N-Acetylpenicillamine (SNAP) (10 nM-30 µM), in order to determine whether SK3/IK1 channels might be involved in NO relaxant actions in VSM. Since 5-HT-induced contraction was enhanced in arteries from OZR [26], 5-HT was applied in a range of concentrations (1-3 µM) in order to match the levels of precontraction in LZR and OZR. Arteries were incubated with the inhibitors in the myogragh chamber for 30 minutes.
In order to assess whether SK3/IK1 activation is involved in endothelial NO release in coronary arteries, the effect of the SK3/IK1 channel opener 6,7-dichloro-1H-indole-2,3-dione 3-oxime (NS309) was tested in 5-HT-precontracted coronary arteries in the presence and in the absence of endothelium, and under conditions of NOS blockade with L-NOARG. The compound NS309 exhibits a slight selectivity for IK1 over SK3 channels and has no effect on BKCa channels [34]. NS309 has been reported to block smooth muscle L-type voltage-dependent Ca2+ channels at high concentrations [34], [35] and therefore doses of this compound higher than 3 µM were not used in the present study. The endothelium was mechanically removed by guiding a human hair in the vessel lumen and the absence of functional endothelium in these arteries was confirmed by the lack of relaxation elicited by ACh (10 µM).
1.5. In situ measurements of endothelial [Ca2+] i
Measurements of endothelial [Ca2+]i were performed in intact arterial segments by Fura-2 acetoxymethyl ester (Fura2-AM) fluorescence as previously described [36]. In order to allow access to the endothelial layer, coronary arterial segments were mounted in a wire myograph with a U shape cut out on the wall facing down. The myograph was mounted on an inverted microscope (Axiovert S100 TV) equipped for dual excitation wavelength microfluorimetry (Deltascan, Photon Technology International). For the measurements of endothelial [Ca2+]i, coronary arteries were loaded in the dark in PSS containing 4 µM Fura2-AM, 40 µg/ml pluronic acid and 0.05% Cremophor EL for 30 minutes at 37°C in order to selectively load the endothelial cell layer [36]. After loading, arteries were washed three times with fresh PSS every 10 minutes during 30 minutes and then illuminated with alternating 340 and 380 nm light using a monochromator based system and the intensity of the emitted fluorescence was collected at a wavelength of 510 nm using a photomultiplier and monitored. At the end of each experiment, Ca2+-insensitive signals were determined after quenching with Mn2+ and the values obtained were subtracted from those recorded during the experiment. The ratio (R) of fluorescence at 340 and 380 nm (F340/F380) corrected for autofluorescence was taken as a measure of [Ca2+]i. Ratiometric measurements compensate for the fading in the F340 and F380 fluorescent signals over time. The relative change in the fluorescent signal upon agonist stimulation was calculated as the increase in the ratio baseline prior to addition of the agonist to the level reached after 5-8 min of stimulation (ΔF340/380). Ratio min values were 0.4±0.09 and 0.62±0.08 and ratio max values were 2.17±0.97 and 2.95±0.51 in LZR and OZR respectively (n = 5 and n = 6).The effect of 3 µM ACh and the potent transient receptor potential vanilloid 4 (TRPV4) channel agonist, (N-((1S)-1-{[4-((2S)-2-{[(2,4-Dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790a) [37] was evaluated in endothelial cells from intact coronary arteries, in the absence and in the presence of apamin plus TRAM34.
Measurements of in situ endothelial [Ca2+]i in intact arteries were performed in the continuous presence of nifedipine (0.5 µM) to quench the coronary smooth muscle Ca2+ signal, largely-dependent on L-type Ca2+ channels [33]. In endothelium-denuded segments of the coronary artery there was no measurable FURA-2 signal (n = 3).
1.6. Western blotting
The whole left descending coronary artery was dissected from the myocardium of LZR (n = 7) and OZR (n = 5), snap frozen in liquid nitrogen and homogenized under reducing condition in the presence of DTT and proteases inhibitors. Protein content was determined by Bio-Rad DC Protein Assay Kit (Bio-Rad, CA, USA) and equal amounts of proteins (20 µg) were loaded and subjected to electrophoresis on a SDS-PAGE (7.5%) followed by transference to a PVDF membrane (Bio-Rad). Protein expression was quantified using primary antibodies anti-SK3 (Alomone, Israel, 1∶200 dilution), anti-IK1 (Alomone, Israel, 1∶450 dilution), or anti-β-actin as a loading control (Sigma-Aldrich, Spain, 1∶10000 dilution) and horseradish peroxidase conjugated secondary goat anti-mouse and anti-rabbit antibodies (Santa Cruz Biotech, CA, USA, 1∶10000 dilution). Proteins were detected using ECL-Plus Western blotting reagents (Amersham, CT, USA) and analyzed using Quantity One (BioRad) [32]. Relative intensity for each protein was determined by comparison with the intensity of β-actin staining on blots that were stripped and then reprobed with β-actin primary antibody.
1.7. Immunohistochemistry
Tissue samples from the heart containing the left descending coronary artery were immersion-fixed in 4% paraformaldehyde in 0.1 M sodium phosphate-buffer (PB), cryoprotected in 30% sucrose in PB, embedded and frozen in OCT compound (Tissue-Tek, Sakura Finetek, Europe BV) and stored at −80°C. Cryostat sections 5–10 µm thick were obtained and preincubated in 10% normal goat serum in PB containing 0.3% Triton-X-100 for 2–3 h. Then, sections were incubated with either a rabbit anti-SK3 channel antibody (Alomone Labs, Israel) diluted at 1∶100 or a rabbit anti-IK1 channel antibody (Alomone Labs, Israel) diluted at 1∶100 for 48 h. Endothelial layer was visualized by coimmunostaining for eNOS with a mouse anti-eNOS antibody (Chemicon International Inc, 1∶500 dilution). Sections were then washed and reacted with the second antibodies for 2 h at room temperature. Secondary antibodies used were Alexa Fluor 594 (red) goat-anti-rabbit (Invitrogen, Life Technologies S.A., Spain, 1∶200 dilution) and Alexa Fluor 488 (green) goat-antimouse (Invitrogen, Great Britain, 1∶200 dilution). No immunoreactivity could be detected in sections incubated in the absence of the primary antisera. Preadsorption with IK1 and SK3 channels showed no cross-reactivity for the antibodies.
1.8. Drugs
The sources of the compounds used were as follows: acetylcholine, apamin, GSK101679a and serotonin were obtained from Sigma Aldrich (Spain); NS309, SNAP and TRAM34 were obtained from Tocris (Great Britain); Fura2-AM and ionomycin were obtained from Invitrogen (Great Britain). Stock solutions of GSK101679a, NS309, SNAP and TRAM34 were dissolved in DMSO and further diluted in distilled water. The final volume of DMSO never exceeded 0.01% in organ baths and did not affect smooth muscle tone in control experiments. All the other drugs were dissolved in distilled water.
1.9. Statistical analysis and data presentation
In the functional experiments, mechanical responses of the arteries were measured as force and expressed as active wall tension. Results are expressed as either absolute values (mN/mm of tension or units of R [F340/F380]) or as a percent of constrictor response to 5-HT in each artery. Data are expressed as means ± SEM (n = number of arteries, 1–2 from each animal in the vasoreactivity experiments, 1 from each animal in the measurements of endothelial [Ca2+]i, and 1 from each animal in the western blotting and immunohistochemical studies). pEC50 is −log EC50, EC50 was the concentration of agonist giving half maximal response (Emax). The differences between means were analyzed using one way ANOVA followed by a Bonferroni post-test or paired or unpaired Student's t test when appropriate. Probability levels smaller than 5% were considered significant. All calculations were made using a standard software package (Prism 5.0, GraphPad, CA, USA).
Results
2.1. General parameters
At 17–18 weeks of age, OZR displayed a significant increase in body weight compared with LZR and exhibited mild hyperglycemia and hyperinsulinemia along with elevated total cholesterol and triglyceride levels (Table S1).
The normalized internal lumen diameters, l1, of coronary arteries in the OZR group (332±13 µm, n = 39, 1–2 arterial segments per animal) were not significantly different from those in the LZR group (309±13 µm, n = 43, 1–2 arterial segments per animal). The standard contractions evoked by a high K+ solution were also similar in the LZR (0.55±0.07 Nm−1, n = 39) and in the OZR group (0.63±0.06 Nm−1, n = 43).
2.2. Contribution of SK3 and IK1 channels to ACh-induced relaxation is increased in intramyocardial arteries from OZR
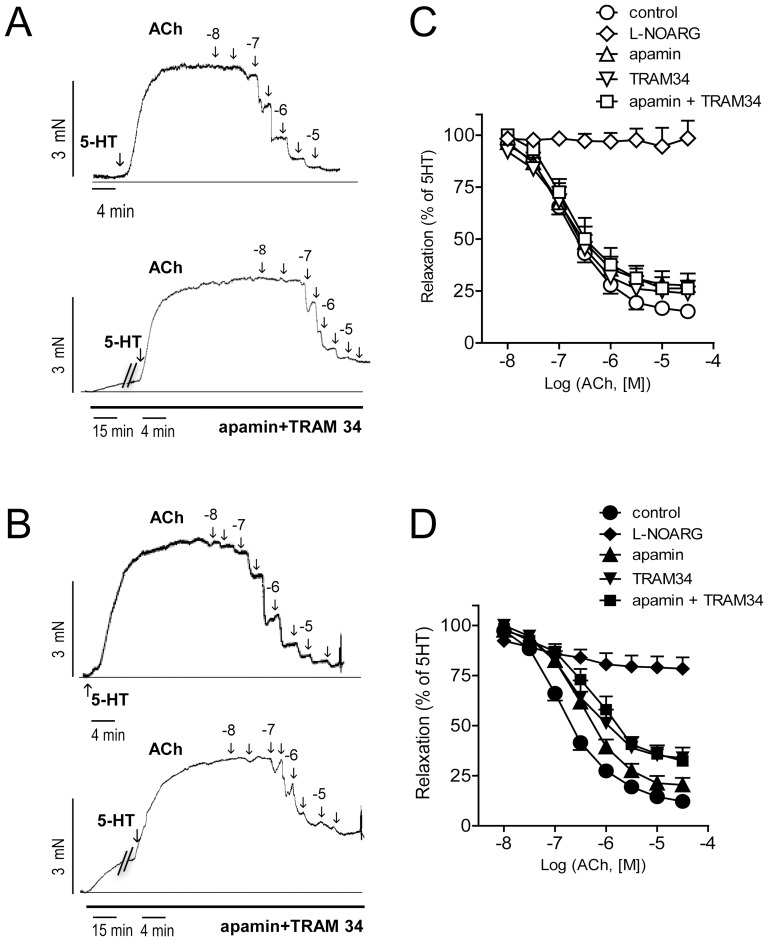
ACh induced relaxations of similar magnitude in coronary arteries from LZR and OZR, as previously reported [25], [26], [32]. NOS inhibition with L-NOARG increased basal tone in both LZR and OZR arteries, and ACh-induced relaxations were abolished in LZR and reduced by about 80% in OZR (Fig. 1, Table 1), suggesting that NO mediates these relaxations in both healthy and obese animals. In this regard, we aimed to explore whether SK3/IK1 channels contribute to the ACh-induced NO-mediated relaxation of coronary arteries, thus the effects of selective inhibitors of SK3 (apamin) and IK1 (TRAM34) channels were assessed. In LZR arteries, incubation with apamin slightly decreased sensitivity and maximal response to ACh, while treatment with TRAM34 or apamin plus TRAM34 affected maximal relaxant response (Fig. 1 C, Table 1). However, apamin and TRAM 34, either alone or in combination, reduced to a larger extent both sensitivity and maximal response to ACh in arteries from obese animals (Fig. 1 D, Table 1). Furthermore, treatment with TRAM34 either alone or in combination with apamin increased basal tone in arteries of OZR (Table 1) indicating an enhanced basal activity of IK1 channels in obese animals.
Figure 1. Increased contribution of SK3 and IK1 channels to the ACh-induced relaxation in coronary arteries from OZR.
(A, B) Isometric tension recordings showing the effects of increasing concentrations of acetylcholine (ACh) on coronary arteries precontracted with 5-HT (1–3 µM) and effect of treatment with the SK3 channel inhibitor apamin (0.5 µM) plus the IK1 channel inhibitor TRAM34 (0.4 µM) in LZR (A) and OZR (B). (C–D) Effects of apamin, TRAM34, apamin plus TRAM34 and L-NOARG (100 µM) on the average concentration-dependent curves for the relaxation to ACh in coronary arteries from LZR (C) and OZR (D). Results are expressed as a percentage of the contraction induced by 5-HT. Points represent mean ± SEM of n = 6–9 arteries (1–2 arteries per animal).
Table 1. Effect of selective inhibitors of NOS (L-NOARG), SK3 (apamin) and IK1 channels (TRAM 34), and combined inhibition of SK3/IK1 channels (A+T) on the ACh and SNAP-induced vasodilation in coronary arteries from LZR and OZR.
| ACh | ||||||||
| LZR | OZR | |||||||
| pEC50 | Emax | ΔBT | n | pEC50 | Emax | ΔBT | n | |
| control | 7.01±0.19 | 91±3 | - | 7 | 6.9±0.15 | 90±5 | - | 6 |
| L-NOARG | - | 2±8 c | 25±14 | 7 | - | 22±6c | 31±13 | 6 |
| control | 6.8±0.16 | 87±6 | - | 7 | 6.8±0.13 | 93±2 | - | 8 |
| apamin | 7.0±0.18 a | 73±5 a | 9±3 | 7 | 6.42±0.10a | 80±4b | 14±5 | 8 |
| control | 7.0±0.2 | 90±2 | - | 6 | 6.9±0.19 | 86±3 | - | 9 |
| TRAM34 | 6.9±0.13 | 73±6 a | 9±6 | 6 | 6.43±0.16a | 65±5c | 25±6† | 9 |
| control | 6.9±0.22 | 89±6 | - | 9 | 6.9±0.25 | 90±4 | - | 6 |
| A+T | 6.8±0.19 | 74±3 b | 10±2 | 9 | 6.1±0.14b | 68±1c | 35±8# | 6 |
Values represent mean ± S.E.M. of the number n of individual arteries. pEC50 is –logEC50. Emax is maximal relaxation expressed as percentage of 5-HT-induced precontraction. ΔBT; is the increase in basal tension expressed as percentage of KPSS-induced contraction. Significant differences from controls were analyzed using paired t test; a P <0.05; b P <0.01; c P <0.001 vs control; or one way ANOVA followed by a Bonferroni test for across-group comparisons; # P <0.05 vs A+T-treated LZR; † P <0.05 vs TRAM34-treated LZR.
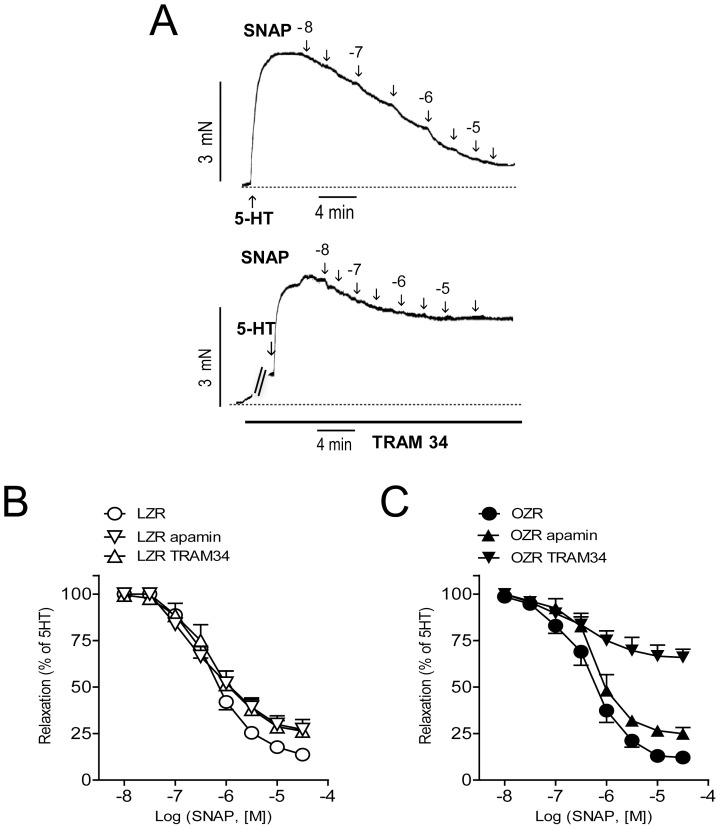
A possible contribution of SK3 and IK1 channels to the NO-mediated coronary relaxation was assessed by testing the effect of SK3/IK1 channel blockers on relaxations to the NO donor SNAP. Apamin modestly decreased SNAP-elicited relaxations in arteries from both LZR and OZR, whereas TRAM34 induced a marked decrease of NO relaxant responses in arteries from OZR (Fig 2, Table 1).
Figure 2. Increased contribution of IK1 channels to the SNAP-induced relaxations in coronary arteries from OZR.
(A) Isometric tension recordings showing the effects of increasing concentrations of SNAP on coronary arteries precontracted with 5-HT (1–3 µM) and the effect of treatment with TRAM34 (0.4 µM) in OZR. (B, C) Effect of apamin (0.5 µM) and TRAM34 on the average concentration-dependent curves for the relaxation to SNAP in coronary arteries of LZR (B) and OZR (C). Results are expressed as a percentage of the contraction induced by 5-HT. Points represent mean ± SEM of n = 6–8 arteries (1–2 arteries per animal).
2.3. Role of SK3 and IK1 channels activation in the NO-induced coronary relaxation
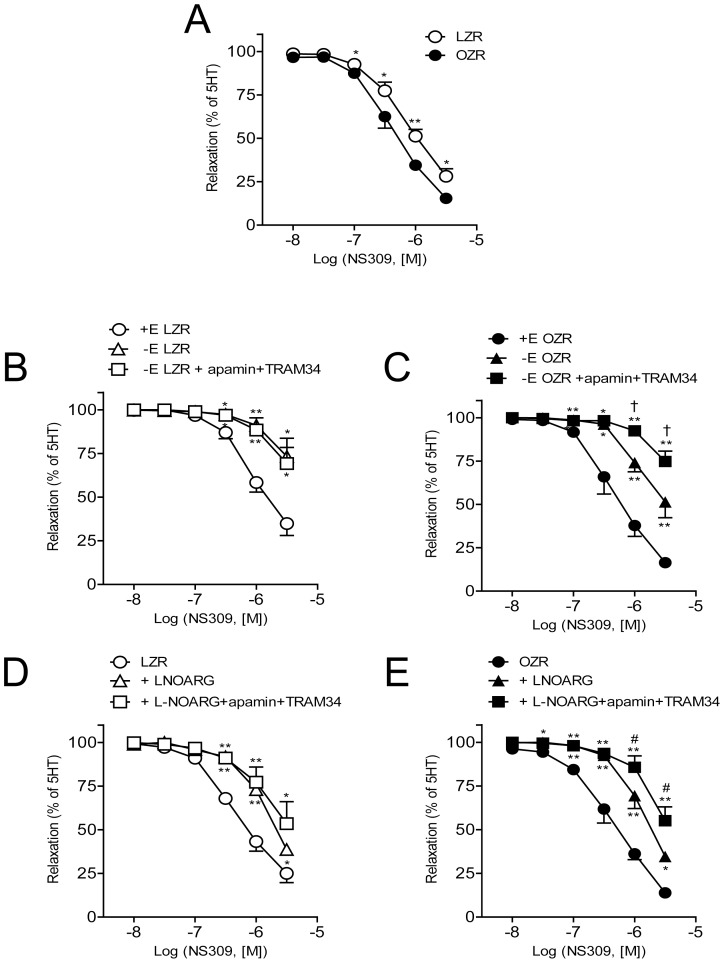
To further elucidate the role of SK3/IK1 channels in the coronary relaxations mediated by endothelial NO, arteries pre-contracted with 5-HT were stimulated with increasing concentrations of the selective opener of SK3/IK1 channels NS309. This drug elicited relaxations in arteries from both LZR and OZR, and both sensitivity and responses to the maximal concentration of NS309 were enhanced in arteries from OZR (Fig 3 A).
Figure 3. Endothelial SK3/IK1 channels activation contributes to NO-induced relaxation in coronary arteries from LZR and OZR.
(A) The selective opener of SK3 and IK1 channels NS309 induces a larger relaxant effect on coronary arteries of OZR. (B, C) Effect of endothelium removal and further blockade of SK3/IK1 channels with apamin (0.5 µM) plus TRAM (0.4 µM) on the relaxations induced by NS309 in coronary arteries of LZR (B) and OZR (C). (D, E) Effect of NOS blockade with L-NOARG (100 µM) and further inhibition of SK3/IK1 channels with apamin plus TRAM on the relaxations elicited by NS309 in coronary arteries of LZR (D) and OZR (E). Results are expressed as a percentage of the contraction induced by serotonin. Points represent mean ± SEM of n = 9–13 arteries (1–2 arteries per animal). Significant differences from controls were analyzed using unpaired t-test *P<0.05; **P<0.01 vs control (Fig 3 A); or one way ANOVA followed by a Bonferroni test *P<0.05 and **P<0.01 vs control; † P<0.05 vs –E; #P<0.05 vs L-NOARG (Fig 3 B–E).
Mechanical removal of endothelium was performed to explore the contribution of endothelial SK3/IK1 channels to the NS309-induced relaxations. Endothelium removal blunted NS309-induced relaxations in arteries of LZR and to a lesser extent in OZR (Fig 3 B–C). Inhibition of SK3/IK1 channels with a combination of apamin plus TRAM34 did not further decrease NS309-induced relaxations in endothelium-denuded arteries from LZR whereas this procedure induced an additional blockade in arteries of OZR (Fig 3 B–C). Treatment with apamin plus TRAM34 increased basal tone in endothelium-denuded arteries of OZR by 24±5 of the KPSS-induced contraction (n = 6).
Since relaxations to ACh in coronary arteries from OZR are mostly dependent on NO release [26], the contribution of SK3 and IK1 channel activation to endothelial NO-mediated coronary relaxations was assessed. Inhibition of NOS with L-NOARG largely reduced NS309-induced relaxations in both lean and obese animals (Fig. 3 D–E) whereas further inhibition of SK3/IK1 channels induced an additional modest inhibition only in OZR (Fig. 3 D–E). This suggests that the relaxation induced by activation of SK3/IK1 channels with NS309 is mostly mediated by NO in coronary arteries, although other non-NO related mechanism(s) may exist in obese animals.
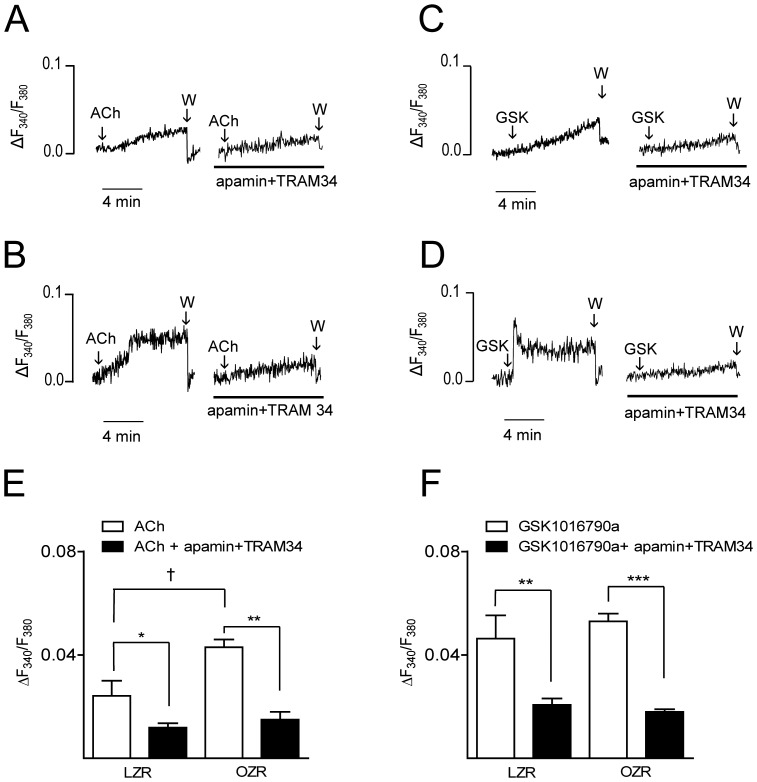
2.4. Role of SK3 and IK1 channels in the ACh-induced changes in [Ca2+]i in coronary endothelium from LZR and OZR
Measurements of endothelial [Ca2+]i were performed in intact coronary arteries to elucidate whether ACh-induced Ca2+ signaling is changed in coronary endothelium under conditions of obesity. Basal F340/F380 ratios were not significantly different in the coronary endothelium from LZR (0.82±0.02, n = 5) and OZR (0.94±0.11, n = 6). Stimulation of endothelial cells with ACh (3 µM) induced an increase in [Ca2+]i that was significantly higher in arteries from OZR compared to LZR (Fig. 4 A, B, E). Treatment with apamin plus TRAM34 reduced to the same level the increase in [Ca2+]i induced by ACh suggesting that enhanced activity of SK3/IK1 channels in the coronary endothelium may contribute to the larger endothelial [Ca2+]i mobilization in response to ACh in obese animals (Fig. 4 A, B, E).
Figure 4. SK3 and IK1 channels are involved in the increased ACh-induced [Ca2+]i mobilization in endothelial cells from OZR.
Original recordings showing (A, B) the acetylcholine (ACh) (3 µM) and (C, D) GSK1016790a (1 µM)-induced increases in endothelial cell [Ca2+]i in the presence and in the absence of apamin (0.5 µM) plus TRAM34 (0.4 µM) in intact coronary arteries from LZR (A, C) and OZR (B, D). (E, F) Summarized data showing the changes in endothelial cell [Ca2+]i in response to ACh (E) and GSK1016790a (F) in the presence and in the absence of apamin plus TRAM34 in LZR and OZR. Results are expressed as absolute values and are means ± SEM of 5–6 arteries, (1 artery from 1 animal). Significant differences were analyzed using paired t-test *P<0.05, **P<0.01, ***P<0.001 or one way ANOVA followed by a Bonferroni test for across-group comparisons; † P<0.05 ACh OZR vs ACh LZR.
The selective agonist of TRPV4 channels GSK1016790a [37] induced increases in endothelial cell [Ca2+]i that were of similar magnitude in arteries from LZR and OZR, and also significantly reduced after treatment with apamin plus TRAM34 in both lean and obese animals (Fig. 4 C, D, F).
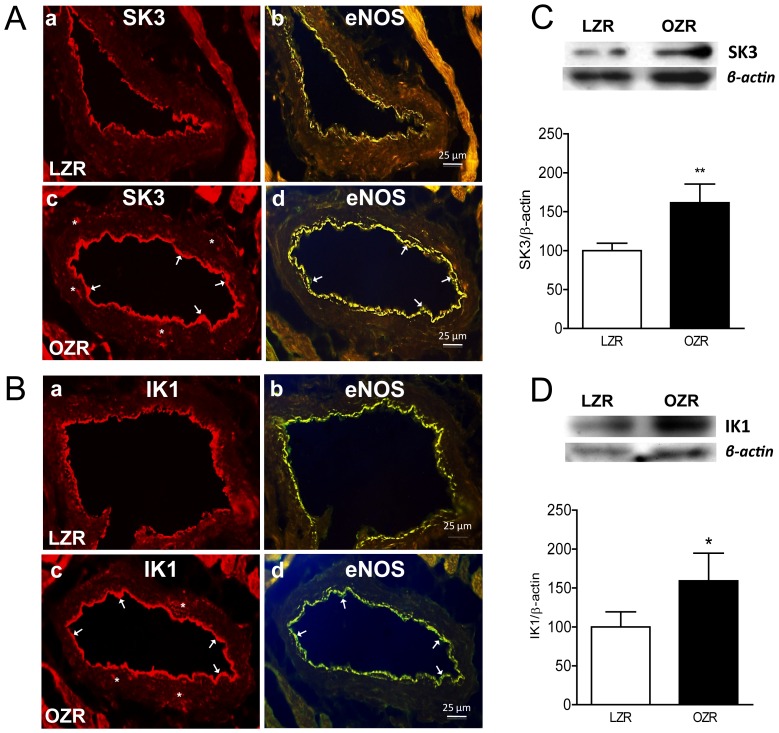
2.5. Immunohistochemical localization and expression of SK3 and IK1 channels in coronary arteries
Figure 5 illustrates the expression and localization of SK3 (Fig 5 A–C) and IK1 (Fig 5B–D) channels in cross sections of coronary arteries from LZR and OZR. Immunoreactivity for SK3 and IK1 channels was found mostly in the endothelial layer of both LZR and OZR and was higher in arteries from obese animals. SK3 and IK1 channel immunoreactivity was occasionally found in small clusters distributed in the smooth muscle layer mainly in arteries from OZR. To confirm these findings using more quantitative methods, we next assessed SK3 and IK1 channel protein levels using western blot analysis, which were significantly increased in coronary arteries from OZR (Fig. 5 C and Fig. 5 D).
Figure 5. SK3 and IK1 channel expression in coronary arteries from LZR and OZR.
(A, B) Immunohistochemical labeling of SK3 (A) and IK1 (B) channel in coronary arteries from LZR (top pannel) and OZR (bottom pannel). SK3 (Aa, Ac) and IK1 (Ba, Bc) channel immunoreactivity (arrows) was mainly located in the endothelium and eventually in the smooth muscle layer (asterisk) and was higher in arteries from OZR compared with LZR (Ac and Bc, respectively). Sections are representative of n = 3 OZR and n = 3 LZR, (1 artery per animal). (C, D) SK3 and IK1 channel expression is increased in coronary arteries from OZR. Western blot analysis of SK3 (C) and IK1 (D) channel expression in coronary arteries from LZR and OZR. Results were quantified by densitometry. Data are shown as means ± SEM of 5–7 animals. Significant differences from controls were analyzed using unpaired t-test *P<0.05; **P<0.01 vs LZR.
Discussion
This study provides new findings coupling activation of SK3 and IK1 channels to endothelial cell Ca2+ and NO-mediated relaxations of coronary arteries in obesity. The contribution of these channels to the ACh relaxant responses is markedly increased in coronary arteries of obese animals. SK3/IK1 channels are involved in the ACh-induced [Ca2+]i mobilization in the endothelial cell that is also increased in obese animals in part due to the enhanced activity of SK3/IK1 channels. Enhanced function is accompanied by upregulation of SK3/IK1 channels in coronary arteries in obesity.
The role of endothelial SK3 and IK1 channels in the EDH-mediated responses has generally been assumed as a parallel or compensatory pathway to NO-mediated endothelium-dependent dilation, suggesting the importance of these endothelial channels for the EDH-derived relaxation when NO and prostacyclin are compromised [1], [2], [4]. However, SK3/IK1 channels can modulate the availability of endothelium-derived NO, since their opening is associated with the activation of endothelial NOS [8]–[11]. The present study demonstrates that while the contribution of SK3/IK1 to the endothelium-dependent NO-mediated ACh relaxations was minor in coronary arteries from LZR, this contribution was significantly augmented in arteries from obese animals. Furthermore, ACh relaxant responses were confirmed to be largely dependent on NO release in both LZR and OZR [26], [32]. The fact that the relaxations induced by NS309 were blunted by endothelial removal and by NOS blockade in LZR further indicates that activation of endothelial SK3/IK1 channels might be involved in the endothelial NO-mediated relaxations of healthy coronary arteries. This is supported by recent studies showing that NS309 and the new selective opener of SK3 and IK1 channels SKA-31 markedly enhanced coronary blood flow [38], [39], these vasodilator responses being mediated by NO [38].
Obesity is associated with endothelial dysfunction and diminished NO bioavailability, mainly as a consequence of oxidative stress and the proinflammatory state of the vascular wall [15]–[17]. However, EDH-mediated responses seem to be preserved at least in the early stages of obesity due to maintained function of SK3/IK1 channels [18]–[21], [40]. In this regard, unchanged [19] or even increased [18] EDH-mediated relaxations evoked by ACh in peripheral arteries of obese mice have been reported. Moreover, vascular IK1 channel function and expression were found to be upregulated in obese rats [21] and endothelium-dependent relaxation was shown to undergo a shift from entirely NO-mediated mechanisms to IK1-dependent pathways that accounted for EDH activity in obese animals [20]. In contrast, a reduced EDH-mediated response was found in OZR due to decreased myoendothelial gap junction expression, but SK3/IK1 channel function remained likewise unaltered [41].
In the present study, we have also found a shift in the contribution of SK3 and IK1 channels to the endothelium-dependent relaxations of coronary arteries in obese animals. Thus, blockade of these channels produced a significantly greater inhibition of the ACh relaxant responses in OZR compared to healthy controls. Moreover, relaxations induced by NS309 were enhanced and Western blot analysis showed a marked increase in protein expression of both SK3 and IK1 channels in the coronary artery wall of obese animals. In contrast to that reported in obesity [21] and type 2 diabetes [42], augmented contribution to the endothelial vasodilator responses was not restricted to IK1 but also to SK3 channels, this preservation suggesting the functional relevance of the latter in the coronary circulation. Both SK3 and IK1 channels are involved in the non-chemical EDH-mediated response that is electrotonically transferred to the vascular myocytes in coronary arteries [43]. IK1 channels are usually found in the endothelial projections through the inner elastic lamina and SK3 channels are preferentially located within the endothelial caveolae and nearby homocellular gap junctions in the arterial wall [3], [44]. These different subcellular localizations might explain the diverging roles reported for SK3 and IK1 channels: the IK1 channel seems to be of pivotal importance in the EDH-mediated response, whereas SK3 channel deficiency preferably impairs ACh-induced NO-mediated relaxation [45]. Accordingly, upregulation of SK3 channels found in the coronary endothelium of obese animals would be consistent with the preserved NO-mediated relaxations reported in these arteries [26], [32].
The mechanism underlying increased expression of SK3 and IK3 channels in coronary arteries of obese rats remains to be elucidated. Recent studies have shown that laminar shear-stress increased expression of SK3 and IK1 channel mRNAs in cultured human coronary artery endothelial cells via the Akt signalling cascade [46]. Moreover, advanced glycation end products up-regulated IK1 channels in cardiac fibroblasts through a pathway also including PI3K/Akt signals [47]. Although OZR exhibit mild hyperglycemia, hyperinsulinemia in this strain has been found associated to up-regulation of the endothelial PI3K/Akt signalling pathway [32], which might account for the increased expression of SK3 and IK1 channels shown in the present study.
Whereas SK3 channels have earlier been reported in VSM of coronary arteries [48], is generally accepted that healthy contractile VSM cells do not express IK1 channels [49]. Up-regulation of the latter has been reported to be involved in VSM proliferation [49] and also to be related with the development of coronary atherosclerosis in vivo [50]. In this regard, functional experiments in the present study indicate an enhanced IK1 and to a lesser extent SK3 channel function involved in the relaxations induced by exogenous NO in coronary arteries of obese animals, which might additionally account for the preserved ACh-induced relaxation observed in OZR [25], [26]. In support of these findings, the immunohistochemical images revealed that despite expression of SK3/IK1 channels takes place mainly in the endothelial layer, immunoreaction for both channel proteins can be found scattered in the VSM layer in OZR. Moreover, enhanced endothelium-independent SK3/IK1 activity involved in the maintenance of basal tone was found in OZR. Relaxations elicited by activation of both channels with NS309, are mostly dependent on the endothelium and on the release of NO in healthy coronary arteries. Strikingly, these relaxations exhibited an endothelium-independent NOS inhibitor-resistant component that was blocked by apamin plus TRAM34 in obese animals. Hence, these findings suggest a role of VSM SK3/IK1 channels in the relaxations of coronary arteries in obese animals. The pronounced inhibitory effect of TRAM34 on the relaxant responses to the NO donor SNAP in OZR further suggests a role for VSM IK1 channels in the NO relaxant responses of coronary arteries from obese animals. Technical limitations in the present study, due to the restricted amount of protein obtained from the rat left descending coronary artery, prevented the assessment of the differential increase in SK3/IK1 channel expression in endothelium and VSM of coronary arteries from obese rats. The mechanism(s) by which NO may activate VSM SK3/IK1 channels to contribute to the NO-induced smooth muscle relaxation in OZR is unknown but the NO/cGMP signaling pathway might be involved, as in the case of VSM BKCa channels [51]. Accordingly, this pathway has recently been reported for the agonist of the alpha estrogen receptor propyl pyrazole triol that causes vascular relaxation via activation of VSM IK1 channels through the NO/cGMP signaling cascade [52].
Taken together, the present findings demonstrate an enhanced contribution of SK3 and IK1 channels to the endothelium-dependent relaxations of coronary arteries in insulin resistant obese rats, through both increased NO release and EDH-mediated responses. This is in agreement with the enhanced activity and contribution of VSM BKCa channels to the maintained EDH-mediated responses of coronary arterioles recently reported in a rat model of high-fat diet-induced obesity [31], which suggests that up-regulation of KCa channels is a mechanism to preserve coronary vasodilatation in obesity.
Stimulation of cultured endothelial cells with ACh has been shown to be associated with an initial transient rise in [Ca2+]i due to inositol 1,4,5-trisphosphate-mediated Ca2+ released from intracellular stores followed by a sustained elevation due to Ca2+ influx via TRP channels [53]. Rise in [Ca2+]i in endothelial cells is a key signaling event that triggers the synthesis and release of endothelium-derived relaxing factors including NO and prostacyclin and activates the EDH-mediated response [2]. In the present study, ACh induced an increase in endothelial [Ca2+]i of coronary arteries that was markedly enhanced in arteries from OZR. Increased agonist-induced [Ca2+]i mobilization has been reported in cultured endothelial cells in response to hyperglycemia [54], although impaired rises in endothelial [Ca2+]i coupled to reduced expression and activity of SK3 were recently reported in arteries of type 1 diabetic rats [55].
Upon stimulation with endothelial agonists, endothelial SK3 and IK1 channels are activated by local Ca2+ events from the inositol 1,4,5-trisphosphate receptors [56] and by Ca2+ signals due to Ca2+ influx through TRPV4 channels [37], [57]. Levels of endothelial cell [Ca2+]i have been reported to be both independent [8], [58]–[60] or dependent on changes in membrane potential [5]–[7], [61]. In support of the former, endothelial [Ca2+]i levels were shown to be unaltered by changes in membrane potential in response to activators of SK3/IK1 channels in intact segments of retinal arteries [60]. Furthermore, a combination of SK3/IK1 channels inhibitors neither affected agonist-induced increases in endothelial [Ca2+]i in intact rat mesenteric arteries [8], [58], [59]. The idea that activation of both SK3 and IK1 channels is coupled to NO release is based on the assumption that SK3/IK1 channels-induced hyperpolarization in endothelial cells might increase the driving force for the influx of Ca2+ via TRP channels thereby sustaining the activating Ca2+ signal [5]–[7], [61]. Thus, inhibition of SK3/IK1 channels was associated with a decreased agonist-induced [Ca2+]i rise and NO release in cultured endothelial cells [9]. In addition, selective activation of these channels with NS309 increased [Ca2+]i and NO formation in human umbilical vein endothelial cells and freshly isolated endothelial cells [10], [62]. Moreover, recent reports of intact arteries have confirmed the involvement of endothelial IK1 and to some extent SK3 channels in the positive feedback mechanism that influences endothelial Ca2+ dynamics upon agonist stimulation [7]. In addition, both pharmacological blockade and SK3/IK1 deficiency reduced the frequency of ACh-stimulated Ca2+ dynamics in mice mesenteric arteries [7]. Accordingly, in the present study a combination of apamin plus TRAM34 reduced the profile of rises in endothelial [Ca2+]i in response to ACh stimulation in coronary arteries. Hence, this suggests that activation of SK3/IK1 channels in the present study is involved in the ACh-induced mobilization of endothelial [Ca2+]i and it is consistent with the idea that Ca2+ influx in endothelial cells is driven, to a significant extent, by SK3/IK1 channel-induced membrane hyperpolarization.
On the other hand, SK3 and IK1 channels might be in part responsible for the higher ACh-induced endothelial [Ca2+]i mobilization observed in coronary arteries from obese animals, since treatment with apamin plus TRAM34 decreased endothelial [Ca2+]i to a similar level in OZR and LZR arteries. This larger inhibition along with the increased expression of SK3 and IK1 channels in the coronary vasculature of obese animals suggest that activation of these channels might induce a larger endothelium hyperpolarization, therefore increasing the electrochemical driving force for Ca2+ entry into endothelial cells. This in turn would explain the higher rise in endothelial [Ca2+]i upon ACh stimulation in arteries from obese rats. Among all TRP channels, TRPV4 is ubiquitously expressed in endothelial cells in close proximity to KCa channels and has been involved in agonist-induced Ca2+ influx and NO production [37] in endothelial cells leading to relaxation [7], [57], [63]. In the present study, stimulation of the quiescent coronary endothelium with a specific agonist of TRPV4 channels induced a rise in endothelial [Ca2+]i of similar magnitude in arteries from both lean and obese rats that was blunted by blockade of SK3/IK1 channels. This is in agreement with recent studies demonstrating that endothelial agonist-induced Ca2+ influx through TRPV4 channels leads to IK1 channel activation and endothelial cell hyperpolarization [57]. Thus, TRPV4-mediated Ca2+ influx and IK1-hyperpolarization are essential to the Ca2+ amplifier feedback mechanism that sustains the driving force for Ca2+ influx into endothelial cells [7]. Whether Ca2+ entry through TRPV4 channels contributes to the enhanced endothelial [Ca2+] mobilization in response to ACh observed in coronary arteries from obese rats requires further elucidation.
In summary, the observed upregulation of SK3 and IK1 channels might contribute for the signaling pathway that compensates and preserves coronary relaxation by increasing endothelial [Ca2+]i in early stages of obesity. Whether upregulation of SK3/IK1 channels continues to be beneficial in the long term, or by contrast it might contribute to endothelial damage needs to be answered. In this regard, a limited spread of relaxation mediated by increased activation of SK3/IK1 channels in aging has recently been reported [64]. Moreover, up-regulation/induction of IK1 channels has been involved in VSM proliferation[49] and also has been related with the development of coronary atherosclerosis in vivo[50].
Supporting Information
Supplementary material. Metabolic parameters of LZR and OZR.
(DOC)
Acknowledgments
We thank Manuel Perales and Francisco Puente for expert technical assistance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by: 1. Grant n° SAF2009-10448 from the Spanish Ministry of Science and Innovation (http://www.idi.mineco.gob.es/portal/site/MICINN/). 2. Grant n° SAF2012-31631 of the Spanish Ministry of Economy (http://www.mineco.gob.es/portal/site/mineco/). 3. LM was supported by MS program (http://www.isciii.es/ISCIII/es/contenidos/fd-el-instituto/fd-comunicacion/fd-notas-prensa/15mar011XIIIPromocionMiguelServet.shtml). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Félétou M, Köhler R, Vanhoutte P (2011) Nitric oxide: Orchestrator of endothelium-dependent responses. Ann Med 44: 1–23. [DOI] [PubMed] [Google Scholar]
- 2. Garland C, Hiley C, Dora K (2011) EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dora K, Gallagher N, McNeish A, Garland C (2008) Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res 102: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards G, Félétou M, Weston A (2010) Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch Eur J Physiol 459: 863–879. [DOI] [PubMed] [Google Scholar]
- 5. Laskey R, Adams D, Johns A, Rubanyi G, van Breemen C (1990) Membrane potential and Na+-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem 265: 2613–2619. [PubMed] [Google Scholar]
- 6. Lückhoff A, Busse R (1990) Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol 342: 94–99. [DOI] [PubMed] [Google Scholar]
- 7. Qian X, Francis M, Köhler R, Solodushko V, Lin M, et al. (2014) Positive feedback regulation of agonist-stimulated endothelial Ca2+ dynamics by KCa3.1 channels in mouse mesenteric arteries. Arterioscler Thromb Vasc Biol 34: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stankevicius E, Lopez-Valverde V, Rivera L, Hughes D, Mulvany M, et al. (2006) Combination of Ca2+ -activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br J Pharmacol 149: 560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheng J, Braun A (2007) Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol 293: C458–67. [DOI] [PubMed] [Google Scholar]
- 10. Stankevicus E, Dalsgaard T, Kroigaard C, Beck L, Boedtkjer E, et al. (2011) Opening of Small and Intermediate Calcium-Activated Potassium Channels Induces Relaxation Mainly Mediated by Nitric-Oxide Release in Large Arteries and Endothelium- Derived Hyperpolarizing Factor in Small Arteries from Rat. J Pharmacol Exp Ther 339: 842–850. [DOI] [PubMed] [Google Scholar]
- 11. Climent B, Schubert R, Stankevicius E, García-Sacristán A, Simonsen U, et al. (2012) Large conductance Ca2+-activated K+ channels modulate endothelial cell outward currents and nitric oxide release in the intact rat superior mesenteric artery. Biochem Biophys Res Commun 417: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 12. Cersosimo E, DeFronzo R (2006) Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev 22: 423–436. [DOI] [PubMed] [Google Scholar]
- 13. Campia U, Tesauro M, Cardillo C (2012) Human obesity and endothelium-dependent responsiveness. Br J Pharmacol 165: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erdös B, Miller A, Busija D (2002) Alterations in KATP and KCa channel function in cerebral arteries of insulin-resistant rats. Am J Physiol Heart Circ Physiol 283: H2472–7. [DOI] [PubMed] [Google Scholar]
- 15. Frisbee J, Maier K, Stepp D, Jefferson C (2002) Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Hear Circ Physiol 53226: 2160–2168. [DOI] [PubMed] [Google Scholar]
- 16. Bagi Z, Feher A, Cassuto J (2012) Microvascular responsiveness in obesity: implications for therapeutic intervention. Br J Pharmacol 165: 544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prieto D, Contreras C, Sánchez A, Sanchez A (2014) Endothelial Dysfunction, Obesity and Insulin Resistance. Curr Vasc Pharmacol 12: 1–15. [DOI] [PubMed] [Google Scholar]
- 18. Ellis A, Cheng Z, Li Y, Jiang Y, Yang J, et al. (2008) Effects of a Western diet versus high glucose on endothelium-dependent relaxation in murine micro- and macro-vasculature. Eur J Pharmacol 601: 111–117. [DOI] [PubMed] [Google Scholar]
- 19. Wölfle S, De Wit C (2005) Intact endothelium-dependent dilation and conducted responses in resistance vessels of hypercholesterolemic mice in vivo. J Vasc Res 42: 475–482. [DOI] [PubMed] [Google Scholar]
- 20. Chadha P, Haddock R, Howitt L, Morris M, Murphy T, et al. (2010) Obesity Up-Regulates Intermediate Conductance Calcium- Activated Potassium Channels and Myoendothelial Gap Junctions to Maintain Endothelial Vasodilator Function. J Pharmacol Exp Ther 335: 284–293. [DOI] [PubMed] [Google Scholar]
- 21. Haddock R, Grayson T, Morris M, Howitt L, Chadha P, et al. (2011) Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS One 6: e16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Motivala A, Rose P, Kim H, Smith Y, Bartnik C, et al. (2008) Cardiovascular risk, obesity, and myocardial blood flow in postmenopausal women. J Nucl Cardiol 15: 510–517. [DOI] [PubMed] [Google Scholar]
- 23. Fulop T, Jebelovszki E, Erdei N, Szerafin T, Forster T, et al. (2007) Adaptation of vasomotor function of human coronary arterioles to the simultaneous presence of obesity and hypertension. Arterioscler Thromb Vasc Biol 27: 2348–2354. [DOI] [PubMed] [Google Scholar]
- 24. Katakam P, Tulbert C, Snipes J, Miller A, Busija D, et al. (2005) Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Hear Circ Physiol 288: 854–860. [DOI] [PubMed] [Google Scholar]
- 25. Oltman C, Richou L, Davidson E, Coppey L, Lund D, et al. (2006) Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Hear Circ Physiol 291: 1780–1787. [DOI] [PubMed] [Google Scholar]
- 26. Villalba N, Martínez P, Bríones A, Sánchez A, Salaíces M, et al. (2009) Differential structural and functional changes in penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol 297: H696–707. [DOI] [PubMed] [Google Scholar]
- 27. Bagi Z (2009) Mechanisms of coronary microvascular adaptation to obesity. Am J Physiol Regul Integr Comp Physiol 10595: 556–567. [DOI] [PubMed] [Google Scholar]
- 28. Danser A, Tom B, Vries R, Saxena P (2000) L -NAME-resistant bradykinin-induced relaxation in porcine coronary arteries is NO-dependent: effect of ACE inhibition. Br J Pharmacol 131: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Batenburg W, Popp R, Fleming I, de Vries R, Garrelds I, et al. (2004) Bradykinin-induced relaxation of coronary microarteries: S-nitrosothiols as EDHF? Br J Pharmacol 142: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borbouse L, Dick G, Asano S, Bender B, Dincer U, et al. (2009) Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Hear Circ Physiol 297: H1629–H1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feher A, Rutkai I, Beleznai T, Ungvari Z, Csiszar A, et al. (2010) Caveolin-1 limits the contribution of BK(Ca) channel to EDHF-mediated arteriolar dilation: implications in diet-induced obesity. Cardiovasc Res 87: 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Contreras C, Sánchez A, García-Sacristán A, Martínez M, Andriantsitohaina R, et al. (2011) Preserved insulin vasorelaxation and up-regulation of the Akt/eNOS pathway in coronary arteries from insulin resistant obese Zucker rats. Atherosclerosis 217: 331–339. [DOI] [PubMed] [Google Scholar]
- 33. Santiago E, Contreras C, García-Sacristán A, Sánchez A, Rivera L, et al. (2013) Signaling pathways involved in the H2O2-induced vasoconstriction of rat coronary arteries. Free Radic Biol Med 60: 136–146. [DOI] [PubMed] [Google Scholar]
- 34. Strøbaek D, Teuber L, Jørgensen T, Ahring P, Kjaer K, et al. (2004) Activation of human IK and SK Ca2+ −activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim Biophys Acta 1665: 1–5. [DOI] [PubMed] [Google Scholar]
- 35. Morimura K, Yamamura H, Ohya S, Imaizumi Y (2006) Voltage-Dependent Ca2+-Channel Block by Openers of Intermediate and Small Conductance Ca2+-Activated K + Channels in Urinary Bladder Smooth Muscle Cells. J Pharmacol SciPharmacol Sci 241: 237–241. [DOI] [PubMed] [Google Scholar]
- 36. Schuster A, Oishi H, Bény JL, Stergiopulos N, Meister JJ (2001) Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am J Physiol Heart Circ Physiol 280: H1088–96. [DOI] [PubMed] [Google Scholar]
- 37. Adapala R, Talasila P, Bratz I, Zhang D, Suzuki M, et al. (2011) PKCalfa mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Hear Circ Physiol 301: H757–H765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurian M, Berwick Z, Tune J (2011) Contribution of IKCa channels to the control of coronary blood flow. Exp Biol Med 1: 621–627. [DOI] [PubMed] [Google Scholar]
- 39. Mishra R, Belke D, Wulff H, Braun A (2012) SKA-31, a novel activator of SKCa and IKCa channels, increases coronary flow in male and female rat hearts. Cardiovasc Res 97: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Climent B, Simonsen U, Rivera L (2014) Effects of Obesity on Vascular Potassium Channels. CurrVasc Pharmacol 12: 1–15. [DOI] [PubMed] [Google Scholar]
- 41. Young E, Hill M, Wiehler W, Triggle C, Reid J (2008) Reduced EDHF responses and connexin activity in mesenteric arteries from the insulin-resistant obese Zucker rat. Diabetologia 51: 872–881. [DOI] [PubMed] [Google Scholar]
- 42. Burnham M, Johnson I, Weston A (2006) Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in Type II diabetic ZDF rats. Bristish J Pharmacol 148: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weston A, Félétou M, Vanhoutte P, Falck J, Campbell W, et al. (2005) Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol 145: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sandow S, Neylon C, Chen M, Garland C (2006) Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat 209: 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brähler S, Kaistha A, Schmidt V, Wölfle S, Busch C, et al. (2009) Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119: 2323–2332. [DOI] [PubMed] [Google Scholar]
- 46. Takai J, Santu A, Zheng H, Koh S, Ohta M, et al. (2013) Laminar shear stress upregulates endothelial Ca(2)(+)-activated K(+) channels KCa2.3 and KCa3.1 via a Ca(2)(+)/calmodulin-dependent protein kinase kinase/Akt/p300 cascade. Am J Physiol Hear Circ Physiol 305 H484–93: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao L, Zhang W, Wang L, Li G, Deng X (2012) Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflügers Arch Eur J Physiol 464: 613–621. [DOI] [PubMed] [Google Scholar]
- 48. Simonsen U, García-Sacristán A, Prieto D (1997) Apamin-sensitive K+ channels involved in the inhibition of acetylcholine-induced contractions in lamb coronary small arteries. Eur J Pharmacol 329: 153–163. [PubMed] [Google Scholar]
- 49. Köhler R, Kaistha B, Wulff H (2010) Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets 14: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tharp DL, Bowles DK (2009) The intermediate-conductance Ca2+ -activated K+ channel (KCa3.1) in vascular disease. Cardiovasc Hematol Agents Med Chem 7: 1–11. [DOI] [PubMed] [Google Scholar]
- 51. Schubert R, Nelson MT (2001) Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22: 505–512. [DOI] [PubMed] [Google Scholar]
- 52. Alda J, Valero M, Pereboom D, Gros P, Garay R (2009) Endothelium-independent vasorelaxation by the selective alpha estrogen receptor agonist propyl pyrazole triol in rat aortic smooth muscle. J Pharm Pharmacol 61: 641–646. [DOI] [PubMed] [Google Scholar]
- 53. Nilius B, Droogmans G (2001) Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459. [DOI] [PubMed] [Google Scholar]
- 54. Bishara NB, Ding H (2010) Glucose enhances expression of TRPC1 and calcium entry in endothelial cells. Am J Physiol Hear Circ Physiol 298: 171–178. [DOI] [PubMed] [Google Scholar]
- 55. Ma X, Du J, Zhang P, Deng J, Liu J, et al. (2013) Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension 62: 134–139. [DOI] [PubMed] [Google Scholar]
- 56. Ledoux J, Taylor M, Bonev A, Hannah R, Solodushko V, et al. (2008) Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sonkusare S, Bonev A, Ledoux J, Liedtke W, Kotlikoff I, et al. (2012) Elementary Ca2+ Signals Through Endothelial TRPV4 Channels Regulate Vascular Function. Science (80-) 336: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghisdal P, Morel N (2001) Cellular target of voltage and calcium-dependent K(+) channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery. Br J Pharmacol 134: 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McSherry I, Spitaler M, Takano H, Dora K (2005) Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium 38: 23–33. [DOI] [PubMed] [Google Scholar]
- 60. Dalsgaard T, Kroigaard C, Misfeldt M, Bek T, Simonsen U (2010) Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles. Br J Pharmacol 160: 1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Daut J, Standen N, Nelson M (1994) The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol 5: 154–181. [DOI] [PubMed] [Google Scholar]
- 62. Sheng J, Ella S, Davis M, Hill M, Braun A (2009) Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J 23: 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland C, et al. (2012) Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci U S A 109: 18174–18179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Behringer E, Shaw R, Westcott E, Socha M, Segal S (2013) Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arterioscler Thromb Vasc Biol 33: 1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. Metabolic parameters of LZR and OZR.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.