Abstract
In addition to the clinicopathological parameters, molecular biomarkers are becoming increasingly important in the prognostic evaluation of cancer patients. This study aimed to determine the molecular alterations in the RAS association domain family protein1A gene (RASSF1A) in salivary adenoid cystic carcinoma (ACC) and to evaluate the potential of such alterations as prognostic markers. One hundred and sixty-seven ACC tumor tissues and 50 samples of matched normal salivary gland tissues from the same patients were analyzed for RASSF1A promoter methylation status by bisulfite sequencing PCR (BSP) and/or methylation-specific PCR (MSP). Fifty ACC tumor tissues and matched normal salivary gland tissues were analyzed for loss of heterozygosity (LOH) by examining two microsatellite markers (D3S1478, D3S1621) at 3p21. RASSF1A gene mutations were detected by direct sequencing of all six exons in 50 tumor and normal tissue specimens. Over-all, RASSF1A promoter hypermethylation was detected in 35.3% (59/167) of ACC tissues and was associated with histologically solid tumor pattern (P = 0.002) and advanced TNM stage (P = 0.014). RASSF1A LOH was observed in 18.0% (9/50) of cases, and no somatic mutation of RASSF1A was detected in any cases. RASSF1A promoter methylation was associated with the poor over-all survival (Log-rank test, P <0.001) and disease-free survival (Log-rank test, P <0.001) and identified as an independent predicator of over-all patient survival (P = 0.009) and disease-free survival (P <0.001). It was concluded that RASSF1A methylation is involved in the development, differentiation and progression of ACC and is a strong independent biomarker of poor survival in ACC patients in a Chinese population.
Introduction
Salivary adenoid cystic carcinoma (ACC), the most common malignant tumor of salivary glands in our database [1], [2], has certain unique characteristics, such as slow but aggressive growth, nerve and blood invasion, distant metastasis to the lungs at early or late stages and very poor long-term survival [3], [4], [5]. These features make it one of the most characteristic malignant tumors arising from salivary glands. Solid histological pattern and later TNM stage have been confirmed as valid factors for predicting poor prognosis in patients with salivary ACC in our data [6] and other reports [7], [8]. We hypothesized that the clinicopathological parameters of tumor development are accompanied by alterations at the molecular level. Thus, the aim of this study was to understand the molecular basis of ACC tumor development and thereby expand the prospects for developing effective diagnostic and prognostic markers.
Over the past decade, molecular studies have contributed significantly to the identification of genetic and epigenetic changes associated with ACC. In our previous study of 60 ACC tumor tissues [9], we identified the frequent occurrence of promoter methylation of E-cadherin, p16INK4a, RAS association domain family protein 1A (RASSF1A), and death association protein kinase (DAPK) genes. However, RASSF1A promoter methylation was detected with lower frequency in ACC than in some other tumors [10], [11], [12]. Furthermore, its significant association with tumor histological grade and TNM stage [9], [13] implicated RASSF1A as a critical gene in the development of salivary ACC.
RASSF1A is a putative tumor suppressor gene located on 3p21 and functions in cell cycle control, microtubule stabilization, cellular adhesion, motility and apoptosis [14]. Promoter methylation, which is an epigenetic change, is the predominant mechanism of RASSF1A gene inactivation, and has been recognized in many human solid tumors, including non-small cell lung carcinoma (NSCLC) [15], [16], [17], breast carcinoma [10], [18], [19] and colorectal carcinoma [20], [21]; thus, RASSF1A promoter methylation may be a prognostic indicator in such tumors. Loss of heterozygosity (LOH), which is among the genetic changes in regions of the RASSF1A gene, has been detected in cervical squamous cell carcinoma and adenocarcinoma [22], [23] and gallbladder carcinoma [24]. RASSF1A mutation, another genetic abnormality, has also been found in primary nasopharyngeal carcinoma though no obvious mutational hot-spots were observed [25]. These reports demonstrated that various mechanisms are involved in RASSF1A gene inactivation, and that abnormalities in the RASSF1A gene may be a crucial event in the development and progression of some types of malignant tumors. To date, there are no reports describing LOH or RASSF1A mutations in ACC tumors. Furthermore, the association between molecular changes in the RASSF1A gene and tumor survival remains to be clarified. This study, conducted in 167 Chinese patients with salivary ACC, aimed to determine the molecular alterations of RASSF1A in salivary ACC and to evaluate the potential of such alterations as prognostic markers. We detected the promoter methylation status, LOH and RASSF1A gene mutations in ACC tumors and further elucidated the association of the RASSF1A abnormalities with patient outcome.
Results
Clinical data
Of the 167 patients with salivary ACC included in this study, 78 were women and 89 were men, and the medium age was 52.5 years (range 27–83). Eighty-one percent (135/167) of the cases exhibited cribriform and tubular patterns, and 19% (32/167) exhibited the solid pattern. Twenty-two of 167 cases (13%) were classified as TNM stage I, 74 cases (44%) as stage II, 53 cases (32%) as stage III and 18 cases (11%) as stage IV (Table S2 in File S1).
Promoter methylation, LOH and mutation of RASSF1A and correlations between these indexes
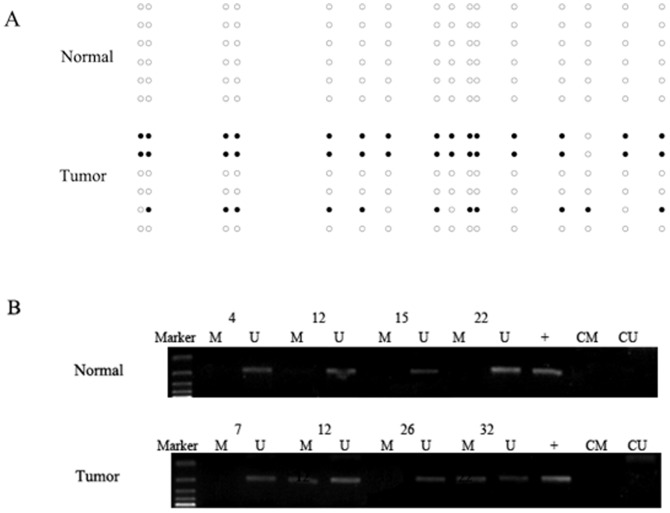
Among 15 paired ACC tumor and normal tissues, methylation of the RASSF1A gene promoter was detected in four cases (27%) (cases 4, 5, 9 and 12) by BSP. By MSP, methylation of the RASSF1A gene promoter was detected in 59 of 167 (35%) tumor tissues but not in salivary gland tissues (Fig. 1). The results of BSP and MSP were consistent
Figure 1. RASSF1A promoter methylation analyses in salivary adenoid cystic carcinoma and normal salivary gland tissues.
A. RASSF1A promoter methylation in case 4 was detected by bisulfite sequencing. Each circle indicates a CpG dinucleotide and each row indicates the sequencing result of one clone. Black and white dots indicate methylation and unmethylation of a CpG dinucleotide, respectively. There was no RASSF1A promoter methylation in normal tissue and partial promoter methylation in tumor tissue in case 4. B. MSP analysis of the promoter methylation status of RASSF1A in normal and tumor tissues. M indicates DNA amplified with methylation-specific primers; U, DNA amplified with unmethylation-specific primers; +, positive control; CM, blank control for methylation; CU, blank control for unmethylation.
LOH was detected in 9 of 50 (18%) tumors in the RASSF1A regions (Fig. 2).
Figure 2. Representative results of detection of LOH in salivary adenoid cystic carcinoma.
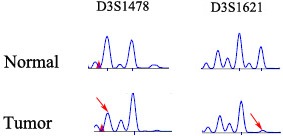
The markers D3S1478, D3S1621 flanking 3p21 regions were analyzed. Arrows indicate alleles that showed LOH in the tumor samples.
In the 50 cases in which both methylation and LOH were explored, methylation of the RASSF1A gene promoter was detected in 17 cases, with concurrent LOH of RASSF1A detected in six cases. Three cases with LOH of RASSF1A without methylation of the RASSF1A gene promoter were found. No association was identified between LOH and methylation of the RASSF1A gene promoter (P = 0.467).
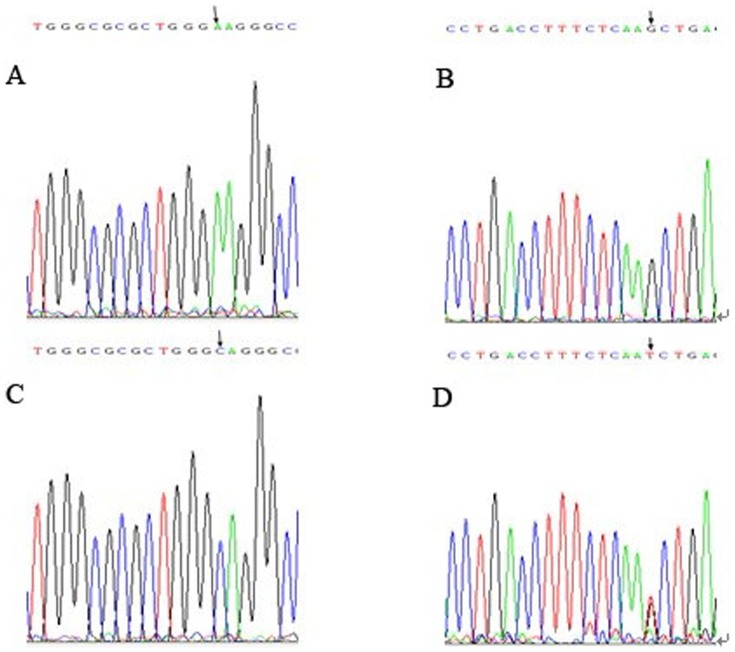
In 27 of 50 (54%) ACC tumors and matched salivary gland tissues and also in 13 of 20 (65%) peripheral blood cells from healthy donors, an identical transversion from A to C was found in exon 1 of the RASSF1A gene. This corresponds to a sequence change from AAG to CAG at codon 21 (Fig. 3), resulting in a lysine to glutamine amino acid substitution. Similarly, a sequence change from GCT to TCT at codon 133 (Fig. 3), resulting in an alanine to serine substitution, was identified in exon 3 of the RASSF1A gene in 5 of 50 (10%) ACC tumors and matched salivary gland tissues, and in 2 of 20 (10%) peripheral blood cells of healthy donors. No nucleotide alterations were found in exons 2, 4, 5 and 6 of the RASSF1A gene, either in salivary ACC patients or in healthy volunteers. No statistical differences in the nucleotide alterations in exon 1 and exon 3 between salivary ACC patients and healthy individuals (P = 0.560, P = 0.680, respectively) indicated that these changes were germline polymorphisms rather than somatic mutations. No somatic mutations were detected in any tumor or normal specimen.
Figure 3. Mutational analyses of RASSF1A in salivary adenoid cystic carcinoma.
A and B, Wild-type sequences. C and D, Polymorphisms at codon 21 (AAG to CAG) and codon 133 (GCT to TCT) were noted (arrow).
The association between the promoter methylation, LOH of RASSF1A and clinicopathological parameters
According to the results of MSP, RASSF1A promoter methylation was correlated with the solid histological pattern (P = 0.002) and advanced clinical stage (P = 0.014) (Table 1).
Table 1. Association between RASSF1A promoter hypermethylation status and clinicopathologic characteristics.
| Clinicopathologic characteristics | Methylation | P | ||
| Positive | Negative | |||
| Age | <60 y | 42 | 75 | 0.861 |
| ≥60 y | 17 | 33 | ||
| Sex | Male | 35 | 54 | 0.261 |
| Female | 24 | 54 | ||
| Location | Minor | 38 | 76 | 0.488 |
| Major | 21 | 32 | ||
| Histological pattern | Cribriform and tubular | 40 | 95 | 0.002 |
| Solid | 19 | 13 | ||
| Stage | I and II | 26 | 70 | 0.014 |
| III and IV | 33 | 38 | ||
| Neural/vascular invasion | Yes | 54 | 94 | 0.453 |
| No | 5 | 14 | ||
No correlation was identified between LOH of RASSF1A and the age (P = 0.442), and sex (P = 0.479) of patients, and the location (P = 0.127), histological pattern (P = 0.595), TNM stage (P = 0.281) and perineural/vascular invasion (P = 1.000) of the tumors.
Survival analysis
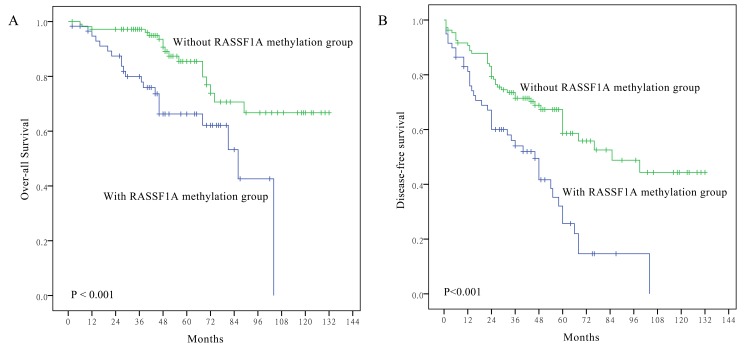
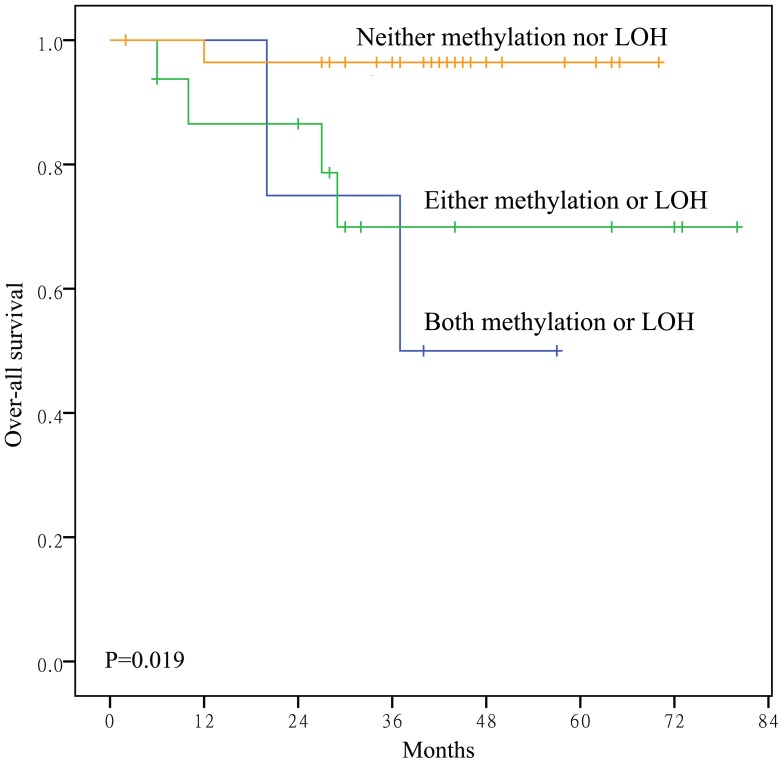
Survival curves were generated for all 167 salivary ACC cases. Methylation of the RASSF1A gene promoter was significantly associated with over-all survival (Log-rank test, P <0.001) and disease-free survival (Log-rank test, P <0.001) (Fig. 4). Based on RASSF1A methylation and LOH status, patients were divided into three groups: neither methylation nor LOH, either methylation or LOH, and both methylation and LOH. Statistically significant differences in over-all patient survival were identified among these three groups (Fig. 5) when analyzed with the Cox proportional hazards model. In univariate analyses, older patients (P <0.001), solid histological pattern (P <0.001), advanced TNM stage (P = 0.045) and RASSF1A promoter methylation (P = 0.001) were associated with poorer over-all survival (Table 2). Furthermore, older patients (P = 0.002), solid histological pattern (P = 0.004), advanced TNM stage (P = 0.045) and RASSF1A promoter methylation (P <0.001) were associated with worse disease-free survival (Table 3).
Figure 4. Kaplan–Meier survival curves according to RASSF1A promoter methylation status.
Patients with RASSF1A promoter methylation showed poorer over-all survival (A) (P <0.001) and disease-free survival (B) than those without (P <0.001).
Figure 5. Kaplan–Meier survival curves according to promoter methylation status and LOH of RASSF1A.
Patients with concurrent promoter methylation and LOH of RASSF1A showed the poorest over-all survival (P = 0.019) among the three groups.
Table 2. Summary of Cox model estimates for over-all survival in 167 patients with ACC.
| Variables | Hazard ratio | 95% Hazard ratio confidence limits | P |
| Univariate | |||
| Age <60 vs. ≥60 | 0.311 | 0.165–0.583 | <0.001 |
| Sex male vs. female | 1.529 | 0.803–2.913 | 0.196 |
| Location major vs. minor | 1.450 | 0.706–2.977 | 0.312 |
| Histological type c/t vs. s | 0.297 | 0.156–0.566 | <0.001 |
| TNM Stage I/II vs. III/IV | 0.522 | 0.277–0.985 | 0.045 |
| Margin negative vs. positive | 0.857 | 0.440–1.671 | 0.651 |
| Neural/vascular invasion positive vs. negative | 1.165 | 0.453–2.996 | 0.751 |
| Radiotherapy and/or chemotherapy yes vs. no | 0.963 | 0.437–2.118 | 0.924 |
| RASSF1A methylation positive vs. negative | 3.051 | 1.608–5.788 | 0.001 |
| Multivariate | |||
| Age <60 vs. ≥60 | 0.315 | 0.166–0.596 | <0.001 |
| Histological type c/t vs. s | 0.350 | 0.178–0.688 | 0.002 |
| TNM Stage I/II vs. III/IV | 0.822 | 0.417–1.618 | 0.570 |
| RASSF1A methylation positive vs. negative | 3.146 | 1.618–6.119 | 0.001 |
Table 3. Summary of Cox model estimates for disease-free survival in 167 patients with ACC.
| Variables | Hazard ratio | 95% Hazard ratio confidence limits | P |
| Univarate | |||
| Age <60 vs. ≥60 | 2.031 | 1.298–3.180 | 0.002 |
| Sex male vs. female | 0.727 | 0.467–1.131 | 0.158 |
| Location major vs. minor | 0.725 | 0.446–1.179 | 0.195 |
| Histological type c/t vs. s | 2.067 | 1.264–3.378 | 0.004 |
| TNM Stage I/II vs. III/IV | 1.562 | 1.009–2.417 | 0.045 |
| Margin negative vs. positive | 1.618 | 1.030–2.541 | 1.618 |
| Neural/vascular invasion positive vs. negative | 0.550 | 0.252–1.201 | 0.134 |
| Radiotherapy and/or chemotherapy yes vs. no | 1.079 | 0.640–1.816 | 0.776 |
| RASSF1A methylation positive vs. negative | 0.415 | 0.267–0.644 | <0.001 |
| Multivariate | |||
| Age <60 vs. ≥60 | 1.892 | 1.206–2.907 | 0.006 |
| Histological type c/t vs. s | 1.600 | 0.961–2.665 | 0.071 |
| TNM Stage I/II vs. III/IV | 1.136 | 0.715–1.807 | 0.589 |
| Margin negative vs. positive | 1.507 | 0.949–2.392 | 0.082 |
| RASSF1A methylation positive vs. negative | 0.434 | 0.275–0.684 | <0.001 |
To determine whether the association between RASSF1A promoter methylation and survival was independent of other parameters, multivariate analysis was performed in which patient age, histological type, TNM stage and RASSF1A promoter methylation were included as co-factors. Older patients (P <0.001), solid histological pattern (P = 0.002) and RASSF1A promoter methylation (P = 0.001) were independently associated with over-all survival (Table 2). Older patients (P = 0.006) and RASSF1A promoter methylation (P <0.001) were also independently associated with disease-free survival (Table 3).
Discussion
In this study, solid tumor pattern, later TNM stage and greater age were identified as predicators of poor outcome in salivary ACC patients, which is consistent with our previous report [6]. However, in this study, we investigated whether the genetic and epigenetic alterations of RASSF1A could be used in addition to the clinicopathological features as prognostic biomarkers in salivary ACC. One of the characteristics of our study was the combination of the BSP and MSP techniques to determine the methylation status of RASSF1A. BSP, which has served as the “gold standard” for determining DNA methylation, has the advantage that it allows the extent of methylation to be determined by detecting each CpG site. However, this approach is quite costly and time-consuming if a large number of cases and clones are sequenced [26]. MSP is a rapid and sensitive technique for assessing the methylation status in CpG islands and can be used for lower quantity and/or quality DNA samples [27], [28]. However, due to the experimental design and variables, MSP is associated with a high false positive and negative detection rate [29]. Therefore, in this study, we employed both BSP and MSP to evaluate DNA methylation; the results of the two techniques were consistent, indicating the reliability of our data.
Our previous report describing the analysis of 60 salivary ACC from our 1999–2002 cohort showed that RASSF1A gene promoter methylation was correlated with the grade and the TNM stage of salivary ACC [9]. In the current study, we used a larger sample group (167 cases) from our 2003–2008 data to further confirm and investigate the role of RASSF1A in ACC tumors. RASSF1A promoter methylation was detected in 35% of salivary ACC tumors in this study by BSP and MSP, which is consistent with our earlier findings [9]. Our study indicated that the methylation ratio of RASSF1A in ACC was lower than in many other tumors, such as the primary lung cancer (88%) [30], breast carcinoma (65%–95%) [31], [32], [33] and colorectal carcinoma(52%) [21]. However, the fact that gene methylation was associated with solid tumor pattern and advanced ACC stage suggested that the RASSF1A gene may be particularly important in cell differentiation and tumor progression in ACC. Similar conclusions have been reported in bladder cancer [34] and Wilms' tumor [35].
According to the two-hit theory, biallelic abnormalities of tumor suppressor genes can result in complete inactivation of the gene and promote tumorigenesis. The protein encoded by RASSF1A modulates a broad range of cellular functions. Although RASSF1A can be inactivated by LOH or point mutation, the most common contributor to loss or reduction of RASSF1A function is the transcriptional silencing of the gene by inappropriate promoter methylation [36]. Our results confirmed that promoter methylation was the most common (35%) molecular abnormality of the RASSF1A gene in salivary ACC. Additionally, LOH (9/50, 18%) was involved in RASSF1A gene inactivation in some ACC tumors. The two-hit phenomenon, which is constituted by RASSF1A gene promoter methylation and LOH at 3p21 was detected in six ACC samples (6/50, 12%). The relatively lower chance of the two-hit phenomenon in the RASSF1A gene has also been reported by Choi et al. and Yu et al. in cervical cancers [22], [23].
Somatic mutation of the RASSF1A gene has rarely been reported in human cancers. Only 9.5% of primary nasopharyngeal carcinomas [37] and 10% of lung tumors [11] carry missense mutations. In this study, no somatic mutations were detected by sequencing all six exons of the RASSF1A gene for the 50 matched cancerous and noncancerous tissues. However, a single nucleotide germline polymorphism at codon 133 of exon 3 was detected in five of the tumor tissues. This polymorphism has also been reported in cervical cancer [23], nasopharyngeal cancer [37]. Moreover, in many cases (27/50, 54%) of in this study, a single germline polymorphism was detected at codon 21 of exon 1, which has not been reported in other tumors.
As a characteristic tumor, there are many reports [7], [8] describing the association of the clinicopathological features of salivary ACC and the prognosis of patients. It is equally important to determine characteristic molecular abnormalities that represent prognostic molecular biomarkers in ACC patients. Our study confirmed the role of clinicopathological parameters such as solid tumor pattern, later TNM stage and greater age in predicting the outcome of ACC patients. Our study is the first to report a correlation between RASSF1A gene promoter methylation and outcome in salivary ACC patients. In our data, RASSF1A promoter methylation was a strong predicator of disease-free survival and over-all survival in patients with salivary ACC, both in univariate and multivariate survival analyses. RASSF1A gene promoter methylation has also been reported to confer poorer survival in surgically treated NSCLC [15], breast cancer [19], [33], stage I and II lung cancer [38] and Wilms' tumor [35]. Our findings provide evidence of the potential usefulness of RASSF1A promoter methylation as an informative prognostic biomarker in patients with ACC.
Our study also demonstrated the potential of promoter methylation and LOH of RASSF1A as molecular markers to predict salivary ACC patient outcome. Survival differences were compared among the 50 cases following allocation to three groups according to RASSF1A methylation and LOH status. Patients with concurrent RASSF1A methylation and LOH at 3p21 had the poorest prognosis compared to patients with either RASSF1A methylation or LOH at 3p21 and those with neither RASSF1A methylation nor LOH at 3p21. This suggested that inactivation of both RASSF1A alleles had the greatest influence on tumor progression, whereas partial RASSF1A gene function is provided by one wild-type allele. However, it should be noted the sample size for detection of LOH of RASSF1A in this study was relatively small; therefore, LOH frequency in ACC may be biased. Further studies are necessary to determine the roles of RASSF1A LOH in ACC.
In summary, the present study indicated that DNA promoter methylation is the most common molecular abnormity of the RASSF1A gene in salivary ACC. Moreover, RASSF1A gene promoter methylation may play a significant role in ACC carcinogenesis progression and may be a reliable prognostic biomarker of poor patient survival. Further studies on the correlation between abnormalities in the RASSF1A gene and protein expression as well as interactions with other genes in salivary ACC are therefore warranted.
Materials and Methods
Ethics statement
This study was approved by the Human Research Ethics Committee of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from all patients.
Tissue samples
One hundred and sixty-seven salivary ACC tumors and 50 samples of matched normal salivary gland tissues from the same patients were collected from ACC surgical patients at the Shanghai 9th People's Hospital, Shanghai Jiao Tong University, Shanghai, China, from 2003 to 2008. All samples were fixed in formalin and embedded in paraffin. The tissue sections (4 µm) were stained with hematoxylin and eosin and all cases were reviewed by two pathologists to confirm the diagnosis. For cases with non typical morphology, the diagnosis was based primarily on immunohistochemical staining of CK7 (Gene Tech, USA), CK8 (Gene Tech, USA), c-kit(Gene Tech, USA), S-100(Gene Tech, USA), SMA (Gene Tech, USA), Calp (Gene Tech, USA), MYB(abcam, UK) and fluoresence in situ hybridization (FISH) for MYB using a dual-color MYB break-apart probe (Zytovision, German).
The histological pattern of each tumor was determined according to the histological typing of salivary gland tumors defined by the World Health Organization [39], and the ACC tumors were subclassified with cribriform, tubular and solid patterns. When more than 30% of tumor had a solid component, it was classified as having a solid histological pattern. Tumors were staged in accordance with the AJCC cancer staging criteria based on clinical findings and preoperative imaging [40].
To identify the most frequently occurring methylation sites of the CpG islands located in the RASSF1A gene in ACC tumors, 15 tumors and their matched normal tissues were selected at random from the 167 cases and subjected to bisulfite sequencing PCR (BSP). The primer pairs for methylation-specific PCR (MSP) were designed according to the results of bisulfite sequencing. In many cases, the normal tissues were not available; therefore, 167 ACC tumors and 50 matched normal tissues were subjected to MSP analysis.
Tumor tissue and matched normal tissue are required for LOH and mutation analysis; therefore, of the 167 cases, only those 50 cases for which the tumor tissues and matched normal tissues were available were analyzed for DNA methylation, LOH and mutations of the RASSF1A gene. DNA extracted from peripheral blood cells of 20 healthy volunteers was used as a normal control for DNA polymorphism evaluation of the germline cells.
DNA extraction
DNA was extracted from formalin-fixed, paraffin-embedded tissues using the QIAamp DNA FFPE Tissue kit (Qiagen) according to the manufacturer's instructions.
Methylation analysis by bisulfite sequencing PCR (BSP) and methylation-specific PCR (MSP)
For BSP, genomic DNA was modified by sodium bisulfite treatment and purified using EpiTect Bisulfite Kit (Qiagen) according to the recommendations of the manufacturer. The sequences of the primers and the amplification products, which included 16 CpG islands of the promoter region of RASSF1A gene, are listed in Table S1 in File S1. The amplified fragments were subcloned. Six clones of each sample were selected for sequencing. The 16 CpG islands were screened for hot-spot methylation sites.
For MSP, genomic DNA was modified by sodium bisulfite treatment. The detailed sequences of primers are listed in Table S1 in File S1. The PCR amplification was performed in a 12.5 µl reaction volume with HotStar Taq DNA polymerase (Qiagen) according to the manufacturer's instructions. Water was substituted for DNA in the blank control. DNA extracted from normal lymphocytes and treated with SssI methylase (New England Biolabs, Beverly, MA, USA) was used as the positive control. The PCR products were subjected to electrophoresis on 2% agarose gels, stained with ethidium bromide, and visualized directly under ultraviolet illumination.
LOH assay
For LOH analysis, two polymorphic microsatellite markers (D3S1478, D3S1621) encompassing the chromosomal region 3p21 were evaluated in 50 tumor tissues and their matched normal salivary gland tissues using fluorescent dye-labeled specific primers (the primer sequences, annealing temperature and estimated product size for each primer pair are available in the National Center for Biotechnology Information UniSTS database). The amplified PCR products for multiple loci were detected with an ABI 3730 Genetic Analyzer (Applied Biosystems) and analyzed with GeneMapper software (version 4.0) and Peak Scanner Software (version 1.0). A given informative marker was considered to display LOH when a ≥1.5-fold difference was detected in the relative allele height ratio between the tumor and the normal tissues. LOH was considered to have occurred in tumors with locus loss both upstream and downstream of the RASSF1A gene.
Mutation analysis by PCR and direct sequencing
To determine the presence of mutation in tissues, all six exons of the RASSF1A gene were amplified using six primer pairs (sequences are listed in Table S1 of File S1). PCR was carried out in 25 µl reaction volume with TaKaRa Ex Taq (TaKaRa Biotechnology). For exons 2, 5 and 6, three-step PCR was used, and for exons 1, 3 and 4, touch-down PCR was adopted. All PCR products were subjected to direct sequencing using an ABI 3730XL Sequencer (Applied Biosystems).
Follow-up information
The follow-up information of the 167 cases was collected from 2 to 132 months (mean 55 months). At the end of the follow-up, 40 patients (24%) had died (37 died of ACC and three died of other causes) and 127 patients (76%) were still alive. Sixty-six patients (40%) showed distant metastases (of lungs, liver, bone and brain) and 23 patients (14%) showed local recurrences of the disease.
Statistical analysis
χ2 or Fisher exact tests were used to study the association between the categorical parameters and the clinicopathological parameters. Time-to-event analysis involved a log-rank testing of a Kaplan-Meier curve. The association between clinicopathological factors and patient outcome was estimated by a Cox proportional hazards regression model. The statistical analyses were performed with the software system of SPSS version 13.0. A 2-tailed P-value ≤0.05 was regarded as statistically significant.
Supporting Information
Table S1. PCR primer sequences for bisulfite sequencing, MSP and mutation. Table S2. Clinicopathological details of 167 patients with ACC and promoter methylation, LOH of RASSF1A in tumor tissues.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: 1. The Natural Science Fund of China (Nos. 81372910, 81302360) to JL and CYZ (http://isisn.nsfc.gov.cn/egrantindex/funcindex/prjsearch-list); 2. The Research Fund for the Doctoral Program of Ministry of Education of China (No. 20120073110085) to JL (http://www.cutech.edu.cn/cn/zxgz/2012/12/1354173228992896.htm); and 3. Youth Research Foundation of Shanghai Municipal Health Bureau (20134Y057) (http://www.wsjsw.gov.cn/wsj/index.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tian Z, Li L, Wang L, Hu Y, Li J (2009) Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg 39: 235–242. [DOI] [PubMed] [Google Scholar]
- 2. Li J, Wang BY, Nelson M, Li L, Hu Y, et al. (2004) Salivary adenocarcinoma, not otherwise specified: a collection of orphans. Arch Pathol Lab Med 128: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 3. Rapidis AD, Givalos N, Gakiopoulou H, Faratzis G, Stavrianos SD, et al. (2005) Adenoid cystic carcinoma of the head and neck. Clinicopathological analysis of 23 patients and review of the literature. Oral Oncol 41: 328–335. [DOI] [PubMed] [Google Scholar]
- 4. Nascimento AG, Amaral AL, Prado LA, Kligerman J, Silveira TR (1986) Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer 57: 312–319. [DOI] [PubMed] [Google Scholar]
- 5. Spiro RH, Huvos AG, Strong EW (1979) Adenoid cystic carcinoma: factors influencing survival. Am J Surg 138: 579–583. [DOI] [PubMed] [Google Scholar]
- 6. Zhang CY, Xia RH, Han J, Wang BS, Tian WD, et al. (2013) Adenoid cystic carcinoma of the head and neck: clinicopathologic analysis of 218 cases in a Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol 115: 368–375. [DOI] [PubMed] [Google Scholar]
- 7. Shen C, Xu T, Huang C, Hu C, He S (2012) Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol 48: 445–449. [DOI] [PubMed] [Google Scholar]
- 8. Gondivkar SM, Gadbail AR, Chole R, Parikh RV (2011) Adenoid cystic carcinoma: a rare clinical entity and literature review. Oral Oncol 47: 231–236. [DOI] [PubMed] [Google Scholar]
- 9. Li J, El-Naggar A, Mao L (2005) Promoter methylation of p16INK4a, RASSF1A, and DAPK is frequent in salivary adenoid cystic carcinoma. Cancer 104: 771–776. [DOI] [PubMed] [Google Scholar]
- 10. Karray-Chouayekh S, Trifa F, Khabir A, Boujelbane N, Sellami-Boudawara T, et al. (2010) Aberrant methylation of RASSF1A is associated with poor survival in Tunisian breast cancer patients. J Cancer Res Clin Oncol 136: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dammann R, Li C, Yoon JH, Chin PL, Bates S, et al. (2000) Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 25: 315–319. [DOI] [PubMed] [Google Scholar]
- 12. Dreijerink K, Braga E, Kuzmin I, Geil L, Duh FM, et al. (2001) The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21.3 is involved in kidney tumorigenesis. Proc Natl Acad Sci U S A 98: 7504–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang CY, Mao L, Li L, Tian Z, Zhou XJ, et al. (2007) Promoter methylation as a common mechanism for inactivating E-cadherin in human salivary gland adenoid cystic carcinoma. Cancer 110: 87–95. [DOI] [PubMed] [Google Scholar]
- 14. Agathanggelou A, Cooper WN, Latif F (2005) Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res 65: 3497–3508. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Wang B, Chen X, Bi J (2011) The prognostic value of RASSF1A promoter hypermethylation in non-small cell lung carcinoma: a systematic review and meta-analysis. Carcinogenesis 32: 411–416. [DOI] [PubMed] [Google Scholar]
- 16. Saito K, Kawakami K, Matsumoto I, Oda M, Watanabe G, et al. (2010) Long interspersed nuclear element 1 hypomethylation is a marker of poor prognosis in stage IA non-small cell lung cancer. Clin Cancer Res 16: 2418–2426. [DOI] [PubMed] [Google Scholar]
- 17. Buckingham L, Penfield Faber L, Kim A, Liptay M, Barger C, et al. (2010) PTEN, RASSF1 and DAPK site-specific hypermethylation and outcome in surgically treated stage I and II nonsmall cell lung cancer patients. Int J Cancer 126: 1630–1639. [DOI] [PubMed] [Google Scholar]
- 18.Gobel G, Auer D, Gaugg I, Schneitter A, Lesche R, et al.. (2011) Prognostic significance of methylated RASSF1A and PITX2 genes in blood- and bone marrow plasma of breast cancer patients. Breast Cancer Res Treat. [DOI] [PubMed]
- 19. Jiang Y, Cui L, Chen WD, Shen SH, Ding LD (2012) The Prognostic Role of RASSF1A Promoter Methylation in Breast Cancer: A Meta-Analysis of Published Data. PLoS One 7: e36780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miranda E, Destro A, Malesci A, Balladore E, Bianchi P, et al. (2006) Genetic and epigenetic changes in primary metastatic and nonmetastatic colorectal cancer. Br J Cancer 95: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliveira C, Velho S, Domingo E, Preto A, Hofstra RM, et al. (2005) Concomitant RASSF1A hypermethylation and KRAS/BRAF mutations occur preferentially in MSI sporadic colorectal cancer. Oncogene 24: 7630–7634. [DOI] [PubMed] [Google Scholar]
- 22. Choi CH, Lee KM, Choi JJ, Kim TJ, Kim WY, et al. (2007) Hypermethylation and loss of heterozygosity of tumor suppressor genes on chromosome 3p in cervical cancer. Cancer Lett 255: 26–33. [DOI] [PubMed] [Google Scholar]
- 23. Yu MY, Tong JH, Chan PK, Lee TL, Chan MW, et al. (2003) Hypermethylation of the tumor suppressor gene RASSFIA and frequent concomitant loss of heterozygosity at 3p21 in cervical cancers. Int J Cancer 105: 204–209. [DOI] [PubMed] [Google Scholar]
- 24. Riquelme E, Tang M, Baez S, Diaz A, Pruyas M, et al. (2007) Frequent epigenetic inactivation of chromosome 3p candidate tumor suppressor genes in gallbladder carcinoma. Cancer Lett 250: 100–106. [DOI] [PubMed] [Google Scholar]
- 25. Pan ZG, Kashuba VI, Liu XQ, Shao JY, Zhang RH, et al. (2005) High frequency somatic mutations in RASSF1A in nasopharyngeal carcinoma. Cancer Biol Ther 4: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 26. Reed K, Poulin ML, Yan L, Parissenti AM (2010) Comparison of bisulfite sequencing PCR with pyrosequencing for measuring differences in DNA methylation. Anal Biochem 397: 96–106. [DOI] [PubMed] [Google Scholar]
- 27. Ku JL, Jeon YK, Park JG (2011) Methylation-specific PCR. Methods Mol Biol 791: 23–32. [DOI] [PubMed] [Google Scholar]
- 28. Licchesi JD, Herman JG (2009) Methylation-specific PCR. Methods Mol Biol 507: 305–323. [DOI] [PubMed] [Google Scholar]
- 29. Warnecke PM, Stirzaker C, Song J, Grunau C, Melki JR, et al. (2002) Identification and resolution of artifacts in bisulfite sequencing. Methods 27: 101–107. [DOI] [PubMed] [Google Scholar]
- 30. Grote HJ, Schmiemann V, Geddert H, Bocking A, Kappes R, et al. (2006) Methylation of RAS association domain family protein 1A as a biomarker of lung cancer. Cancer 108: 129–134. [DOI] [PubMed] [Google Scholar]
- 31. Shinozaki M, Hoon DS, Giuliano AE, Hansen NM, Wang HJ, et al. (2005) Distinct hypermethylation profile of primary breast cancer is associated with sentinel lymph node metastasis. Clin Cancer Res 11: 2156–2162. [DOI] [PubMed] [Google Scholar]
- 32. Yeo W, Wong WL, Wong N, Law BK, Tse GM, et al. (2005) High frequency of promoter hypermethylation of RASSF1A in tumorous and non-tumourous tissue of breast cancer. Pathology 37: 125–130. [DOI] [PubMed] [Google Scholar]
- 33. Buhmeida A, Merdad A, Al-Maghrabi J, Al-Thobaiti F, Ata M, et al. (2011) RASSF1A methylation is predictive of poor prognosis in female breast cancer in a background of overall low methylation frequency. Anticancer Res 31: 2975–2981. [PubMed] [Google Scholar]
- 34. Kim JS, Chae Y, Ha YS, Kim IY, Byun SS, et al. (2012) Ras Association Domain Family 1A: A Promising Prognostic Marker in Recurrent Nonmuscle Invasive Bladder Cancer. Clin Genitourin Cancer 10: 114–120. [DOI] [PubMed] [Google Scholar]
- 35.Ohshima J, Haruta M, Fujiwara Y, Watanabe N, Arai Y, et al.. (2012) Methylation of the RASSF1A promoter is predictive of poor outcome among patients with Wilms tumor. Pediatr Blood Cancer. [DOI] [PubMed]
- 36. Donninger H, Vos MD, Clark GJ (2007) The RASSF1A tumor suppressor. J Cell Sci 120: 3163–3172. [DOI] [PubMed] [Google Scholar]
- 37. Lo KW, Kwong J, Hui AB, Chan SY, To KF, et al. (2001) High frequency of promoter hypermethylation of RASSF1A in nasopharyngeal carcinoma. Cancer Res 61: 3877–3881. [PubMed] [Google Scholar]
- 38. de Fraipont F, Levallet G, Creveuil C, Bergot E, Beau-Faller M, et al. (2012) An Apoptosis Methylation Prognostic Signature for Early Lung Cancer in the IFCT-0002 Trial. Clin Cancer Res 18: 2976–2986. [DOI] [PubMed] [Google Scholar]
- 39.EI-Naggar A, Huvos A (2005) Adenoid cystic carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and genetics of Head and Neck Tumors. Lyon: IARC Press. pp. 221–222. [Google Scholar]
- 40.Ellis GL AP (2008).Tumors of the salivary glands. AFIP atlas of tumor pathology. Maryland:ARP Press.36 p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PCR primer sequences for bisulfite sequencing, MSP and mutation. Table S2. Clinicopathological details of 167 patients with ACC and promoter methylation, LOH of RASSF1A in tumor tissues.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.