Abstract
Background
Recent studies in children have demonstrated that frequent occurrence of parasomnias is related to increased sleep disruption, mental disorders, physical harm, sleep disordered breathing, and parental duress. Although there have been several cross-sectional and clinical studies of parasomnias in children, there have been no large, population-based studies using full polysomnography to examine the association between parasomnias and sleep disordered breathing. The Tucson Children's Assessment of Sleep Apnea study is a community-based cohort study designed to investigate the prevalence and correlates of objectively measured sleep disordered breathing (SDB) in pre-adolescent children six to 11 years of age. This paper characterizes the relationships between parasomnias and SDB with its associated symptoms in these children.
Methods
Parents completed questionnaires pertaining to their child's sleep habits. Children had various physiological measurements completed and then were connected to the Compumedics PS-2 sleep recording system for full, unattended polysomnography in the home. A total of 480 unattended home polysomnograms were completed on a sample that was 50% female, 42.3% Hispanic, and 52.9% between the ages of six and eight years.
Results
Children with a Respiratory Disturbance Index of one or greater were more likely to have sleep walking (7.0% versus 2.5%, p < 0.02), sleep talking (18.3% versus 9.0%, p < 0.006), and enuresis (11.3% versus 6.3%, p < 0.08) than children with an Respiratory Disturbance Index of less than one. A higher prevalence of other sleep disturbances as well as learning problems was observed in children with parasomnia. Those with parasomnias associated with arousal were observed to have increased number of stage shifts. Small alterations in sleep architecture were found in those with enuresis.
Conclusions
In this population-based cohort study, pre-adolescent school-aged children with SDB experienced more parasomnias than those without SDB. Parasomnias were associated with a higher prevalence of other sleep disturbances and learning problems. Clinical evaluation of children with parasomnias should include consideration of SDB.
Background
Sleepwalking, sleep terrors, and sleep talking are parasomnias associated with arousal that usually occur during the first third of sleep [1-3]. In contrast, enuresis may occur during non-rapid eye movement (NREM) or rapid eye movement (REM) sleep [4,5]. Recent studies in children have demonstrated that frequent occurrence of parasomnias is related to increased sleep disruption, mental disorders, physical harm, sleep disordered breathing (SDB), and parental duress [6-10]. Although there have been several cross-sectional and clinical studies of these parasomnias in children, there have been no large, population-based studies using full polysomnography to examine the association between parasomnias and sleep disordered breathing [2,7,8,11]. Epidemiological surveys investigating parasomnias in the general population are uncommon, perhaps because sleepwalking and sleep terrors are usually considered harmless childhood occurrences.
The prevalence of parasomnias in the general population of children has been estimated at approximately 5%–15% for sleepwalking [12-14], 1%–6.5% for sleep terrors [15,16], 5%–18% for enuresis [17-19], and 5%–10% for sleep talking [15,20-22]. These estimates vary greatly because rarely are the same definitions for the frequency of events used and there are no commonly accepted definitions currently in use for these disorders, particularly relating to the frequency of events. Furthermore, there is also no commonly accepted definition for SDB in children. Therefore, reports of associations between SDB and parasomnias have not been done in a standardized manner [23,24].
The Tucson Children's Assessment of Sleep Apnea study (TuCASA) is a prospective cohort study designed to determine the prevalence of objectively documented SDB in pre-adolescent children and to investigate its relationship to symptoms, performance on neurobehavioral measures, and physiologic and anatomic risk factors. This report describes the prevalence of parasomnias in Hispanic and Caucasian children and their association with objective polysomnographic assessment of SDB.
Methods
The design of the TuCASA study specified recruitment of Hispanic and Caucasian children aged six to 11 years to undergo unattended home polysomnography, complete a pediatric sleep habits questionnaire, and perform a neurocognitive assessment. Subjects were recruited through the Tucson Unified School District (TUSD), a very large district with a substantial elementary school population. Detailed recruitment methods have been described previously [25,26]. Typically, parents were asked to complete a short sleep habits screening questionnaire and to provide their contact information if they would allow study personnel to call and schedule a polysomnogram for their child. Over the time period of recruitment, 7,055 screening questionnaires were sent and 2,327 were returned (33%). Of those returning questionnaires, 1,219 (52%) supplied recruitment information from whom we selected children to undergo unattended home polysomnography. Less than 10% of those parents approached declined to have the procedure. An unattended home polysomnogram was scheduled as soon as possible after recruitment.
Methods for obtaining polysomnographic data have been described previously [26]. Briefly, a two-person, mixed gender team arrived at the home approximately one hour prior to the child's normal bedtime. Informed consent was obtained from the parent, and an Institutional Review Board (IRB) approved assent form was signed by the child. Questionnaires were administered, and anthropometric and other physiological measurements were completed. Unattended overnight polysomnograms were obtained using the Compumedics PS-2 system (Abbotsford, Victoria, Australia). The following signals were acquired as part of the TuCASA montage: C3/A2 and C4/A1 electroencephalogram, right and left electrooculogram, a bipolar submental electromyogram, thoracic and abdominal displacement (inductive plethysmography bands), airflow (nasal/oral thermister), nasal pressure cannula, finger pulse oximetry, ECG (single bipolar lead), snoring microphone, body position (Hg gauge sensor), and ambient light (sensor attached to the vest to record on/off).
On the night of the home visit, the parent was asked to complete a detailed Sleep Habits Questionnaire (SHQ). Included were the questions: "Does this child sleepwalk?", and "Does this child talk in his or her sleep? (Talk without being fully awake?)". Responses were "Never", "Less than three times per month", "three to five times per month", or "More than five times per month". The occurrence of these parasomnias was defined as follows: sleepwalking (SW) was present if it was reported more than three times per month, and sleep talking (ST) was present if it was reported more than five times per month. Additionally, the parent was asked "How often does this child awaken at night afraid or appearing tearful?". If the parent answered that the child had more than five fearful awakenings per month then the child was classified as having sleep terrors (TR). Enuresis (EN) was present if it was reported as occurring more than five times per month. The SHQ was also used to define the occurrence of habitual snoring (SN), excessive daytime sleepiness (EDS), witnessed apnea (WITAP), difficulty initiating and maintaining sleep (INSOM), and learning problems (LP). These findings were considered present if they were reported 'frequently' or more [25]. Although the specific range and order of questions used on the TuCASA SHQ and screening questionnaires have not been previously validated, key questions in the questionnaire have face validity and were taken from those used by Carroll and colleagues [27].
The Compumedics software system was used to process all polysomnograms. Scoring has been described in detail previously [26]. Briefly, sleep stages were scored according to Rechtshaffen and Kales' criteria [28]. Arousals were identified using criteria published by the American Academy of Sleep Medicine [29]. Apneas were scored if the amplitude (peak to trough) of the airflow signal using the thermister decreased below at least 25% of the amplitude of 'baseline' breathing (identified during a period of regular breathing with stable oxygen levels), if this change lasted for more than six seconds or two breath cycles. Hypopneas were designated if the amplitude of any respiratory signal decreased below (approximately) 70% of the amplitude of 'baseline' and if the thermister signal did not meet the criterion for apnea. 'Central' events were marked if no displacement was noted on both the chest and abdominal inductance channels. Otherwise, events were scored as 'obstructive'. After full scoring, analysis software was used to link each event to data from the oxygen saturation and EEG channels. In this manner, the Respiratory Disturbance Index (RDI) was defined as the number of respiratory events (apneas and hypopneas) per hour of the total sleep time. A 3% associated oxygen desaturation was required for the event to be counted in the total RDI. Use of this definition is supported by previous evidence that an RDI of one, based on events with a 3% oxygen desaturation, was clinically significant [2,30].
All studies were scored by a single registered polysomnographic technologist who was required to demonstrate a complete understanding of the study's scoring rules and to articulate reasons for assigning epoch by epoch codes for sleep and respiratory scoring. The initial 'pass' rate for polysomnograms was 90%; the overall pass rate was 97%. Approximately 5% of studies were re-scored by the same scorer on a blinded basis to determine consistency in scoring. No systematic differences were observed between initial and re-scored studies [26]. A night-to-night variability study in 10 children showed no statistically significant differences in key sleep parameters between two different nights of recording [26]. The TuCASA protocol was approved by both the University of Arizona Human Subjects Committee and the TUSD Research Committee.
Statistical analysis was done using SPSS 11.5 for Windows 2000 (SPSS®, Inc., Chicago, IL, USA). Comparisons of proportions were made using contingency tables with statistical significance (p < 0.05) determined using the Pearson chi-square test statistic. Because the continuous data were not normally distributed, the Mann-Whitney U test was used to compare medians.
Results
A total of 480 polysomnograms were completed on a sample that was 50.0% female and 42.3% Hispanic (Table 1). Approximately 53% of the children were between the ages of six and eight years, and 9.2% were classified as obese because their body mass index (BMI) exceeded the 95th percentile for their age, gender and ethnicity [31]. Parental report shows that 3.5% of these children had sleepwalking, 11.3% had sleep talking, 6.3% had terrors, and 7.5% had enuresis. Children aged six to eight years were more likely to have enuresis (10.6% versus 4.0%, p < 0.006), and there was trend for boys to have more enuresis than girls (9.6% versus 5.4%, p < 0.08). However, prevalence rates for sleepwalking, sleep talking, and terrors were not different with respect to age, gender and ethnicity. To further describe the sample, 15.0% of these children had habitual snoring, 16.3% had report of excessive daytime sleepiness, 5.2% had witnessed apnea, 29.4% had insomnia, and 5.5% had learning problems. Polysomnographic evidence of SDB (RDI>1) was found in 24% of children (n = 115). The mean RDI in these children was 2.6 (sd = 3.4) in comparison to 0.38 (sd = 0.28) in those without SDB.
Table 1.
Baseline characteristics
| Male (50.0%) (n = 240) | Female (50.0%) (n = 240) | Total (n = 480) | |||||
| n | % of total* | n | % of total* | n | % of total* | ||
| Ethnicity | Caucasian | 129 | 26.9 | 148 | 30.8 | 277 | 57.7 |
| Hispanic | 111 | 23.1 | 92 | 19.2 | 203 | 42.3 | |
| Age | 6–8 | 130 | 27.1 | 124 | 25.8 | 254 | 52.9 |
| 9–11 | 110 | 22.9 | 116 | 24.2 | 226 | 47.1 | |
| BMI >95% | Yes | 28 | 5.8 | 16 | 3.3 | 44 | 9.2 |
| No | 212 | 44.2 | 224 | 46.7 | 436 | 90.8 | |
| Sleepwalk | Yes | 8 | 1.7 | 9 | 1.9 | 17 | 3.5 |
| No | 232 | 48.3 | 231 | 48.1 | 463 | 96.5 | |
| Sleep talk | Yes | 26 | 5.4 | 28 | 5.8 | 54 | 11.3 |
| No | 214 | 44.6 | 212 | 44.2 | 426 | 88.8 | |
| Terrors | Yes | 15 | 3.1 | 15 | 3.1 | 30 | 6.3 |
| No | 225 | 46.9 | 225 | 46.9 | 450 | 93.8 | |
| Enuresis | Yes | 23 | 4.8 | 13 | 2.7 | 36 | 7.5 |
| No | 217 | 45.2 | 227 | 47.3 | 444 | 92.5 | |
| Snore | Yes | 40 | 8.3 | 32 | 6.7 | 72 | 15.0 |
| No | 200 | 41.7 | 208 | 43.3 | 408 | 85.0 | |
| EDS | Yes | 33 | 6.9 | 45 | 9.4 | 78 | 16.3 |
| No | 207 | 43.1 | 195 | 40.6 | 402 | 83.8 | |
| WITAP | Yes | 15 | 3.1 | 10 | 2.1 | 25 | 5.2 |
| No | 225 | 46.9 | 230 | 47.9 | 455 | 94.8 | |
| Insomnia | Yes | 76 | 15.8 | 65 | 13.5 | 141 | 29.4 |
| No | 164 | 34.2 | 175 | 36.5 | 339 | 70.6 | |
| LP | Yes | 16 | 3.3 | 12 | 2.5 | 28 | 5.8 |
| No | 224 | 46.7 | 228 | 47.5 | 452 | 94.2 | |
*may not sum to 100% due to rounding.
Some common characteristics of sleep architecture in these children are shown in Table 2. The mean sleep time was approximately 487 minutes, with a sleep efficiency of approximately 90%. In general, sleep architecture was typical for school-age children with stage 2 sleep accounting for 52% of the sleep period, REM approximately 21%, and slow-wave sleep approximately 22% of total sleep time. Mean sleep and REM latencies were 18.5 and 130.9 minutes, respectively.
Table 2.
Sleep architecture (n = 480)
| Mean | SD | |
| Sleep Time | 487 minutes | 79.7 minutes |
| Sleep Efficiency | 89.8% | 5.8% |
| Stage 1 (%) | 4.6% | 3.3% |
| Stage 2 (%) | 52.0% | 6.1% |
| Stage 3/4 (%) | 21.9% | 6.1% |
| REM (%) | 21.5% | 5.0% |
| Sleep Latency | 18.5 minutes | 21 minutes |
| REM Latency | 130.9 minutes | 50.8 minutes |
Children with SDB were more likely to have sleepwalking (7.0% versus 2.5%, p < 0.02), sleep talking (18.3% versus 9.0%, p < 0.006), and enuresis (11.3% versus 6.3%, p < 0.08) than children without SDB (Figure 1). There was no association between RDI and terrors at an RDI of one, however, this association became significant if a cutpoint of two events per hour was used (15.9% versus 5.3%, p < 0.005).
Figure 1.
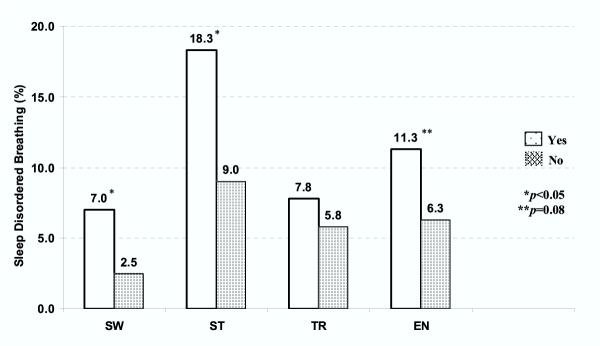
Associations between sleep disordered breathing and parasomnias. Sleep disordered breathing (SDB) was defined as an RDI of more than one per hour of total sleep time. See text for complete definition.
As shown in Figure 2, there was an association between parasomnias with parent report of sleep disturbances and learning problems. Children with sleepwalking were more likely to have report of EDS, insomnia and learning problems as well as a tendency to have more habitual snoring. Children with sleep talking were more likely to have habitual snoring, insomnia, and learning problems, but not EDS. Children with terrors were more likely to have habitual snoring, EDS, insomnia, and learning problems. Enuresis was strongly associated with habitual snoring and witnessed apnea.
Figure 2.
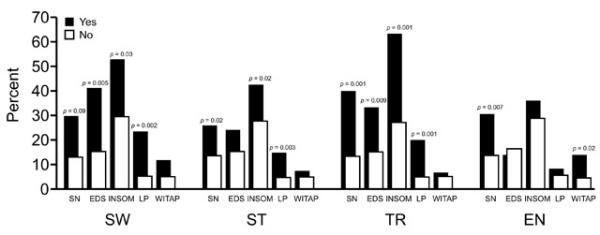
Associations between parasomnias, and symptoms of sleep disordered breathing, and learning problems. SW, sleepwalking; ST, sleep talking; TR, sleep terrors; EN, enuresis; SN, snoring; EDS, excessive daytime sleepiness; INSOM, insomnia; LP, learning problems; WITAP, witnessed apnea. See text for complete definitions.
As shown in Table 3, children frequently were observed to have more than one type of parasomnia. Children with sleepwalking were also more likely to have sleep talking, and those with sleep talking also had sleep terrors more often. However, there were no associations between arousal parasomnias and enuresis.
Table 3.
Associations between parasomniac events (n = 480)
| ST (n = 54) | TR (n = 30) | EN (n = 36) | ||||||||
| Yes | No | p | Yes | No | p | Yes | No | p | ||
| SW | Yes | 8 | 9 | 0.001* | 4 | 13 | n/a | 0 | 17 | n/a |
| No | 46 | 417 | 29 | 437 | 36 | 427 | ||||
| ST | Yes | 9 | 45 | 0.001* | 7 | 47 | 0.11 | |||
| No | 21 | 405 | 29 | 397 | ||||||
| TR | Yes | 3 | 27 | n/a | ||||||
| No | 33 | 417 | ||||||||
n/a indicates insufficient n. *p # 0.05.
Table 4 shows the relationships between parasomnias and sleep architecture. With the exception of enuresis, there were no differences in sleep stage distribution between those children with a parasomnia and those without. Children with enuresis had significantly greater sleep time (522 versus 500 min, p < 0.05), shorter sleep latency (7 versus 11 min, p < 0.008), and greater time in stage 2 (277 versus 258 min, p < 0.05) than children without enuresis.
Table 4.
Associations between sleep architecture (median) and parasomnias (n = 480)
| SW | ST | TR | EN | |||||||||
| Yes | No | p | Yes | No | p | Yes | No | p | Yes | No | p | |
| Total sleep time (min) | 498 | 502 | 0.79 | 489 | 503 | 0.48 | 510 | 502 | 0.48 | 522 | 500 | 0.05* |
| Total time in bed (min) | 562 | 558 | 0.52 | 548 | 561 | 0.32 | 563 | 558 | 0.40 | 572 | 558 | 0.22 |
| Sleep efficiency (%) | 89.8 | 91.6 | 0.19 | 91.6 | 91.4 | 0.48 | 92.1 | 91.4 | 0.56 | 92.6 | 91.3 | 0.09 |
| Sleep latency (min) | 20 | 11 | 0.07 | 12 | 11 | 0.92 | 8 | 11 | 0.19 | 7 | 11 | 0.008* |
| REM latency (min) | 137 | 130 | 0.63 | 145 | 130 | 0.35 | 135 | 130 | 0.68 | 145 | 129 | 0.29 |
| Stage 1 (min) | 17 | 19 | 0.78 | 16 | 19 | 0.42 | 21 | 19 | 0.37 | 17 | 19 | 0.59 |
| Stage 1 (%) | 3.4 | 3.8 | 0.81 | 3.1 | 3.8 | 0.43 | 3.8 | 3.8 | 0.38 | 3.2 | 3.8 | 0.39 |
| Stage 2 (min) | 264 | 259 | 0.58 | 262 | 260 | 0.93 | 249 | 260 | 0.93 | 277 | 258 | 0.05* |
| Stage 2 (%) | 53 | 52.3 | 0.69 | 51.6 | 52.4 | 0.70 | 50.7 | 52.5 | 0.26 | 52.6 | 52.4 | 0.54 |
| Stage 3/4 (min) | 101 | 103 | 0.93 | 101 | 103 | 0.55 | 99.5 | 103 | 0.73 | 109 | 102 | 0.13 |
| Stage 3/4 (%) | 20.3 | 21.6 | 0.79 | 20.3 | 21.7 | 0.80 | 21.0 | 21.6 | 0.94 | 22.6 | 21.6 | 0.63 |
| REM (min) | 113 | 110 | 0.89 | 110 | 111 | 0.95 | 124 | 110 | 0.48 | 114 | 110 | 0.51 |
| REM (%) | 21.0 | 21.8 | 0.61 | 21.9 | 21.7 | 0.83 | 21.5 | 21.7 | 0.90 | 22.3 | 21.7 | 0.80 |
| Shifts stage 2 to stage 1 | 2 | 3 | 0.78 | 4 | 3 | 0.06 | 2 | 3 | 0.73 | 4 | 3 | 0.14 |
| Shifts stage 3 to stage 2 | 4 | 7 | 0.78 | 7 | 7 | 0.98 | 10 | 7 | 0.08 | 7 | 7 | 0.77 |
| Shifts REM to stage 1 | 14 | 12 | 0.51 | 15.5 | 11.0 | 0.006* | 14.5 | 11 | 0.05* | 150.5 | 11.0 | 0.19 |
| Shifts REM to stage 2 | 32 | 24 | 0.40 | 22.5 | 24 | 0.35 | 23.5 | 24 | 0.81 | 26.5 | 24.0 | 0.16 |
| Shifts REM to stage 3/4 | 6 | 5 | 0.40 | 8 | 5 | 0.02* | 5 | 5 | 0.66 | 6.5 | 5 | 0.48 |
| Shifts REM to wake | 4 | 6 | 0.19 | 7 | 6 | 0.39 | 7 | 6 | 0.01* | 7 | 6 | 0.50 |
| Total stage shifts | 77 | 66 | 0.52 | 78.5 | 65 | 0.005* | 79.5 | 66 | 0.06 | 72 | 66 | 0.08 |
| Total arousals | 29 | 27 | 0.24 | 26 | 27 | 0.39 | 25 | 27 | 0.24 | 27 | 27 | 0.48 |
| Arousal index | 3.5 | 3.3 | 0.39 | 3.2 | 3.3 | 0.58 | 3.1 | 3.3 | 0.05* | 3.3 | 3.3 | 0.82 |
*p ≤ 0.05.
Some parasomnias were associated with increased numbers of stage shifts during sleep (Table 4). Shifts from REM to stage 1 (15.5 versus 11.0, p < 0.006), REM to stage 3 (8 versus 5, p < 0.02) as well as total shifts throughout the night (78.5 versus 65, p < 0.005) were significantly greater in those with sleep talking versus those without this behavior. A similar finding was present for sleep terrors with more frequent shifts from REM to stage 1 (14.5 versus 11, p < 0.05), and REM to wake (7 versus 6, p < 0.01). However, there was a slightly lower arousal index (total number of arousals per hour of sleep time) in those with sleep terrors. For those children with sleepwalking, no sleep characteristics were significantly different between those with SW and those without.
Discussion
The TuCASA study has documented the relationships among objectively obtained polysomnographic variables, parental report of parasomnias and SDB-related symptoms in a population-based sample of six- to 11-year-old children. We found that children with SDB were more likely to have parasomnias. Furthermore, there was a greater prevalence of other sleep symptoms and learning problems among children with parasomnias. Finally, children with parasomnias associated with arousal also exhibited more sleep fragmentation, but were not observed to have any alterations in sleep stage distribution. In contrast, children with enuresis did exhibit some minor changes in sleep architecture. These data provide evidence from a general population sample that SDB is a risk factor for parasomnias in children.
The major finding of our study is that children with SDB in a general population cohort were more likely to have sleepwalking and sleep talking. This observation is similar to those noted in a general adult population in whom those with SDB had a higher prevalence of nightmares and other parasomnias than those without SDB [32,33]. Studies in children performed in clinical populations also indicate that parasomnias such as sleep terrors and sleepwalking occur more often in children with obstructive sleep apnea (OSA) [8]. Our results extend these results to a general population not referred for evaluation of a sleep disorder.
The linkage between SDB and parasomnias most likely involves the triggering of parasomnias by arousals related to respiratory events. Recently, Espa et al. found that respiratory events occur more frequently in parasomniacs than in controls, with an arousal reaction probably involved in triggering the parasomnia episode [3]. In addition, a study in pre-pubertal children by Guilleminault et al. showed a clear, prompt improvement in sleepwalking and sleep talking with treatment for SDB and periodic limb movements (PLMS) [2]. It was suggested that both SDB and PLMS induced parasomnias by producing brief arousals from sleep. In our study, we noted more evidence of sleep fragmentation manifested by a greater number of stage shifts amongst those with sleep talking and sleep terrors, but not sleepwalking. However, there was a slight decrease in arousal index amongst those with sleep terrors. The explanation for this latter finding is not readily apparent. Although stage shifts were not increased in those with sleepwalking, our findings nevertheless support the contention that SDB initiates parasomnias in children by promoting a greater nocturnal sleep disruption. Furthermore, initiation of parasomnias by SDB-induced sleep fragmentation suggests that clinical evaluation of children with parasomnias needs to include consideration of the simultaneous presence of SDB.
Our data show significant associations between parasomnias and frequent loud snoring, daytime sleepiness and learning problems – symptoms known to be associated with SDB in children. These observations are consistent with our finding of a correlation between polysomnographic SDB and parasomnias. The association between parasomnias and learning problems is particularly noteworthy because of the relationships between SDB and parasomnias, and between SDB and learning problems. It is possible that parasomnias are an independent risk factor for learning problems. Alternatively, because SDB is associated with both parasomnias and learning problems, this finding is actually an effect attributable to SDB. Unfortunately, our study had an insufficient amount of data to further investigate either of these explanations. Nevertheless, our results are compatible with the report by Ipsiroglu et al. who recently showed that snoring children 11–15 years of age also had more frequent sleepwalking, night terrors, nightmares, and other nocturnal awakenings than non-snorers [34]. In addition, we have shown that children with parasomnias were more likely to have insomnia. Similar findings have been reported by Gau et al. in adolescents [6]. The association between parasomnias and clinical symptoms of other sleep disturbances suggest that the health consequences of parasomnias extend beyond their nocturnal manifestations.
Although enuresis is not usually considered a parasomnia associated with arousal, we provide some population-based evidence for an association between parent report of enuresis and objectively measured SDB. In previous studies in children, the relationship between nocturnal enuresis and SDB in children has been inconsistent, with some studies finding an association [35,36] and others not finding one [37]. In a clinic sample using a definition of SDB similar to ours but with a requirement for an oxygen desaturation of 4%, Brooks et al. found that children with RDI>1 were at higher risk for enuresis than children with an RDI<1 [38]. Moreover, treatment of SDB in children may improve or eliminate enuresis [36,39,40]. In TuCASA, we found that children with SDB were more likely to have enuresis than children without SDB (11.3% versus 6.3%), although statistical significance was borderline (p < 0.08). However, enuresis was strongly associated with frequent loud snoring (p < 0.007), a known correlate of SDB in children.
There have been relatively few studies of the sleep of enuretic children in which there have been concordant recordings of non-enuretic controls. Although one study suggested that their enuretic children exhibited greater amounts of delta sleep [41], others have found little discrepancy between those with enuresis and controls [4,42]. Although in our study those with enuresis had a longer sleep time, greater time in Stage 2 and shorter sleep latency, these differences were relatively small. Thus, our data would tend to support the contention that the sleep of enuretic children is not greatly altered from those who are not enuretic.
The prevalence of parasomnias in this population-based study is similar to that found in previous samples of this age group. However, comparisons are difficult, since no standard method of assessing the frequency of parasomnias exists. Our definitions are more stringent than those employed in most studies. Our requirement that these events occurred more than three to five times per month exceeded reports where events were recorded if 'ever' occurred [14,15]. In studies where the definition of parasomniac events was similar to ours, the prevalence of events is approximately the same [43-45].
Although our study is the first to evaluate the association between childhood parasomnias and SDB using polysomnography in a large general population, it is not without some limitations. Firstly, it is possible that parents underestimated the actual number of parasomnias that occurred. Depending on the bedtime of the child and the severity of the event, it is probable that parents are not awake during every occurrence. Owens et al. found that four- to 11-year-old children tend to self-report more sleep problems, particularly night wakings, than their parents [46]. Secondly, our assessment of RDI was based on one night of study. However, several studies have shown that there is little night-to-night variability in polysomnograms done in children [47,48]. Nevertheless, the sleep of some children may have been slightly hindered by the recording equipment, especially the nasal thermister [30], thus accounting for a slightly longer sleep latency. Thirdly, our definition of sleep terrors, based upon parental report instead of a tape recording, contains potential ambiguity. It would have been desirable to ask more specific questions about the night time awakening of the child, however, our questionnaire did not specify between an early- or late-night awakening. We did ask if the child could remember a dream during or after the frightful awakening, however, there were insufficient numbers of these to analyze. Since most of the events that a parent would report presumably happen earlier in the child's sleep period while the parent is still awake (when sleep terrors would be most likely to occur), we have counted these as terrors. This could account for the 6.3% prevalence of sleep terrors observed in this study being on the higher end of those reported by previous studies [21,34]. Nonetheless, whether terrors or nightmares, the association between SDB and these frightful awakenings is significant. Fourthly, home polysomnography does not allow for concurrent observation of sleep behavior to confirm the report of a parasomnia. However, laboratory sleep studies also do not always directly observe the patient during a parasomniac episode. Additionally, we were unable to record leg EMG and thus monitor periodic limb movements, which may be responsible for some sleep fragmentation unrelated to SDB [49]. Finally, recruitment may have incurred a selection bias so that parents who participated might be more likely to have symptomatic children than those who do not participate. However, the sleep architecture and other sleep-related characteristics of our sample are similar to those found in 'normal' populations, so we do not believe that a large selection bias has occurred.
Conclusions
In summary, the TuCASA study has demonstrated that there are significant associations between parental report of parasomnias and polysomnographically documented SDB in a large general population of pre-adolescent school children. Furthermore, they suggest that parasomnias occurring amongst those with SDB are triggered by sleep fragmentation produced by SDB. These results extend previous clinical findings to a population-based sample that had objective determination of SDB and suggest that evaluation of children with parasomnias should include consideration of concomitant SDB.
Competing interests
None declared.
Authors' contributions
JG conceived the manuscript, performed the primary analyses, produced the first rough draft, and is the corresponding author. KK contributed in the design and implementation of the study, and read and approved the manuscript. RF contributed in the design and implementation of the study, and read and approved the manuscript. GR contributed in the design and implementation of the study and reviewed scoring procedures for polysomnography. TS contributed database design and management. WM contributed in the design and implementation of the study. SQ is the primary investigator for TuCASA and has participated in all aspects of manuscript preparation, data analyses, and revision. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This work was supported by grant HL 62373 from the National Heart Lung and Blood Institute in addition to a grant from the Max and Victoria Dreyfus Foundation. The authors wish to thank the University of Arizona General Clinical Research Center as well as the principals, teachers, parents and students from the Tucson Unified School District for their ongoing support of this research.
Contributor Information
James L Goodwin, Email: jamieg@resp-sci.arizona.edu.
Kris L Kaemingk, Email: kaemingk@peds.arizona.edu.
Ralph F Fregosi, Email: fregosi@u.arizona.edu.
Gerald M Rosen, Email: rosen052@gold.tc.umn.edu.
Wayne J Morgan, Email: wmorgan@resp-sci.arizona.edu.
Terry Smith, Email: tsmith6@u.arizona.edu.
Stuart F Quan, Email: squan@resp-sci.arizona.edu.
References
- Broughton R Sleep disorders: disorders of arousal? Science. 1968;159:1070–1078. doi: 10.1126/science.159.3819.1070. [DOI] [PubMed] [Google Scholar]
- Guilleminault C. Palombini L. Pelayo R. Chervin R Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics. 2003;111:e17–e25. doi: 10.1542/peds.111.1.e17. [DOI] [PubMed] [Google Scholar]
- Espa F. Dauvilliers Y. Ondze B. Billiard M. Besset A Arousal reactions in sleepwalking and night terrors in adults: the role of respiratory events. Sleep. 2002;25:871–875. [PubMed] [Google Scholar]
- Gillin J. Rapoport J. Langer D. Vanskiver C. Mendelson W EEG sleep patterns in enuresis: a further analysis and comparison with normal controls. Biol Psychiatry. 1982;17:947–953. [PubMed] [Google Scholar]
- Mikkelsen E. Rapoport J. Nee L. Gruenau C. Mendelson W. Gillin J Childhood enuresis I: sleep patterns and psychopathology. Arch Gen Psychiatry. 1980;37:1139–1144. doi: 10.1001/archpsyc.1980.01780230057008. [DOI] [PubMed] [Google Scholar]
- Gau S. Soong W Psychiatric comorbidity of adolescents with sleep terrors or sleep walking: a case-control study. Aust N Z J Psychiatry. 1999;33:734–739. doi: 10.1046/j.1440-1614.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- Mehlenbeck R. Spirito A. Owens J. Boergers J The clinical presentation of childhood partial arousal parasomnias. Sleep Med. 2000;1:307–312. doi: 10.1016/S1389-9457(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Owens J. Spirito A. Nobile C. Arrigan M Incidence of parasomnias in children with obstructive sleep apnea. Sleep. 1997;20:1193–1196. [PubMed] [Google Scholar]
- Guilleminault C, Pelayo R, Leger D, Clerk A, Bocian R. Recognition of sleep-disordered breathing in children. Pediatrics. 1996;98:871–882. [PubMed] [Google Scholar]
- Mahowald M. Rosen G Parasomnias in children. Pediatrician. 1990;17:21–31. [PubMed] [Google Scholar]
- Klackenberg G Somnambulism in childhood: prevalence, course and behavioral correlation. Acta Paediatr Scand. 1982;71:495–499. doi: 10.1111/j.1651-2227.1982.tb09458.x. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Kales D. Kales A . Clinical and electrophysiological correlates of sleep disorders in children. In: Kales A, editor. Sleep: Physiology and Pathology. Philadelphia, Lippincott; 1969. pp. 109–118. [Google Scholar]
- Bakwin H Sleep-walking in twins. The Lancet. 1970;2:446–447. doi: 10.1016/s0140-6736(70)90058-9. [DOI] [PubMed] [Google Scholar]
- Archbold K. Pituch K. Panahi P. Chervin R Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97–102. doi: 10.1067/mpd.2002.119990. [DOI] [PubMed] [Google Scholar]
- Laberge L. Tremblay R. Vitaro F. Montplaisir J Development of parasomnias from childhood to early adolescence. Pediatrics. 2000;106:67–74. doi: 10.1542/peds.106.1.67. [DOI] [PubMed] [Google Scholar]
- Simonds JF. Parraga H Prevalence of sleep disorders and sleep behaviours in children and adolescents. J Am Acad Child Psychlatry. 1982;21:383–388. doi: 10.1016/s0002-7138(09)60942-0. [DOI] [PubMed] [Google Scholar]
- Bower W. Moore K. Shepherd R. Adams The epidemiology of childhood enuresis in Australia. Br J Urol. 1996;78:602–606. doi: 10.1046/j.1464-410x.1996.13618.x. [DOI] [PubMed] [Google Scholar]
- Foxman B. Valdez R. Brook R Childhood enuresis: prevalence, perceived impact, and prescribed treatments. Pediatrics. 1986;77:482–487. [PubMed] [Google Scholar]
- Byrd R. Weitzman M. Lanphear N. Auinger P Bed-wetting in US children: epidemiology and related behavior problems. Pediatrics. 1996;98:414–419. [PubMed] [Google Scholar]
- Reimao R. Lefevre A Prevalence of sleep-talking in childhood. Brain Dev. 1980;2:353–357. doi: 10.1016/s0387-7604(80)80047-7. [DOI] [PubMed] [Google Scholar]
- Sadeh A. Raviv A. Gruber R Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36:291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Salzavulo P. Chevalier A Sleep problems in children and their relationship with early disturbances of the waking-sleeping rhythms. Sleep. 1983;6:47–51. doi: 10.1093/sleep/6.1.47. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society, editor. Am J Respir Crit Care Med. Vol. 160. 1999. Cardiorespiratory sleep studies in children. Establishment of normative data and polysomnographic predictors of morbidity. American Thoracic Society. pp. 1381–1387. [DOI] [PubMed] [Google Scholar]
- Schechter MS Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- Goodwin J. Babar S. Kaemingk K. Rosen G. Morgan W. Sherrill D. Quan S Symptoms related to sleep disordered breathing in Caucasian and Hispanic children - the Tucson Children's Assessment of Sleep Apnea study (TuCASA) Chest. 2003;124:196–203. doi: 10.1378/chest.124.1.196. [DOI] [PubMed] [Google Scholar]
- Goodwin J. Enright P. Morgan W. Kaemingk K. Rosen G. Fregosi R. Quan S Feasibility of using unattended polysomnography in children for research: report of the Tucson Children's Assessment of Sleep Apnea Study (TuCASA) Sleep. 2001;24:937–944. doi: 10.1093/sleep/24.8.937. [DOI] [PubMed] [Google Scholar]
- Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndromes in children. Chest. 1995;108::610–618. doi: 10.1378/chest.108.3.610. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A. Kales A . UCLA Brain Information Service/Brain Research Institute. Los Angeles, CA; 1968. A Manual of Standardized Terminology: Techniques and Scoring Systems for Sleep Stages of Human Subjects. [Google Scholar]
- EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- Goodwin J. Kaemingk K. Fregosi R. Rosen G. Morgan W. Sherrill D. Quan S Clinical outcomes associated with sleep disordered breathing in Caucasian and Hispanic children - The Tucson Children's Assessment of Sleep Apnea Study (TuCASA) Sleep. 2003;26:587–591. doi: 10.1093/sleep/26.5.587. [DOI] [PubMed] [Google Scholar]
- Rosner B. Prineas R. Loggie J. Daniels SR Percentiles for body mass index in U.S. children 5 to 17 years of age. J Pediatr. 1998;132:211–222. doi: 10.1016/s0022-3476(98)70434-2. [DOI] [PubMed] [Google Scholar]
- Ohayon M. Guilleminault C. Priest R Night terrors, sleepwalking, and confusional arousals in the general population: their frequency and relationship to other sleep and mental disorders. J Clin Psychiatry. 1999;60:268–276. doi: 10.4088/jcp.v60n0413. [DOI] [PubMed] [Google Scholar]
- Guilleminault C. Pelayo R Sleep-disordered breathing in children. Ann Med. 1998;30:350–356. doi: 10.3109/07853899809029934. [DOI] [PubMed] [Google Scholar]
- Ipsiroglu O. Fatemi A. Werner I. Ekkehart P. Schwarz B Self-reported organic and nonorganic sleep problems in schoolchildren aged 11 to 15 years in Vienna. J Adol Health. 2002;31:436–442. doi: 10.1016/S1054-139X(02)00423-8. [DOI] [PubMed] [Google Scholar]
- Frank Y. Kravath R. Pollak C. Weitzman E Obstructive sleep apnea and it's therapy: clincial and polysomnographic manifestations. Pediatrics. 1983;71:737–742. [PubMed] [Google Scholar]
- Weider D. Sateia M. West R Nocturnal enuresis in children with upper airway obstruction. Otolaryngol Head Neck Surg. 1991;105:427–432. doi: 10.1177/019459989110500314. [DOI] [PubMed] [Google Scholar]
- Brouillette R. Hanson D. David R. Klemka L. Hunt C A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr. 1984;105:10–14. doi: 10.1016/s0022-3476(84)80348-0. [DOI] [PubMed] [Google Scholar]
- Brooks L. Topol H Enuresis in children with sleep apnea. J Pediatr. 2003;142:515–518. doi: 10.1067/mpd.2003.158. [DOI] [PubMed] [Google Scholar]
- Ng D , Chau K. Kwok K Nocturnal enuresis and obstructive sleep apnoea in two children. Singapore Med J. 2001;42:590–591. [PubMed] [Google Scholar]
- Cinar U. Vural C. Cakir B. Topuz E. Karaman M. Turgut S Nocturnal enuresis and upper airway obstruction. Int J Pediatr Otorhinolaryngol. 2001;59:115–118. doi: 10.1016/S0165-5876(01)00463-3. [DOI] [PubMed] [Google Scholar]
- Hunsballe J Increased delta component in computerized sleep electronencephalographic analysis suggests abnormally deep sleep in primary Monosymptomatic nocturnal enuresis. Scand J Urol Nephrol. 2000;34:294–302. doi: 10.1080/003655900750048305. [DOI] [PubMed] [Google Scholar]
- Bader G. Neveus T. Kruse S. Sillen U Sleep of primary enuretic children and controls. Sleep. 2002;25:573–577. [PubMed] [Google Scholar]
- Fisher C. Kahn E. Edwards A. Davis DM A psychophysiological study of nightmares and night-terrors I.Physiological aspects of the stage 4 night terror. J Nerv Ment Dis. 1973;157:75–98. doi: 10.1097/00005053-197308000-00001. [DOI] [PubMed] [Google Scholar]
- Beltramini A. Hertzing M Sleep and bedtime behavior in preschool-aged children. Pediatrics. 1983;71:153–158. [PubMed] [Google Scholar]
- Kales A. Soldatos C. Bixler E. Ladda R. Charney D. Weber G. Schweitzer P Hereditary factors in sleepwalking and night terrors. Br J Psychiatry. 1980;137:111–118. doi: 10.1192/bjp.137.2.111. [DOI] [PubMed] [Google Scholar]
- Owens J. Spirito A. McGuinn M. Nobile C Sleep habits and sleep disturbances in elementary school-aged children. J Dev Behav Pediatr. 2000;21:27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- Owen G. Canter R. Maw R Screening for obstructive sleep apnoea in children. Int J Pediatr Otorhinolaryngol. 1995;32 Suppl:S67–9. doi: 10.1016/0165-5876(94)01145-N. [DOI] [PubMed] [Google Scholar]
- Messner AH. Pelayo R Pediatric sleep-related breathing disorders. Am J Otolaryngol. 2000;21:98–107. doi: 10.1016/s0196-0709(00)85005-x. [DOI] [PubMed] [Google Scholar]
- Brouillette RT. Jacob SV. Waters KA. Morielli A. Mograss M. Ducharme FM Cardiorespiratory sleep studies for children can often be performed in the home. Sleep. 1996;19:S278–S280. [PubMed] [Google Scholar]


