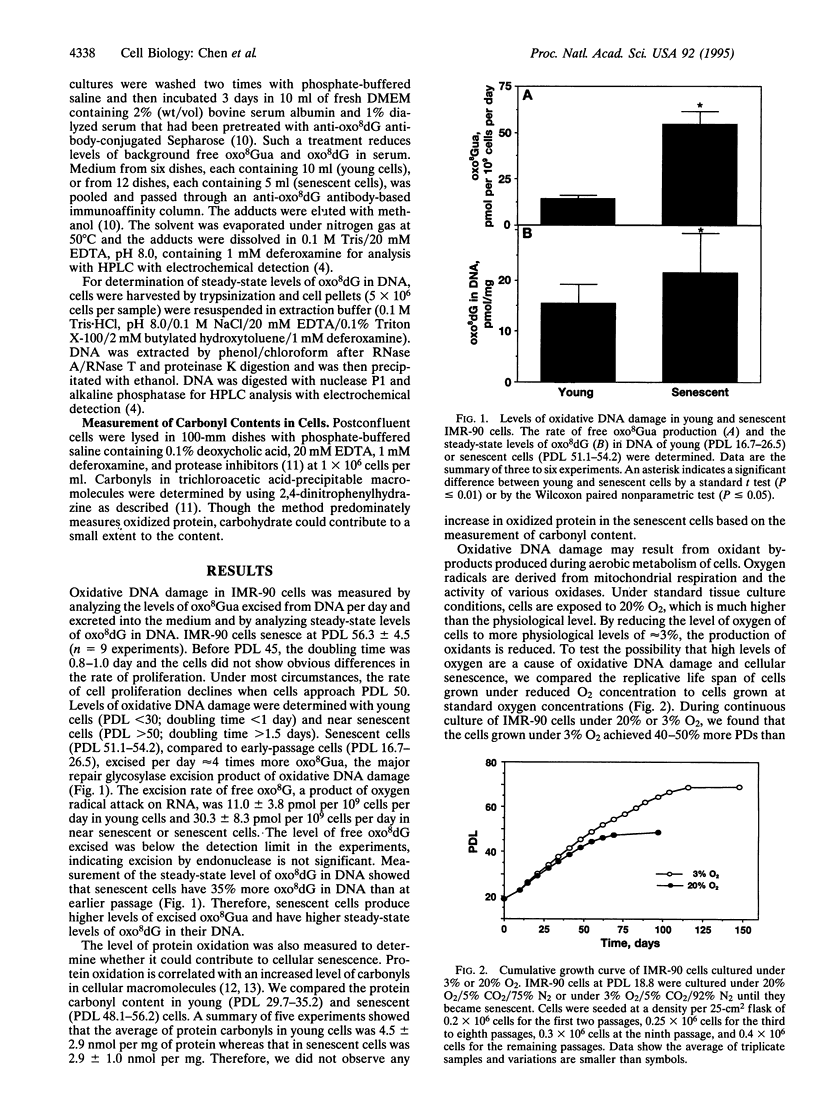
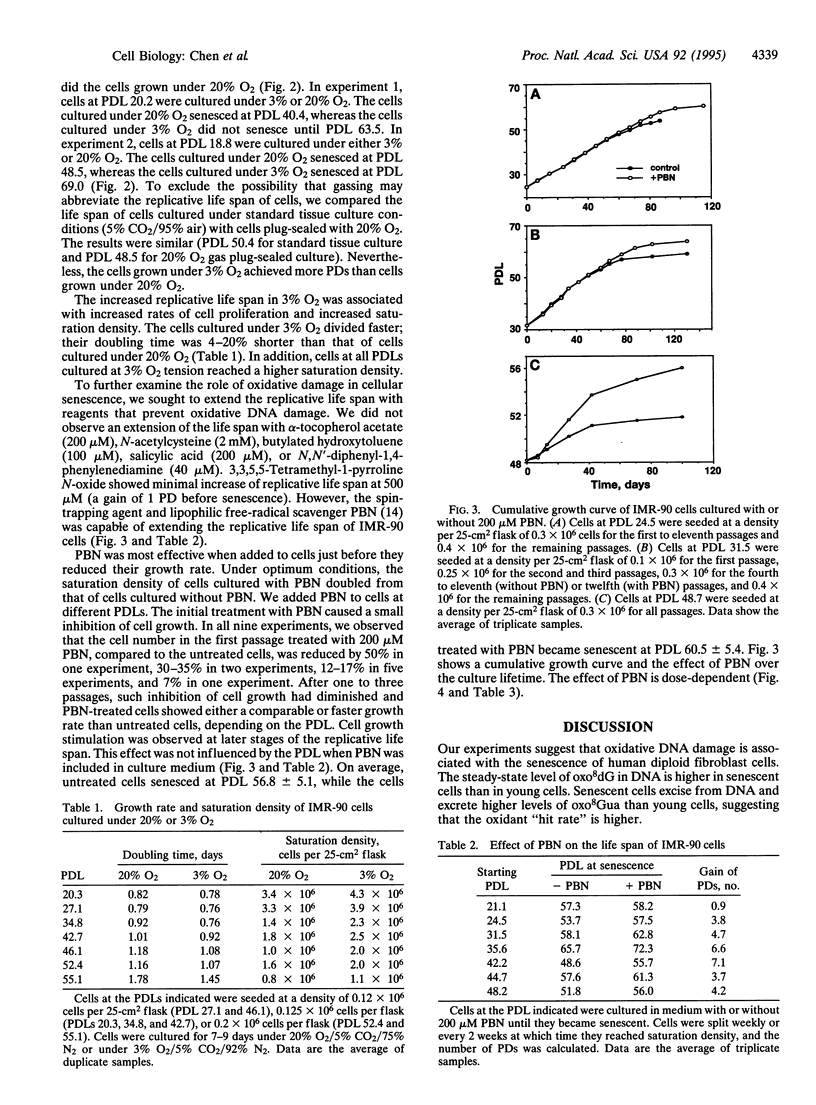
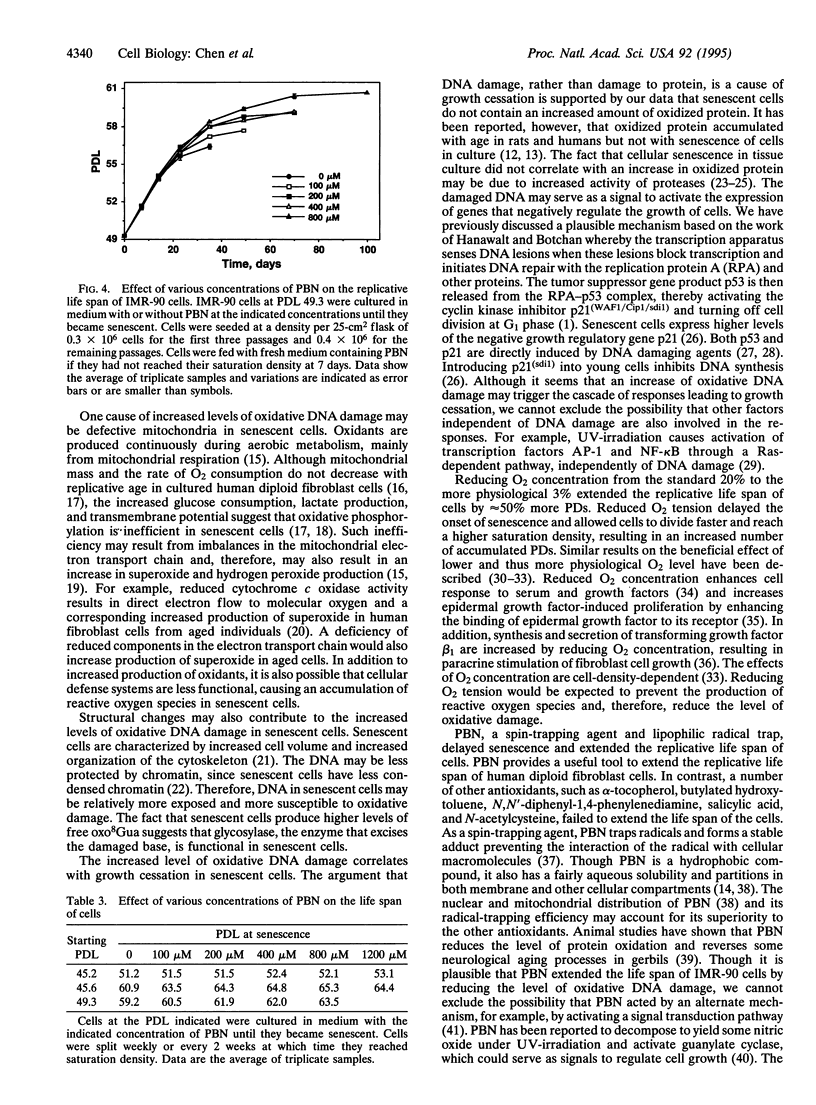
Abstract
Human diploid fibroblast cells cease growth in culture after a finite number of population doublings. To address the cause of growth cessation in senescent IMR-90 human fibroblast cells, we determined the level of oxidative DNA damage by using 8-oxoguanine excised from DNA and 8-oxo-2'-deoxyguanosine in DNA as markers. Senescent cells excise from DNA four times more 8-oxoguanine per day than do early-passage young cells. The steady-state level of 8-oxo-2'-deoxyguanosine in DNA is approximately 35% higher in senescent cells than in young cells. Measurement of protein carbonyls shows that senescent cells did not appear to have elevated protein oxidation. To reduce the level of oxidative damage, we cultured cells under a more physiological O2 concentration (3%) and compared the replicative life span to the cells cultured at the O2 concentration of air (20%). We found that cells grown under 3% O2 achieved 50% more population doublings during their lifetime. Such an extension of life span resulted from the delayed onset of senescence and elevation of growth rate and saturation density of cells at all passages. The spin-trapping agent alpha-phenyl-t-butyl nitrone (PBN), which can act as an antioxidant, also effectively delayed senescence and rejuvenated near senescent cells. The effect is dose-dependent and is most pronounced for cells at the stage just before entry into senescence. Our data support the hypothesis that oxidative DNA damage contributes to replicative cessation in human diploid fibroblast cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Oxygen radicals and 8-hydroxyguanine in DNA. Jpn J Cancer Res. 1991 Dec;82(12):1460–1461. [PubMed] [Google Scholar]
- Angello J. C., Pendergrass W. R., Norwood T. H., Prothero J. Cell enlargement: one possible mechanism underlying cellular senescence. J Cell Physiol. 1989 Aug;140(2):288–294. doi: 10.1002/jcp.1041400214. [DOI] [PubMed] [Google Scholar]
- Balin A. K., Fisher A. J., Carter D. M. Oxygen modulates growth of human cells at physiologic partial pressures. J Exp Med. 1984 Jul 1;160(1):152–166. doi: 10.1084/jem.160.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin A. K., Goodman B. P., Rasmussen H., Cristofalo V. J. The effect of oxygen tension on the growth and metabolism of WI-38 cells. J Cell Physiol. 1976 Oct;89(2):235–249. doi: 10.1002/jcp.1040890207. [DOI] [PubMed] [Google Scholar]
- Blumenthal E. J., Miller A. C., Stein G. H., Malkinson A. M. Serine/threonine protein kinases and calcium-dependent protease in senescent IMR-90 fibroblasts. Mech Ageing Dev. 1993 Nov;72(1):13–24. doi: 10.1016/0047-6374(93)90127-d. [DOI] [PubMed] [Google Scholar]
- Carlin C., Phillips P. D., Brooks-Frederich K., Knowles B. B., Cristofalo V. J. Cleavage of the epidermal growth factor receptor by a membrane-bound leupeptin-sensitive protease active in nonionic detergent lysates of senescent but not young human diploid fibroblasts. J Cell Physiol. 1994 Sep;160(3):427–434. doi: 10.1002/jcp.1041600305. [DOI] [PubMed] [Google Scholar]
- Carney J. M., Floyd R. A. Protection against oxidative damage to CNS by alpha-phenyl-tert-butyl nitrone (PBN) and other spin-trapping agents: a novel series of nonlipid free radical scavengers. J Mol Neurosci. 1991;3(1):47–57. doi: 10.1007/BF02896848. [DOI] [PubMed] [Google Scholar]
- Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamulitrat W., Jordan S. J., Mason R. P., Saito K., Cutler R. G. Nitric oxide formation during light-induced decomposition of phenyl N-tert-butylnitrone. J Biol Chem. 1993 Jun 5;268(16):11520–11527. [PubMed] [Google Scholar]
- Chen Q., Ames B. N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova D., De Angelis L., Monti E., Piccinini F. Subcellular distribution of two spin trapping agents in rat heart: possible explanation for their different protective effects against doxorubicin-induced cardiotoxicity. Free Radic Res Commun. 1992;15(6):353–360. doi: 10.3109/10715769209049151. [DOI] [PubMed] [Google Scholar]
- Devary Y., Rosette C., DiDonato J. A., Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993 Sep 10;261(5127):1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- Falanga V., Qian S. W., Danielpour D., Katz M. H., Roberts A. B., Sporn M. B. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. J Invest Dermatol. 1991 Oct;97(4):634–637. doi: 10.1111/1523-1747.ep12483126. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Ballantyne S. R., Robson A. L., Moerman E. J. Energy metabolism in cultured human fibroblasts during aging in vitro. J Cell Physiol. 1982 Sep;112(3):419–424. doi: 10.1002/jcp.1041120316. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Moerman E. J., Porter K. High-voltage electron microscopy of human diploid fibroblasts during ageing in vitro. Morphometric analysis of mitochondria. Exp Cell Res. 1984 Sep;154(1):101–111. doi: 10.1016/0014-4827(84)90671-2. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kagawa Y., Kondo H., Kaneda H., Yonekawa H., Takai D., Miyabayashi S. Nuclear but not mitochondrial genome involvement in human age-related mitochondrial dysfunction. Functional integrity of mitochondrial DNA from aged subjects. J Biol Chem. 1994 Mar 4;269(9):6878–6883. [PubMed] [Google Scholar]
- Honda S., Matsuo M. Shortening of the in vitro lifespan of human diploid fibroblasts exposed to hyperbaric oxygen. Exp Gerontol. 1983;18(5):339–345. doi: 10.1016/0531-5565(83)90012-8. [DOI] [PubMed] [Google Scholar]
- Johnson M., Dimitrov D., Vojta P. J., Barrett J. C., Noda A., Pereira-Smith O. M., Smith J. R. Evidence for a p53-independent pathway for upregulation of SDI1/CIP1/WAF1/p21 RNA in human cells. Mol Carcinog. 1994 Oct;11(2):59–64. doi: 10.1002/mc.2940110202. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Macieira-Coelho A. Chromatin reorganization during senescence of proliferating cells. Mutat Res. 1991 Mar-Nov;256(2-6):81–104. doi: 10.1016/0921-8734(91)90003-t. [DOI] [PubMed] [Google Scholar]
- Martinez A. O., Over D., Armstrong L. S., Manzano L., Taylor R., Chambers J. Separation of two subpopulations of old human fibroblasts by mitochondria (rhodamine 123) fluorescence. Growth Dev Aging. 1991 Fall;55(3):185–191. [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Okada A. A., Dice J. F. Altered degradation of intracellular proteins in aging human fibroblasts. Mech Ageing Dev. 1984 Aug;26(2-3):341–356. doi: 10.1016/0047-6374(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Oliver C. N., Ahn B. W., Moerman E. J., Goldstein S., Stadtman E. R. Age-related changes in oxidized proteins. J Biol Chem. 1987 Apr 25;262(12):5488–5491. [PubMed] [Google Scholar]
- Packer L., Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977 Jun 2;267(5610):423–425. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- Park E. M., Shigenaga M. K., Degan P., Korn T. S., Kitzler J. W., Wehr C. M., Kolachana P., Ames B. N. Assay of excised oxidative DNA lesions: isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3375–3379. doi: 10.1073/pnas.89.8.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacocke M., Campisi J. Cellular senescence: a reflection of normal growth control, differentiation, or aging? J Cell Biochem. 1991 Feb;45(2):147–155. doi: 10.1002/jcb.240450205. [DOI] [PubMed] [Google Scholar]
- Reznick A. Z., Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- Sen S., Phillis J. W. alpha-Phenyl-tert-butyl-nitrone (PBN) attenuates hydroxyl radical production during ischemia-reperfusion injury of rat brain: an EPR study. Free Radic Res Commun. 1993;19(4):255–265. doi: 10.3109/10715769309056513. [DOI] [PubMed] [Google Scholar]
- Sherwood S. W., Rush D., Ellsworth J. L., Schimke R. T. Defining cellular senescence in IMR-90 cells: a flow cytometric analysis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9086–9090. doi: 10.1073/pnas.85.23.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga M. K., Aboujaoude E. N., Chen Q., Ames B. N. Assays of oxidative DNA damage biomarkers 8-oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Ames B. N. Assays for 8-hydroxy-2'-deoxyguanosine: a biomarker of in vivo oxidative DNA damage. Free Radic Biol Med. 1991;10(3-4):211–216. doi: 10.1016/0891-5849(91)90078-h. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Hagen T. M., Ames B. N. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. R., Lincoln D. W., 2nd Aging of cells in culture. Int Rev Cytol. 1984;89:151–177. doi: 10.1016/s0074-7696(08)61303-0. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Brunk U. T. Mitochondrial production of pro-oxidants and cellular senescence. Mutat Res. 1992 Sep;275(3-6):295–304. doi: 10.1016/0921-8734(92)90033-l. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Storch T. G., Talley G. D. Oxygen concentration regulates the proliferative response of human fibroblasts to serum and growth factors. Exp Cell Res. 1988 Apr;175(2):317–325. doi: 10.1016/0014-4827(88)90195-4. [DOI] [PubMed] [Google Scholar]
- Wing D. A., Talley G. D., Storch T. G. Oxygen concentration regulates EGF-induced proliferation and EGF-receptor down regulation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):952–958. doi: 10.1016/s0006-291x(88)81320-2. [DOI] [PubMed] [Google Scholar]



