Abstract
Retinal gene therapy with adeno-associated viral (AAV) vectors is safe and effective in humans. However, the limited cargo capacity of AAV prevents their use for therapy of those inherited retinopathies (IRs) due to mutations in large (>5kb) genes. Viral vectors derived from Adenovirus (Ad), Lentivirus (LV) and Herpesvirus (HV) can package large DNA sequences but do not target efficiently retinal photoreceptors (PRs) where the majority of genes responsible for IRs are expressed.
Here, we have evaluated the mouse retinal transduction profiles of vectors derived from 16 different Ad serotypes, 7 LV pseudotypes, and from a bovine HV. Most of the vectors tested transduced efficiently the retinal pigment epithelium (RPE). We found that LV-GP64 tends to transduce more PRs than the canonical LV-VSVG albeit this was restricted to a narrow region. We observed more extensive PR transduction with HdAd1, 2 and 5/F35++ than with LV, although none of them outperformed the canonical HdAd5 or matched the extension of PR transduction achieved with AAV2/8.
Keywords: retina, adenoviral vectors, lentiviral vector
INTRODUCTION
Gene therapy has great potential for treatment for many inherited retinopathies (IRs), which are mostly monogenic blinding diseases. Viral vectors based on adeno-associated viruses (AAV) have demonstrated to be safe and efficient in clinical trials for Leber Congenital Amaurosis type 2 (LCA2) (reviewed in 1). LCA2 is an IR due to mutations in RPE65, a gene expressed in the retinal pigment epithelium (RPE)1. Most of the genes mutated in IRs are expressed in photoreceptors (PRs)2. Notably, AAV serotypes 5, 7, 8 and 9 target PRs of various species efficiently3-7. However, the cloning capacity of AAV vectors is close to its wild-type genome length (~4.7kb) and this represents a limitation for gene therapies of several IRs due to defects in larger genes, such as Usher1B (USH1B, OMIM entry #276900), Stargardt disease 1 (STGD1, OMIM entry #248200), and LCA10 (OMIM entry #611755). Moreover, AAV limited capacity may preclude the delivery of cassettes with large promoters that maintain transgene expression levels close to physiological as well as cell type specificity.
Viral vectors that transduce post-mitotic cells with transgene capacity higher than AAVs include adenovirus (Ad, with a wild-type genome length of ~36kb), lentivirus (LV, with a wild-type genome length of ~9kb), and herpesvirus (HV, with a wild-type genome length of ~150kb). The retinal transduction potential of each of them has been evaluated in vivo in animal models: recombinant vectors based on Ad5, the most studied Ad serotype in the context of gene therapy, transduce the RPE primarily when injected subretinally in the mouse retina8-10. LV vectors are enveloped and have been pseudotyped predominantly with the vesicular stomatitis viral glycoprotein (VSVG) since it confers tropism for a broad range of tissues11. The LV-VSVG whether based on the human immunodeficiency virus (HIV-1)12-15, simian (SIV)16-17 or feline immunodeficiency viruses (FIV)18-19 transduces the RPE mainly when delivered in adult retinas. LV-VSVG vectors based on the equine infectious anaemia virus (EIAV) have been reported to transduce PRs efficiently20. However, LV transduction appears mostly limited to PRs in the newborn retina21-22 which has a less compact structure than the adult retina23-24. Only a few reports are available on retinal transduction mediated by HV vectors based on Herpes simplex 1 (HSV-1) which is mostly limited to the RPE following subretinal injection25. Thus, none of the high-capacity viral vectors tested so far transduce PRs efficiently.
Here, we aimed at identifying high-capacity Ad, LV and HV vectors with more robust PR transduction efficiency than those described so far.
Evolution has modeled the innate ability of viruses to deliver genes to a cell. Ads are divided into subgroups (A to F) and recognize different cellular receptors26. In addition, since Ad entry requires high-affinity binding to cell receptors via the knob portion of the fiber, the Ad capsid can be genetically engineered to exchange surface proteins such as the knob (K) or fiber (F) among different serotypes or subgroups, potentially giving rise to modified tropism. Previous works reported that a modified Ad5 (Ad5ΔRGD27-28) and Ad5-based vectors containing heterologous fibers (Ad5/F3729 and Ad5/F3530-31) are efficient for PR targeting. In this work, in addition to naturally-occurring heterologous Ad capsids, we have analyzed PR transduction by 8 vectors based on Ad5 mutant capsids32-38 (Table 1).
Table 1. Viral vectors based on Adenovirus (Ad and HdAd), Lentivirus (LV) or Herpesvirus (HV) evaluated in this study.
| Vector | Subgroup | Reference | |
|---|---|---|---|
|
Ad naturally-occurring
n=8 |
HdAd1 | C | 75 |
| HdAd2 | C | 74 | |
| HdAd5 | C | 73 | |
| Ad6 | C | ATCC® VR-6™ | |
| AdC1 | B | ATCC® VR-20™ | |
| ChAd7 | E | 42 | |
| ChAd30 | B | 42 | |
| ChAd63 | E | 42 | |
|
Ad genetically-modified
n=8 |
External surface protein | ||
| HdAd5/F35 | Ad35 fiber cloned in an Ad5 context | 33 | |
| HdAd5/F35++ | Mutant Ad35 fiber with increased affinity for the CD46 ligand in an Ad5 context | 35 and this publication | |
| Ad5/F2+pK | Heptalysin tract incorporation in the H-I loop of Ad2 fiber cloned in an Ad5 context | 36 | |
| Ad5/F2+RGD | RGD-motif incorporation in the H-I loop of Ad2 fiber cloned in an Ad5 context | 36 | |
| Ad5ΔRGD | RGD-motif deleted from penton base proteins of Ad5 capsid | 37 | |
| Ad5/F3 | Fiber from Ad3 cloned in an Ad5 context | Generous gift from David T. Curiel, 34 | |
| HdAd5/K3 | Ad3 knob cloned in an Ad5 context | 32 | |
| HdAd5/6 | Ad6 hexon hypervariable regions in Ad5 capsid | 38 | |
|
LV
n=7 |
VSVG | Vesicular Stomatitis Virus Glycoprotein | 85 |
| JSRV | Jaagsiekte Sheep Retrovirus | 86 | |
| RRV | Ross River Virus | 87 | |
| EBOLAΔO | Ebola mucin domain-deleted glycoprotein | 77 | |
| LCMV | Lymphocytic Choriomeningitis Virus | 85 | |
| MOKOLA | Mokola virus envelope glycoprotein | 13 | |
| GP64 | Baculovirus GP64 envelope glyprotein | 88 | |
| HV, n=1 | BoHV-4 | Bovine herpesvirus type 4 | 55 |
On the other side, heterologous glycoproteins11 can be easily exchanged among enveloped LV thus generating pseudotyped LV vectors. These may have different transduction properties than vectors with the native envelope glycoproteins. Indeed, it has been shown that HIV may be redirected to the endocytic pathway from its normal route of entry when pseudotyped with VSVG39. Thus, we have tested 7 HIV-1 based LV vectors pseudotyped with various non-native envelope glycoproteins (Table 1), seeking for improved PR transduction.
HV vectors present the highest cloning capacity and naturally infect neurons. They are enveloped, but the attachment and entry processes are complex and not fully understood yet, resulting in few pseudotypes available40. Here we have evaluated the ability of a naturally-occurring bovine herpes virus (Table 1), which belongs to a different subfamily (gammaherpesviridae) than HSV-1 (alphaherpesviridae) that was previously investigated in the retina25.
RESULTS
We evaluated a total of 16 Ad vectors (8 derived from human and chimpanzee naturally-occurring Ad and 8 genetically-modified Ad vectors) for PR transduction after subretinal delivery in the adult mouse retina (see Table 1). The ubiquitous cytomegalovirus (CMV) promoter was included in all vectors to investigate broad cellular tropism on retinal histological sections. The vectors contained either EGFP or β-galactosidase (lacZ) as reporter genes, and were subretinally injected in pigmented C57BL/6 or in albino CD-1 or BALB/c mice. Ad vectors were injected at doses ranging from 106 to 109 viral particles (vp)/eye. Depending on the availability, we used either first-generation (Ad, E1/E3-deleted) or helper-dependent adenoviral vectors (HdAd, deleted of all viral genes and containing only the cis sequences required for vector genome encapsidation41), as indicated in Table 1. Because we were interested in vector tropism and eyes were harvested 3-14 days after vector injection, HdAd and Ad can be directly compared for transduction efficiency. This comparison is possible given the rapid onset of transgene expression of Ad vectors, in contrast to AAV vectors. Retinal sections were analyzed for direct EGFP fluorescence or after X-gal staining. We tested the serotype 1 HdAd (HdAd1), serotype 2 HdAd (HdAd2), and serotype 6 Ad (Ad6) vectors from subgroup C that recognize the CAR receptor for initial attachment, followed by internalization through an interaction between the RGD motif in the Ad penton base with αvβ3-5 integrins26. The genetically modified HdAd5/F35, HdAd5/F35++, Ad5/F3 and HdAd5/K3 contain fiber or knobs from serotypes reported to recognize the cellular CD46 receptor26. Vectors derived from Chimpanzee viruses were classified into subgroup B (AdC1 and ChAd30) or subgroup E (ChAd7 and 63) based on sequence alignments of the hypervariable regions of the hexon gene42. Notably, the Ad receptors CAR, αv-integrin and CD46 are expressed by PRs30
Consistent with previous published studies8-10, we found that Ad5 targets mostly the RPE and Müller cells along with some PRs following subretinal injection (Figure 1 and Figure 2). Similar to Ad5, most of the Ad vectors tested targeted efficiently the RPE and some PRs (Figure 1 and Figure 2). However, HdAd1, HdAd2 and HdAd5/F35++ transduced the outer nuclear layer (ONL), which contains PRs, at levels that in some eyes appeared higher and more extensive than with HdAd5 (see Figure 1 and Table 1 for the vector descriptions). No β-galactosidase staining was detected in the retinas of control PBS-injected animals (data not shown).
Figure 1. Mouse retinal transduction after subretinal delivery of adenoviral vectors.
Ad vectors containing the CMV-lacZ cassette were delivered subretinally in adult CD-1, BALB/c or C57BL/6 mice. Retinas injected with Ad vectors were analyzed 4-14 days later. Magnification=20×; scale bar=100μm. The vector serotypes used are indicated above each panel. The n of retinas, the dose of each vector and the relative transduction observed in photoreceptors, retinal pigment epithelium and Müller cells are indicated below each panel. Abbreviations: rpe: retinal pigment epithelium; onl: outer nuclear layer; inl: inner nuclear layer, gcl: ganglion cell layer; n: number of eyes; vp: viral particles; PR: photoreceptors; NT: not transduced.
Figure 2. Mouse retinal transduction after subretinal delivery of adenoviral vectors.
Ad vectors containing the CMV-EGFP cassette were delivered subretinally in adult C57BL/6 mice. Retinas were analyzed 3-6 days later. Magnification=20×; scale bar=50μm. The vector serotypes used are indicated above each panel. The n of retinas, the dose of each vector and the relative transduction observed in photoreceptors, retinal pigment epithelium and Müller cells are indicated below each panel. Abbreviations: rpe: retinal pigment epithelium; onl: outer nuclear layer; inl: inner nuclear layer, gcl: ganglion cell layer; n: number of eyes; vp: viral particles; PR: photoreceptors; NT: not transduced.
To confirm that HdAd1, HdAd2 and HdAd5/F35++ transduce adult mouse PR efficiently as it appears using the CMV-lacZ expression cassette, we produced HdAd vectors containing the PR-specific rhodopsin (Rho) promoter which drives the expression of the EGFP reporter gene. The HdAd vectors were all injected subretinally in C57BL/6 mice at a dose of 5.5×108 genome copies (GC)/eye, and compared to HdAd5 and AAV2/8, containing the same expression cassette and injected at the same dose. Eyes were harvested 14 days or one month later and retinas cryosectioned for further histological analysis. HdAd1, HdAd2 and HdAd5/F35++ showed an extension of PR transduction which was lower than with HdAd5, which was similar to that of AAV2/8 (see Figure 3a). The pattern of PR transduction mediated by HdAd1 and 2 was patchy, as observed in a previous work using a different Ad vector containing a Rho-EGFP cassette28. HdAd5-mediated PR transduction appeared patchy in some eyes, and continuous in others. In Figure 3a and b, the four best transduced retinas with each serotype are shown. HdAd1 and 2 seemed to transduce PRs more intensely than HdAd5, although along a narrower area.
Figure 3. Mouse photoreceptor transduction after subretinal delivery of HdAd5, HdAd1, HdAd2, HdAd5/F35++ and AAV2/8.
Vectors expressing EGFP from the PR-specific rhodopsin promoter where injected subretinally in adult C57BL/6 mice (5.5×108 GC/eye), and analyzed 14 days or one month post injection. a) Montage of whole retinal cross-sections from the four best transduced eyes for each serotype. The extension of PR transduction is indicated by arrows. The n of retinas showing a transduction pattern similar to those shown in the pictures are n=8/12 for AAV2/8, n=6/8 for HdAd5, n=8/9 for HdAd1, n=6/9 for HdAd2 and n=5/8 for HdAd5/F35++. In the remaining retinas, the ONL transduction was either significantly lower than in the retinas shown in the pictures or undetectable. Magnification=10×. b) High-magnification images of the retinas shown in a). Magnification: 40×; scale bar=25μm. A faint, off-target EGFP signal is observed in the RPE of all retinas (arrows). Abbreviations: rpe: retinal pigment epithelium; onl: outer nuclear layer; inl: inner nuclear layer; gcl: ganglion cell layer.
LV vectors have a larger cloning capacity compared to AAVs. However, VSVG-pseudotyped LV vectors efficiently transduced newborn14-15 but not adult PRs12-15. Here, we have evaluated six HIV-1-based vectors pseudotyped with various envelopes (Table 1) and compared them to the more studied VSVG. In previous works, the mokola pseudotype was shown to transduce RPE13,43 after subretinal delivery, and RRV to transduce the RPE of Sprague-Dawley rats44.
LCMV and GP64 pseudotyped vectors transduced the RPE of Wistar rats, although the vectors were based on a simian (SIV) and bovine immunodeficiency viruses (BIV), respectively45-46. To our knowledge, neither the EbolaΔO nor the JSRV envelopes had been previously tested in the retina. All vectors contained the CMV-EGFP expression cassette, they were injected subretinally in C57BL/6 mice and retinas were harvested 14 days later. The injected doses range between 2×103 and 1×105 transducing units (TU)/eye (Figure 4) and they were the maximum allowed for each viral preparation given that 1 μl is the maximum volume that can be delivered subretinally in adult mice47. All vectors, excluding JSRV, transduced the RPE (Figure 4). The baculovirus GP64-pseudotyped LV (6×104 TU/eye) appeared to drive a more robust PR transduction (Figure 4, see inset) than the standard VSVG (1×105 TU/eye), which transduced PRs in one eye out of five (not shown). However, the transduction was restricted to a narrow area. To evaluate whether LV-GP64 drives PR transduction to levels that can be attractive for therapeutic applications, we generated LV-GP64 vectors containing the Rho-EGFP expression cassette. C57BL/6 retinas were subretinally injected with 4.5×105 GC/eye of either LV-GP64 or -VSVG vectors for comparison and analyzed at 2 weeks post-injection. No EGFP fluorescence was observed with either vector (not shown).
Figure 4. Mouse retinal transduction after subretinal administration of LV vectors.
LV vectors containing the CMV-EGFP expression cassette were injected subretinally in adult C57BL/6 mice, and retinas were analyzed 2 weeks post injection. The dose used was the maximum possible for each LV vector based on the prep titers. The n of retinas, the dose of each vector and the relative transduction observed in photoreceptors and retinal pigment epithelium are indicated below each panel. RPE was the only target of most LV pseudotypes but LV-GP64, which transduced some photoreceptors (see inset). Abbreviations: rpe: retinal pigment epithelium; onl: outer nuclear layer; inl: inner nuclear layer; gcl: ganglion cell layer; PR: photoreceptors; TU: transducing units; NT: not transduced. Magnification=20×; scale bar=50μm.
Among the various high-capacity vectors available, we finally evaluated the herpevirus-based BoHV-4-CMV-EGFP. At 2, 7 and 28 days after subretinal injection of 8×106 GC/eye, BoHV-4-CMV-EGFP resulted in efficient RPE but modest PR transduction in adult C57BL/6 retinas (Figure 5). To properly evaluate the specific PR transduction of BoHV-4 vectors, we produced one containing the Rho-EGFP cassette and we injected it subretinally in C57BL/6 mice at a dose of 2.5×108 GC/eye. AAV2/8 Rho-EGFP was injected in contralateral eyes at 5×108 GC/eye for comparison. Retinal cryosections analyzed one month later by fluorescence microscopy showed that only scattered PRs were transduced (Figure 5) by BoHV-4-Rho-EGFP in contrast to the wide transduction achieved with the AAV2/8 vector.
Figure 5. Mouse retinal transduction mediated by BoHV-4.
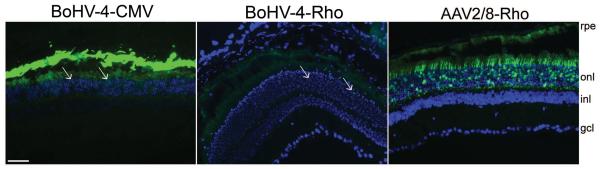
The BoHV-4-CMV-EGFP vector was injected subretinally (8×106 GC/eye) in adult C57BL/6 mice (left panel). Histological analyses performed one month post-injection revealed that both the RPE and some photoreceptors (arrows) were targeted (n=2/4 retinas with a transduction pattern similar to that shown in the picture, the remaining retinas show fewer transduced photoreceptors). The BoHV-4 vector containing the photoreceptor-specific Rho-EGFP expression cassette was injected subretinally (2.5×108 GC/eye, middle panel, n=10/10) in adult C57BL/6 mice. AAV2/8 containing the same expression cassette was injected alongside at 5×108 GC/eye (right panel, n=4/4), showing that AAV2/8 targets PRs more efficiently than BoHV-4. Abbreviations: rpe: retinal pigment epithelium; onl: outer nuclear layer; inl: inner nuclear layer, gcl: ganglion cell layer. Magnification=20×; scale bar=50μm.
To test the longevity of mouse retinal transduction mediated by high-capacity viral vectors, we injected subretinally C57BL/6 mice with vectors expressing EGFP under the control of the CMV promoter to monitor transgene expression in vivo by non-invasive fluorescent fundoscopy. Fluorescence was detected for 20 months in HdAd5-injected retinas (Figure 6), which almost equals the mouse lifespan and is the most sustained expression reported thus far in the retina for this vector. EGFP expression was maintained for up to: i. 14 months post-injection in the eyes injected with LV-VSVG (Figure 6), -GP64, -RRV or -EbolaΔO (not shown); ii. 4 months post-injection in those containing BoHV-4. These were the latest time points analyzed (Figure 6).
Figure 6. Long term transgene expression following subretinal delivery of HdAd5, LV and BoHV-4 vectors.
Non-invasive fluorescent fundoscopies were obtained at various time points after subretinal injection of HdAd5-, LV-VSVG- or BoHV-4-CMV-EGFP in adult C57BL/6 mice. The fundus photographs show EGFP expression along time in a representative eye injected with HdAd5 (3×108 vp/eye, n=2/3 eyes showing a fluorescent signal at the fundoscopy similar to the image), LV-VSVG (1×105 TU/eye, n=2/2) and BoHV-4 (8×106 GC/eye, n=16/16). PI: post-injection.
To investigate the safety of subretinal delivery of high-capacity viral vectors, we monitored retinal electrical activity by Ganzfeld electroretinogram (ERG) (Figure 7) before sacrificing the animals at the time points of the last ophthalmoscopy (shown in Figure 6). The maximum b-wave amplitude values in retinas that were injected with HdAd5, LV-VSVG, -RRV, -EbolaΔO, -GP64 or BoHV-4 are shown in Figure 7, and compared with the maximum b-wave amplitude of PBS-injected or non-injected controls of similar age. The amplitude of the b-waves were not significantly lower in eyes injected with viral vectors than in control eyes.
Figure 7. ERG responses following subretinal injection of HdAd5, LV or BoHV-4.

Ganzfeld electroretinogram (ERG) maximum b-wave amplitudes (±SE) in retinas injected with: HdAd5-, LV- or BoHV-4-CMV-EGFP vectors. The b-wave ERG values of eyes injected with HdAd5 at 1-3×108 vp/eye were compared to those of non-injected C57BL/6 mice of similar age. LV-VSVG, -RRV, -EbolaΔO, -GP64 were pooled together in the LV column (n=2 eyes for each LV pseudotype). LV were injected at 1×104-1×105 TU/eye and the ERG values were compared to those of non-injected C57BL/6 mice. ERG values of eyes that received BoHV-4 at 8×106 GC/eye were compared to those of eyes injected with PBS at the same age. Abbreviations: NI: not injected.
DISCUSSION
In the present work, we compared a large series of 24 different high-capacity viral vectors for their efficiency at transducing the adult mouse retina following subretinal delivery. Finding an efficient vector, capable of carrying large genes, would be a significant step forward for the development of gene therapy approaches for retinal blinding conditions due to defects in large genes such as STGD1, LCA10, and USH1B and for which no therapy is currently available.
We investigated 16 different Ad vectors and found that HdAd5, 1, 2 and 5/F35++ transduced PRs. AdC1, Ad5/F3 and HdAd5/6 targeted the RPE exclusively, and the rest of the tested vectors transduced RPE along with some PRs. Previous reports had shown that Ad5/F3530-31 and Ad5ΔRGD27-28 are efficient for PR targeting. However, in our hands these vectors did not perform better than Ad5 in terms of transduction efficiency when containing the ubiquitous CMV promoter. The PR transduction levels of HdAd1, HdAd2 and HdAd5/F35++ appeared higher than those of HdAd5 when using the CMV-lacZ cassette. Even if HdAd 1, 2 and 5 belong to the same C subgroup and recognize CAR receptor for initial binding, it has been reported that subgenus C Ads have differences in the fiber knob region48, which could account for different affinities to a target receptor. Differences in transduction capabilities among subgroup C Ads were also found in liver gene transfer studies where Ad2 was found to be a weaker transducer than Ad549. Ad2 retinal transduction had been studied previously, showing β-galactosidase expression in RPE and ganglion cells50, but not in PRs. This different outcome could be explained by the short 24 hour post-injection time-point that was evaluated by the authors whereas in our experiments PR expression was analyzed four days after injection. However, the use of HdAd1, HdAd2, and HdAd5/F35++ vectors containing the Rho-EGFP cassette resulted in levels of ONL transduction which were overall similar to the most commonly used HdAd5. The transduction differences observed using the CMV-lacZ and the Rho-EGFP expression cassettes could be due to the presence of “pseudotransduction” after injection of lacZ-expressing vectors. Some β-galactosidase enzyme may diffuse from the RPE to the inter-photoreceptor matrix (IPM) and to the ONL, as previously suggested13, 19, 51.
Among the various LV pseudotypes tested, LV-GP64 appeared to have a greater PR transduction efficiency than LV-VSVG. However this was limited to a narrow area. The interaction of GP64 with phospholipids on the cell surface was first reported to play a role in baculovirus infection into mammalian cells52 and lately, binding to heparan sulfate was also found to be involved53. Both pathways may have played a role in LV-GP64-mediated retinal transduction. In our case LV-Mokola failed to transduce PRs as it has been previously shown 13, 43. In the case of LV-LCMV, even if the RPE was transduced it is possible that we have underestimated its PR transduction properties since its infectious titer could not be assessed on HT-1080 cells, the target cells used to titer the LV vectors used in this study.
Vectors derived from herpesvirus are interesting for PR targeting because they naturally infect neurons11. Studies with HSV-1 containing the CMV-EGFP expression cassette showed that only the RPE was transduced in most cases25 although in one report PR outer segments were also found to be transduced51. Here, we evaluated whether the vector based on the bovine BoHV-4, isolated in Europe from respiratory and ocular diseases54, could have an innate tropism for PRs. This vector is not pathogenic, it is unlikely to be oncogenic, and can infect several cell types from different animal species55-56. Here, we show that BoHV-4-based CMV-EGFP vectors efficiently target RPE and some PRs as early as 2 days post injection and up to one month post injection (the latest time point evaluated) (Figure 5). When PR-specific Rho-EGFP cassette was used, very rare PRs were positive for transgene expression (Figure 5). It is possible that the strong transcriptional activity of the CMV promoter is requested for PR transduction by a BoHV-4-based vector.
The large capacity vector platforms we have used result in long-term transgene expression in the retina. We show that EGFP expression mediated by HdAd5 is present by fundus ophthalmoscopy for 20 months after injection (Figure 6), while first generation Ad5 transduction shut s off at 3 months (not shown). Retinal transduction mediated by HdAd5 was previously found to be sustained for up to 12 months57. Here, we report a duration that largely exceeds prior studies (20 months) that is close to the lifespan of a mouse and is consistent with previous studies showing multi-year HdAd-mediated expression in the liver of animal models58. Consistent with previous studies59, we also show that both LV-VSVG and -GP64 express EGFP long-term in the mouse retina for up to 14 months post-injection. In vivo retinal transduction mediated by HSV-1 vectors has been transient with loss of transgene expression by 6 weeks post-injection25, representing a drawback for their applications in genetic diseases requiring long term expression of the therapeutic gene. In contrast, we show that subretinal delivery of BoHV-4-based vectors results in stable EGFP fluorescence detectable up to 4 months post-injection by ophthalmoscopy. This finding is the first report of long-term retinal transduction mediated by an HV vector.
Subretinal injection of HdAd5, LV-VSVG, -GP64, -RRV- and EbolaΔO or BoHV-4 was not associated with significant reduction of the ERG b-wave amplitude when compared to control eyes (see Figure 7). However, additional studies including a careful histopathological examination are required to rule out potential toxicity resulting from subretinal delivery of high-capacity vectors. It is somehow puzzling that none of the high-capacity vectors investigated significantly outperforms the others, despite the wide difference in their capsid proteins. In the case of Ad, we have tested capsids recognizing different receptors, covering almost all the reported repertoire (CAR, αvβ3-5 integrins, CD46, and others) which are localized in the adult mouse ONL30; yet we were not able to detect levels of PR transduction dramatically higher than those achieved with the canonical HdAd5. The larger size of the high-capacity particle we have investigated compared to AAV might play a role. Indeed, several AAV serotypes (AAV5, AAV7, AAV8 and AAV9) efficiently transduce PRs4, and while the diameter of an AAV particle is around 25nm60, the Ad, LV and HV viral vector particles all have diameters >80nm61-63. Thus, diffusion of larger viral particles towards PRs may be limited by anatomical barriers, which might prevent their interaction with their cognate receptors64-65. In the retina, hindrance may be exerted by the IPM and the outer limiting membrane (OLM), which may limit access to PR bodies from the subretinal space where the large vectors are injected. This is indirectly suggested by the higher levels of PR transduction observed for both Ad and LV vectors in the developing mouse retinas where these barriers may be more permissive to the vectors. Indeed, Ad5 transduces efficiently PRs when delivered to neonatal retinas8, 24 and LV-VSVG transduced14-15 and rescued21, 66 PR degeneration when administered to neonatal but not to mature mouse PRs12, 14-15. As a matter of fact, the IPM was found to be an effective barrier that impaired LV-VSVG from transducing the adult PR layer23. Notably, a LV vector based on the equine infectious anemia virus (EIAV), which was originally shown to transduce67-68 and rescue neonatal mouse PRs affected by STGD122 has been recently reported to transduce adult rabbit and macaque PRs20 with satisfactory safety profile. However, thus far there is no evidence that these levels of adult PR transduction are therapeutically relevant. The two clinical trials which are currently being carried out for both Usher1B (NCT01505062, NCT02065011) and STGD1 (NCT01367444, NCT01736592) using EIAV vectors will help to understand whether this class of vectors effectively transduces adult human PRs. Weakened physical barriers due to PR loss are observed in animal models of retinal degeneration such as rd and Rho−/−24, 69. PR transduction mediated by Ad5 and LV-VSVG was shown to be more efficient in rd than in wild type retinas15, 24, while more Müller cells were transduced by LV-Mokola in Rho−/− than in control retinas69. These results suggest that disruption of anatomical barriers may improve Ad- and LV-mediated penetration and thus transduction24, 69, or as well indicate that retinal remodeling during disease may modify the tropism of high-capacity vectors69. A recent work carried out in adult mouse retinal explants, which lack physical barriers since they are compromised during the process of explantation, have shown that LV-VSVG is not able to transduce mouse PR70. Surprisingly, when a human retinal explant was transduced with LV-VSVG, limited PR transduction was observed, which suggests that transduction may also be species-dependent 70.
In summary, among the 24 different high-capacity viral vectors investigated, we found HdAd1, 2 and 5/F35++ to transduce mouse PRs with similar efficiencies to HdAd5, but with an overall PR transduction efficiency that appears lower than the one of AAV2/8 that remains the golden standard among naturally-occurring AAV serotypes for inherited PR diseases. Recently, effective gene transfer to mouse PRs has been shown with dual AAV vectors which significantly expand AAV cargo capacity in the retina71-72. In the future, it will be interesting to compare side-by-side the PR transduction efficiency of high-capacity HdAd-based vectors to that of dual AAV vectors, which allow efficient PR transduction although at levels which are lower than those obtained with normal size AAV vectors71-72.
MATERIALS AND METHODS
First Generation Ad vectors
Chimpanzee ChAd7, ChAd30 and ChAd63 preAd plasmid were obtained as described previously42. Ad5, AdC1 and Ad6 plasmids were constructed by following the same procedure as described in42 starting from wild type viruses obtained from ATCC. These plasmids were first digested with PmeI to release the viral ITRs. Then, 3-5×106 HEK293/PER.C6 cells were transfected with 10 μg of Ad viral plasmids using Lipofectamine (Invitrogen). This generated recombinant Ad vectors which were then expanded using 2×109 cells. Purification was performed by two-step cesium chloride gradient42. The Ad5, Ad6, AdC1, ChAd7, ChAd30 and ChAd63 vectors containing the CMV-EGFP expression cassette were provided by S. C.
Ad5/F2+pK36, Ad5/F2+RGD36 and Ad5ΔRGD37 viruses were constructed using a combination of conventional cloning and bacterial RecA-mediated recombination. Ad5-, Ad5/F2+pK- and Ad5/F2+RGD-CMV-lacZ plasmids were digested with PacI and recovered as viruses in 293 cells. Virus were propagated, purified and titered as described36. R. J. P provided these vectors. Ad5ΔRGD37 was transfected and recovered as a virus in 293 cells, banded in CsCl gradients and collected, dialyzed, and aliquoted as described37. This vector was provided by D. M. S.
The Tigem Adenoviral Vector Core (P.P. and N.B.P.) expanded in 293 cells seed vectors provided by other collaborators, which included Ad5-CMV-lacZ, Ad5ΔRGD-CMV-EGFP, Ad5/F2+pK-CMV-lacZ and Ad5/F2+RGD-CMV-lacZ.
The E1-deleted Ad vector FGAd5F3-CMV-LacZ34 was a generous gift from Dr. David T. Curiel, Washington University, St. Louis, MO, USA (and provided to us by P. N.).
The viral particles (vp)/mL titer of the first generation Ad vectors, obtained by measuring the absorbance at 260nm following virion lysis and correction for vector genome size, was provided for each vector by our collaborators.
Helper-dependent Ad vectors
All HdAd vectors were produced in 116 cells as previously described41. The helper virus AdNG16373 was used to produce HdAd5-CMV-lacZ and HdAd5-Rho-EGFP-WPRE-bGHpA. The helper virus Ad2LC8cCARP74 was used to produce HdAd2-CMV-LacZ and HdAd2-Rho-EGFP-WPRE-bGHpA. The helper virus Ad1LC8cCEVS-175 was used to produce HdAd1-CMV-LacZ and HdAd1-Rho-EGFP-WPRE-bGHpA. The helper virus AdHPBGF3533 was used to produce HdAd5F35-CMV-LacZ. The helper virus AdNG163-5F35++ (modified as described in 35) was used to produce HdAd5F35++CMV-LacZ and HdAd5F35++-Rho-EGFP-WPRE-bGHpA. The helper virus Ad5/3-NG16332 was used to produce HdAd5K3-CMV-LacZ. The helper virus AdNG17738 was used to produce HdAd5/6-CMV-LacZ. The HdAd vectors were provided by P. N. The Tigem Adenoviral Vector Core supplied HdAd5-CMV-lacZ and expanded HdAd5-Rho-EGFP-WPRE-bGHpA.
For the generation of HdAd-Rho-EGFP-WPRE-bGHpA vectors, the Rho-EGFP-WPRE-bGHpA cassette was PCR-amplified from the pAAV2.1-Rho-EGFP4 plasmid using primers with AscI restriction enzyme site at each end. The primer sequences are: Fw 5′-GGC GCG CCC TAG CAG ATC TTC CCC ACC TAG CC-3′ and Rv 5′-GGC GCG CCT CCC CAG CAT GCC TGC TAT TG-3′. The PCR product was inserted in pCR®2.1-TOPO® (Invitrogen, S.R.L., Milan, Italy), sequenced and then sent to Eurofins MWG Operon (Ebersberg, Germany) that performed the subcloning in the adenoviral pΔ25.3E4 plasmid (derived from pΔ28E476, and generously provided by the Tigem Adenoviral Vector Core). For HdAd-Rho-EGFP-WPRE-bGHpA vectors, titers in genome copies (GC)/mL were determined by PCR quantification using TaqMan (PerkinElmer Life and Analytical Sciences, Boston, MA, USA). The FAM-labeled TaqMan probe used was bGH Prb 5′-TCC CCC GTG CCT TCC TTG ACC-3′ and the primers were bGH Fwd 5′-TCT AGT TGC CAG CCA TCT GTT GT-3′ and bGH Rev 5′-TGG GAG TGG CAC CTT CCA-3′.
LV vectors
The HIV-1-based lentiviral vectors used for this work were produced through a quadruple transfection of a shuttle and two backbone plasmids (pCMV/HIV gag-pol, pCMV/Rev, and pHIVψeGFP)77 as well as an envelope plasmid that changed each time to generate each of the different 7 pseudotypes (see Table 1). The DNA was transfected into 15-cm diameter dishes of 80% confluent HEK293T cells using calcium phosphate transfection. After 12 h the cells were washed, and fresh medium was added (DMEM, 2% [vol/vol] FBS, 1% [vol/vol] Pen-Strep). Supernatants were collected at 24, 36, 48, 60, and 72 h posttransfection and frozen at −80°C. The supernatants were thawed, filtered through a 0.45-μm-pore-size filter, and pelleted by a 16-h centrifugation step (7,700 × g at 4°C in a Sorvall GSA rotor). The viral pellet was resuspended in DMEM for an approximate 200-fold concentration, and the virus was stored at −80°C until use. Titers were calculated on HT-1080 cells (ATCC) and given in transducing units (TU)/mL.
The Rho-EGFP lentiviral plasmid was generated as follows: Rho-EGFP was amplified by PCR from pAAV2.1-Rho-EGFP4 using the primer Fw 5′-AAT TCA ATT GCT AGC AGA TCT TCC CCA CCT AGC CAC-3′ which contained the MfeI restriction enzyme site and the primer Rv 5′-TTA ACT CGA GCT TGT ACA GCT CGT CCA TGC CG-3′ that contained the XhoI site. The PCR product was subcloned in pCR®2.1-TOPO® (Invitrogen, S.R.L., Milan, Italy) and sequenced. The Rho-EGFP fragment was then digested with MfeI/XhoI, purified and ligated with the LV plasmid backbone. The backbone consisted of the pSMPUW Universal Lentiviral Expression Vector (Promoterless) VPK211 (Cell Biolabs, CA, USA) digested with EcoRI/SalI (sharing compatible cohesive ends with MfeI and XhoI of the insert, respectively). The obtained LV-Rho-EGFP plasmid was then sequenced and sent to the Gene Transfer Vector Core (University of Iowa, IA, USA) for production of LV vectors pseudotyped with either VSVG or GP64 envelope glycoproteins. Physical titers (GC/mL) of vectors with the Rho-EGFP cassette were obtained with the Lentivirus qPCR Titer Kit (Applied Biological Materials Inc, Richmond, BC, Canada). Transducing/infectious titers on HT1080 cells could not be calculated since the Rho promoter is inactive in this cell type. The LV vectors were provided by B. L. D.
BoHV-4 vectors
BoHV-4-A78, BoHV-4-A-EGFPΔTK55 and BoHV-4-A-Rho-EGFP-WPREΔTK were propagated by infecting confluent monolayers of Madin Darby Bovine Kidney [(MDBK) ATCC, CCL-22], bovine embryo kidney [(BEK) provided by Dr. M. Ferrari, Istituto Zooprofilattico Sperimentale, Brescia, Italy; (BS CL-94)] or BEK expressing cre recombinase (BEKcre)78 at a multiplicity of infection (M.O.I.) of 0.5 TCID50 (50% tissue culture infectious dose) per cell and maintained in minimal essential medium (MEM; SIGMA) with 2% FBS for 2 h. The medium was then removed and replaced with fresh MEM containing 10% FBS. When approximately 90% of the cell monolayer exhibited cytopathic effect (CPE) (72 h post infection), the virus was prepared by freezing and thawing cells three times and pelleting the virions through 30% sucrose, as described previously79. Virus pellets were resuspended in cold MEM without FBS. TCID50 were determined by limited dilution in MDBK cells. The physical titer of the CMV-EGFP vector was calculated as GC/mL by RT-PCR with a probe recognizing EGFP. The sequence of the FAM-labeled TaqMan probe used is: 5′-TTC AAG TCC GCC ATG CCC GAA-3′ and that of the primers is: Fw 5′-CCA CAT GAA GCA GCA CGA CTT-3′; Rv 5′-GGT GCG CTC CTG GAC GTA-3′.
For BoHV-4-A-Rho-EGFP-WPREΔTK cloning, Rho-EGFP-WPRE-bGHpA was excised from the pAAV2.1-Rho-EGFP plasmid4 with NheI/XhoI, blunted with T4 DNA polymerase and sub-cloned in a SmaI digested pINT2 shuttle vector55, a plasmid vector containing two BoHV-4 TK gene sequences, to obtain pTK-Rho-EGFP-WPRE-TK. Next, TK-Rho-EGFP-WPRE-TK was cut out from the plasmid backbone and electroporated in SW102 E. coli containing the pBAC-BoHV-4-A-KanaGalKΔTK78. pBAC-BoHV-4-A-KanaGalKΔTK is a BoHV-4 genome clone coming from a non-pathogenic strain of BoHV-4 isolated from the milk cell fraction of an healthy cow, whose genome was cloned as a Bacterial Artificial Chromosome (BAC), pBAC-BoHV-4-A; and where its TK locus was targeted with a KanaGalK selectable cassette78. TK-Rho-EGFP-WPRE-TK electroporated E. coli containing the pBAC-BoHV-4-A-KanaGalKΔTK were first heat-induced, then negatively selected on deoxygalactose minimal plates. The resulting clones were additionally negatively selected with medium containing kanamycin. Then, to obtain pBAC-BoHV-4-A-Rho-EGFP-WPREΔTK, the retargeting was performed to the same site to replace the KanaGalK cassette with the TK-Rho-EGFP-WPRE-TK cassette. Retargeted clones (pBAC-BoHV-4-A-Rho-EGFP-WPRE ΔTK) were distinguished from the un-retargeted control clone (pBAC-BoHV-4-A-KanaGalKΔTK), by HindIII digestion. The selected clones’ stability was assessed by serially passaging over 25 days and analysis by HindIII restriction enzyme digestion. Infectious BoHV-4-A-Rho-EGFP-WPREΔTK virus was reconstituted in both BEK and BEKcre cells78, which enabled the depletion of the floxed BAC cassette from pBAC-BoHV-4-A-Rho-EGFP-WPRE ΔTK. Furthermore, BoHV-4-A-Rho-EGFP-WPREΔTK, BoHV-4-A-EGFPΔTK and BoHV-4-A were compared in terms of replication and no differences were observed among them. The Rho-EGFP vector physical titer was calculated by RT-PCR with a probe recognizing the bGHpA sequence, as explained in the Materials and Methods section for the Ad vectors. All BoHV-4 vectors were provided by G. D.
AAV Vectors
AAV2/8 vectors were produced by the TIGEM AAV Vector Core (Napoli, Italy) using the pAAV2.1-CMV-EGFP80 and -Rho-EGFP4 expression plasmids, and the packaging pAAV2/8 plasmid81. Vectors were produced by triple transfection of 293 cells followed by cesium chloride purification43. For each viral preparation, physical titers (GC/mL) were determined by both PCR quantification using TaqMan (PerkinElmer Life and Analytical Sciences, Boston, MA, USA) and dot-blot analysis82.
Animal Procedures and Vector Administration
Ethics Statement
All studies on mice were conducted in strict accordance with: i. the institutional guidelines for animal research; ii. the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research; iii. the Italian Ministry of Health regulation for animal procedures. All procedures on mice were submitted to the Italian Ministry of Health; Department of Public Health, Animal Health, Nutrition and Food Safety on October 17th, 2011. The Ministry of Health approved the procedures by silence/consent, as per article 7 of the 116/92 Ministerial Decree. Surgery was performed under anesthesia and all efforts were made to minimize suffering.
Mice
Four to five week-old male C57BL/6, CD-1 or BALB/c mice (Harlan, S. Pietro al Natisone, Italy) were anesthetized with an intraperitoneal injection of 2 mL/100 g body weight of avertin [1.25% w/v of 2,2,2-tribromoethanol and 2.5% v/v of 2-methyl-2-butanol (Sigma-Aldrich, Milan, Italy)]83, then viral vectors were delivered subretinally via a trans-scleral trans-choroidal approach as described47. A volume of 1 μl of viral vectors diluted in phosphate buffered saline (PBS) was delivered subretinally.
Histological Analysis
Mice were sacrificed and their eyeballs were harvested and fixed overnight by immersion in 2% or 4% paraformaldehyde. Before harvest, the temporal aspect of the sclerae was marked by cautery to orient the eyes with respect to the injection site at the moment of the inclusion. The eyeballs were cut so that the lens and vitreous could be removed leaving the eyecup intact. Mice eyecups were infiltrated with 30% sucrose for cryopreservation and embedded in tissue freezing medium (O.C.T. matrix, Kaltek, Padua, Italy). For each eye, 150 to 200 serial sections (10 μm-thick) were cut along the horizontal plane and the sections were progressively distributed on 10 slides so that each slide contained 15 to 20 sections, each section representing the whole eye at different levels. The sections were stained with 49,69-diamidino-2-phenylindole (Vectashield, Vector Lab Inc., Peterborough, UK) if the vector contained EGFP as a reporter protein. If the viral vector contained lacZ as the reporter gene, the cryosections were treated for X-gal staining (see below). EGFP and X-gal were monitored with a Leica DM 5000B microscope at various magnifications.
X-gal staining
Cryosections were fixed in 0.5% glutaraldehyde in PBS+ buffer (1 mM CaCl2 and 0.5 mM MgCl2 in PBS [pH 7.4]) for 10 minutes at room temperature. After fixation, three 5-minute rinses were performed with PBS+ buffer. Sections were stained using 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mM MgCl2 and 0.5 mg/mL X-gal powder in PBS+ buffer) at room temperature with protection from light. The reaction was stopped when staining was observed in the sample cryosections, and were analyzed only if the negative control (cryosections from non-injected or PBS-injected retinas) were X-gal negative. After they were X-gal stained, the sections were counterstained with eosin, dehydrated and coverslipped.
Fundus Photography
Fundus imaging was performed from 4 days to 20 months post viral injection (PI) using a Topcon TRC-50IX retinal camera connected to a charge-coupled-device NikonD1H digital camera (Topcon Medical System). Mice ocular fundi were photographed using a 300 W flash with a fluorescein filter, after dilating the pupils with a drop of tropicamide 1% (Visufarma, Roma, Italy),.
Electroretinogram Recordings
Electrophysiological recordings in mice were performed as detailed84.
ACKNOWLEDGEMENTS
We thank the TIGEM AAV Vector Core for AAV vector production and the TIGEM Ad Vector Core for expanding some adenoviral preps and providing some Ad controls (see Materials and Methods for details). We are also grateful to Graciana Diez-Roux for the critical reading of this manuscript. This work was supported by the European Research Council/ERC Grant agreement n° 282085 “RetGeneTx”; the NIH (grant R24 EY019861-01A); the Italian Telethon Foundation (grant TGM11MT1).
Footnotes
CONFLICT OF INTERESTS:
The authors declare no conflict of interest.
REFERENCES
- 1.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29(5):398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daiger SP, Sullivan LS, Bowne SJ. RetNet. Retinal Information Network. 2014 [Google Scholar]
- 3.Vandenberghe LH, Bell P, Maguire AM, Xiao R, Hopkins TB, Grant R, et al. AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One. 2013;8(1):e53463. doi: 10.1371/journal.pone.0053463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M, et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 2007;81(20):11372–80. doi: 10.1128/JVI.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mussolino C, della Corte M, Rossi S, Viola F, Di Vicino U, Marrocco E, et al. AAV-mediated photoreceptor transduction of the pig cone-enriched retina. Gene Ther. 2011;18(7):637–45. doi: 10.1038/gt.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3(88):88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manfredi A, Marrocco E, Puppo A, Cesi G, Sommella A, Della Corte M, et al. Combined rod and cone transduction by adeno-associated virus 2/8. Hum Gene Ther. 2013;24(12):982–92. doi: 10.1089/hum.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Adamian M, Roof DJ, Berson EL, Dryja TP, Roessler BJ, et al. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest Ophthalmol Vis Sci. 1994;35(5):2543–9. [PubMed] [Google Scholar]
- 9.Bennett J, Wilson J, Sun D, Forbes B, Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci. 1994;35(5):2535–42. [PubMed] [Google Scholar]
- 10.Kreppel F, Luther TT, Semkova I, Schraermeyer U, Kochanek S. Long-term transgene expression in the RPE after gene transfer with a high-capacity adenoviral vector. Invest Ophthalmol Vis Sci. 2002;43(6):1965–70. [PubMed] [Google Scholar]
- 11.Brunetti-Pierri N, Auricchio A. Gene Therapy of Human Inherited Diseases. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Scriver CR, Childs B, Sly WS, Bunz F, Gibson KM, Mitchell G, editors. The Online Metabolic & Molecular Bases of Inherited Disease. McGraw-Hill; New York: Part 2: Perspectives. Chapter 5.2: Gene Therapy of Human Inherited Diseases. DOI: 10.1036/ommbid.12. [Google Scholar]
- 12.Bainbridge JW, Stephens C, Parsley K, Demaison C, Halfyard A, Thrasher AJ, et al. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 2001;8(21):1665–8. doi: 10.1038/sj.gt.3301574. [DOI] [PubMed] [Google Scholar]
- 13.Bemelmans AP, Bonnel S, Houhou L, Dufour N, Nandrot E, Helmlinger D, et al. Retinal cell type expression specificity of HIV-1-derived gene transfer vectors upon subretinal injection in the adult rat: influence of pseudotyping and promoter. J Gene Med. 2005;7(10):1367–74. doi: 10.1002/jgm.788. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci U S A. 1997;94(19):10319–23. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang J, Cheng M, Haire SE, Barker E, Planelles V, Blanks JC. Efficiency of lentiviral transduction during development in normal and rd mice. Mol Vis. 2006;12:756–67. [PubMed] [Google Scholar]
- 16.Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Sakamoto T, Tabata T, et al. Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther. 2003;10(17):1503–11. doi: 10.1038/sj.gt.3302028. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Yonemitsu Y, Miyazaki M, Kohno R, Murakami Y, Murata T, et al. Stable retinal gene expression in nonhuman primates via subretinal injection of SIVagm-based lentiviral vectors. Hum Gene Ther. 2009;20(6):573–9. doi: 10.1089/hum.2009.009. [DOI] [PubMed] [Google Scholar]
- 18.Derksen TA, Sauter SL, Davidson BL. Feline immunodeficiency virus vectors. Gene transfer to mouse retina following intravitreal injection. J Gene Med. 2002;4:463–9. doi: 10.1002/jgm.267. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L, Toyoguchi M, Looney DJ, Lee J, Davidson MC, Freeman WR. Efficient gene transfer to retinal pigment epithelium cells with long-term expression. Retina. 2005;25(2):193–201. doi: 10.1097/00006982-200502000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binley K, Widdowson P, Loader J, Kelleher M, Iqball S, Ferrige G, et al. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci. 2013;54(6):4061–71. doi: 10.1167/iovs.13-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto T, Gibbs D, Lillo C, Azarian SM, Legacki E, Zhang XM, et al. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther. 2007;14(7):584–94. doi: 10.1038/sj.gt.3302897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15(19):1311–20. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruter O, Kostic C, Crippa SV, Perez MT, Zografos L, Schorderet DF, et al. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005;12(11):942–7. doi: 10.1038/sj.gt.3302485. [DOI] [PubMed] [Google Scholar]
- 24.Pang J, Cheng M, Stevenson D, Trousdale MD, Dorey CK, Blanks JC. Adenoviral-mediated gene transfer to retinal explants during development and degeneration. Exp Eye Res. 2004;79(2):189–201. doi: 10.1016/j.exer.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Fraefel C, Mendes-Madeira A, Mabon O, Lefebvre A, Le Meur G, Ackermann M, et al. In vivo gene transfer to the rat retina using herpes simplex virus type 1 (HSV-1)-based amplicon vectors. Gene Ther. 2005;12(16):1283–8. doi: 10.1038/sj.gt.3302553. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Bergelson JM. Adenovirus receptors. J Virol. 2005;79(19):12125–31. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cashman SM, McCullough L, Kumar-Singh R. Improved retinal transduction in vivo and photoreceptor-specific transgene expression using adenovirus vectors with modified penton base. Mol Ther. 2007;15(9):1640–6. doi: 10.1038/sj.mt.6300203. [DOI] [PubMed] [Google Scholar]
- 28.Sweigard JH, Cashman SM, Kumar-Singh R. Adenovirus vectors targeting distinct cell types in the retina. Invest Ophthalmol Vis Sci. 2010;51(4):2219–28. doi: 10.1167/iovs.09-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Seggern DJ, Aguilar E, Kinder K, Fleck SK, Gonzalez Armas JC, Stevenson SC, et al. In vivo transduction of photoreceptors or ciliary body by intravitreal injection of pseudotyped adenoviral vectors. Mol Ther. 2003;7(1):27–34. doi: 10.1016/s1525-0016(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 30.Mallam JN, Hurwitz MY, Mahoney T, Chevez-Barrios P, Hurwitz RL. Efficient gene transfer into retinal cells using adenoviral vectors: dependence on receptor expression. Invest Ophthalmol Vis Sci. 2004;45(6):1680–7. doi: 10.1167/iovs.03-0730. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S-H, Wu J-H, Wu X-B, Dong X-Y, Liu X-J, Li C-Y, et al. Distinctive Gene Transduction Efficiencies of Commonly Used Viral Vectors in the Retina. Curr Eye Res. 2008;33(1):81–90. doi: 10.1080/02713680701799408. [DOI] [PubMed] [Google Scholar]
- 32.Guse K, Suzuki M, Sule G, Bertin TK, Tyynismaa H, Ahola-Erkkila S, et al. Capsid-modified adenoviral vectors for improved muscle-directed gene therapy. Hum Gene Ther. 2012;23(10):1065–70. doi: 10.1089/hum.2012.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shayakhmetov DM, Li ZY, Gaggar A, Gharwan H, Ternovoi V, Sandig V, et al. Genome size and structure determine efficiency of postinternalization steps and gene transfer of capsid-modified adenovirus vectors in a cell-type-specific manner. J Virol. 2004;78(18):10009–22. doi: 10.1128/JVI.78.18.10009-10022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takayama K, Reynolds PN, Short JJ, Kawakami Y, Adachi Y, Glasgow JN, et al. A mosaic adenovirus possessing serotype Ad5 and serotype Ad3 knobs exhibits expanded tropism. Virology. 2003;309(2):282–93. doi: 10.1016/s0042-6822(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Liu Y, Li Z, Tuve S, Stone D, Kalyushniy O, et al. In vitro and in vivo properties of adenovirus vectors with increased affinity to CD46. J Virol. 2008;82(21):10567–79. doi: 10.1128/JVI.01308-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bramson JL, Grinshtein N, Meulenbroek RA, Lunde J, Kottachchi D, Lorimer IA, et al. Helper-dependent adenoviral vectors containing modified fiber for improved transduction of developing and mature muscle cells. Hum Gene Ther. 2004;15(2):179–88. doi: 10.1089/104303404772679986. [DOI] [PubMed] [Google Scholar]
- 37.Shayakhmetov DM, Eberly AM, Li ZY, Lieber A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J Virol. 2005;79(2):1053–61. doi: 10.1128/JVI.79.2.1053-1061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, et al. Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol Ther. 2011;19(7):1254–62. doi: 10.1038/mt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol. 1998;9(5):457–63. doi: 10.1016/s0958-1669(98)80029-3. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, Yang T, Ghosh HP, Geller AI. Helper virus-free HSV-1 vectors packaged both in the presence of VSV G protein and in the absence of HSV-1 glycoprotein B support gene transfer into neurons in the rat striatum. J Neurovirol. 2001;7(6):548–55. doi: 10.1080/135502801753248132. [DOI] [PubMed] [Google Scholar]
- 41.Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8(5):846–52. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4(115):115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auricchio A, Kobinger G, Anand V, Hildinger M, O’Connor E, Maguire AM, et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10(26):3075–81. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg KP, Geller SF, Schaffer DV, Flannery JG. Targeted transgene expression in muller glia of normal and diseased retinas using lentiviral vectors. Invest Ophthalmol Vis Sci. 2007;48(4):1844–52. doi: 10.1167/iovs.05-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duisit G, Conrath H, Saleun S, Folliot S, Provost N, Cosset FL, et al. Five recombinant simian immunodeficiency virus pseudotypes lead to exclusive transduction of retinal pigmented epithelium in rat. Mol Ther. 2002;6(4):446–54. doi: 10.1006/mthe.2002.0690. [DOI] [PubMed] [Google Scholar]
- 46.Molina RP, Ye HQ, Brady J, Mech CA, Kaleko M, Luo T. Baculovirus GP64 Pseudotyped Bovine Immunodeficiency Virus-Based Lentiviral Vectors Efficiently Transduce Retinal Cells In Vivo. Abstract, Molecular Therapy. 2004;9(Suppl 1):S277. abstract 728. [Google Scholar]
- 47.Liang FQ, Anand V, Maguire AM, Bennett J. Intraocular delivery of recombinant virus. Methods Mol Med. 2001;47:125–39. doi: 10.1385/1-59259-085-3:125. [DOI] [PubMed] [Google Scholar]
- 48.Adhikary AK, Banik U, Numaga J, Suzuki E, Inada T, Okabe N. Heterogeneity of the fibre sequence in subgenus C adenoviruses. J Clin Pathol. 2004;57(6):612–7. doi: 10.1136/jcp.2003.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weaver EA, Hillestad ML, Khare R, Palmer D, Ng P, Barry MA. Characterization of species C human adenovirus serotype 6 (Ad6) Virology. 2011;412(1):19–27. doi: 10.1016/j.virol.2010.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jomary C, Piper TA, Dickson G, Couture LA, Smith AE, Neal MJ, et al. Adenovirus-mediated gene transfer to murine retinal cells in vitro and in vivo. FEBS Lett. 1994;347(2-3):117–22. doi: 10.1016/0014-5793(94)00512-5. [DOI] [PubMed] [Google Scholar]
- 51.Kaufman PL, Jia WW, Tan J, Chen Z, Gabelt BT, Booth V, et al. A perspective of gene therapy in the glaucomas. Surv Ophthalmol. 1999;43(Suppl 1):S91–7. doi: 10.1016/s0039-6257(99)00028-4. [DOI] [PubMed] [Google Scholar]
- 52.Tani H, Nishijima M, Ushijima H, Miyamura T, Matsuura Y. Characterization of cell-surface determinants important for baculovirus infection. Virology. 2001;279(1):343–53. doi: 10.1006/viro.2000.0699. [DOI] [PubMed] [Google Scholar]
- 53.Wu C, Wang S. A pH-sensitive heparin-binding sequence from Baculovirus gp64 protein is important for binding to mammalian cells but not to Sf9 insect cells. J Virol. 2012;86(1):484–91. doi: 10.1128/JVI.06357-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartha A, Juhasz M, Liebermann H. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjunctivitis. A preliminary report. Acta Vet Acad Sci Hung. 1966;16(3):357–8. [PubMed] [Google Scholar]
- 55.Donofrio G, Cavirani S, Simone T, van Santen VL. Potential of bovine herpesvirus 4 as a gene delivery vector. J Virol Methods. 2002;101(1-2):49–61. doi: 10.1016/s0166-0934(01)00419-0. [DOI] [PubMed] [Google Scholar]
- 56.Donofrio G, Manarolla G, Ravanetti L, Sironi G, Cavirani S, Cabassi CS, et al. Assessment of bovine herpesvirus 4 based vector in chicken. J Virol Methods. 2008;148(1-2):303–6. doi: 10.1016/j.jviromet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Lamartina S, Cimino M, Roscilli G, Dammassa E, Lazzaro D, Rota R, et al. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: stable gene transfer, regulated gene expression and therapeutic efficacy. J Gene Med. 2007;9(10):862–74. doi: 10.1002/jgm.1083. [DOI] [PubMed] [Google Scholar]
- 58.Brunetti-Pierri N, Ng T, Iannitti D, Cioffi W, Stapleton G, Law M, et al. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum Gene Ther. 2013;24(8):761–5. doi: 10.1089/hum.2013.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balaggan KS, Binley K, Esapa M, MacLaren RE, Iqball S, Duran Y, et al. EIAV vector-mediated delivery of endostatin or angiostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. Gene Ther. 2006;13(15):1153–65. doi: 10.1038/sj.gt.3302769. [DOI] [PubMed] [Google Scholar]
- 60.Kronenberg S, Kleinschmidt JA, Bottcher B. Electron cryo-microscopy and image reconstruction of adeno-associated virus type 2 empty capsids. EMBO Rep. 2001;2(11):997–1002. doi: 10.1093/embo-reports/kve234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar-Singh R. Barriers for retinal gene therapy: separating fact from fiction. Vision Res. 2008;48(16):1671–80. doi: 10.1016/j.visres.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sastry L, Miller CA, Johnson T, Jasti A, Gattone VH, Cornetta K. Negative Staining and Immunocytochemistry of HIV-1 Vectors. Microsc Microanal. 2005;11(S02):972–3. [Google Scholar]
- 63.Forest T, Barnard S, Baines JD. Active intranuclear movement of herpesvirus capsids. Nat Cell Biol. 2005;7(4):429–31. doi: 10.1038/ncb1243. [DOI] [PubMed] [Google Scholar]
- 64.Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, et al. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6(9):1520–35. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 65.Pickles RJ, Fahrner JA, Petrella JM, Boucher RC, Bergelson JM. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J Virol. 2000;74(13):6050–7. doi: 10.1128/jvi.74.13.6050-6057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi M, Miyoshi H, Verma IM, Gage FH. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol. 1999;73(9):7812–6. doi: 10.1128/jvi.73.9.7812-7816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balaggan KS, Binley K, Esapa M, Iqball S, Askham Z, Kan O, et al. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8(3):275–85. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- 68.Nicoud M, Kong J, Iqball S, Kan O, Naylor S, Gouras P, et al. Development of photoreceptor-specific promoters and their utility to investigate EIAV lentiviral vector mediated gene transfer to photoreceptors. J Gene Med. 2007;9(12):1015–23. doi: 10.1002/jgm.1115. [DOI] [PubMed] [Google Scholar]
- 69.Calame M, Cachafeiro M, Philippe S, Schouwey K, Tekaya M, Wanner D, et al. Retinal degeneration progression changes lentiviral vector cell targeting in the retina. PLoS One. 2011;6(8):e23782. doi: 10.1371/journal.pone.0023782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lipinski DM, Barnard AR, Charbel Issa P, Singh MS, De Silva SR, Trabalza A, et al. Vesicular stomatitis virus glycoprotein- and venezuelan equine encephalitis virus-derived glycoprotein-pseudotyped lentivirus vectors differentially transduce corneal endothelium, trabecular meshwork, and human photoreceptors. Hum Gene Ther. 2014;25(1):50–62. doi: 10.1089/hum.2013.009. [DOI] [PubMed] [Google Scholar]
- 71.Trapani I, Colella P, Sommella A, Iodice C, Cesi G, de Simone S, et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med. 2014;6(2):194–211. doi: 10.1002/emmm.201302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colella P, Trapani I, Cesi G, Sommella A, Manfredi A, Puppo A, et al. Efficient gene delivery to the cone-enriched pig retina by dual AAV vectors. Gene Ther. 2014 doi: 10.1038/gt.2014.8. e-pub ahead of print 27 February 2014; doi: 10.1038/gt.2014.8. [DOI] [PubMed] [Google Scholar]
- 73.Palmer DJ, Ng P. Physical and infectious titers of helper-dependent adenoviral vectors: a method of direct comparison to the adenovirus reference material. Mol Ther. 2004;10(4):792–8. doi: 10.1016/j.ymthe.2004.06.1013. [DOI] [PubMed] [Google Scholar]
- 74.Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6(9):1565–73. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- 75.Weaver EA, Nehete PN, Buchl SS, Senac JS, Palmer D, Ng P, et al. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS One. 2009;4(3):e5059. doi: 10.1371/journal.pone.0005059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toietta G, Pastore L, Cerullo V, Finegold M, Beaudet AL, Lee B. Generation of helper-dependent adenoviral vectors by homologous recombination. Mol Ther. 2002;5(2):204–10. doi: 10.1006/mthe.2002.0532. [DOI] [PubMed] [Google Scholar]
- 77.Quinn K, Brindley MA, Weller ML, Kaludov N, Kondratowicz A, Hunt CL, et al. Rho GTPases modulate entry of Ebola virus and vesicular stomatitis virus pseudotyped vectors. J Virol. 2009;83(19):10176–86. doi: 10.1128/JVI.00422-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donofrio G, Sartori C, Franceschi V, Capocefalo A, Cavirani S, Taddei S, et al. Double immunization strategy with a BoHV-4-vectorialized secreted chimeric peptide BVDV-E2/BoHV-1-gD. Vaccine. 2008;26(48):6031–42. doi: 10.1016/j.vaccine.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 79.Donofrio G, Cavaggioni A, Bondi M, Cavirani S, Flammini CF, Mucignat-Caretta C. Outcome of bovine herpesvirus 4 infection following direct viral injection in the lateral ventricle of the mouse brain. Microbes Infect. 2006;8(3):898–904. doi: 10.1016/j.micinf.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 80.Auricchio A, Hildinger M, O’Connor E, Gao GP, Wilson JM. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther. 2001;12(1):71–6. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- 81.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doria M, Ferrara A, Auricchio A. AAV2/8 vectors purified from culture medium with a simple and rapid protocol transduce murine liver, muscle, and retina efficiently. Hum Gene Ther Methods. 2013;24(6):392–8. doi: 10.1089/hgtb.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci. 1993;43(2):189–92. [PubMed] [Google Scholar]
- 84.Allocca M, Manfredi A, Iodice C, Di Vicino U, Auricchio A. AAV-mediated gene replacement, either alone or in combination with physical and pharmacological agents, results in partial and transient protection from photoreceptor degeneration associated with betaPDE deficiency. Invest Ophthalmol Vis Sci. 2011;52(8):5713–9. doi: 10.1167/iovs.10-6269. [DOI] [PubMed] [Google Scholar]
- 85.Stein CS, Martins I, Davidson BL. The lymphocytic choriomeningitis virus envelope glycoprotein targets lentiviral gene transfer vector to neural progenitors in the murine brain. Mol Ther. 2005;11(3):382–9. doi: 10.1016/j.ymthe.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Sinn PL, Burnight ER, Shen H, Fan H, McCray PB., Jr. Inclusion of Jaagsiekte sheep retrovirus proviral elements markedly increases lentivirus vector pseudotyping efficiency. Mol Ther. 2005;11(3):460–9. doi: 10.1016/j.ymthe.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 87.Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD, et al. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol. 2002;76(18):9378–88. doi: 10.1128/JVI.76.18.9378-9388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sinn PL, Arias AC, Brogden KA, McCray PB., Jr. Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J Virol. 2008;82(21):10684–92. doi: 10.1128/JVI.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]







