Abstract
Maternal obesity is a global epidemic affecting the developed and developing world. Human and animal studies indicate that maternal obesity programs development predisposing offspring to later-life chronic diseases. Several mechanisms act together to produce these adverse health problems. There is a need for effective interventions that prevent these outcomes and guide management in human pregnancy. We report here dietary and exercise intervention studies in both altricial and precocial species, rats and sheep, designed to prevent adverse offspring outcomes. Both interventions present exciting opportunities to at least in part prevent adverse metabolic and other outcomes in mother and offspring.
INTRODUCTION
Worldwide nearly 1.5 billion people are overweight (body mass index – BMI - greater than 25 kg/m2) or obese (BMI greater than 30 kg/m2) 1. Almost every country is affected: Mexico,32% of women of reproductive years are obese 2, 3; USA, 35% of women of reproductive years are obese 4; Brazil 50% of the population is overweight or obese 5, 6; United Kingdom 33% of pregnant women are overweight or obese 7; India 26% of women of reproductive years are overweight and 8% obese 8; China 16% of women are overweight or obese 9; Ghana 64.7% of women are either overweight or obese10. The WHO (www.who.int/nut/obs.htm) has declared obesity one of the top ten adverse health risk conditions in the world and one of the top five in developed nations.
As pointed out in our previous review “Animal Studies that Reveal Mechanisms of Programming of Offspring Outcomes of Maternal Obesity”, maternal obesity programs offspring predisposition to a wide variety of chronic, later-life diseases. A better understanding of developmental programming requires integration of factors involved in challenges, mechanisms and outcomes involved. Reports of experimental interventions in animal models in the setting of obesity are scarce 11, 12. Using nutritional or targeted therapeutic interventions during windows of developmental programming studies in some experimental animal models have shown potential reversibility of unwanted offspring metabolic outcomes 12. For example, leptin treatment of neonatal female rats born to undernourished mothers prevents development of metabolic compromise in adulthood 13; maternal antioxidant supplementation in rats fed with the Western diet partially prevents offspring adiposity and normalizes glucose tolerance 14. In other studies it has been shown that genistein supplementation in mice during gestation protects offspring susceptibility to obesity 15. This chapter will focus on animal studies designed to illuminate mechanisms by which interventions involving a change in maternal diet or level of exercise may act to improve maternal and offspring outcomes.
There are many reasons why animal intervention studies are needed. Importantly, animal studies are much more controllable than human clinical interventions which is the parallel human approach to hypothesis driven animal research. In addition a greater depth of mechanistic interrogation is possible resulting from tissue retrieval and multiple testing in animal studies and results are obtained much more quickly to guide management in human pregnancy. Reproducibility and independent confirmation, the indispensible requirements of scientific certainty, are also generally easier to achieve in animal studies. Carefully designed clinical trials to determine effects of interventions and improve maternal health in pregnancy and offspring outcomes are now in progress 16. However, in addition to the length of time needed to obtain the required data, clinical trials have to contend with multiple confounds related to the mother’s socioeconomic status and pre-pregnancy health that not only make their analysis and interpretation difficult but also may limit their usefulness in determining mechanisms. There is a pressing need, as the recent IOM report indicates 17, for the development of evidence-based interventions that inform and motivate pregnant women to adopt a healthy lifestyle before and during pregnancy. Currently there is much interest in both maternal diet and exercise as potentially modifiable factors to use as interventions 18-20. The optimal timing and extent to which adverse effects of the maternal metabolic phenotype resulting from maternal obesity and associated high calorie diets can be prevented and/or possibly reversed by these interventions remain unanswered questions of considerable physiological interest and importance in clinical obstetric management. Most authorities believe that interventions introduced before conception will have the best results. It should always be born in mind that poor maternal nutrition also programs adverse offspring outcomes 21-23 and sudden and excessive restriction of maternal and fetal nutrient availability may well introduce new dangers. Thus firm scientific data are needed to guide interventions.
When considering the goals of specific interventions to beneficially impact developmental programming outcomes, a distinction must always be made between interventions designed to prevent negative offspring outcomes and interventions conducted at later stages of an offspring’s life to reverse adverse health outcomes. Clearly prevention is a better strategy than to try to reverse problems. The present review focuses on maternal intervention to prevent negative offspring outcomes by maternal obesity.
STUDIES ON PRE-PREGNANCY DIETARY INTERVENTION IN THE OBESE RAT
Investigation of programming of offspring by maternal obesity requires that investigators ensure initial phenotypic homogeneity of the different groups of mothers studied - controls, obese mothers and mothers in which interventions are introduced. Care is necessary to achieve this important goal when females who will be the study mothers are purchased from commercial vendors without information on their background. Specific information should be obtained as to the lineage of all females to avoid inclusion of sibling females in the same sub-group. To avoid this and related problems we maintain our own colony of non-pregnant females which are bred to deliver the female pups that will be recruited as the mothers in our studies. All rats are maintained on the same laboratory chow unless exposed to an experimental diet 24. At delivery (postnatal day – PND- 0) litters are culled to 10 pups, each litter containing at least four female pups. This standardization is important since programming effects have been shown in offspring according to different litter sizes reared by mothers during lactation 25.
At weaning (PND 21) female offspring are randomly assigned to either a control (C) group fed normal laboratory chow or to a maternal obesity group (MO) fed a high-energy, obesogenic diet containing 23.5% protein, 20% animal lard, 5% fat, 20.2% polysaccharide, 20.2% simple sugars, 5% fiber, 5% mineral mix, 1% vitamin mix (w/w), energy 4.9 Kcal/g. Only one female from any one litter is assigned to any study group. At PND 90, one month before breeding, half of the obese females are selected at random for the dietary intervention (DINT) group and placed back on the C diet for the rest of the study including pregnancy and lactation. The remaining obese females continue on the high fat diet during pregnancy and lactation. We have chosen to breed females at 120 days as we have shown that at younger breeding ages the mothers are still growing – albeit not as fast as earlier in life - and offspring outcomes of key factors such as growth, triglycerides and leptin are affected by maternal age as well as the nutritional challenge (unpublished data). At PND 120 all three groups C, MO and DINT are bred and fed their pre-pregnancy diet throughout pregnancy and lactation. All mothers deliver by spontaneous vaginal delivery. Day of delivery is considered as post natal day 0 24, 26 (Figure 1).
Figure 1.

Time line of the dietary intervention studies in maternal obesity.
Changes in maternal and offspring phenotypes resulting from MO and DINT
At breeding MO females were 16% heavier than controls, equivalent to a pregnant women increasing her BMI from 25 (the top of the normal BMI range) to 30.5 (the lower end of the obese range). DINT females were 9 percent heavier than controls (equivalent to BMI in the mid-overweight range) at breeding. Maternal serum leptin at the end of lactation was higher in MO than C. Leptin levels in the DINT group were similar to controls 27.
The effects of MO and DINT have only been reported in male offspring 27. No differences in body weight were seen between pups at birth and at weaning. At weaning MO offspring had more subcutaneous fat tissue, and higher serum triglycerides and leptin than C offspring – showing dysregulation of lipid metabolism; pre-pregnancy maternal dietary intervention prevented these increases offspring measures. This important finding shows the limitations of weight alone at any age as an assessment of outcomes. Body composition is much more important in predicting future offspring health. Serum glucose did not differ between the three offspring groups, but offspring serum insulin was elevated in MO and returned to C levels in DINT offspring indicating the presence of insulin resistance in MO offspring 27.
At PND 120, male MO offspring had elevated resting serum glucose and insulin and increased insulin resistance compared with C offspring. Insulin remained elevated in DINT compared with C offspring while blood glucose did not differ from either of the two other groups 27. It is important to note that all offspring were on the same post-weaning diet. Thus there was no opportunity for effects of an increase in offspring dietary intake acting as a “second hit” on the background of developmental programming that had already occurred.
STUDIES ON PRE-PREGNANCY EXERCISE INTERVENTION IN THE OBESE RAT
We have recently completed a study of effects of an exercise intervention on mother and offspring of obese and control rats. General management of the pregnancies and lactation were as described above for the dietary intervention. At PND 90, one month before breeding, one half of the group of C non-pregnant females and half of the MO female groups were selected at random to continue on their diet and begin wheel-running exercise (C exercised – CEx; MO exercised - MOEx) (Figure 2). Mothers continued to be placed in the wheel through pregnancy. All females continued on their respective diets. CEx and MOEx rats were trained to wheel run on two separate days the week before they reach PND 90. A training session lasted 15 min which we established was the optimum running schedule that was always completed, followed by a 15 minute rest period and a second 15 minute run. Rats were allowed two days rest every seven days. Before pregnancy, all rats completed the 30 minutes running while during pregnancy rats were placed in the wheel for only one 15 minute session per day and the amount of voluntary exercise completed varied between animals especially in late gestation. During lactation mothers nursed their pups and did not exercise. Therefore, lactating mothers were maintained on their pregnancy diet and not placed in the wheel.
Figure 2.

Time line study of the exercise intervention studies in maternal obesity.
Changes in maternal phenotype and fertility resulting from maternal exercise prior to and during pregnancy
Calorie and food intake per day were similar in all four groups and exercise did not affect calorie or food intake in either group. Exercise had no effect on maternal weight at any stage except for the CEx group in which exercise decreased body weight in comparison with C at parturition. There were no differences between groups in average distance run per session before pregnancy or in the first 15 days of pregnancy but interestingly MO rats that were exercised ran further than control rats in the last few days of pregnancy. We hypothesize that this difference was a result of their lower circulating estrogen levels since a negative correlation has been shown to exist between estrogen levels and physical activity 28.
As in the DINT study mentioned above, at the end of lactation maternal insulin, glucose, HOMA, leptin, triglycerides and cholesterol were all elevated in MO mothers. Exercise did not alter these variables in C mothers but prevented the changes in all these variables except leptin in MO.
Effect of maternal obesity and exercise on male offspring metabolism at postnatal day 36
We evaluated offspring outcomes since the goal of interventions in MO pregnancy is to improve both maternal and offspring outcomes. At offspring PND 36, one male offspring from each litter (n= 8) was chosen at random, fasted for 4 hours and euthanized by decapitation, trunk blood samples obtained and fat depots excised and weighed. Litter size, litter weight individual pup body weight or sex ratio at birth was not affected by either MO or exercise. In male offspring body weight, cholesterol and insulin were not different at PND 36 between the four groups. Maternal obesity increased offspring leptin, triglycerides and fat mass in males. Exercise in MO prevented the male offspring MO leptin increase and partially prevented the increased triglycerides. Maternal exercise in C reduced male offspring glucose and male HOMA. The importance of paying attention to the phenotype of study mothers is shown by the interesting observation that maternal exercise decreased weight and cholesterol in control offspring indicating that even animals recruited as controls may be affected by experimental protocols.
Maternal voluntary exercise intervention has been previously reported in lean pregnant rats in two different models. Male, but not female, offspring of exercised mothers show increased percent lean mass and decreased fat mass percent compared to male offspring from controls, showing that maternal exercise can affect offspring 29, 30. These effects on offspring metabolic phenotype show similarities to the effects with our present study in control mothers. In another study normal, lean, pregnant rats performed voluntary exercise 31, 32 training from 42 days before pregnancy and continued on to day 19 of gestation with the result that maternal plasma antioxidant status was improved. Both of these studies provide important data for design of studies in obese mothers.
Comparison of DINT and Exercise models
One of the important differences between the DINT and the exercise intervention model is the maternal weight. In the DINT model 27 the increased maternal body weight of the DINT group at breeding was partially prevented, and there were no differences in maternal body weight during pregnancy and lactation between DINT and C. In contrast, maternal exercise intervention did not modify the maternal body weight at any stage. The weight of MOEx mothers was the same as MO before and during pregnancy as well as during lactation. However, for both models, mothers undertaking the intervention presented a better metabolic and hormonal maternal environment than MO with regards to offspring outcomes.
In a completely independent study we compared all groups with both interventions (maternal dietary and exercise interventions. Corticosterone was increased in serum of MO mothers prior to breeding. Maternal DINT and exercise intervention before pregnancy decreased maternal corticosterone concentration but MOEx values were not returned to those in C (Fig 3 A). A similar picture was seen at the end of lactation (Fig 3 B) and in the neonate and young adult male offspring (Fig 3 C and D). These changes may be protective mechanisms of future metabolic problems in the offspring by MO.
Figure 3.
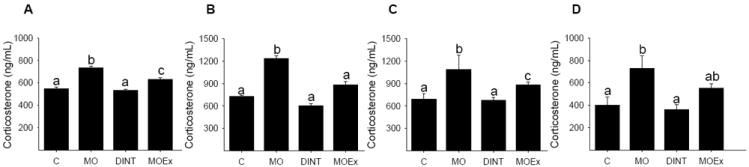
Rat serum corticosterone levels: (A) maternal pre-pregnant, (B) maternal end of lactation (C) 2 days offspring male and female combined neonate and (D) 110 d young adult male. Mean ± SEM; n = 6. C control diet, MO maternal obesity, DINT maternal dietary intervention, MOEx maternal obesity exercise intervention; p<0.05 for groups not sharing at least one letter.
Both maternal interventions improved the adverse offspring metabolism outcomes produced by MO, but the improvement was different in the two models. Maternal DINT partially prevents glucose, insulin, HOMA, fat and fat cell size, and completely prevents leptin increases offspring by MO. Maternal exercise intervention partially prevents fat and insulin and completely glucose, HOMA and fat cell size increase in offspring by MO.
For both models the data indicate that there were different changes in metabolism in various tissues since no differences in food intake and body weight were found in the young adult offspring. The two intervention models appear to benefit offspring metabolism in diverse ways suggesting dissimilar maternal mechanisms. Excessive gestational weight gain has been associated with adverse maternal pregnancy outcomes. Catalano has clearly indicated that maternal pre-pregnancy BMI is a major factor in determining maternal and offspring outcome 33. One important finding of the exercise intervention study was that the adverse offspring outcomes produced by MO are the result of maternal metabolic changes and/or in increased maternal corticosterone and not due to maternal body weight since MOEx prevented many of the MO offspring outcomes offspring without any change in maternal calorie intake or body weight. This finding again shows that outcomes are more related to body composition rather than body weight. The overwhelming evidence in favor of the importance of body composition clearly indicates that the most successful interventions will at least contain a component of intervention prior to pregnancy. Addition of interventions during pregnancy will further build on the interventions prior to pregnancy.
In our report of effects of maternal dietary intervention on offspring outcomes in the setting of obesity we wrote: “There is a need to determine optimal timing, nature and extent of interventions. We have taken the view – as others have done - that the optimal time for recuperation would be prior to pregnancy and have sought to develop a model to show the ability and extent of the simplest of interventions, reducing global intake, to produce beneficial results. … The available evidence indicates that women do not spontaneously alter their dietary patterns when they discover they are pregnant 34. Interventions in pregnancy, as in any other major health area, therefore need to be based on firm, reproducible scientific evidence”27.
Obese women contemplating pregnancy need to be provided with firm information as to the benefits that accrue from decreasing their BMI both before and during pregnancy for at least two reasons. First, they need to be aware of the biological reasons that maternal obesity is harmful to themselves and their baby in many ways. Secondly they need to be confident that appropriately lowering their BMI and food intake will provide significant benefit to themselves and their children.
Influence of interventions on offspring Aging
Maternal low protein diets accelerate aging in rat offspring 35. We have shown increased adiposity index, leptin, and triglycerides (TG) in male OFF of MO mothers in young adult life (postnatal day110) 27 with no changes in body weight. However by 650 days MO results in a more rapid aging of some metabolic indices such as body weight (Figure 4, 5 and 6), fat and adiposity index, increases which were prevented by maternal dietary and exercise intervention (Figure 4).
Figure 4.

Rat male offspring (A) body weight (B) fat and (C) adiposity index at 650 days. Mean ± SEM; n = 6. C control diet, MO maternal obesity, DINT maternal dietary intervention, MOEx, maternal obesity exercise intervention; p<0.05 for groups not sharing at least one letter.
Figure 5.

Representative pictures of male offspring at postnatal day 650. C control diet, MO maternal obesity and DINT maternal dietary intervention.
Figure 6.

Representative pictures of male offspring at postnatal day 650. C control diet, CEx control diet + maternal exercise intervention, MO maternal obesity and MOEx maternal obesity + maternal exercise intervention.
One good example of positive features of programming is seen in the offspring of CEx mothers which, at this early stage of aging (PND 650), had a better metabolic phenotype than the rest of the groups, including the control group.
STUDIES ON DIETARY INTERVENTION IN OBESE PREGNANT SHEEP
As discussed in our earlier review of developmental programming (“Animal Studies that Reveal Mechanisms of Programming of Offspring Outcomes of Maternal Obesity”, there are differences between pregnancy in altricial, polytocous mammals such as the rat, and precocial, monotocous species such as humans, sheep and nonhuman primates. The pregnant sheep has been extensively investigated to determine the impacts of decreased maternal nutrition but fewer studies have been conducted on effects of maternal overnutrition/obesity on fetuses and offspring in this important, precocial, experimental species 36-44. Although there are differences in some capabilities – locomotion for example - both sheep and pregnant women produce well-developed, precocial offspring, exhibit similar newborn to maternal weight ratios, and temporal pattern of fetal tissue and organ development. Further, investigators worldwide have utilized the fetal sheep as a biomedical model to design studies on human pregnancy such as fetal behavior, heart rate and sleep states 45-48.
Our studies on the impact of maternal overnutrition/obesity in the ewe on fetal growth and development and offspring health are conducted with animals from a well-characterized, closed flock at the Center for the Study of Fetal Programming, University of Wyoming. Ewes of similar, size and breeding are maintained in the source flock developed from lambs born within the flock whose mothers were fed National Research Council (NRC) feed requirements throughout pregnancy and lactation. The ewe lambs are then maintained on the same diet and are used as the mothers in all studies and are housed together and fed only to NRC requirements from weaning to maturity. This management policy provides assurance that animals have not been exposed to highly variable environments prior to any investigation and thus limits the chance of markedly different environmental (epigenetic) influences on study results, and other influences such as sibships within groups.
We have developed and characterized a model of maternal overnutrition/obesity (MO) where ewes are fed a highly palatable pelleted diet at 150% of requirements from 60 days before conception through pregnancy. On this diet, ewes become obese by the time they are bred and continue to gain additional weight throughout pregnancy and fetuses show a definitive endophenotype 36-39, 43, 44, 49, 50. Overweight and obesity at conception in pregnant women has been shown to have the greatest impact on increasing adiposity of infants at birth, leading to insulin resistance and exhibit obesity in later life. In our model of diet–induced MO, lambs are born with increased adiposity, and by 19 months of age they exhibit hyperphagia, glucose and insulin dysregulation and increased adiposity compared to offspring of ewes fed only to requirements 38. Previous studies in our laboratory demonstrated that maternal undernutrition (50% global undernutrition) starting at day 28 of gestation resulted in delivery of offspring who exhibited metabolic disturbances (i.e. they were hyperphagic, insulin resistant, and were obese) as adults 51. We therefore hypothesized that a dietary intervention in which the obesogenic diet is reduced from 150% to 100% of NRC requirements (MO intervention, MOI) beginning on day 28 of gestation would be early enough to at least in part prevent the negative impacts of maternal overnutrition/obesity on the fetus and resulting offspring. Further, day 28 in the sheep is equivalent to ~day 50 in human pregnancy, which is about the time when women confirm they are pregnant and early enough for their obstetrician to provide overweight/obese women with a corrective dietary regimen if deemed necessary.
MOI eliminated MO-induced fetal macrosomia at mid-gestation, and either reduced (right ventricular weight, liver weight) or prevented MO-induced increases in organ weight (left ventricular weight, total kidney weight, pancreatic weight, and total perirenal fat weight). At day 135, while fetal weight was similar between CON, MO and MOI fetuses, MO fetuses exhibited greater left ventricular weights and thicknesses, right ventricular thicknesses, total kidney weight, and total perirenal fat, and reduced pancreatic weight than CON fetuses. Weights and thicknesses of these organs and tissues were returned to CON levels in the MOI fetuses. The data provide the first indication that alterations in fetal organ and tissue growth as well as endocrine changes (see below) can at least in part, be prevented by early pregnancy MOI in the face of maternal obesity.
To date we have only evaluated the cortisol changes in the MOI model in order to observe any similarities with our findings in the obese rat model described above (Fig 3). MO increased both maternal and fetal cortisol at 0.9G and this increase was prevented by MOI at both ages. Similar results were obtained at 0.9G. Interestingly, while the maternal increases in cortisol were accompanied by increased ACTH, this was not so in the fetus where cortisol but not ACTH, was increased above CON in MO at both ages. We hypothesize two possible mechanisms for this finding. First, MO may change adrenal sensitivity to ACTH. The second hypothesis is that much of the fetal cortisol in the setting of maternal obesity is produced in peripheral fetal tissues by increased activity of 11BHSD1, converting inactive cortisone to active cortisol. We have shown that the 11BHSD1 system is up-regulated in fetal female perirenal fat and fetal male liver in the setting of maternal under-nutrition supporting a potential role for increased 11BSD1 activity in response to maternal dietary challenges 52. Importantly, it remains to be seen to what extent inhibition of the increase in fetal cortisol will prevent adverse side effects of MO on offspring. These findings illustrate clearly the value of the combining studies in precocial and altricial species. We are currently evaluating CON, MO and MOI offspring to determine whether reducing maternal nutrition to recommended levels in early pregnancy of overnourished/obese ewes prevents endocrine and metabolic disturbances in offspring in adult life.
How do we get the message of the need for interventions to the general public? Lessons from the anti-smoking campaign
Improved women’s health and especially the institution of effective corrective measures is vitally important to obtain the optimal obstetric outcomes in the face of the current epidemic of obesity in women of reproductive years. There may be lessons that can be learned from the success in the developed world in decreasing the incidence of smoking. The anti-smoking campaign has met with considerable success in the developed world at least. This success suggests that even the strongest of compulsive behaviors can be modified when firm, incontrovertible information on benefit is provided. It has taken over 50 years since Sir Richard Bradford Doll demonstrated the connection between cigarette smoking and lung cancer 53. Changing this self-destructive behavior has taken decades but the decrease in smoking has saved thousands of lives. One of the most persuasive pieces of scientific evidence in the antismoking campaign was the demonstration in that while smoking from early adult life tripled mortality rates, giving up smoking at age 50 halved the risk and stopping at age 30 removed virtually all the risk. Human studies indicate that maternal pre-pregnancy BMI in women is a major determinant of adverse offspring metabolic outcomes resulting from maternal obesity 54. The parallel between smoking and the adverse effects of MO would be the suggestion from the evidence given here that life-style adjustment of life-style aimed at reducing obesity would have the potential to avoid the maternal and offspring hazards.
OVERALL CONCLUSIONS
There are epidemiological studies that shown that once a woman knows is pregnant, she does not modify her life style 34. Clearly the earlier the intervention the better the outcome. As the important IOM report and reviews by several clinical and basic science leaders indicate that studies such as those reported here are essential to the determination of mechanistic targets in the mother to develop predictive and clinical tools in human pregnancy 55. Other potential interventions remain to be investigated to permit evidence-based changes in clinical management. These include supplemented diets with polyunsaturated fatty acids or anti-oxidants. Different interventions to improve outcomes may act through different mechanisms. If so, a combination of approaches may lead to even better results for the mother and the offspring.
Figure 7.

Sheep fetal A) ACTH and B) cortisol, and maternal C) ACTH and D) cortisol at 75 and 135 days gestation (0.5 and 0.9G; Term 150 days gestation). Control (CON - open), maternal obesity (MO - solid), maternal obesity dietary intervention (MOI - striped); Mean ± SEM; n = 6; p<0.05 ** vs CON and MO, * vs CON and MOI.
Acknowledgments
This work was supported by CONACyT (Consejo Nacional de Ciencia y Tecnología) 155166, México, Sociedad Mexicana de Nutrición y Endocrinología and HD 21350 from the National Institute of Child Health and Human Development.
References
- 1.Nguyen T, Lau DC. The obesity epidemic and its impact on hypertension. Can J Cardiol. 2012;28:326–333. doi: 10.1016/j.cjca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Encuesta Nacional de Nutricion 1999-2006. Encuesta Nacional de Nutricion 1999-2006. Mexico: 2006. [Google Scholar]
- 3.Colchero MA, Sosa-Rubi SG. Heterogeneity of income and lifestyle determinants of body weight among adult women in Mexico, 2006. Soc Sci Med. 2012;75:120–128. doi: 10.1016/j.socscimed.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Correia LL, da Silveira DM, e Silva AC, et al. Prevalence and determinants of obesity and overweight among reproductive age women living in the semi-arid region of Brazil. Cien Saude Colet. 2011;16:133–145. doi: 10.1590/s1413-81232011000100017. [DOI] [PubMed] [Google Scholar]
- 6.Seabra G, Padilha Pde C, de Queiroz JA, Saunders C. Pregestational overweight and obesity: prevalence and outcome associated with pregnancy. Rev Bras Ginecol Obstet. 2011;33:348–353. [PubMed] [Google Scholar]
- 7.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989-2007. Int J Obes (Lond) 2010;34:420–428. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 8.Sahu MT, Agarwal A, Das V, Pandey A. Impact of maternal body mass index on obstetric outcome. J Obstet Gynaecol Res. 2007;33:655–659. doi: 10.1111/j.1447-0756.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 9.Leung TY, Leung TN, Sahota DS, et al. Trends in maternal obesity and associated risks of adverse pregnancy outcomes in a population of Chinese women. BJOG. 2008;115:1529–1537. doi: 10.1111/j.1471-0528.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 10.Benkeser RM, Biritwum R, Hill AG. Prevalence of overweight and obesity and perception of healthy and desirable body size in urban, Ghanaian women. Ghana Med J. 2012;46:66–75. [PMC free article] [PubMed] [Google Scholar]
- 11.Gavard JA, Artal R. Effect of exercise on pregnancy outcome. Clin Obstet Gynecol. 2008;51:467–480. doi: 10.1097/GRF.0b013e31816feb1d. [DOI] [PubMed] [Google Scholar]
- 12.Vickers MH, Sloboda DM. Strategies for reversing the effects of metabolic disorders induced as a consequence of developmental programming. Front Physiol. 2012;3:242. doi: 10.3389/fphys.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005 Oct;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 14.Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes. 2010;59:3058–3065. doi: 10.2337/db10-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006 Apr;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson SM, Poston L. Intervention strategies to improve outcome in obese pregnancies: insulin resistance and gestational diabetes. In: Gillman MW, editor. Maternal Obesity. Cambridge University Press; New York: 2012. pp. 179–198. [Google Scholar]
- 17.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21:521–526. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCance RA, Widdowson EM. Fat. Pediatr Res. 1977;11:1081–1083. [PubMed] [Google Scholar]
- 19.Oken E, Gillman MW. Interventios strategies to improve outcome in obese pregnancies: focus on gestational weight gain. In: Poston L, editor. Maternal Obesity. Cambridge University Press; New York: 2012. pp. 151–178. [Google Scholar]
- 20.Stern JS, Johnson PR. Spontaneous activity and adipose cellularity in the genetically obese Zucker rat (fafa) Metabolism. 1977;26:371–380. doi: 10.1016/0026-0495(77)90104-4. [DOI] [PubMed] [Google Scholar]
- 21.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- 23.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zambrano E, Rodriguez-Gonzalez GL, Guzman C, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- 26.Zambrano E, Bautista CJ, Deas M, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrano E, Martinez-Samayoa PM, Rodriguez-Gonzalez GL, Nathanielsz PW. Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J Physiol. 2010;588:1791–1799. doi: 10.1113/jphysiol.2010.190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyllenhammer LE, Vanni AK, Byrd-Williams CE, et al. Objective Habitual Physical Activity and Estradiol Levels in Obese Latina Adolescents. J Phys Act Health. 2012 doi: 10.1123/jpah.10.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter LG, Lewis KN, Wilkerson DC, et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab. 2012;303:E1061–1068. doi: 10.1152/ajpendo.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter LG, Qi NR, de Cabo R, Pearson KJ. Maternal Exercise Improves Insulin Sensitivity in Mature Rat Offspring. Med Sci Sports Exerc. 2012 doi: 10.1249/MSS.0b013e31827de953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert JS, Banek CT, Bauer AJ, et al. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol. 2012;303:R520–526. doi: 10.1152/ajpregu.00253.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham K. Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat. Hypertension. 2012;60:1545–1551. doi: 10.1161/HYPERTENSIONAHA.112.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. J Nutr. 2009;139:1956–1963. doi: 10.3945/jn.109.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morimoto S, Calzada L, Sosa TC, et al. Emergence of ageing-related changes in insulin secretion by pancreatic islets of male rat offspring of mothers fed a low-protein diet. Br J Nutr. 2011;107:1562–1565. doi: 10.1017/S0007114511004855. [DOI] [PubMed] [Google Scholar]
- 36.Dong M, Zheng Q, Ford SP, et al. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J Mol Cell Cardiol. 2012;55:111–116. doi: 10.1016/j.yjmcc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Zhao JX, Yan X, et al. Maternal obesity enhances collagen accumulation and cross-linking in skeletal muscle of ovine offspring. PLoS One. 2012;7:e31691. doi: 10.1371/journal.pone.0031691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long NM, George LA, Uthlaut AB, et al. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci. 2010;88:3546–3553. doi: 10.2527/jas.2010-3083. [DOI] [PubMed] [Google Scholar]
- 39.Long NM, Rule DC, Zhu MJ, Nathanielsz PW, Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J Anim Sci. 2012;90:2201–2210. doi: 10.2527/jas.2011-4343. [DOI] [PubMed] [Google Scholar]
- 40.Muhlhausler BS, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Fetal leptin is a signal of fat mass independent of maternal nutrition in ewes fed at or above maintenance energy requirements. Biol Reprod. 2002;67:493–499. doi: 10.1095/biolreprod67.2.493. [DOI] [PubMed] [Google Scholar]
- 41.Philp LK, Muhlhausler BS, Janovska A, Wittert GA, Duffield JA, McMillen IC. Maternal overnutrition suppresses the phosphorylation of 5’-AMP-activated protein kinase in liver, but not skeletal muscle, in the fetal and neonatal sheep. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1982–1990. doi: 10.1152/ajpregu.90492.2008. [DOI] [PubMed] [Google Scholar]
- 42.Rattanatray L, MacLaughlin SM, Kleemann DO, et al. Impact of maternal periconceptional overnutrition on fat mass and expression of adipogenic and lipogenic genes in visceral and subcutaneous fat depots in the postnatal lamb. Endocrinology. 2010;151:5195–5205. doi: 10.1210/en.2010-0501. [DOI] [PubMed] [Google Scholar]
- 43.Yan X, Huang Y, Zhao JX, et al. Maternal obesity-impaired insulin signaling in sheep and induced lipid accumulation and fibrosis in skeletal muscle of offspring. Biol Reprod. 2011;85:172–178. doi: 10.1095/biolreprod.110.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Long NM, Hein SM, et al. Maternal obesity in ewes results in reduced fetal pancreatic beta-cell numbers in late gestation and decreased circulating insulin concentration at term. Domest Anim Endocrinol. 2011;40:30–39. doi: 10.1016/j.domaniend.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalton KJ, Dawes GS, Patrick JE. Diurnal, respiratory, and other rhythms of fetal heart rate in lambs. Am J Obstet Gynecol. 1977;127:414–424. doi: 10.1016/0002-9378(77)90500-2. [DOI] [PubMed] [Google Scholar]
- 46.Dawes GS. Breathing before birth in animals and man. An essay in developmental medicine. N Engl J Med. 1974;290:557–559. doi: 10.1056/NEJM197403072901010. [DOI] [PubMed] [Google Scholar]
- 47.Dawes GS, Moulden M, Redman CW. The advantages of computerized fetal heart rate analysis. J Perinat Med. 1991;19:39–45. doi: 10.1515/jpme.1991.19.1-2.39. [DOI] [PubMed] [Google Scholar]
- 48.Patrick J. The physiological basis for fetal assessment. Semin Perinatol. 1989;13:403–408. [PubMed] [Google Scholar]
- 49.George LA, Uthlaut AB, Long NM, et al. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod Biol Endocrinol. 2010;8:75. doi: 10.1186/1477-7827-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long NM, Ford SP, Nathanielsz PW. Maternal obesity eliminates the neonatal lamb plasma leptin peak. J Physiol. 2011;589:1455–1462. doi: 10.1113/jphysiol.2010.201681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford SP, Hess BW, Schwope MM, et al. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci. 2007;85:1285–1294. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- 52.Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2012;62:1175–1185. doi: 10.2337/db12-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maternal Obesity. Cambridge University Press; New York: 2012. [Google Scholar]


