Abstract
Stimulated Raman Scattering microscopy allows label-free chemical imaging and has enabled exciting applications in biology, material science, and medicine. It provides a major advantage in imaging speed over spontaneous Raman scattering and has improved image contrast and spectral fidelity compared to coherent anti-Stokes Raman. Wider adoption of the technique has, however, been hindered by the need for a costly and environmentally sensitive tunable ultra-fast dual-wavelength source. We present the development of an optimized all-fibre laser system based on the optical synchronization of two picosecond power amplifiers. To circumvent the high-frequency laser noise intrinsic to amplified fibre lasers, we have further developed a high-speed noise cancellation system based on voltage-subtraction autobalanced detection. We demonstrate uncompromised imaging performance of our fibre-laser based stimulated Raman scattering microscope with shot-noise limited sensitivity and an imaging speed up to 1 frame/s.
In coherent Raman scattering (CRS) microscopy1–6, the sample is illuminated with two synchronized laser beams, which are commonly referred to as pump and Stokes. If their frequency difference matches a molecular vibrational frequency of the sample, the targeted population is excited from the ground to the vibrational state (Fig. 1a)7. In contrast to spontaneous Raman scattering, the stimulated transition from the virtual to the vibrational state by the presence of the Stokes field results in an amplification of the molecular transition rate and allows label-free chemical imaging at speeds up to video-rate8, 9.
Figure 1. Schematic of the fibre-laser system and SRS microscope.
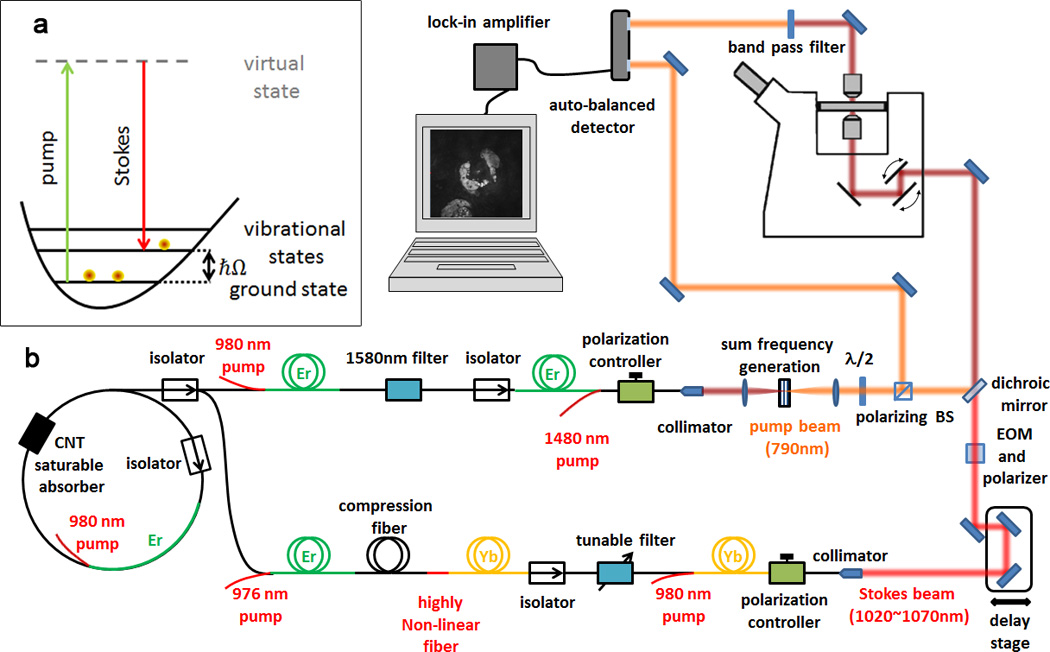
a, Energy diagram of SRS. When the difference in photon energy between the pump and the Stokes beam matches the energy of a vibrational state of the target molecule, ħΩ, molecules are efficiently excited from the ground to the corresponding excited state and a pump photon is absorbed (stimulated Raman loss, SRL) and a Stokes photon is generated (stimulated Raman gain, SRG). b, Schematic of the fibre laser. The laser system starts with an Er-doped fibre oscillator, which is mode-locked with a carbon nanotube (CNT) saturable absorber. The output is split into two arms to generate the pump (upper arm) and Stokes (lower arm) beams. The Stokes beam is modulated at 10MHz with an electro optic modulator (EOM), temporally and spatially combined with the pump beam, and aligned into to a beam-scanning microscope. The transmitted beams are collected with a condenser. The pump beam is detected by the autobalanced detector after the Stokes beam is blocked with an optical filter. The reference beam is sampled in front of the microscope with a polarizing beam splitter (BS).
Various detection schemes have been developed to probe this amplified Raman signal in microscopy. The easier to implement coherent anti-Stokes Raman scattering (CARS)1, 2 suffers from a nonresonant background signal that limits sensitivity for dilute species10, 11. Stimulated Raman scattering (SRS) is free from this background and its excitation spectra match well-documented spontaneous Raman spectra3–6. The typical implementation of SRS12 detects the small intensity loss of the transmitted pump beam with a high-frequency modulation transfer scheme that takes advantage of the fact that laser noise of solid-state lasers commonly reduces to shot-noise at high frequencies13.
SRS has been applied extensively in bio-medical research14 and achieving high sensitivity under biocompatible excitations conditions is a key challenge. As a nonlinear optical process, SRS benefits from pulsed near-infrared lasers, which generate high signal levels at moderate average power. The best signal levels are obtained by narrowband excitation of a single vibrational frequency. Species with spectrally mixed bands can be distinguished by multispectral imaging, where the frequency difference of the pump and Stokes beams is tuned between image frames15, 16 or lines17. An ideal light source for SRS provides two tightly synchronized and quickly tunable pulse trains with bandwidths narrower than a typical Raman linewidth (<20cm−1).
The current gold-standard laser system for SRS is a synchronously pumped picosecond optical parametric oscillator (OPO)18, which has vanishing timing jitter compared to electronically synchronized lasers19. Its cost is high and the free-space cavity is sensitive to environmental changes. Fibre laser technology has the potential to overcome these limitations as its components are inexpensive and light-guiding by the fibre core avoids misalignment. Different concepts have been proposed20–26. From the free-space OPO system, we have learned that optical synchronization avoids timing jitter and improves long-term stability. However, previous approaches to fibre-based optically synchronized systems are less than optimal: super-continuum generation (SC)20–22 does not scale to sufficiently high power, implementation of a fibre-based OPO has proven challenging24, and unseeded four-wave mixing requires low repetition rates23, which ultimately limit the imaging speed. Here we combine the advantages of optical synchronization with straight-forward power scaling in fibre amplifiers.
A remaining challenge of amplified fibre lasers compared to high-power free-space lasers is the presence of high-frequency laser noise22, 26. We present the implementation of a noise suppression scheme based on autobalanced detection27, 28. Compared to previous implementations that suffer from low speed21 or a limited noise cancellation bandwidth26, we have developed detection electronics optimized for high-speed microscopy. We present sensitive SRS imaging with uncompromised performance with our novel fibre laser source.
Results
The laser system is based on the key realization that the difference frequency of the two major fibre gain media, Erbium and Ytterbium, corresponds to the high-wavenumber region of Raman spectra25, where most SRS imaging is performed. We have implemented an all-fibre dual-wavelength laser system based on optical synchronization of two picosecond amplifiers using a broadband super-continuum and have optimized the pulse parameters for high-speed SRS imaging.
Fig. 1b shows a detailed schematic of our tunable dual-wavelength fibre laser source. It starts with an Er-doped fibre oscillator at 1,560nm that is mode-locked with a carbon nanotube (CNT) saturable absorber29 at a repetition rate of 59MHz. The output power of the oscillator is 7mW and is split into two arms to generate the pump and Stokes beams for SRS.
The first arm is amplified to 80mW in a normal dispersion Er-doped preamplifier. Its output is broadened due to self-phase modulation (SPM) and we use a narrowband filter to reduce the bandwidth for SRS. The power (about 2.2mW after filtering) is restored in a low-nonlinearity Er-doped power amplifier. Its output could directly provide the Stokes beam for SRS. However, most multi-photon microscopes are optimized for transmission in the wavelength range from 400–1,100nm and we frequency double the output in a periodically poled lithium niobate (PPLN) crystal (Covesion, MSHG1550-1.0-10). With a conversion efficiency of ~45%, we obtain a near-infrared pump beam for SRS with up to 75mW average power.
The second arm is frequency shifted in a super-continuum generator unit based on a highly nonlinear fibre (HNLF). Stable super-continuum generation requires the use of a short piece of HNLF. By careful optimization of the splice between the single-mode fibre and the HNLF, it is possible to reduce the spice loss to <1dB, enabling an all-fibre implementation. The output covers a range from 950nm to >1700nm (the detection limit of the optical spectrum analyzer)30. We then use an Yb-doped preamplifier to increase the power of the spectral range around 1 µm to 40mW30, 31 and a narrowband filter to produce a picosecond pulse train that can be amplified in a low-nonlinearity Yb-doped power amplifier. We now have a narrowband Stokes beam with up to 120mW average power that is synchronized to the oscillator, and thus the pump beam.
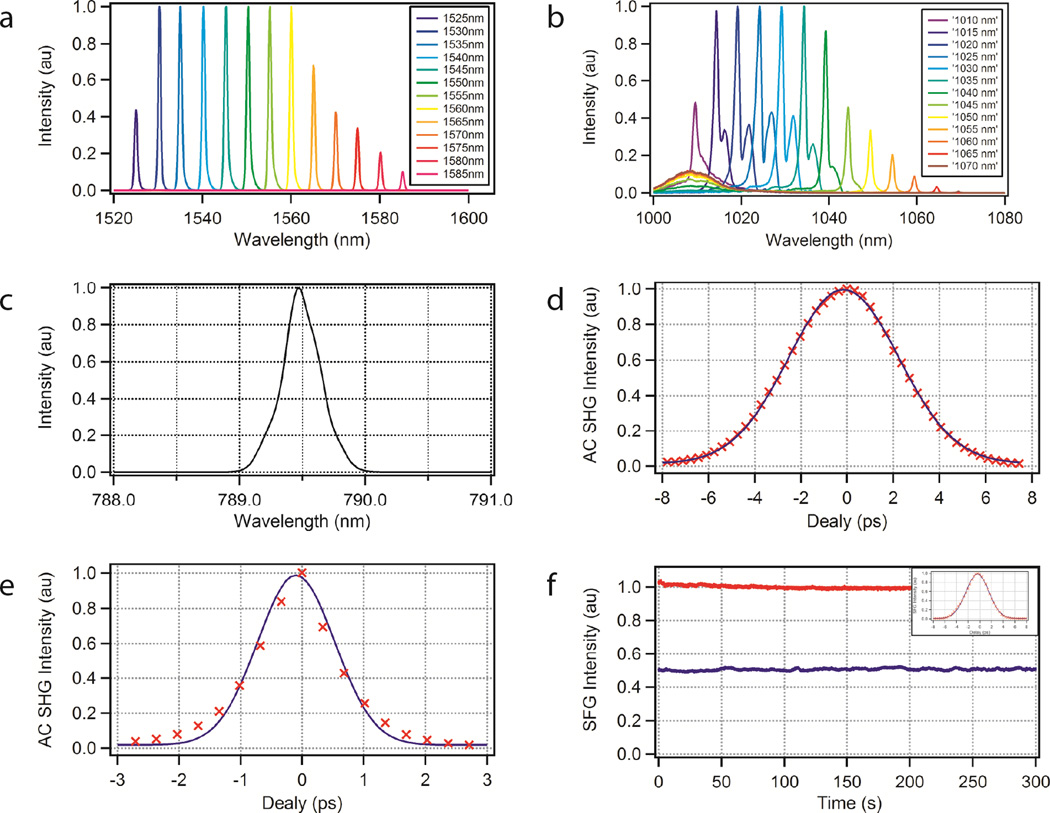
Precise tuning of the difference frequency between pump and Stokes beams is achieved with motorized tunable filters. The outputs of the Er- and Yb-doped amplifiers are tunable from 1530nm to 1590nm (Fig. 2a) and from 1010nm to 1060nm (Fig. 2b), respectively. For fast tuning, we keep the pump beam fixed (the phase-matching wavelength of the PPLN crystal is temperature controlled) and only tune the Stokes beam. Specifically, to access Raman spectra in the high-wavenumber region from 2800 to 3100 cm−1, we frequency double the fixed wavelength Er-arm at 1580nm to 790 nm (Fig. 2c) and tune the Yb-arm from 1,015nm to 1,045nm with <0.1nm tuning accuracy (Agiltron, FOTF-01-6).
Figure 2. Characterization of the fibre laser source.
a, b,c, Tuning ranges of the Er- and Yb-doped fibre power amplifiers. The Er-arm is frequency doubled to provide the pump beam. d, e, Optical auto-correlations of the pump and Stokes pulses. f, Measurement of the timing jitter. Intensity noise at the peak (red) and half maximum (blue) of the optical cross-correlation (inset) over 5 minutes.
The pulse parameters were optimized specifically for SRS imaging of biological specimens by choosing the bandwidths of the narrowband filters and the length of the oscillator cavity. Our theoretical modeling (see Supplementary Section ‘Interpretation of the ANSI standard’) indicates that the signal-to-nonise ratio (SNR) does not depend on the exact laser duty factor (Supplementary Fig. 1a) as nonlinear photo-damage limits the maximum permissible peak power32–34. However, at lower duty factors, higher average power is required to achieve the same SNR (Supplementary Fig. 1b). With the American National Standards Institute (ANSI) safety standard for clinical use in mind (see Supplementary Section ‘Interpretation of the ANSI standard’), it is thus advantageous to increase the duty factor compared to the solid-state OPO laser system, which has a pulse duration of 6 ps and repetition rate of 80MHz.
We chose filters with a bandwidth of 1.2nm (Fig. 2b), which approaches the required spectral resolution limit for SRS while still providing high peak power. Figure 2d shows the autocorrelation of the Stokes beam at 1,030 nm. The pulse duration of 1.0 ps was calculated by dividing the full width at half maximum (FWHM) by . This corresponds to a time-bandwidth product of a transform-limited pulse and, at 59 MHz repetition rate, provides a duty factor increase of about 8× compared to the solid-state OPO laser system. Figure 2e shows the autocorrelation of the pump beam with a pulse duration of 4.0ps. The time-bandwidth product of 0.68 is about 2× larger than the transform limit, which is not fundamental. In a further optimized version of the laser source, we will aim to match the pulse duration of pump and Stokes pulses.
The two beams are spatially overlapped with a free-space dichroic mirror. The rough time delay is adjusted by adding undoped fibre before the Yb-doped power amplifier. Fine delay is obtained by adjusting a mechanical delay line (Fig. 1b) while monitoring the sum-frequency generation (SFG) signal from a beta barium borate (BBO) crystal. This setup was also used to measure the cross-correlation of the two pulse trains and determine the timing jitter. Figure 2f shows a 5 minutes time trace of the SFG signal at the half-maximum of the cross-correlation. Using the slope of the cross-correlation to convert from amplitude to timing fluctuations, the timing jitter is estimated to be <24fs. Such an approach only provides an upper limit estimate as laser intensity fluctuations can be falsely interpreted as timing jitter. However, it is sufficient to demonstrate that the timing jitter is much smaller than the cross-correlation bandwidth of 4.4 ps and does not affect the SRS imaging capabilities.
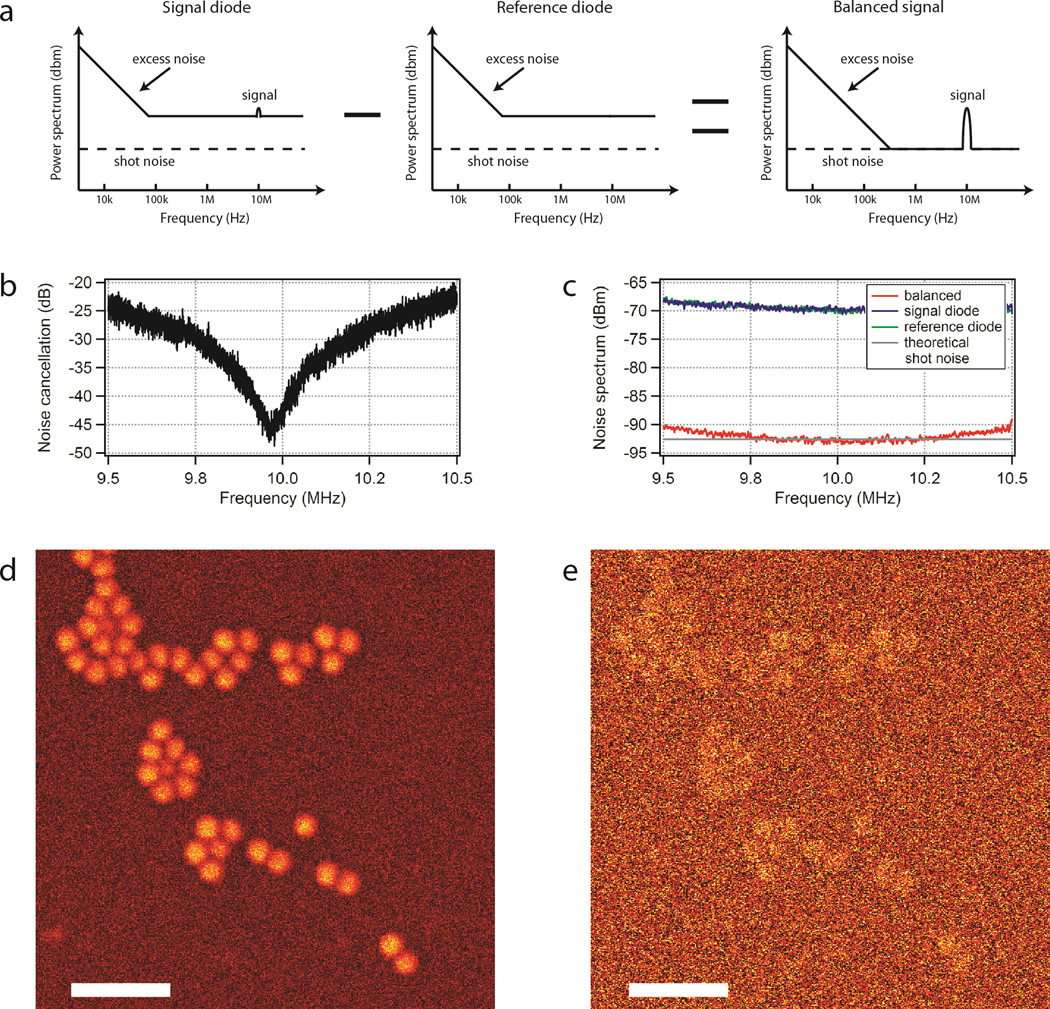
After careful optimization of the noise performance of the Er-arm, we obtained a pump beam that is about 27dB above the shot-noise floor at 28mW average power (Supplementary Fig. 3). Anything other than shot-noise limited performance is not acceptable for SRS microscopy, as it limits the ability to detect low-concentration molecular species. The general idea of balanced detection (Fig. 3a) is that a portion of the pump beam can be sampled at the laser output (Fig. 1b). Laser noise other than the shot-noise is common to both the sample and reference arms and subtracting the two results in suppression of such common mode noise. As the modulated signal only appears in the sample arm, it is not subtracted and can be detected with demodulation electronics (for example, a lock-in amplifier) with shot-noise limited sensitivity (Fig. 3a).
Figure 3. Autobalanced detection.
a, Principle of balanced detection. b Noise suppression of the home-built auto-balanced detector optimized for 10MHz signal. c Noise spectrum of the fibre laser with (red) and without (blue) autobalanced detection. The theoretical shot-noise is indicated in grey. d,e, SRS images of 1.1 polystyrene beads with (d) and without (e) auto-balanced detection. Scale bars, 5.
In theory, balanced detection can reduce the laser noise to 3dB above the shot-noise because it also introduces the shot-noise of the reference arm. In practice, noise cancellation depends on the exact balance of the two arms. For example, if the intensity of the sample arm is only 90% of the reference arm, noise is suppressed by a factor of 10 or 20dB. Application of the technique to microscopy would thus be limited by varying sample transmission. To ensure maximum suppression independent of sample transmission we implement ‘auto’-balanced detection and rely on the d.c. signals from both arms to control the gain of the reference arm to balance the signals before subtraction. This approach takes advantage of the time-scale separation of the modulation, the autobalancing speed, and the pixel dwell time. However, commercially available balanced detectors do not meet these timing requirements and their use drastically reduces the imaging speed22.
To overcome this limitation, we have developed a voltage-subtraction autobalanced detector for the specific requirements in high-speed microscopy. A detailed schematic is shown in Supplementary Fig. 2. Identical large-area silicon diodes are used for the sample and reference arms to accommodate the optical power. The a.c. and d.c. signals of each arm are then separated. The a.c. signals are filtered around the modulation frequency (10MHz) and preamplified by trans-impedance amplifiers to overcome electronic noise. A four-channel variable gain amplifier (VGA) amplifies the filtered a.c. and d.c. signals of the two arms and a proportional integral derivative loop feedback circuit is used to control the gain of the reference arm to lock the d.c. levels. Because a.c. and d.c. signals experience the same gain in each channel, this generates the auto-balancing of the a.c. signals before subtraction in a differential amplifier.
Our autobalanced detector allows a noise suppression of >40dB at 10MHz (Fig. 3b), which is significantly more than the laser excess noise. We thus achieve shot-noise-limited detection sensitivity (Fig. 3c). The noise suppression bandwidth of our implementation is currently limited by the dispersion of the specific phase-shifters, which is not fundamental and is sufficiently large (500kHz) to allow removal of all excess noise at the sampling speeds required for 1 frame/s imaging. The 3dB point of the auto-balancing loop (data not show) is chosen at about 500kHz to compensate for pixel-to-pixel transmission variation.
For SRS imaging, the Stokes beam is modulated at 10MHz with an electro-optic modulator (Thorlabs, EO-AM-NR-C2) and combined with the pump beam using a dichroic mirror. The collinear beams are directed into a beam-scanning microscope (Olympus, IX71) and focused into the sample with a water immersion objective (Olympus, UPLANSAPO 60XW, 1.2NA). The transmitted beams are collected with an oil immersion condenser (Nikon, 1.4NA), the Stokes beam is blocked with a high-optical-density filter (Chroma, CARS890/220), and the pump beam is detected with the signal arm of the auto-balanced detector. The reference beam is generated with a polarization beam splitter and the rough balance is set with a half wave plate prior to imaging. The output of the auto-balanced detector is then sent to a custom lock-in amplifier9 to detect the 10MHz SRS signal.
Figures 3d,e show SRS images of 1.1µm polystyrene beads acquired at an imaging speed of 1 frame/s and with 24mW power at the focus with (Fig. 3d) and without auto-balancing (Fig. 3e). Balanced detection improves SNR by 10.6× from 0.5 to 5.3. No image artifact due to varying sample transmission is visible even at the edges of the beads (Supplementary Fig. 4).
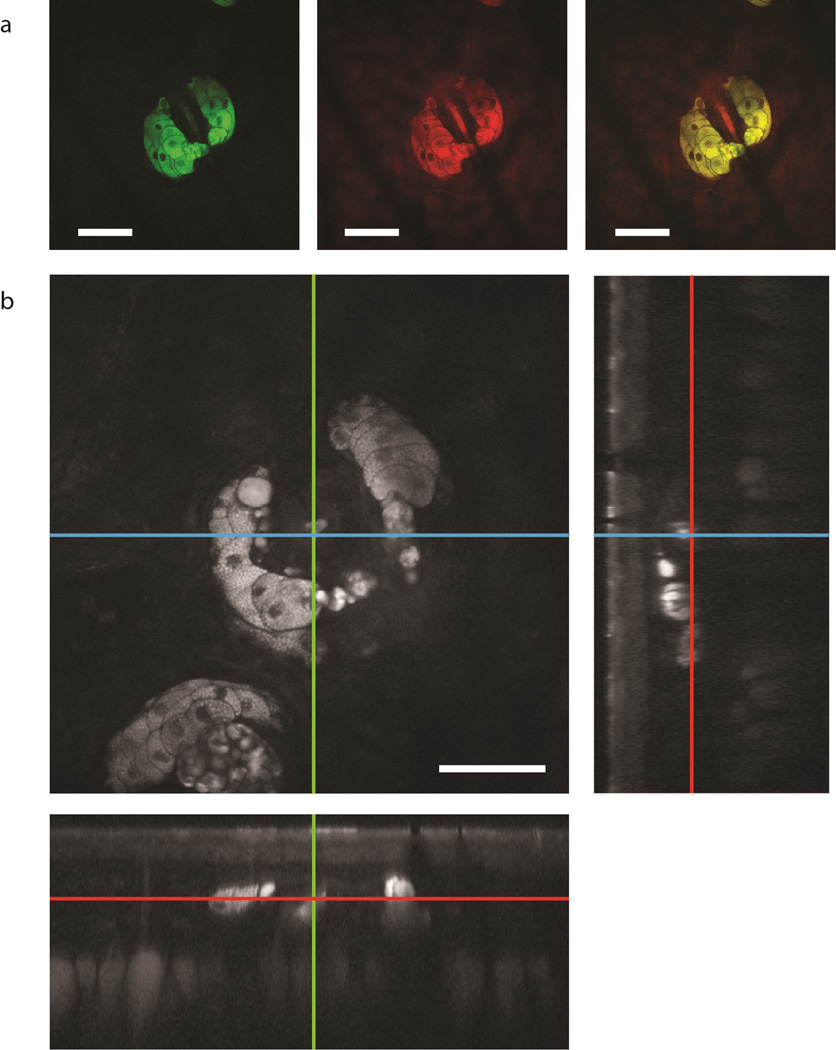
Figure 4a shows multicolor SRS imaging of mouse skin samples. The green channel in Fig. 4a was acquired at the CH2-streching vibration of lipids (2850cm−1) and the red channel at the CH3-streaching vibration of proteins (2950cm−1). The images show a lipid-rich sebaceous gland wrapped around a protein-rich hair. Figure 4b shows a 100µm depth stack acquired every 1µm at a frame-rate of 1 frame/s (Supplementary Movie 1 shows the complete z-stack). This is fastest SRS imaging experiment performed with a fibre laser source to date, reaching a performance equivalent to that of the more expensive state-of-the-art solid-state laser systems.
Figure 4. SRS spectral imaging with the fibre-laser source.
a, Two color image of a sebaceous gland in mouse skin acquired at 2850cm−1 (mainly lipids, green), 2950cm−1 (mainly proteins, red) and the composite of the two colours. b, z-stack acquired at 1 frame/s and cross sections from different directions. Scale bars, 50µm.
To demonstrate the ‘robustness’ of the laser system, we performed imaging on a table that was not vibration isolated and in an environment that was not climate controlled (Supplementary Fig. 5). We also demonstrated the performance of the laser in response to external shock (Supplementary Movie 2) and ultra-long-term imaging over 44 hours without adjusting the laser (Supplementary Movie 3).
Discussion
We have developed a dual-wavelength fibre laser source for SRS microscopy that is precisely tunable over the entire high-wavenumber region of Raman spectra, where most SRS imaging is performed. To reach the performance of solid-state laser systems, we optimized the laser amplifiers and developed a broadband voltage-subtraction autobalanced detector. We demonstrate SRS imaging at an imaging speed up to 1 frame/s with shot-noise limited sensitivity. The major advantages of our fibre laser SRS system are (1) operation that does not require vibration isolated optical table, (2) operation in environments that are not climate controlled, and (3) reduced cost. It has the promise to extend the use of SRS microscopy to non-expert users and enable clinical applications. Toward this goal, demonstrating high-quality imaging at excitation powers that are compliant with the ANSI safety standard is a major milestone.
The major limitation of the current proof-of-concept implementation is the use of non-polarization maintaining (PM) components and the fact that adjustment of the polarization state of the output is required to maintain maximum SRS signal. All components are available as PM version and an all-PM design is conceivable based on the same setup. The use of large-mode area (LMA) components will further reduce SPM broadening and increase spectral fidelity. Care will need to be taken to achieve better matching of the pump and Stokes beam pulse durations. All-fibre delivery is further required to fully take advantage of the properties of fibre-lasers. Finally, system integration of the microscope and the fibre laser will enable automated spectral SRS imaging, and the development of an integrated hand-held SRS scanner is under way.
Methods
Laser spectra were measured with an optical spectrum analyzer (HP, 70951A) with a spectral resolution of 0.1nm. Optical auto-correlations were measured with a home-built background-free autocorrelator based on a 1 mm BBO crystal (Witcore, BBO 6-6-1mm) and a manual delay stage. The timing jitter measurements were acquired with the same setup. Relative intensity noise (RIN) spectra were acquired with an radiofrequency spectrum analyzer (Siganlhound,USB-SA44B) following reference35. The bandwidth of the spectrum analyzer for the measurements of Fig. 3b,c is 400Hz. We used a large-area Si photodiode (OSI Optoelectronics, S-100CL) back-biased at 48V to provide both dynamic range as well as response time. The d.c. signal was separated with a bias-T (Minicircuits, ZFBT-4R2GW+) and recorded with an oscilloscope to provide the intensity calibration of the relative intensity noise measurement (Supplementary Fig. 3). The shot-noise level was estimated following reference35 given the measured d.c. signal. The suppression ratio of the balanced detector was measured with the radiofrequency spectrum analyzer by artificially increasing the noise of the fibre laser with an acousto-optic modulator driven by a white noise signal from an radiofrequency function generator.
Supplementary Material
Acknowledgements
We thank Philip Hobbs, James McArthur and Jay Trautman for discussions. We thank Dan Fu and Fa-Ke Lu for help with sample preparation. This material is based upon work supported by the National Science Foundation (NSF; grant No. 1214848 to CWF) and by the National Institute of Health (NIH; grant No. 5R01EB010244 to XSX). This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the NSF (under grant No. ECS-0335765). CNS is part of Harvard University. KK and NP would like to acknowledge the support from the CIAN NSF ERC under grant #EEC-0812072 and the State of Arizona’s TRIF funding.
Footnotes
Author contributions: CWF and KQK designed and characterized the fibre laser system. WY and GRH designed and characterized the auto-balanced detector. CWF and WY performed the imaging experiments. CWF, XSX, NP and KQK conceived the project and supervised its implementation. CWF, WY, KQK, NP and XSX wrote the manuscript and all authors commented on it.
Competing financial interests: Harvard University and University of Arizona has filed a patent application based on the current work. CWF and XSX have financial interests in INVENIO IMAGING INC. KQK has financial interests in KPhotonics LLC. Any opinions, findings, conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF or NIH.
References
- 1.Zumbusch A, Holtom GR, Xie XS. Three-dimensional vibrational imaging by coherent anti-stokes raman scattering. Phys. Rev. Lett. 1999;82:4142–4145. [Google Scholar]
- 2.Evans CL, Xie XS. Coherent anti-stokes raman scattering microscopy: Chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 2008;1:883–909. doi: 10.1146/annurev.anchem.1.031207.112754. [DOI] [PubMed] [Google Scholar]
- 3.Ploetz E, Laimgruber S, Berner S, Zinth W, Gilch P. Femtosecond stimulated raman microscopy. Appl. Phys. B-Lasers O. 2007;87:389–393. [Google Scholar]
- 4.Freudiger CW, et al. Label-free biomedical imaging with high sensitivity by stimulated raman scattering microscopy. Science. 2008;322:1857–1861. doi: 10.1126/science.1165758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozeki Y, Dake F, Kajiyama S, Fukui K, Itoh K. Analysis and experimental assessment of the sensitivity of stimulated raman scattering microscopy. Opt. Express. 2009;17:3651–3658. doi: 10.1364/oe.17.003651. [DOI] [PubMed] [Google Scholar]
- 6.Nandakumar P, Kovalev A, Volkmer A. Vibrational imaging based on stimulated raman scattering microscopy. New J. Phys. 2009;11:033026. [Google Scholar]
- 7.Bloembergen N. The stimulated raman effect. Am.J. Phys. 1967;35:989–1023. [Google Scholar]
- 8.Evans CL, et al. Chemical imaging of tissue in vivo with video-rate coherent anti-stokes raman scattering microscopy. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16807. doi: 10.1073/pnas.0508282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saar BG, et al. Video-rate molecular imaging in vivo with stimulated raman scattering. Science. 2010;330:1368–1370. doi: 10.1126/science.1197236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganikhanov F, Evans CL, Saar BG, Xie XS. High-sensitivity vibrational imaging with frequency modulation coherent anti-stokes raman scattering (fm cars) microscopy. Opt. Lett. 2006;31:1872–1874. doi: 10.1364/ol.31.001872. [DOI] [PubMed] [Google Scholar]
- 11.Min W, Freudiger CW, Lu S, Xie XS. Coherent nonlinear optical imaging: Beyond fluorescence microscopy. Annu. Rev. Phys. Chem. 2011;62:507–530. doi: 10.1146/annurev.physchem.012809.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owyoung A. Sensitivity limitations for cw stimulated raman spectroscopy. Opt. Commun. 1977;22:323–328. [Google Scholar]
- 13.Ozeki Y, et al. Stimulated raman scattering microscope with shot noise limited sensitivity using subharmonically synchronized laser pulses. Opt. Express. 2010;18:13708–13719. doi: 10.1364/OE.18.013708. [DOI] [PubMed] [Google Scholar]
- 14.Ji MB, et al. Rapid, label-free detection of brain tumors with stimulated raman scattering microscopy. Sci. Transl. Med. 2013;5:201ra119. doi: 10.1126/scitranslmed.3005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CY, et al. Picosecond spectral coherent anti-stokes raman scattering imaging with principal component analysis of meibomian glands. J. Biomed. Opt. 2011;16:021104–021109. doi: 10.1117/1.3533716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozeki Y, et al. High-speed molecular spectral imaging of tissue with stimulated raman scattering. Nat. Photonics. 2012;6:845–851. [Google Scholar]
- 17.Kong LJ, et al. Multicolor stimulated raman scattering microscopy with a rapidly tunable optical parametric oscillator. Opt. lett. 2013;38:145–147. doi: 10.1364/OL.38.000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganikhanov F, et al. Broadly tunable dual-wavelength light source for coherent anti-stokes raman scattering microscopy. Opt. Lett. 2006;31:1292–1294. doi: 10.1364/ol.31.001292. [DOI] [PubMed] [Google Scholar]
- 19.Jones DJ, et al. Synchronization of two passively mode-locked, picosecond lasers within 20 fs for coherent anti-stokes raman scattering microscopy. Rer. Sci. Instrum. 2002;73:2843–2848. [Google Scholar]
- 20.Pegoraro AF, et al. Optimally chirped multimodal cars microscopy based on a single ti: Sapphire oscillator. Opt. Express. 2009;17:2984–2996. doi: 10.1364/oe.17.002984. [DOI] [PubMed] [Google Scholar]
- 21.Krauss G, et al. Compact coherent anti-stokes raman scattering microscope based on a picosecond two-color er: Fibre laser system. Opt. Lett. 2009;34:2847–2849. doi: 10.1364/OL.34.002847. [DOI] [PubMed] [Google Scholar]
- 22.Gambetta A, et al. Fibre-format stimulated-raman-scattering microscopy from a single laser oscillator. Opt. Lett. 2010;35:226–228. doi: 10.1364/OL.35.000226. [DOI] [PubMed] [Google Scholar]
- 23.Baumgartl M, et al. All-fibre laser source for cars microscopy based on fibre optical parametric frequency conversion. Opt. Express. 2012;20:4484–4493. doi: 10.1364/OE.20.004484. [DOI] [PubMed] [Google Scholar]
- 24.Lefrancois S, et al. Fibre four-wave mixing source for coherent anti-stokes raman scattering microscopy. Opt. Lett. 2012;37:1652–1654. doi: 10.1364/OL.37.001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bégin S, et al. Coherent anti-stokes raman scattering hyperspectral tissue imaging with a wavelength-swept system. Biomed. Opt. Express. 2011;2:1296–1306. doi: 10.1364/BOE.2.001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nose K, et al. Sensitivity enhancement of fibre-laser-based stimulated raman scattering microscopy by collinear balanced detection technique. Opt. Express. 2012;20:13958–13965. doi: 10.1364/OE.20.013958. [DOI] [PubMed] [Google Scholar]
- 27.Hobbs PCD. Shot noise limited optical measurements at baseband with noisy lasers. Proc. SPIE vol. 1376, Laser Noise. 1990:216–221. [Google Scholar]
- 28.Hobbs PCD. Building electro-optical systems : Making it all work. New York: Wiley; 2000. [Google Scholar]
- 29.Kieu K, Mansuripur M. Femtosecond laser pulse generation with a fibre taper embedded in carbon nanotube/polymer composite. Opt. Lett. 2007;32:2242–2244. doi: 10.1364/ol.32.002242. [DOI] [PubMed] [Google Scholar]
- 30.Kieu K, Jones RJ, Peyghambarian N. High power femtosecond source near 1 micron based on an all-fibreer-doped mode-locked laser. Opt. Express. 2010;18:21350–21355. doi: 10.1364/OE.18.021350. [DOI] [PubMed] [Google Scholar]
- 31.Andrianov A, Anashkina E, Muravyev S, Kim A. All-fibre design of hybrid er-doped laser/yb-doped amplifier system for high-power ultrashort pulse generation. Opt. Lett. 2010;35:3805–3807. doi: 10.1364/OL.35.003805. [DOI] [PubMed] [Google Scholar]
- 32.Hopt A, Neher E. Highly nonlinear photodamage in two-photon fluorescence microscopy. Biophys. J. 2001;80:2029–2036. doi: 10.1016/S0006-3495(01)76173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Y, Wang H, Shi R, Cheng JX. Characterization of photodamage in coherent anti-stokes raman scattering microscopy. Opt. Express. 2006;14:3942–3951. doi: 10.1364/oe.14.003942. [DOI] [PubMed] [Google Scholar]
- 34.Nan X, Potma EO, Xie XS. Nonperturbative chemical imaging of organelle transport in living cells with coherent anti-stokes raman scattering microscopy. Biophys. J. 2006;91:728–735. doi: 10.1529/biophysj.105.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obarski GE, Hale PD. How to measure relative intensity noise in lasers. Laser Focus World. 1999;35:273–278. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.