Abstract
It has been shown that TLR7 and TLR9 signaling play a role in SLE pathogenesis. Our recent study revealed that estrogen receptor α knockout mice have impaired inflammatory responses to TLR3, TLR4, TLR7 and TLR9 ligand stimulation in DCs, B cells and whole spleen cells. These findings indicate that estrogen receptor mediated signaling may impact universal TLR responsiveness. Whether estrogen has a direct or indirect effect on TLR responsiveness by immune cells is not clear. There is evidence of a role of TLR4 in SLE disease pathogenesis, such as the kidney damage, the induction of CD40 and autoantibodies, the suppression of regulatory T cells, and the role of pro-inflammatory cytokines (e.g., IL-6, IL-1β, TNF-α) in SLE pathogenesis that can be induced by TLR4-mediated monocyte activation, suggesting that TLR4 and TLR4 responsiveness are also important for SLE disease. This review will focus on TLR4 responses and monocytes, which are understudied in systemic autoimmune diseases such as SLE.
Keywords: Toll-like receptor, monocytes, sex, autoimmunity, SLE
Women exhibit stronger cellular-mediated and humoral-mediated immune responses compared to men, and a higher risk of autoimmune disease [1]. The ratio of female to male disease prevalence of systemic lupus erythematosus (SLE), for example, is 9:1 [2]. Although several mechanisms, such as Toll-like receptor (TLR) 7 expression, activity of T regulatory cells, or genetic and environmental factors [1–10], could account for heightened immune responses and increased incidences of autoimmune disease in women, the exact mechanisms are not fully understood. Though sex chromosomes partially account for the sex differences in autoimmune diseases; sex hormones and their receptors are also a likely major determinant as the onset of SLE most often occurs in women at the age of child-bearing potential [11].
In the periphery, human B cells express estrogen receptor β, while plasmacytoid dendritic cells (pDCs) and CD4 T cells express estrogen receptor α. CD8 T cells and monocytes express low to undetectable levels of estrogen receptors [12]. Studies from Guery’s group [13] showed that pDCs from premenopausal women have heightened responses to TLRs compared to men, while pDCs from postmenopausal women do not. Adding estrogen in vitro to pDC cultures had no effect on TLR7 responses. When postmenopausal women were given estrogen replacement, their pDCs had responses similar to premenopausal women. Again, in vitro addition of estrogen had no effect. These effects were mediated via ERα and were pDC centric, and indicate that the effect of estrogen on TLR responses of pDCs from women is via an indirect mechanism [13]. Our previous study showed that TLR3, TLR4, TLR7 and TLR9 responsiveness was decreased in immune cells from estrogen receptor α knockout mice [14], suggesting that not only TLR7 responsiveness, but other TLR responsiveness is modified by estrogen receptor signaling. But whether estrogen has a direct effect on TLR responsiveness in peripheral lymphocytes is not clear. Dendritic cells (DCs), especially pDCs produce a large amount of IFN-α in response to TLR7 and TLR9 ligands, which play an important role in the pathogenesis of SLE disease [15,16]. pDCs produce more IFN-α in women than men in response to TLR7 ligands, perhaps due to TLR7 being located on the X chromosome with variable expression of TLR7 between men and women leading to variable responsiveness [8,17]. The universal heightened TLR7 responsiveness in women versus men would argue against variable TLR7 expression in individual women being the proximate mechanism. To expand the scope of sex differences in TLR responsiveness beyond TLR7 and dendritic cells, this review will focus on sex differences in TLR4 responsiveness and monocyte populations in healthy individuals and patients with SLE.
Monocytes
Human monocytes represent 5–10% of peripheral blood mononuclear cells (PBMCs) and are progenitors of macrophages and DCs [18–20]. They express high levels of TLR1, TLR2 and TLR4 compared to lymphocytes [21], and produce pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-1β) triggered through TLR activation [16]. Little is known about the effect of sex hormones on the modulation of monocyte activation, maturation, subset differentiation, and antigen-presentation function. Previous studies showed increased total monocyte numbers in the periphery in the luteal phase compared to the follicular phase in women [22]. The data on estrogen receptor expression on monocytes is controversial [12,23–26]. Progesterone and testosterone receptors are not expressed in monocytes.
Monocyte subsets
Monocytes can be defined into two subsets (CD14+CD16+, CD14+CD16−) or three subsets (CD14++CD16−, CD14++CD16+, CD14+CD16++) as identified recently [27–31]. CD14++CD16− classic monocytes produce IL-10; and CD16-expressing non-classic monocytes (either intermediate or non-classical subset) produce TNF-α, IL-6 and IL-1β in response to a variety of TLR ligands [32,33]. The non-classic monocyte subset (CD14+CD16++) expresses a unique pattern of chemokine receptors and produces pro-inflammatory cytokines, and plays a role in cardiovascular risk in chronic kidney disease [31]. Moreover, these cells have distinct effector responses to virus and immune complexes containing nucleic acids, via a TLR7 or TLR8 pathway [29]. Virus and certain pro-inflammatory cytokines (e.g., type I IFN) have the function of regulating the expression of CD16 on monocytes [29,34,35]. Elevated levels of CD16-expressing monocytes are seen in blood during inflammatory conditions, such as HIV disease, atherosclerosis [27,30,36], sepsis [37], rheumatoid arthritis, SLE [38–40], and cancer [41], suggesting that inflammation (including microbial TLR ligands) promotes monocyte differentiation into a CD16-expressing subset in vivo. CD16 expression on monocytes can be regulated by estradiol in vitro, but the results are controversial [42,43].
Monocyte activation and maturation
LPS activates and promotes maturation of monocytes [44,45]. After activation and maturation, monocytes increase expression of CD80, CD40, CD86 and HLA-DR, secrete pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β and sCD14), and change their ability for phagocytosis and antigen presenting and processing function [44,45]. These cells can differentiate to macrophages and DCs under certain conditions [46–48]. DCs are professional antigen presenting cells due to their ability to prime naïve T cells and cross present to CD8 T cells [49,50]. On the other hand, monocytes, as another type of antigen-presenting cells, account for 5–10% of cells in the peripheral blood, compared to 1% of DCs. Although less ability to present antigens to T cells compared to DCs, monocytes are important in antigen presentation overall due to their large number in the periphery and their roles as DC progenitors.
Monocytes in SLE
An increased number of monocytes and increased activation of monocytes are present in the periphery in SLE patients compared to controls [51]. Monocytes spontaneously release pro-inflammatory cytokines such as IL-6 and are a predominant source of IL-6 in SLE [52]. CD16+DR++ monocytes are also the major source of TNFα in response to TLR stimulation [33]. Treatments targeting such pro-inflammatory cytokines (e.g., TNFα, IL-6 and IL-1β) are effective in animal models of SLE and patients with SLE [53–56]. These results suggest that monocytes are activated in vivo, produce pro-inflammatory cytokines (e.g., IL-6), and play a key role in chronic inflammation and disease pathogenesis in SLE [57]. Moreover, LPS also may account for kidney damage in SLE disease [58,59]. Therefore, there may be a link between TLR4 signaling, LPS-mediated monocyte activation, subset differentiation, and SLE pathogenesis. An elevated plasma level of soluble CD14, which is released by monocytes in response to LPS, is present in SLE patients [60]. Our previous work indicated that TLR9 ligands, bacterial CpG ODNs, induce monocytes to express CD80, CD86, CD40 and HLA-DR, and drive monocytes to be better antigen-presenting cells; this effect is through type I IFN [61]. Moreover, in vitro IFN-α induces monocyte activation as measured by expression of CD80, CD86, CD40 and HLA-DR [61]. Levels of IFN-α are elevated and involved in the pathogenesis of SLE [62]. In vitro, these monocytes have impaired ability to up-regulate CD80 and CD86 expression following stimuli such as IFN-γ, [51,63], suggesting that in vivo monocytes in SLE are pre-activated. Therefore they may be desensitized to be activated again in vitro. Moreover, Fc gamma receptor genes, associated with monocyte activation, partially account for the underlying immune mechanisms resulting in SLE [64–69]. Patients with SLE have increased levels of pro-inflammatory cytokines in plasma, implying that heightened levels of innate immune responses, including TLR signaling, may contribute to the etiology and pathogenesis in SLE.
TLRs
TLRs play an important role in innate immunity and recognize pathogens through pathogen associated molecular patterns (PAMPs). In the periphery, antigen-presenting cells (monocytes/macrophages, DCs and B cells) are the predominant cell populations to express TLRs, and directly respond to TLR ligands [16,70]. TLR signals are essensal for maintaining normal immunity, and certain TLR ligands such as CpG ODNs and imiqimod are used as vaccine adjuvants to increase vaccine-specific responses [71,72]. Cytoplasmic Toll-IL-1 receptor (TIR) domains are activated by TLR signaling pathways initially. The TLR-activated TIR domain is associated with MyD88, which recruits IL-1 receptor associated kinase (IRAK) to TLRs upon activation. MyD88 knockout mice have no response to stimulation by TLR5, TLR7 and TLR9 ligands [73–76]. These evidences indicate that the TIR domain-associated adaptor MyD88 is nessessary for these TLR mediated responses [77]. Consistently, responses to TLR2, TLR3, TLR4, and TLR9 agonists are almost abolished in IRAK-4 knockout mice [78]. There are, however, also MyD88-independent TLR signaling pathways [79]. These findings in animal models suggest that TLR signaling knockout results in immune-deficiencies, while, robust TLR-mediated hyperactivity could drive autoimmune diseases.
It is well recognized that females have heightened responses to TLR7 ligands [8,17]. Human B cells, pDCs and myeloid dendritic cells (mDCs) express TLR7, and respond to its ligands, imiquimod, HIV viral sequences, gardiquimod or loxoribine et al [21]. TLR7 signaling is involved in SLE disease largely due to its downstream cytokine IFN-α production by pDCs, resulting in higher levels of IFN-α in cells from females compared to males, and in SLE patients compared to controls [17,80–82]. TLR7, TLR8 and TLR9 signaling pathways in pDCs in SLE are extensively studied, and treatment with inhibitors against TLR7/8 and TLR9 are in Phase I trials in patients with SLE [83–86].
TLR4 expression and responsiveness
In the periphery, human monocytes express the highest TLR4 levels of PBMCs. MDCs are the other cell type to express TLR4; both cells produce pro-inflammatory cytokines such as IL-1β, TNF-α or IL-6 in response to the TLR4 ligand LPS [21,87,88]. Human pDCs, B cells, T cells, and NK cells do not express TLR4, and do not directly respond to its ligand LPS [21]. TLR4 knockout or mutations in mice exhibit defects in responses to LPS, including pro-inflammatory cytokine production, susceptibility to bacterial infections, tissue injury induced ischemia, myocardial infarction, neuro-degeneration, and cancer related immunities [77,89–95].
Sex differences in TLR4 responsiveness in monocytes are listed as follows: TNF-α
Monocytes from males produce higher levels of TNF-α in response to LPS compared to females [96–99]. However, the results from in vitro experiments are conflicting [96,97,99–102]. IL-1β. There are increased plasma levels of IL-1β and LPS-induced IL-1β-producing monocytes in the luteal phase compared to the follicular phase [25,103,104]. IL-12. LPS induced IL-12 production by monocytes was higher in men compared to women, but similar in women in luteal phase versus follicular phase [25,96], suggesting that androgens may affect IL-12 production by monocytes through TLR4. IL-6. Results related to sex differences in IL-6 production in response to LPS in controls and patients with SLE are conflicting [102,105–109]. Aulock’s group reported that TNFα, IL-1β, IL-6 and IL-8 production by monocytes in response to LPS was similar or less in women compared to men [99]. Conflicting data were also reported whether estrogen/progesterone affect cytokine production by LPS-stimulated monocytes in humans [96]. The sex differences in monocyte activation and maturation may include both differences in quantity and quality (e.g., genetic factors). It is difficult to draw a conclusion on sex differences in TLR4 responsiveness in monocytes; nevertheless, women seemingly have a reduced TLR4 responsiveness to LPS in monocytes in vitro. This could be due to pre-activation and desensitization in monocytes in women, or due to sex differences in TLR expression and signaling responses in monocytes.
The responses to the TLR4 ligand LPS involve the LPS binding protein (LBP), HDL particles, MD2, TLR4 and soluble CD14 [110–113]. Peripheral LPS is mainly cleared in the liver [114]; LBP transfers LPS to HDL particles, which leads to sequestration of LPS-induced responses [112,115]. A previous study showed that plasma sCD14 also plays a role in the inactivation of LPS-induced host responses [110]. There is no clear evidence of a sex difference in TLR4 expression on monocytes. Although these results were not always consistent, as we stated in the last paragraph, in vivo the levels of several cytokines and in vitro monocyte responses to LPS are different based on female menstrual cycles, suggesting that there is an effect of sex hormones on TLR4 responses and monocytes in vivo. However, it is not clear in vitro whether monocytes have a direct response to estrogen.
Several mechanisms could be accounting for the effect of estrogen on TLR4 effects, including direct effects through levels of estrogen and estrogen receptors, and indirect effects through sex mediated differences in cytokine patterns. TLR4-responding cells may express estrogen receptors and directly respond to estrogen. As a result, estrogen activation may change TLR expression or signaling transduction at the single cell level. In contrast, TLR-responding cells may not express estrogen receptors. Their response to estrogen is through indirect activation by estrogen-responsive cells. For example, pDCs express ERα and TLR7/TLR9. Estrogen increased TLR7 or TLR9-mediated IFNα production in pDCs, but there is no evidence that this is a direct response of pDCs to estrogen [116].
TLR4 responsiveness in SLE
TLR4 responses play a role in SLE pathogenesis in murine models [117–120]; the potential mechanisms include TLR4-mediated suppression of regulatory T cells [121], induction of CD40 expression on antigen-presenting cells [122], and induction of autoantibodies [123]. There is no reported difference in the TLR4 expression in PBMCs from controls and SLE patients [124]. Monocytes spontaneously secrete TNF-α or IL-6 in SLE disease [57]. In vivo, plasma levels of IL-6, IL-10 and TNF-α are elevated in SLE patients compared to controls. Moreover, SLE is associated with increased numbers of monocytes in the periphery, increased expression of Fc receptors, increased levels of IgG production, decreased function of phagocytes in response to LPS, and increased levels of soluble CD14 and LBP [60,125–128]. LPS has a reported pathogenic role in SLE pathogenesis [58,117,129]. In vitro, IL-1β and IL-6 production by monocytes from SLE patients in response to LPS is reduced regardless of disease activity, but TNF-α production remains the same in monocytes compared to controls [57,130]. In murine macrophages, TLR4 expression and pro-inflammatory cytokine production are decreased after removal of endogenous estrogens, and exogenous replacement of 17β-estradiol reverses this effect [131]. Moreover, treatment with low dose steroids or chloroquine did not have a significant effect on TLR4 expression and signaling activation. High dose corticosteroids decrease cytokine production (TNF-α and IL-6) in response to LPS [57,124]. These results suggest that monocytes from SLE are activated, release pro-inflammatory cytokines, and contribute to disease pathogenesis, especially at target tissue sites (e.g., kidney) [58,117,129].
TLR4 responsiveness and monocyte activation in other autoimmune diseases
TLR4 responsiveness is reported to play a role in the pathogenesis of other autoimmune diseases besides SLE including coxsackievirus-induced autoimmune myocarditis [132] collagen-induced arthritis [133], primary biliary cirrhosis [134], experimental autoimmune uveitis (EAU) [135], antibody-mediated glomerulonephritis [136] and autoimmune destructive arthritis [137]. However, most data were in mice; data in humans are largely lacking. Furthermore, the subset of CD14+/CD16+ blood monocytes is expanded in autoimmune diseases such as rheumatoid arthritis, [38], and plays a role in the pathogenesis of experimental autoimmune encephalomyelitis (EAE) [138]. Importantly, TLR4 downstream pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6 are key mediators in several autoimmune diseases [139–142] besides SLE. Therefore, TLR4 signaling is involved in the pathogenesis of several autoimmune diseases, and needs to be studied further.
Genetic predisposition in SLE
There are variant genes associated with the etiology and pathogenesis of SLE, including antigen presentation molecules (HLA-DQ, HLA-DR alleles) [143,144], complement related genes (C1q, C2 and C4) [145–148], Fc gamma receptors (CD64, CD32 and CD16), [64–69], the programmed cell death 1 gene (PDD1) [149–151], IFN and TNF related genes [152–154], and the C-reactive protein (CRP) gene [155,156].
Female cells carry both maternal and paternal X chromosomes, whereas male cells carry only the maternal X chromosome. The inactivation of X chromosomes is not random. However, roughly 16% of healthy females aged 50 or older are shown to have a skewed X-chromosome inactivation [157,158]. Furthermore, certain frequencies of X-linked genes are known to escape inactivation, and express both alleles on the X chromosomes [159–161]. It is possible that perturbation in X-chromosome inactivation results in the breakdown of tolerance and the induction of autoimmunity.
Anti-estrogen treatment in SLE
In female (NZB × NZW) F1 and MRLlpr/lpr mice, anti-estrogen had beneficial effects on experimental SLE, including reduction of anti-DNA production and immune complex–mediated glomerulonephritis, and prolonged survival [162–164]. Clearly, estrogen treatment in mice not only enhances disease progression but also drives increased serum anti-dsDNA antibody titers [162,163,165]. However, in SLE patients, treatment with anti-estrogens, has led to mixed responses [165–168]. Treatment of female lupus patients with estrogen containing birth control pills premenopausal or use of hormone replacement therapy post menopausal had minimal to no effect on disease. There is no evidence that use of estrogens increases the risk for developing lupus.
Summary
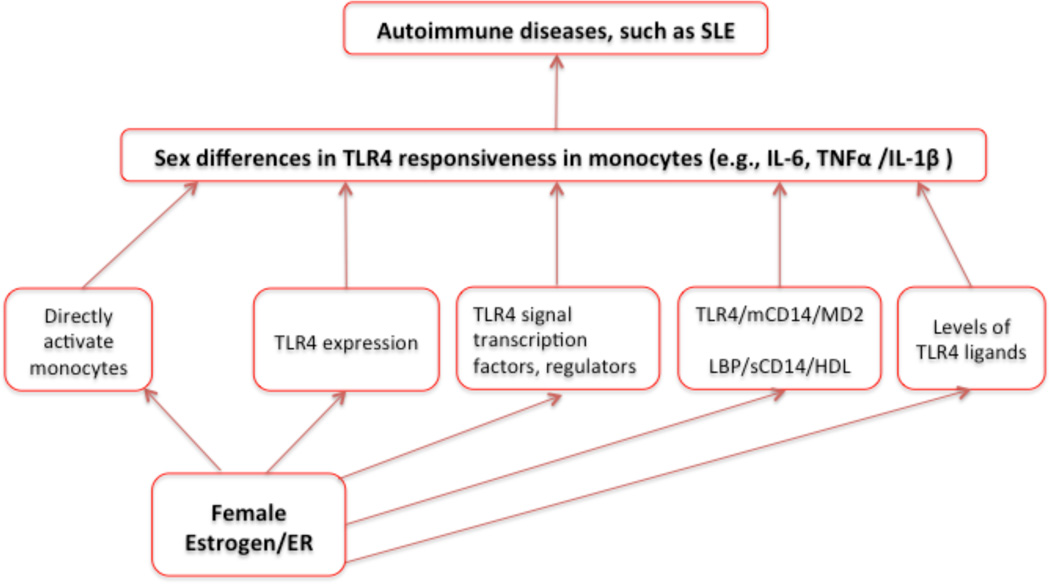
The subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients, and also elevated in SLE. This subset of monocytes is a major source of pro-inflammatory cytokines such as TNF-α. Data in mice indicate that LPS and TLR4 play a role in mediating autoimmunity, pro-inflammatory cytokine production, and other immune activation. Sex hormones impact TLR4-associated innate immune responses in monocytes in healthy individuals and in patients with SLE (Figure 1). In our model, sex hormones (e.g., estrogen) could directly activate or indirectly activate monocytes, and change TLR4 responsiveness in monocytes through several mechanisms, such as sex hormone associated changes in the levels of TLR4 ligands, TLR4 expression, TLR4 signaling pathway, and LPS-TLR4 interaction cofactors (Figure 1). Monocytes in SLE patients are activated and produce pro-inflammatory cytokines. Importantly, TLR4-mediated pro-inflammatory cytokines (e.g., IL-6, IL-1β and TNF-α) are increased and play an important role in the etiology and pathogenesis of SLE [169]. Treatments targeting these cytokines or specific TLRs are at least partially effective in animal models of SLE and are in clinical trials in patients with SLE [15,170]. Therefore, further therapeutic strategies should not only focus on TLR7/8 and TLR9 signaling, but also should investigate the contribution of TLR4 signaling in lupus pathogenesis and sex differences in the prevalence of autoimmune diseases.
Figure 1. A model of sex differences in monocytes and TLR4 responsiveness.
The effect of sex hormones (e.g., estrogen) on TLR4 responsiveness and monocyte activation could be through direct activation of monocytes, or through indirect activation of monocytes. These actions include estrogen effects on TLR4 expression, TLR4 signaling pathways, LPS interaction cofactors (e.g., TLR4, CD14, MD2 and LBP), and levels of TLR4 ligands. As a consequence of monocyte activation and altered TLR4 responsiveness, there are increased levels of downstream pro-inflammatory cytokines (e.g, TNFα, IL-1β and IL-6), which play a role in autoimmune diseases such as SLE.
Acknowledgement
This work is supported by NIAID grant AI91526, NIAMS supplemental grant P60AR062755, the Medical Research Service at the Ralph H. Johnson VAMC, and the National 12th Five-Year Major Projects of China (2012ZX10001-003, 2012ZX10001-006).
Abbreviations
- TLR
Toll-like receptor
- TIR
Toll/IL-1 receptor
- ERα
Estrogen receptor alpha
- SLE
systemic lupus erythematosis
- pDCs
plasmacytoid dendritic cells
- IFN-α
Interferon alpha
- PAMPs
pathogen associated molecular patterns
- PBMCs
peripheral blood mononuclear cells
Reference
- 1.Voskuhl R. Sex differences in autoimmune diseases. Biol Sex Differ. 2011;2:1. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin Rev Allergy Immunol. 2011;40:42–49. doi: 10.1007/s12016-009-8192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshan G, Afzal N, Qureshi S. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin Lab. 2012;58:567–571. [PubMed] [Google Scholar]
- 4.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–2550. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Karnell JL, Yin B, Zhang R, Zhang J, et al. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J Clin Invest. 2010;120:2497–2507. doi: 10.1172/JCI42382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Helms C, Liao W, Zaba LC, Duan S, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4:e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenzo N, Barbera A, Dominguez MC, Torres AM, Hernandez MV, et al. Therapeutic effect of an altered peptide ligand derived from heat-shock protein 60 by suppressing of inflammatory cytokines secretion in two animal models of rheumatoid arthritis. Autoimmunity. 2012;45:449–459. doi: 10.3109/08916934.2012.697592. [DOI] [PubMed] [Google Scholar]
- 8.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weckerle CE, Franek BS, Kelly JA, Kumabe M, Mikolaitis RA, et al. Network analysis of associations between serum interferon-alpha activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 2011;63:1044–1053. doi: 10.1002/art.30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Yu Y, Matarese G, La Cava A. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J Immunol. 2012;188:2070–2073. doi: 10.4049/jimmunol.1102835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petri M, Robinson C. Oral contraceptives and systemic lupus erythematosus. Arthritis Rheum. 1997;40:797–803. doi: 10.1002/art.1780400504. [DOI] [PubMed] [Google Scholar]
- 12.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Seillet C, Rouquie N, Foulon E, Douin-Echinard V, Krust A, et al. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor alpha. J Immunol. 2013;190:5459–5470. doi: 10.4049/jimmunol.1203312. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham MA, Naga OS, Eudaly JG, Scott JL, Gilkeson GS. Estrogen receptor alpha modulates Toll-like receptor signaling in murine lupus. Clin Immunol. 2012;144:1–12. doi: 10.1016/j.clim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill JT, Wallace DJ, Petri M, Kirou KA, Yao Y, et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann Rheum Dis. 2011;70:1905–1913. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 16.Krieg AM, Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251–269. doi: 10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 17.Berghofer B, Frommer T, Haley G, Fink L, Bein G, et al. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 18.Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171:2262–2269. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- 19.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 22.Mathur S, Mathur RS, Goust JM, Williamson HO, Fudenberg HH. Cyclic variations in white cell subpopulations in the human menstrual cycle: correlations with progesterone and estradiol. Clin Immunol Immunopathol. 1979;13:246–253. doi: 10.1016/0090-1229(79)90069-2. [DOI] [PubMed] [Google Scholar]
- 23.Suenaga R, Evans MJ, Mitamura K, Rider V, Abdou NI. Peripheral blood T cells and monocytes and B cell lines derived from patients with lupus express estrogen receptor transcripts similar to those of normal cells. J Rheumatol. 1998;25:1305–1312. [PubMed] [Google Scholar]
- 24.Wada K, Itoh T, Nakagawa M, Misao R, Mori H, et al. Estrogen binding sites in peripheral blood monocytes and effects of danazol on their sites in vitro. Gen Pharmacol. 1992;23:693–700. doi: 10.1016/0306-3623(92)90150-i. [DOI] [PubMed] [Google Scholar]
- 25.Bouman A, Moes H, Heineman MJ, de Leij LF, Faas MM. The immune response during the luteal phase of the ovarian cycle: increasing sensitivity of human monocytes to endotoxin. Fertil Steril. 2001;76:555–559. doi: 10.1016/s0015-0282(01)01971-9. [DOI] [PubMed] [Google Scholar]
- 26.McCrohon JA, Nakhla S, Jessup W, Stanley KK, Celermajer DS. Estrogen and progesterone reduce lipid accumulation in human monocyte-derived macrophages: a sex-specific effect. Circulation. 1999;100:2319–2325. doi: 10.1161/01.cir.100.23.2319. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar-Ruiz SR, Torres-Aguilar H, Gonzalez-Dominguez E, Narvaez J, Gonzalez-Perez G, et al. Human CD16+ and CD16− monocyte subsets display unique effector properties in inflammatory conditions in vivo. J Leukoc Biol. 2011;90:1119–1131. doi: 10.1189/jlb.0111022. [DOI] [PubMed] [Google Scholar]
- 28.Amir O, Spivak I, Lavi I, Rahat MA. Changes in the monocytic subsets CD14(dim)CD16(+) and CD14(++)CD16(−) in chronic systolic heart failure patients. Mediators Inflamm. 2012;2012:616384. doi: 10.1155/2012/616384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heine GH, Ortiz A, Massy ZA, Lindholm B, Wiecek A, et al. Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol. 2012;8:362–369. doi: 10.1038/nrneph.2012.41. [DOI] [PubMed] [Google Scholar]
- 32.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–677. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 33.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 34.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong KL, Tai JJ, Wong WC, Han H, Sem X, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 36.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 37.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, et al. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–3176. [PubMed] [Google Scholar]
- 38.Cairns AP, Crockard AD, Bell AL. The CD14+ CD16+ monocyte subset in rheumatoid arthritis and systemic lupus erythematosus. Rheumatol Int. 2002;21:189–192. doi: 10.1007/s00296-001-0165-8. [DOI] [PubMed] [Google Scholar]
- 39.Nakatani K, Yoshimoto S, Iwano M, Asai O, Samejima K, et al. Fractalkine expression and CD16+ monocyte accumulation in glomerular lesions: association with their severity and diversity in lupus models. Am J Physiol Renal Physiol. 2010;299:F207–F216. doi: 10.1152/ajprenal.00482.2009. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto S, Nakatani K, Iwano M, Asai O, Samejima K, et al. Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am J Kidney Dis. 2007;50:47–58. doi: 10.1053/j.ajkd.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Saleh MN, Goldman SJ, LoBuglio AF, Beall AC, Sabio H, et al. CD16+ monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood. 1995;85:2910–2917. [PubMed] [Google Scholar]
- 42.Kramer PR, Winger V, Kramer SF. 17beta-Estradiol utilizes the estrogen receptor to regulate CD16 expression in monocytes. Mol Cell Endocrinol. 2007;279:16–25. doi: 10.1016/j.mce.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004;50:1967–1975. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 44.Guha M, O'Connell MA, Pawlinski R, Hollis A, McGovern P, et al. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- 45.Rossol M, Heine H, Meusch U, Quandt D, Klein C, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 46.Iwamoto S, Iwai S, Tsujiyama K, Kurahashi C, Takeshita K, et al. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J Immunol. 2007;179:1449–1457. doi: 10.4049/jimmunol.179.3.1449. [DOI] [PubMed] [Google Scholar]
- 47.Polancec DS, Munic Kos V, Banjanac M, Vrancic M, Cuzic S, et al. Azithromycin drives in vitro GM-CSF/IL-4-induced differentiation of human blood monocytes toward dendritic-like cells with regulatory properties. J Leukoc Biol. 2012;91:229–243. doi: 10.1189/jlb.1210655. [DOI] [PubMed] [Google Scholar]
- 48.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 49.Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat Immunol. 2013;14:254–261. doi: 10.1038/ni.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mouries J, Moron G, Schlecht G, Escriou N, Dadaglio G, et al. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu MF, Li JS, Weng TH, Lei HY. Differential expression and modulation of costimulatory molecules CD80 and CD86 on monocytes from patients with systemic lupus erythematosus. Scand J Immunol. 1999;49:82–87. doi: 10.1046/j.1365-3083.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 52.Mellor-Pita S, Citores MJ, Castejon R, Yebra-Bango M, Tutor-Ureta P, et al. Monocytes and T lymphocytes contribute to a predominance of interleukin 6 and interleukin 10 in systemic lupus erythematosus. Cytometry B Clin Cytom. 2009;76:261–270. doi: 10.1002/cyto.b.20468. [DOI] [PubMed] [Google Scholar]
- 53.Aringer M, Smolen JS. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. 2008;10:202. doi: 10.1186/ar2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang B, Gardner DB, Griswold DE, Bugelski PJ, Song XY. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119:296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112:397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robak E, Robak T. Monoclonal antibodies in the treatment of systemic lupus erythematosus. Curr Drug Targets. 2009;10:26–37. doi: 10.2174/138945009787122833. [DOI] [PubMed] [Google Scholar]
- 57.Swaak AJ, van den Brink HG, Aarden LA. Cytokine production (IL-6 and TNF alpha) in whole blood cell cultures of patients with systemic lupus erythematosus. Scand J Rheumatol. 1996;25:233–238. doi: 10.3109/03009749609069992. [DOI] [PubMed] [Google Scholar]
- 58.Zhai JX, Zhang ZX, Feng YJ, Ding SS, Wang XH, et al. PDTC attenuate LPS-induced kidney injury in systemic lupus erythematosus-prone MRL/lpr mice. Mol Biol Rep. 2012;39:6763–6771. doi: 10.1007/s11033-012-1501-7. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Liu S, Yu Y, Zhang T, Liu J, et al. Immune complex enhances tolerogenecity of immature dendritic cells via FcgammaRIIb and promotes FcgammaRIIb-overexpressing dendritic cells to attenuate lupus. Eur J Immunol. 2011;41:1154–1164. doi: 10.1002/eji.201040767. [DOI] [PubMed] [Google Scholar]
- 60.Nockher WA, Wigand R, Schoeppe W, Scherberich JE. Elevated levels of soluble CD14 in serum of patients with systemic lupus erythematosus. Clin Exp Immunol. 1994;96:15–19. doi: 10.1111/j.1365-2249.1994.tb06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang W, Lederman MM, Salkowitz JR, Rodriguez B, Harding CV, et al. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in alpha/beta interferon responsiveness and production. J Virol. 2005;79:4109–4119. doi: 10.1128/JVI.79.7.4109-4119.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindau D, Mussard J, Rabsteyn A, Ribon M, Kotter I, et al. TLR9 independent interferon alpha production by neutrophils on NETosis in response to circulating chromatin, a key lupus autoantigen. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-203041. [DOI] [PubMed] [Google Scholar]
- 63.Tsokos GC, Kovacs B, Sfikakis PP, Theocharis S, Vogelgesang S, et al. Defective antigen-presenting cell function in patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:600–609. doi: 10.1002/art.1780390409. [DOI] [PubMed] [Google Scholar]
- 64.Brown EE, Edberg JC, Kimberly RP. Fc receptor genes and the systemic lupus erythematosus diathesis. Autoimmunity. 2007;40:567–581. doi: 10.1080/08916930701763710. [DOI] [PubMed] [Google Scholar]
- 65.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salmon JE, Millard S, Schachter LA, Arnett FC, Ginzler EM, et al. Fc gamma RIIA alleles are heritable risk factors for lupus nephritis in African Americans. J Clin Invest. 1996;97:1348–1354. doi: 10.1172/JCI118552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kyogoku C, Dijstelbloem HM, Tsuchiya N, Hatta Y, Kato H, et al. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 2002;46:1242–1254. doi: 10.1002/art.10257. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Lee PY, Sobel ES, Narain S, Satoh M, et al. Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis Res Ther. 2009;11:R6. doi: 10.1186/ar2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karassa FB, Trikalinos TA, Ioannidis JP, Fcgamma R-SLEM-AI. Role of the Fcgamma receptor IIa polymorphism in susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysis. Arthritis Rheum. 2002;46:1563–1571. doi: 10.1002/art.10306. [DOI] [PubMed] [Google Scholar]
- 70.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 71.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwissa M, Nakaya HI, Oluoch H, Pulendran B. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood. 2012;119:2044–2055. doi: 10.1182/blood-2011-10-388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hacker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, et al. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J Exp Med. 2000;192:595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 75.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 76.Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr Biol. 2000;10:1139–1142. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 77.Bauerfeld CP, Rastogi R, Pirockinaite G, Lee I, Huttemann M, et al. TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J Immunol. 2012;188:2847–2857. doi: 10.4049/jimmunol.1102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 79.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 80.Anders HJ, Krug A, Pawar RD. Molecular mimicry in innate immunity? The viral RNA recognition receptor TLR7 accelerates murine lupus. Eur J Immunol. 2008;38:1795–1799. doi: 10.1002/eji.200838478. [DOI] [PubMed] [Google Scholar]
- 81.Pan ZJ, Maier S, Schwarz K, Azbill J, Akira S, et al. Toll-like receptor 7 (TLR7) modulates anti-nucleosomal autoantibody isotype and renal complement deposition in mice exposed to syngeneic late apoptotic cells. Ann Rheum Dis. 2010;69:1195–1199. doi: 10.1136/ard.2009.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 83.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 84.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 86.Lenert PS. Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like receptors (TLR) 7 and 9. Mediators Inflamm. 2010;2010:986596. doi: 10.1155/2010/986596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andersson LI, Hellman P, Eriksson H. Receptor-mediated endocytosis of particles by peripheral dendritic cells. Hum Immunol. 2008;69:625–633. doi: 10.1016/j.humimm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 88.Pelletier S, Bedard N, Said E, Ancuta P, Bruneau J, et al. Sustained hyperresponsiveness of dendritic cells is associated with spontaneous resolution of acute hepatitis C. J Virol. 2013;87:6769–6781. doi: 10.1128/JVI.02445-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 90.Illario M, Giardino-Torchia ML, Sankar U, Ribar TJ, Galgani M, et al. Calmodulin-dependent kinase IV links Toll-like receptor 4 signaling with survival pathway of activated dendritic cells. Blood. 2008;111:723–731. doi: 10.1182/blood-2007-05-091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61:1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nunez NG, Andreani V, Crespo MI, Nocera DA, Breser ML, et al. IFNbeta produced by TLR4-activated tumor cells is involved in improving the antitumoral immune response. Cancer Res. 2012;72:592–603. doi: 10.1158/0008-5472.CAN-11-0534. [DOI] [PubMed] [Google Scholar]
- 93.Riddell JR, Bshara W, Moser MT, Spernyak JA, Foster BA, et al. Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4-dependent regulation of tumor vasculature. Cancer Res. 2011;71:1637–1646. doi: 10.1158/0008-5472.CAN-10-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, et al. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhuang L, Jung JY, Wang EW, Houlihan P, Ramos L, et al. Pseudomonas aeruginosa lipopolysaccharide induces osteoclastogenesis through a toll-like receptor 4 mediated pathway in vitro and in vivo. Laryngoscope. 2007;117:841–847. doi: 10.1097/MLG.0b013e318033783a. [DOI] [PubMed] [Google Scholar]
- 96.Bouman A, Schipper M, Heineman MJ, Faas MM. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol. 2004;52:19–26. doi: 10.1111/j.1600-0897.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 97.Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, et al. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 2001;16:340–343. doi: 10.1097/00024382-200116050-00003. [DOI] [PubMed] [Google Scholar]
- 98.Schwarz E, Schafer C, Bode JC, Bode C. Influence of the menstrual cycle on the LPS-induced cytokine response of monocytes. Cytokine. 2000;12:413–416. doi: 10.1006/cyto.1999.0570. [DOI] [PubMed] [Google Scholar]
- 99.Aulock SV, Deininger S, Draing C, Gueinzius K, Dehus O, et al. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J Interferon Cytokine Res. 2006;26:887–892. doi: 10.1089/jir.2006.26.887. [DOI] [PubMed] [Google Scholar]
- 100.Rogers A, Eastell R. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30–34. doi: 10.1016/s8756-3282(01)00468-9. [DOI] [PubMed] [Google Scholar]
- 101.Ralston SH, Russell RG, Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990;5:983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- 102.Brannstrom M, Friden BE, Jasper M, Norman RJ. Variations in peripheral blood levels of immunoreactive tumor necrosis factor alpha (TNFalpha) throughout the menstrual cycle and secretion of TNFalpha from the human corpus luteum. Eur J Obstet Gynecol Reprod Biol. 1999;83:213–217. doi: 10.1016/s0301-2115(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 103.Cannon JG, Dinarello CA. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985;227:1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- 104.Polan ML, Loukides JA, Honig J. Interleukin-1 in human ovarian cells and in peripheral blood monocytes increases during the luteal phase: evidence for a midcycle surge in the human. Am J Obstet Gynecol. 1994;170:1000–1006. doi: 10.1016/s0002-9378(94)70093-1. discussion 1006–1007. [DOI] [PubMed] [Google Scholar]
- 105.Jilma B, Dirnberger E, Loscher I, Rumplmayr A, Hildebrandt J, et al. Menstrual cycle-associated changes in blood levels of interleukin-6, alpha1 acid glycoprotein, and C-reactive protein. J Lab Clin Med. 1997;130:69–75. doi: 10.1016/s0022-2143(97)90060-3. [DOI] [PubMed] [Google Scholar]
- 106.Kania DM, Binkley N, Checovich M, Havighurst T, Schilling M, et al. Elevated plasma levels of interleukin-6 in postmenopausal women do not correlate with bone density. J Am Geriatr Soc. 1995;43:236–239. doi: 10.1111/j.1532-5415.1995.tb07328.x. [DOI] [PubMed] [Google Scholar]
- 107.McKane WR, Khosla S, Peterson JM, Egan K, Riggs BL. Circulating levels of cytokines that modulate bone resorption: effects of age and menopause in women. J Bone Miner Res. 1994;9:1313–1318. doi: 10.1002/jbmr.5650090821. [DOI] [PubMed] [Google Scholar]
- 108.Rachon D, Mysliwska J, Suchecka-Rachon K, Wieckiewicz J, Mysliwski A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J Endocrinol. 2002;172:387–395. doi: 10.1677/joe.0.1720387. [DOI] [PubMed] [Google Scholar]
- 109.Scuderi F, Convertino R, Molino N, Provenzano C, Marino M, et al. Effect of pro-inflammatory/anti-inflammatory agents on cytokine secretion by peripheral blood mononuclear cells in rheumatoid arthritis and systemic lupus erythematosus. Autoimmunity. 2003;36:71–77. doi: 10.1080/0891693031000079275. [DOI] [PubMed] [Google Scholar]
- 110.Kitchens RL, Thompson PA, Viriyakosol S, O'Keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 112.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitchens RL, Wolfbauer G, Albers JJ, Munford RS. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J Biol Chem. 1999;274:34116–34122. doi: 10.1074/jbc.274.48.34116. [DOI] [PubMed] [Google Scholar]
- 114.Coulthard MG, Swindle J, Munford RS, Gerard RD, Meidell RS. Adenovirus-mediated transfer of a gene encoding acyloxyacyl hydrolase (AOAH) into mice increases tissue and plasma AOAH activity. Infect Immun. 1996;64:1510–1515. doi: 10.1128/iai.64.5.1510-1515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munford RS, Andersen JM, Dietschy JM. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981;68:1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seillet C, Laffont S, Tremollieres F, Rouquie N, Ribot C, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 117.Lee TP, Tang SJ, Wu MF, Song YC, Yu CL, et al. Transgenic overexpression of anti-double-stranded DNA autoantibody and activation of Toll-like receptor 4 in mice induce severe systemic lupus erythematosus syndromes. J Autoimmun. 2010;35:358–367. doi: 10.1016/j.jaut.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 118.Liu B, Yang Y, Dai J, Medzhitov R, Freudenberg MA, et al. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol. 2006;177:6880–6888. doi: 10.4049/jimmunol.177.10.6880. [DOI] [PubMed] [Google Scholar]
- 119.Summers SA, Hoi A, Steinmetz OM, O'Sullivan KM, Ooi JD, et al. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J Autoimmun. 2010;35:291–298. doi: 10.1016/j.jaut.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Lartigue A, Colliou N, Calbo S, Francois A, Jacquot S, et al. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]
- 121.Dai J, Liu B, Ngoi SM, Sun S, Vella AT, et al. TLR4 hyperresponsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J Immunol. 2007;178:3219–3225. doi: 10.4049/jimmunol.178.5.3219. [DOI] [PubMed] [Google Scholar]
- 122.Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN. LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood. 2005;106:3114–3122. doi: 10.1182/blood-2005-02-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harlow L, Fernandez I, Soejima M, Ridgway WM, Ascherman DP. Characterization of TLR4-mediated auto-antibody production in a mouse model of histidyl-tRNA synthetase-induced myositis. Innate Immun. 2012;18:876–885. doi: 10.1177/1753425912446714. [DOI] [PubMed] [Google Scholar]
- 124.Cepika AM, Bendelja K, Vergles JM, Malenica B, Kapitanovic S, et al. Monocyte response to LPS after exposure to corticosteroids and chloroquine with implications for systemic lupus erythematosus. Scand J Immunol. 2010;72:434–443. doi: 10.1111/j.1365-3083.2010.02450.x. [DOI] [PubMed] [Google Scholar]
- 125.Boswell J, Schur PH. Monocyte function in systemic lupus erythematosus. Clin Immunol Immunopathol. 1989;52:271–278. doi: 10.1016/0090-1229(89)90178-5. [DOI] [PubMed] [Google Scholar]
- 126.Carter SD, Bourne JT, Elson CJ, Hutton CW, Czudek R, et al. Mononuclear phagocytes in rheumatoid arthritis: Fc-receptor expression by peripheral blood monocytes. Ann Rheum Dis. 1984;43:424–429. doi: 10.1136/ard.43.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fries LF, Mullins WW, Cho KR, Plotz PH, Frank MM. Monocyte receptors for the Fc portion of IgG are increased in systemic lupus erythematosus. J Immunol. 1984;132:695–700. [PubMed] [Google Scholar]
- 128.Prokopec KE, Berntson L, Oman A, Kleinau S. Up regulated complement and fc receptors in juvenile idiopathic arthritis and correlation with disease phenotype. J Clin Immunol. 2012;32:540–550. doi: 10.1007/s10875-012-9657-4. [DOI] [PubMed] [Google Scholar]
- 129.Shui HA, Ka SM, Wu WM, Lin YF, Hou YC, et al. LPS-evoked IL-18 expression in mesangial cells plays a role in accelerating lupus nephritis. Rheumatology (Oxford) 2007;46:1277–1284. doi: 10.1093/rheumatology/kem136. [DOI] [PubMed] [Google Scholar]
- 130.Andersen LS, Petersen J, Svenson M, Bendtzen K. Production of IL-1beta, IL-1 receptor antagonist and IL-10 by mononuclear cells from patients with SLE. Autoimmunity. 1999;30:235–242. doi: 10.3109/08916939908993804. [DOI] [PubMed] [Google Scholar]
- 131.Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150:3877–3884. doi: 10.1210/en.2009-0098. [DOI] [PubMed] [Google Scholar]
- 132.Roberts BJ, Moussawi M, Huber SA. Sex differences in TLR2 and TLR4 expression and their effect on coxsackievirus-induced autoimmune myocarditis. Exp Mol Pathol. 2013;94:58–64. doi: 10.1016/j.yexmp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park SY, Lee SW, Baek SH, Lee CW, Lee WS, et al. Suppression of PU.1-linked TLR4 expression by cilostazol with decrease of cytokine production in macrophages from patients with rheumatoid arthritis. Br J Pharmacol. 2013;168:1401–1411. doi: 10.1111/bph.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao J, Zhao S, Zhou G, Liang L, Guo X, et al. Altered biliary epithelial cell and monocyte responses to lipopolysaccharide as a TLR ligand in patients with primary biliary cirrhosis. Scand J Gastroenterol. 2011;46:485–494. doi: 10.3109/00365521.2010.539624. [DOI] [PubMed] [Google Scholar]
- 135.Fang J, Fang D, Silver PB, Wen F, Li B, et al. The role of TLR2, TRL3, TRL4, and TRL9 signaling in the pathogenesis of autoimmune disease in a retinal autoimmunity model. Invest Ophthalmol Vis Sci. 2010;51:3092–3099. doi: 10.1167/iovs.09-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brown HJ, Lock HR, Wolfs TG, Buurman WA, Sacks SH, et al. Toll-like receptor 4 ligation on intrinsic renal cells contributes to the induction of antibody-mediated glomerulonephritis via CXCL1 and CXCL2. J Am Soc Nephrol. 2007;18:1732–1739. doi: 10.1681/ASN.2006060634. [DOI] [PubMed] [Google Scholar]
- 137.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–2967. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 138.Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 139.Yang H, Tuzun E, Alagappan D, Yu X, Scott BG, et al. IL-1 receptor antagonist-mediated therapeutic effect in murine myasthenia gravis is associated with suppressed serum proinflammatory cytokines, C3, and anti-acetylcholine receptor IgG1. J Immunol. 2005;175:2018–2025. doi: 10.4049/jimmunol.175.3.2018. [DOI] [PubMed] [Google Scholar]
- 140.Terayama H, Naito M, Qu N, Hirai S, Kitaoka M, et al. Intratesticular expression of mRNAs of both interferon gamma and tumor necrosis factor alpha is significantly increased in experimental autoimmune orchitis in mice. J Reprod Dev. 2011;57:296–302. doi: 10.1262/jrd.10-121n. [DOI] [PubMed] [Google Scholar]
- 141.Chen H, Wen F, Zhang X, Su SB. Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Mol Vis. 2012;18:219–226. [PMC free article] [PubMed] [Google Scholar]
- 142.Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181:8010–8017. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 143.Harley JB, Sestak AL, Willis LG, Fu SM, Hansen JA, et al. A model for disease heterogeneity in systemic lupus erythematosus. Relationships between histocompatibility antigens, autoantibodies, and lymphopenia or renal disease. Arthritis Rheum. 1989;32:826–836. [PubMed] [Google Scholar]
- 144.Marchini M, Antonioli R, Lleo A, Barili M, Caronni M, et al. HLA class II antigens associated with lupus nephritis in Italian SLE patients. Hum Immunol. 2003;64:462–468. doi: 10.1016/s0198-8859(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 145.Agnello V, De Bracco MM, Kunkel HG. Hereditary C2 deficiency with some manifestations of systemic lupus erythematosus. J Immunol. 1972;108:837–840. [PubMed] [Google Scholar]
- 146.Nishino H, Shibuya K, Nishida Y, Mushimoto M. Lupus erythematosus-like syndrome with selective complete deficiency of C1q. Ann Intern Med. 1981;95:322–324. doi: 10.7326/0003-4819-95-3-322. [DOI] [PubMed] [Google Scholar]
- 147.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 148.Yang Y, Chung EK, Wu YL, Savelli SL, Nagaraja HN, et al. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. Am J Hum Genet. 2007;80:1037–1054. doi: 10.1086/518257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin SC, Yen JH, Tsai JJ, Tsai WC, Ou TT, et al. Association of a programmed death 1 gene polymorphism with the development of rheumatoid arthritis, but not systemic lupus erythematosus. Arthritis Rheum. 2004;50:770–775. doi: 10.1002/art.20040. [DOI] [PubMed] [Google Scholar]
- 150.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 151.Prokunina L, Castillejo-Lopez C, Oberg F, Gunnarsson I, Berg L, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32:666–669. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- 152.Sigurdsson S, Nordmark G, Goring HH, Lindroos K, Wiman AC, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taylor KE, Remmers EF, Lee AT, Ortmann WA, Plenge RM, et al. Specificity of the STAT4 genetic association for severe disease manifestations of systemic lupus erythematosus. PLoS Genet. 2008;4:e1000084. doi: 10.1371/journal.pgen.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Delgado-Vega AM, Abelson AK, Sanchez E, Witte T, D'Alfonso S, et al. Replication of the TNFSF4 (OX40L) promoter region association with systemic lupus erythematosus. Genes Immun. 2009;10:248–253. doi: 10.1038/gene.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, et al. C-reactive protein binding to FcgammaRIIa on human monocytes and neutrophils is allele-specific. J Clin Invest. 2000;105:369–376. doi: 10.1172/JCI7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Russell AI, Cunninghame Graham DS, Shepherd C, Roberton CA, Whittaker J, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–147. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharp A, Robinson D, Jacobs P. Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet. 2000;107:343–349. doi: 10.1007/s004390000382. [DOI] [PubMed] [Google Scholar]
- 158.Chagnon P, Provost S, Belisle C, Bolduc V, Gingras M, et al. Age-associated skewing of X-inactivation ratios of blood cells in normal females: a candidate-gene analysis approach. Exp Hematol. 2005;33:1209–1214. doi: 10.1016/j.exphem.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 159.Brown CJ, Robinson WP. The causes and consequences of random and non-random X chromosome inactivation in humans. Clin Genet. 2000;58:353–363. doi: 10.1034/j.1399-0004.2000.580504.x. [DOI] [PubMed] [Google Scholar]
- 160.Hellman A, Chess A. Gene body-specific methylation on the active X chromosome. Science. 2007;315:1141–1143. doi: 10.1126/science.1136352. [DOI] [PubMed] [Google Scholar]
- 161.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 162.Sthoeger ZM, Bentwich Z, Zinger H, Mozes E. The beneficial effect of the estrogen antagonist, tamoxifen, on experimental systemic lupus erythematosus. J Rheumatol. 1994;21:2231–2238. [PubMed] [Google Scholar]
- 163.Sthoeger ZM, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341–346. doi: 10.1136/ard.62.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang Y, Saha S, Rosenfeld G, Gonzalez J, Pepeljugoski KP, et al. Raloxifene modulates estrogen-mediated B cell autoreactivity in NZB/W F1 mice. J Rheumatol. 2010;37:1646–1657. doi: 10.3899/jrheum.090911. [DOI] [PubMed] [Google Scholar]
- 165.Julkunen HA. Oral contraceptives in systemic lupus erythematosus: side-effects and influence on the activity of SLE. Scand J Rheumatol. 1991;20:427–433. doi: 10.3109/03009749109096822. [DOI] [PubMed] [Google Scholar]
- 166.Counihan KA, Vertosick FT, Kelly RH. Anti-estrogen antibodies in systemic lupus erythematosus: a quantitative evaluation of serum levels. Immunol Invest. 1991;20:317–331. doi: 10.3109/08820139109026233. [DOI] [PubMed] [Google Scholar]
- 167.Sturgess AD, Evans DT, Mackay IR, Riglar A. Effects of the oestrogen antagonist tamoxifen on disease indices in systemic lupus erythematosus. J Clin Lab Immunol. 1984;13:11–14. [PubMed] [Google Scholar]
- 168.Mok CC, To CH, Mak A, Ma KM. Raloxifene for postmenopausal women with systemic lupus erythematosus: a pilot randomized controlled study. Arthritis Rheum. 2005;52:3997–4002. doi: 10.1002/art.21477. [DOI] [PubMed] [Google Scholar]
- 169.Ronnblom L, Alm GV. Systemic lupus erythematosus and the type I interferon system. Arthritis Res Ther. 2003;5:68–75. doi: 10.1186/ar625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li J, Wang X, Zhang F, Yin H. Toll-like receptors as therapeutic targets for autoimmune connective tissue diseases. Pharmacol Ther. 2013;138:441–451. doi: 10.1016/j.pharmthera.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]