Abstract
Context
Individuals with cocaine use disorder (CUD) have difficulty monitoring ongoing behavior, possibly stemming from dysfunction of brain regions subserving insight and self-awareness [e.g., anterior cingulate cortex (ACC)].
Objective
To test the hypothesis that CUD with impaired insight (iCUD) would show abnormal (A) ACC activity during error processing, assessed with functional magnetic resonance imaging during a classic inhibitory control task; (B) ACC gray matter integrity assessed with voxel-based morphometry; and (C) awareness of one’s own emotional experiences, assessed with the Levels of Emotional Awareness Scale (LEAS). Using a previously validated probabilistic choice task, we grouped 33 CUD according to insight [iCUD: N=15; unimpaired insight CUD: N=18]; we also studied 20 healthy controls, all with unimpaired insight.
Design
Multimodal imaging design.
Setting
Clinical Research Center at Brookhaven National Laboratory.
Participants
Thirty-three CUD and 20 healthy controls.
Main Outcome Measure
Functional magnetic resonance imaging, voxel-based morphometry, LEAS, and drug use variables.
Results
Compared with the other two study groups, iCUD showed lower (A) error-induced rostral ACC (rACC) activity as associated with more frequent cocaine use; (B) gray matter within the rACC; and (C) LEAS scores.
Conclusions
These results point to rACC functional and structural abnormalities, and diminished emotional awareness, in a subpopulation of CUD characterized by impaired insight. Because the rACC has been implicated in appraising the affective/motivational significance of errors and other types of self-referential processing, functional and structural abnormalities in this region could result in lessened concern (frequently ascribed to minimization and denial) about behavioral outcomes that could potentially culminate in increased drug use. Treatments targeting this CUD subgroup could focus on enhancing the salience of errors (e.g., lapses).
Keywords: cocaine addiction, insight, fMRI, VBM, emotional awareness, anterior cingulate cortex, color-word Stroop
INTRODUCTION
Drug addicted individuals often take drugs despite conscious, well-intentioned plans to abstain. Rather than reflecting deficiencies of will power, we recently suggested that a core symptom of drug addiction involves dysfunction of brain regions subserving insight and self-awareness1. Because impaired insight is marked by reduced sensitivity to negative outcomes, poorer treatment outcome, and lowered treatment compliance across various neuropsychiatric disorders (e.g., schizophrenia, neurological insults) (discussed in2), we reasoned that this deficit could also have important implications for addiction. Discrepancies between self-reports and objective indices of behavior3–5, and compromised monitoring of ongoing behavior6,7 as associated with more severe drug-seeking7, provided the preliminary evidence for impaired insight in addiction. Here we investigated the neural correlates of impaired insight in addiction using a combined functional magnetic resonance imaging (fMRI) and voxel-based morphometry (VBM) approach.
We hypothesized key roles for brain regions underlying self-monitoring, self-awareness, interoception, and error-related processing, especially the anterior cingulate cortex (ACC) and the anterior insula. The ACC is classically implicated in the neural response to errors8 and in cognitive control more generally9, subserving functions that include performance monitoring10, conflict monitoring11, error detection12, and the prediction of post-error slowing13. Abnormal (especially: hypoactive) ACC activity has been documented on selective attention and inhibitory control tasks in users of various addictive substances (reviewed in14). We recently showed that ACC deficits extend to emotionally salient tasks in addiction, with individuals with cocaine use disorder (CUD) showing hypoactivations in dorsal and rostral ACC (dACC and rACC, respectively) during a drug Stroop task15. Of particular relevance, the ACC also participates in consciously mediated behavior. The ACC forms part of a network that is hypoactive during vegetative states, minimally conscious states, seizures, and sleep16; and damage to the ventromedial prefrontal cortex (PFC) and adjacent ACC is associated with unawareness of one’s social impairment17. In cannabis users, dACC (extending into rACC) hypoactivity was associated with unaware errors on an error awareness task18. In further agreement, a study of Alzheimer’s disease showed that patients unaware of their illness-related deficits had reduced activity in the dACC and rACC/PFC during a go/no-go task19. Insula involvement was hypothesized because of its central role in interoception20,21, implicated in conscious drug craving in addicted individuals22–24 and error awareness in health25,26. In one study that targeted both regions, insula and ACC error-related activity during a go/no-go task was associated with individual differences in absentmindedness27, a concept related to self-monitoring/awareness.
Using a previously developed choice task that assesses self-monitoring of behavior7,28, participants in the current study were grouped by insight. In parallel, participants underwent fMRI while performing an event-related color-word Stroop29. Errors on this classical inhibitory control task could have implications for insight because of the need to self-monitor behavior (e.g., upon error commission); of additional relevance, errors reliably engage the ACC and insula, including during Stroop tasks30–36 and other inhibitory control tasks37–42. During these same scanning sessions, structural MRI was collected. Compared with healthy controls and CUD with unimpaired insight (uCUD), we hypothesized that CUD with impaired insight (iCUD) would show abnormal ACC and insula (A) functional activity during error processing and (B) gray matter integrity (with the latter resting on previous studies in which CUD had reduced gray matter volume in the ACC and/or insula43–46), and that these functional and/or structural abnormalities would correlate with increased drug use. We further hypothesized that (C) iCUD would show diminished self-awareness of one’s own emotional experiences, assessed with the Levels of Emotional Awareness Scale (LEAS)47. Inclusion of the LEAS was important to validate our insight measure; it was also intended to extend the insight concept in addiction beyond compromised behavioral-monitoring (e.g., error or choice awareness) and into more complex socioemotional/interpersonal scenarios.
METHODS
Participants
Our main sample included 33 CUD and 20 controls, all right-handed and native English speakers; all provided written informed consent to participate in accordance with the local Institutional Review Board. A psychiatric interview (Supplementary Materials) determined that all CUD met DSM-IV criteria for current cocaine dependence (N=28) or cocaine dependence in early (N=3) or sustained (N=2) remission (Table 1 provides current dependence/remission partitioning; Supplementary Materials provides current/past comorbidities). A triage urine panel for drugs of abuse was conducted in all participants immediately before all other study procedures (i.e., not on a separate screening day) (Table 1 provides cocaine urine status partitioning). Positive urine for drugs other than cocaine in CUD, and positive urine screens for any drugs in controls, were exclusionary (see Supplementary Materials for additional discussion of this variable and for additional exclusion criteria).
Table 1.
Demographics and drug use of all study participants.
| Between- Group Test | Impaired Insight Cocaine (N=15) | Intact Insight Cocaine (N=18) | Intact Insight Controls (N=20) | |
|---|---|---|---|---|
| Gender: Male/Female | χ2=0.4 | 13/2 | 15/3 | 18/2 |
| Race: African-American/Caucasian/Other | χ2=1.0 | 10/4/1 | 11/5/2 | 15/4/1 |
| Age (years) | F=1.9 | 44.1 ± 8.1 | 43.0 ± 8.6 | 39.6 ± 5.5 |
| Education (years) | F=2.9 | 12.2 ± 1.4 | 12.8 ± 1.5 | 13.3 ± 1.0 |
| Verbal IQ: Wide Range Achievement Test III – Scaled Score90 | F=2.4 | 96.9 ± 10.1 | 91.9 ± 13.7 | 100.6 ± 11.7 |
| Non-Verbal IQ: WASI - Matrix Reasoning Scale91 | F=0.2 | 10.2 ± 6.0 | 11.1 ± 2.3 | 10.9 ± 2.6 |
| Depression: Beck Depression Inventory II92 | H=23.5*** | 10.5 ± 6.0C | 9.4 ± 8.5C | 1.7 ± 3.2A,B |
| Socioeconomic Status: Hollingshead Index | F=1.9 | 28.8 ± 5.3 | 35.7 ± 12.3 | 34.7 ± 10.6 |
| Cigarette smokers (current or past/nonsmokers) | χ2=20.4*** | 11/4C | 16/2C | 4/16A,B |
| Daily cigarettes (current smokers: N = 9/13/2) | F=0.0 | 7.4 ± 2.5 | 7.3 ± 5.7 | 7.0 ± 4.2 |
| Time since last cigarette smoking (within 4 hrs./>4 hrs.) | χ2=5.4 | 5/4 | 3/10 | 2/0 |
| Cocaine diagnosis status: current/remission | χ2=0.1 | 13/2 | 15/3 | -- |
| Cocaine urine status: positive/negative (immediately before fMRI) | χ2=3.5 | 7/8 | 3/15 | -- |
| Treatment seeking status: no/yes | χ2=3.9 | 11/4 | 7/11 | -- |
| Cocaine age of onset (years) | t=1.7 | 29.1 ± 7.3 | 24.7 ± 7.3 | -- |
| Cocaine duration of use (years) | t=1.8 | 11.6 ± 6.8 | 16.5 ± 8.2 | -- |
| Cocaine past month use: days/week | z=−2.0 | 1.9 ± 1.9 | 0.8 ± 1.4 | -- |
| Cocaine past month use: $/use | t=0.8 | 42.3 ± 53.1 | 28.0 ± 43.3 | -- |
| Cocaine current abstinence (min – max, median) | z=−1.8 | 0–548, 4 | 0–1825, 31 | -- |
| Cocaine longest abstinence (min – max, median) | z=−1.1 | 18–2920, 210 | 21–3650, 1003.5 | -- |
| Withdrawal symptoms: CSSA (0–126) | t=0.4 | 14.9 ± 10.0 | 16.4 ± 12.0 | -- |
| Severity of Dependence Scale (0–15) | t=0.0 | 8.1 ± 3.3 | 8.1 ± 3.6 | -- |
| Cocaine Craving Questionnaire (0–45) | t=2.9* | 20.7 ± 12.2B | 10.3 ± 8.5A | -- |
Note. Values are frequencies or means ± standard deviation;
p<0.05,
p<0.001
(mean value significantly differs from that of impaired insight cocaine participants;
mean value significantly differs from that of intact insight cocaine participants;
mean value significantly differs from that of controls);
Race: Other (Hispanic/Asian); WASI = Wechsler Abbreviated Scale of Intelligence; CSSA = Cocaine Selective Severity Assessment Scale. With the exception of cocaine craving, impaired insight and intact insight cocaine participants were generally well-matched on drug use variables; any factors that did differ between groups (depression, cigarette smoking history, or craving) did not explain our results as described in Supplementary Materials.
Study Procedures
Insight Assessment
Insight was assessed using established, validated procedures7,28 (Supplementary Materials provides comprehensive description). In brief, participants performed a probabilistic learning choice task, providing their objective preference for viewing standardized48 pleasant (e.g., babies), unpleasant (e.g., disfigurement), neutral (e.g., household objects), and in-house5 cocaine images. After the task, participants’ most selected picture category (actual choice) was compared with participants’ awareness of this choice (self-report of which picture category was chosen most frequently). CUD showing agreement between their behavior and self-reports formed the group of uCUD (N=18); those showing disagreement between these measures formed the group of iCUD (N=15). All included controls (N=20) were selected to have intact insight (only seven controls with completed study procedures had impaired insight, requiring future investigation with larger samples; see Supplementary Materials for additional discussion of these controls). This task’s relevance to insight is in assessing whether CUD have explicit knowledge (awareness) about their drug-seeking. Because human instrumental learning (under conditions similar to the current task) is encoded as explicit causal knowledge49–51, choice on this task is likely goal-driven [i.e., not governed by habitual, implicit responding (see Supplementary Materials for additional discussion)].
Inhibitory Control Task
Participants performed three runs of an event-related fMRI color-word Stroop task, with instructions to press for the ink color of color-words (red, blue, yellow, green) printed in their congruent or incongruent colors. Each task run contained 12 incongruent events (totaling 36 such events per participant) and 188 congruent events (totaling 564 such events per participant). Participants committed an average of 20.4 (range=1–74), 25.6 (range=2–119), and 24.0 (range=1–73) total errors (i.e., summed across congruent and incongruent trials) during runs 1, 2, and 3, respectively (combined M=23.4; SD=16.6). No word or color of an incongruent stimulus mirrored the preceding congruent color-word; otherwise, stimuli were presented randomly. Each word was presented for 1300 ms, which was also the time allotted for response (intertrial interval=350 ms); participants were not given performance feedback. Remuneration for task completion was $25 (fixed). This Stroop task version was adapted from a previous neuroimaging study52 and is comprehensively described elsewhere (including a descriptive task schematic)31,53. Table 2 displays the behavioral data.
Table 2.
Performance on the color-word Stroop fMRI task across all study participants.
| F (between) | Impaired Insight Cocaine N=15 | Intact Insight Cocaine N=18 | Intact Insight Controls N=20 | |
|---|---|---|---|---|
| Accuracy (all trials) (% correct) | 1.1 | .74 ± .03 | .69 ± .04 | .76 ± .03 |
| Accuracy (congruent trials) (% correct) | 2.8 | .92 ± .02 | .87 ± .03 | .93 ± .01 |
| Accuracy (incongruent trials) (% correct) | 0.6 | .57 ± .06 | .51 ± .07 | .59 ± .05 |
| Accuracy (incongruent – congruent trials) (% correct) | 0.1 | −.35 ± .06 | −.36 ± .06 | −.33 ± .04 |
| Reaction time (all trials) (ms) | 0.1 | 804.5 ± 20.2 | 795.4 ± 16.8 | 797.6 ± 16.7 |
| Reaction time (congruent trials) (ms) | 0.5 | 686.4 ± 20.0 | 707.3 ± 20.4 | 685.3 ± 15.9 |
| Reaction time (incongruent trials) (ms) | 0.8 | 922. 6 ± 25.1 | 883.5 ± 18.5 | 909.8 ± 21.8 |
| Reaction time (incongruent – congruent trials) (ms) | 2.6 | 236.2 ± 20.7 | 176.3 ± 19.8 | 224.5 ± 18.7 |
| Post-conflict slowing (ms) | 0.6 | 968.1 ± 34.7 | 926.1 ± 21.2 | 938.9 ± 24.2 |
| Post-error slowing (all trials) (ms) | 1.5 | 14.8 ± 11.7 | 57.4 ± 20.2 | 46.3 ± 16.6 |
Note. Task accuracy, reaction time, post-conflict slowing (slowing after an incongruent, correct trial), and post-error slowing (slowing after an error) were computed for all participants (see Supplementary Materials for further description). Although no group differences were significant, there was an interesting trend for impaired insight cocaine participants compared with the other groups to show lower post-error slowing (p<0.10), suggested previously as a measure of error awareness93. Overall, however, these behavioral results suggest that the groups were well-matched on task performance, and that the MRI group differences described in the Results are not attributable to differences in task-related motivation or interest. Values are M ± SE.
MRI Data Acquisition
MRI scanning was performed on a 4T whole-body Varian/Siemens MRI scanner. The blood-oxygenation-level-dependent (BOLD) fMRI responses were measured as a function of time using a T2*-weighted single-shot gradient-echo planar sequence (TE/TR=20/1600 ms, 3.125×3.125 mm2 in-plane resolution, 4 mm slice thickness, 1 mm gap, typically 33 coronal slices, 20 cm FOV, 64×64 matrix size, 90°-flip angle, 200kHz bandwidth with ramp sampling, 207 time points, and 4 dummy scans to avoid non-equilibrium effects in the fMRI signal). Anatomical images were collected using a T1-weighted 3D-MDEFT (three-dimensional modified driven equilibrium Fourier transform) sequence54 and a modified T2-weighted hyperecho sequence55.
MRI Data Processing
Image processing and analysis were performed with Statistical Parametric Mapping (SPM8) (Wellcome Trust Centre for Neuroimaging, London). Image reconstruction was performed using an iterative phase correction method that produces minimal signal-loss artifacts in echo-planar images56. A six-parameter rigid body transformation (3 rotations, 3 translations) was used for image realignment and correction of head motion. Criteria for acceptable motion were 2 mm displacement and 2° rotation. The realigned datasets were spatially normalized to the standard Montreal Neurological Institute (MNI) stereotactic space using a 12-parameter affine transformation57 and a voxel size of 3×3×3 mm. An 8-mm full-width-half-maximum Gaussian kernel spatially smoothed the data.
BOLD-fMRI Analyses
A general linear model58, which included six motion regressors (3 translation and 3 rotation) and one task condition regressor convolved with a canonical hemodynamic response function and a high-pass (cut-off frequency: 1/90 s) filter, was used to calculate individual BOLD-fMRI maps. Specifically, our design matrix included one regressor collapsed across both error trials (Congruent Incorrect and Incongruent Incorrect), leaving both correct trials (Congruent Correct and Incongruent Correct) to serve as the active, implicit baseline; this implicit baseline was chosen because the task contained mostly correct events. Thus, the beta weights for this incorrect (error) regressor equated to a contrast functionally equivalent to incorrect>‘everything else’ (insofar as ‘everything else’ consisted entirely of correct events), reflecting task-related error processing remaining after the variance related to correct events was removed. For analyses pertaining to a second design matrix that modeled the incongruent events, see Supplementary Materials. Because error is contrasted with an active baseline (correct) and not a neutral baseline (e.g., fixation), BOLD signal values below zero do not necessarily reflect deactivations.
At the 2nd Level, we conducted a whole-brain one-way analysis of variance (ANOVA) in SPM8. Because our regions of interest (ROIs) were relatively large (ACC and insula), and following the recommendation that broader, more diffuse activations are best detected by lower thresholds59, we specified a height threshold of p<0.005 voxel-level uncorrected (T=2.68), a common threshold in psychiatric neuroscience research. We then used a Monte Carlo procedure60 (similar to AlphaSim) to identify the number of contiguous voxels necessary for a p<0.05 cluster-corrected threshold (i.e., given our imaging parameters and a height threshold of T=2.68), which was calculated to be 26 contiguous voxels. One sample t-tests were then conducted on the same 1st Level contrasts to confirm that the regions that differed between groups were indeed engaged during the task. To focus these latter analyses, results were masked by the respective between-group ANOVA contrasts (for results of unmasked one-sample t-tests across all participants, see Supplementary Materials). Nevertheless, to protect against Type I error, statistical significance for these one-sample t-tests was set at p<0.05 family-wise voxel-level-corrected. The average BOLD signals from peaks that met both criteria were extracted as spherical volumes (3-mm radius) to inspect for outliers and for use in correlation analyses (below). MRIcron corroborated anatomical specificity.
Structure
VBM analysis was conducted with the VBM toolbox (VBM8) (Gaser, C, University of Jena, Department of Psychiatry, Germany; http://dbm.neuro.uni-jena.de/vbm/), which combines spatial normalization, tissue segmentation, and bias correction into a unified model. The MDEFT scans, which produce especially precise characterization of gray matter tissue61, were first spatially normalized to standard proportional stereotaxic space (voxel size: 1×1×1 mm) and segmented into gray matter, white matter, and cerebrospinal fluid tissue classes according to a priori tissue probability maps62,63. A hidden Markov random field64 maximized segmentation accuracy. Jacobian modulation compensated for the effect of spatial normalization and restored the original absolute gray matter volume in the gray matter segments. Three uCUD had unusable structural scans; for these participants, structural scans during a six-month follow-up session were substituted (note that removing these three participants did not change any VBM results). After smoothing the normalized and modulated gray matter segments with a 10 mm3 full-width at half maximum Gaussian kernel, we estimated a one-way analysis of covariance (ANCOVA), with age and total brain volume included as covariates of no interest43,44,65–68. We first performed whole-brain analyses, consistent with the functional approach. As an additional test of group differences, we defined spherical ROIs (3-mm radius) at the coordinates from the functional data that were observed for both the between-group ANOVA and one-sample t-tests. These firmly a priori ROIs were then analyzed in SPSS.
LEAS
Participants were presented with 20 emotionally-charged interpersonal scenarios and answered how each person involved would likely feel. For example, “You and your best friend are in the same line of work. There is a prize given annually to the best performance of the year. The two of you work hard to win the prize. One night the winner is announced: your friend. How would you feel? How would your friend feel?” Scoring followed a validated coding scheme (higher scores=higher self-awareness of one’s own emotion)47. Previously, lower LEAS scores were associated with reduced rACC activity during trauma recall in patients with post-traumatic stress disorder, relative to controls who had also experienced trauma69. Because only 15 participants from our main sample had LEAS data (i.e., this measure was not yet in place when the fMRI protocol commenced), data from 20 additional participants (who did not complete the fMRI component) were included in the LEAS analyses to maximize sample size. Importantly, the 15 participants overlapping between both protocols did not differ from the rest of the main sample, and did not differ from these new 20 participants, on any Table 1 demographics (all p>0.05), suggesting that these 20 new participants were comparable to the main sample. An ANCOVA tested for between-group differences while controlling for age (i.e., one anticipates LEAS to increase with age/development70) and verbal IQ (i.e., to produce effective written responses, one anticipates LEAS to increase with higher verbal IQ47). LEAS scoring was blind to insight/participant grouping.
Correlation Analyses
We first tested for functional-structural correspondence (correlations) between regions that showed parallel between-group differences for both methodologies. We then tested correlations between functional activations or gray matter (that also first showed between-group differences) with the 12 cocaine use variables from Table 1. Significance for these drug use correlations was set at p<0.01 to minimize Type I error. Because only 15 total participants from our main sample had LEAS data as described above, we were unable to inspect correlations with this measure.
RESULTS
Function
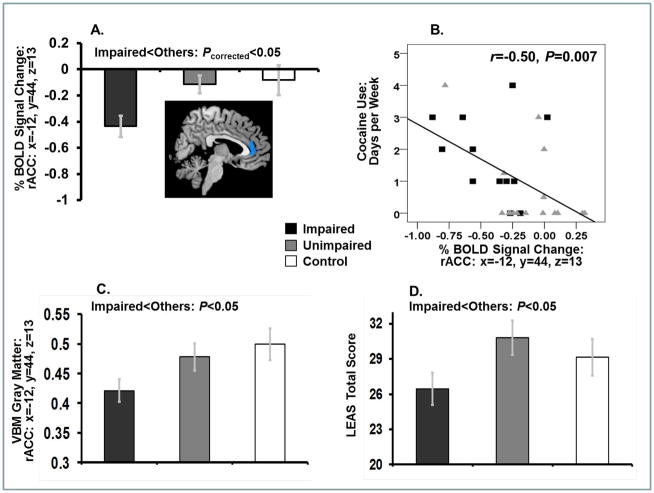
Whole-brain SPM8 analyses revealed iCUD to have less error>correct activity compared with the other two study groups in the rACC (Figure 1A). Although this cluster extended dorsally to include additional ACC subregions (Table 3), a one-sample t-test in iCUD showed that this between-group difference was driven by error>correct lower activations in this group specifically in the rACC [i.e., not in the entire ACC cluster; note that one peak coordinate overlapped across both analytical approaches (x=12, y=44, z=13; Table 3)]. No other between-group differences reached significance.
Figure 1.
(A) Reduced error>correct rACC mean % BOLD signal change in impaired insight cocaine participants (N=15) compared with the other two study groups (unimpaired insight cocaine participants: N=18; healthy controls: N=20) during the color-word Stroop task (with corresponding image, which for display purposes only was thresholded at 2.4≤T≤7.0 and masked by an anatomical ACC region of interest). This reduced error-related rACC activity correlated with (B) more frequent drug use in the last 30 days in all cocaine participants. In parallel, and compared with the other study groups, impaired insight cocaine participants showed lower (C) gray matter volume in the same rACC region; and (D) emotional awareness (LEAS scores). Bar plots show means ± standard errors. Note that BOLD signal values below zero do not necessarily reflect deactivations (as the contrast with error is not with a fixation baseline, but rather with an implicit, active baseline of correct trials; see Methods).
Table 3.
Color-word Stroop between-group differences during error.
| Region | BA | Side | Voxels | Peak T | Peak P | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Impaired Insight < Others | ||||||||
| (Rostral) Anterior Cingulate Cortex | 32, 24,11 | B | 231 | 3.4 | .000 | −6 | 38 | −5 |
| −3 | 38 | 16 | ||||||
| 12 | 44 | 25 | ||||||
| 12 | 44 | 13 | ||||||
| Precentral Gyrus | 6 | R | 82 | 3.0 | .002 | 30 | 7 | 52 |
| Controls > Cocaine | ||||||||
| Inferior Parietal Lobule | 40 | R | 137 | 4.2 | .000 | 30 | −46 | 46 |
| Inferior Frontal Gyrus: Triangularis | 45 | L | 41 | 3.1 | .002 | −39 | 23 | 22 |
| −51 | 23 | 16 | ||||||
Note. Analyses are one-way ANOVAs, thresholded at p<0.05 corrected (p<0.005 voxel-uncorrected and 26 voxels); bolded coordinates were also observed using one-sample t-tests (p<0.05 voxel-level family-wise error corrected, masked by the respective between-group ANOVA contrasts); BA=Brodmann Area; R=right, L=left, B=bilateral.
Structure
Although whole-brain between-group differences were nonsignificant, we extracted two ROIs corresponding to the peak rACC functional coordinate that emerged using both the whole-brain between-group ANOVA and one-sample t-tests (x=12, y=44, z=13; Table 3; extracted on both the ipsilateral and contralateral sides). iCUD had reduced gray matter compared with the other study groups in the contralateral rACC ROI (planned comparison: F(1,50)=4.7, p=0.035) (Figure 1C).
LEAS
iCUD scored lower on the LEAS (total score) than the other two study groups (planned comparison: F(1,31)=4.3, p=0.048) (Figure 1D), suggesting decreased self-awareness of one’s own emotion in iCUD.
Correlations
The lower the error>correct activity in the extracted rACC cluster, the more frequently (days per week, last 30 days) cocaine was used in all CUD (Figure 1B). The other drug use variables did not correlate with rACC activity or structure; structure and function also did not correlate.
DISCUSSION
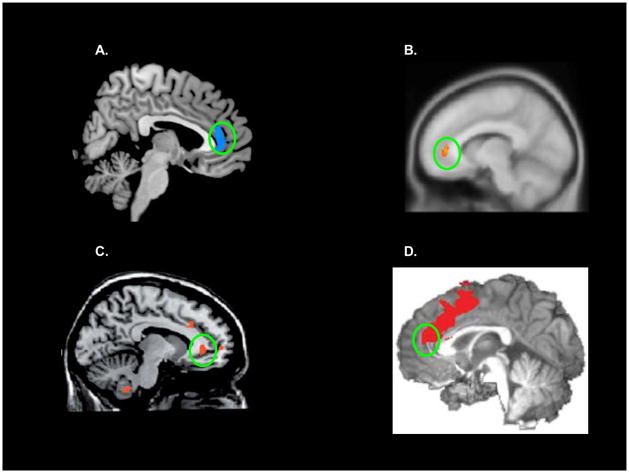
Our data provide novel evidence that impaired insight is associated with rACC dysfunction in cocaine addiction. Compared with controls and even uCUD (both with intact insight), iCUD (with impaired insight) showed lowered rACC (A) error>correct activity during a classical inhibitory control task (uCUD’s pattern of response more closely resembled that of controls) and (B) gray matter volume – effects not attributable to demographic/drug use between-group differences (Supplementary Material). Given the task’s active task baseline (correct trials), our functional results indicate that iCUD show disproportionately reduced activity to error events; in contrast, the other two groups showed relative equivalence of these trial types (see Supplementary Materials for time-series plots, which provide visual evidence that rACC error-related activity, even when not directly contrasted with correct responses, is decreased in iCUD). Interestingly, the rACC (extending into medial PFC) has been previously associated with insight-related compromises in schizophrenia71, cannabis use disorder18, and Alzheimer’s disease19; notably, only the rACC/medial PFC was implicated in all three studies/disorders (Figure 2). Also potentially relevant to insight, this brain area is activated during the experience of negative self-conscious emotions72–74, and during other activities relevant to social cognition (e.g., self-knowledge, person perception, mentalizing75,76).
Figure 2.
rACC involvement in neuropsychiatric illnesses characterized by impaired insight. (A) Current results; (B) activations during reality monitoring (the ability to distinguish internally-generated information from externally-generated information) in health (that do not emerge under the same task conditions in schizophrenia)71 (adapted with permission from Elsevier); (C) activity during a go/no-go task in Alzheimer’s patients with unimpaired insight relative to those with impaired insight19 (adapted with permission from Oxford University Press); and (D) activity during error on an error awareness task, which was lower during unaware errors in cannabis abusers18 (note that although peak ACC activity in this cannabis study is more caudal/dorsal, the cluster indeed extends to the rACC) (adapted with permission from Nature Publishing Group).
Another notable finding was a correlation between lower rACC functional activity to error and more frequent cocaine use. Because iCUD and uCUD did not differ on days of current abstinence, current use frequency, or cocaine urine status (Table 1), this association is unlikely attributable to the residual effects of recent cocaine use (i.e., acute drug effects) and might instead reflect addiction-related symptomatology – an interpretation consistent with previous research. In one relevant study, less cocaine use per week correlated with greater activation in the rACC during a modified Stroop task, measured with positron emission tomography (PET H(2)(15)O)77; because this study was conducted in 23-days abstinent CUD, results suggest that, similarly to the current study, the rACC-drug use association is more likely marking an addiction-related deficit (not acute drug use). If future studies determine that iCUD (with associated rACC dysfunction) also have worse clinical outcomes as we anticipate, then treatments targeting rACC functioning could have clinical viability. This region showed cue-reactivity reductions to pharmacotherapeutic interventions in cigarette smokers78,79, and was suggested through meta-analysis as a marker of treatment response in major depression80. Conversely, future studies should also uncover the mechanisms of continued drug use in uCUD, themselves a highly interesting CUD subgroup insofar as they showed preserved rACC function/structure while still meeting criteria for addiction. One potential explanation could be that while uCUD report lower craving overall (Table 1), there is tighter correspondence between their craving and drug-seeking (Supplementary Materials).
In parallel to these rACC results, the dACC and insula showed informative null results, which were not attributable to the inability of the current task to activate these regions (Supplementary Materials). While both the dACC and rACC participate in error-related processing, the dACC is involved in error detection and is closely interconnected with higher-order frontal brain regions involved in adaptive behavior (e.g., lateral PFC), whereas the rACC is involved in generating the (presumably negative) affective response that occurs shortly after error commission and is interconnected with several limbic brain regions (e.g., amygdala, hypothalamus, insula) (discussed in37). The insula is involved in forming an interoceptive representation of one’s subjective feeling state20, participating in drug craving in addiction22–24 and error awareness in health25,26. Null effects in the dACC and the insula could collectively indicate that although iCUD can recognize (both cognitively and interoceptively) that an error has occurred, this error might fail to elicit the appropriate emotional significance. This interpretation is bolstered by previous findings indicating that error-induced rACC activity tracks autonomic arousal36, increases when error salience is amplified (e.g., when attached to monetary loss)38, and participates in learning of optimal task strategies81. Given that iCUD also showed reduced LEAS scores, our results could indicate that this compromised salience tagging of negative emotional events may generalize to other emotional contexts (i.e., extending beyond task-related errors into more complex socioemotional scenarios of potential relevance to drug-taking). For example, one could speculate that for iCUD attempting to remain abstinent, a lapse (error) may not elicit the requisite salience or aversive valence, increasing the probability of subsequent full relapse into frequent drug use – well-anticipated from our negative correlation between rACC activity and current cocaine use.
A limitation of this study is the relatively small sample size for VBM, possibly explaining the lack of whole-brain results. Although in subsequent ROI analyses we accordingly restricted gray matter group comparisons to the region that first showed (corrected) functional effects (rACC), future studies with larger samples should replicate these results. Another limitation is that we cannot determine the precise neurobiological mechanisms underlying the decreased rACC error response; structure, while a plausible mediator82, did not directly correlate with function. An alternative possibility could involve abnormalities in anterior frontal cortex cerebral blood flow in CUD, as suggested by previous perfusion fMRI studies83; because such frontal blood flow abnormalities are seemingly more pronounced in men than women84, future studies should also replicate these effects in samples including more women. Future studies could also employ novel tasks targeting functioning of other insight/self-awareness-related regions not observed in this study (e.g., anterior insula20, but also somatosensory cortex85).
In conclusion, because the rACC has been implicated in appraising the affective/motivational significance of errors and self-referential processing, and given the association of impaired insight with diminished emotional self-awareness (LEAS), functional and structural abnormalities in this region could be expressed behaviorally as lessened concern regarding behavioral outcomes, potentially resulting in increased drug use. The current research therefore challenges the long-held clinical assumption that impaired insight in addiction is simply a manifestation of minimization and denial; instead, such impaired insight may stem from functional and structural abnormalities of the rACC. Our results extend prior research on compromised error awareness/processing6,18,86 and gray matter abnormalities43,44,66 in drug addiction, offering the intriguing suggestion that impaired insight may drive such effects. Our results also raise the possibility that a specific CUD subgroup (iCUD) might benefit from therapeutic interventions directed at enhancing the neuropsychological mechanisms underlying insight/self-awareness1 [e.g., self-relevant (tailored) motivational interventions87,88]. More broadly, our results can inform other neuropsychiatric disorders (e.g., anosognosia, alexithymia, schizophrenia, mania)89, similarly characterized by impaired insight and disadvantageous, unwanted, or inappropriate behaviors (e.g., leading to violence, self-harm).
Supplementary Material
Acknowledgments
This research was conducted with grant support from the National Institute on Drug Abuse (to RZG: 1R01DA023579; to SJM: 1F32DA030017-01; to MAP: 1F32DA033088). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. S.J.M. and R.Z.G. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors gratefully acknowledge the contributions of Michail Misyrlis, Thomas Maloney, Nelly Alia-Klein, Patricia A. Woicik, and Gene-Jack Wang.
Footnotes
The authors declare no financial conflict of interest.
References
- 1.Goldstein RZ, Craig AD, Bechara A, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009 Sep;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein TA, Ullsperger M, Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Frontiers in human neuroscience. 2013;7(14) doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein RZ, Alia-Klein N, Tomasi D, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164(1):43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein RZ, Parvaz MA, Maloney T, et al. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology. 2008;45:705–713. doi: 10.1111/j.1469-8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moeller SJ, Maloney T, Parvaz MA, et al. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biological Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hester R, Simões-Franklin C, Garavan H. Post-error behavior in active cocaine users: Poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32(9):1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- 7.Moeller SJ, Maloney T, Parvaz MA, et al. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010 May;133(Pt 5):1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehring WJ, Goss B, Coles MG, Meyer DE. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- 9.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004 Oct 15;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 10.van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002 Dec;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 11.Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008 Jun;18(6):1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 12.Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior Medial Frontal Cortex Activity Predicts Post-Error Adaptations in Task-Related Visual and Motor Areas. J Neurosci. 2011;31(5):1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005 Apr;9(4):195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein RZ, Alia-Klein N, Tomasi D, et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci USA. 2009;106(23):9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005 Dec;9(12):556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Bechara A. Disturbances of emotion regulation after focal brain lesions. Int Rev Neurobiol. 2004;62:159–193. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- 18.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amanzio M, Torta DM, Sacco K, et al. Unawareness of deficits in Alzheimer’s disease: role of the cingulate cortex. Brain. 2011 Mar 7; doi: 10.1093/brain/awr020. [DOI] [PubMed] [Google Scholar]
- 20.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 21.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004 Feb;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 22.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010 Jun;214(5–6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009 Jan;32(1):56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007 Jan 26;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. Neuroimage. 2005 Sep;27(3):602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007 Feb 15;34(4):1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004 Sep;14(9):986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- 28.Moeller SJ, Hajcak G, Parvaz MA, Dunning JP, Volkow ND, Goldstein RZ. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain. 2012 Nov;135(Pt 11):3481–3494. doi: 10.1093/brain/aws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 30.Moeller SJ, Beebe-Wang N, Woicik PA, Konova AB, Maloney T, Goldstein RZ. Choice to view cocaine images predicts concurrent and prospective drug use in cocaine addiction. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2012.11.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl Psychiatry. 2012;2:e176. doi: 10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer AR, Teshiba TM, Franco AR, et al. Modeling conflict and error in the medial frontal cortex. Hum Brain Mapp. 2012 Dec;33(12):2843–2855. doi: 10.1002/hbm.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sozda CN, Larson MJ, Kaufman DA, Schmalfuss IM, Perlstein WM. Error-related processing following severe traumatic brain injury: an event-related functional magnetic resonance imaging (fMRI) study. Int J Psychophysiol. 2011 Oct;82(1):97–106. doi: 10.1016/j.ijpsycho.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008 Feb;65(2):179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerns JG, Cohen JD, MacDonald AW, 3rd, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005 Oct;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 36.Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005 Oct 1;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Edwards BG, Calhoun VD, Kiehl KA. Joint ICA of ERP and fMRI during error-monitoring. Neuroimage. 2012 Jan 16;59(2):1896–1903. doi: 10.1016/j.neuroimage.2011.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor SF, Martis B, Fitzgerald KD, et al. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006 Apr 12;26(15):4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR. Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Res. 2006 Aug 11;1105(1):143–154. doi: 10.1016/j.brainres.2006.02.091. [DOI] [PubMed] [Google Scholar]
- 40.Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005 Dec 14;25(50):11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003 Oct;20(2):1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 42.Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001 Dec;14(6):1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- 43.Franklin TR, Acton PD, Maldjian JA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002 Jan 15;51(2):134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 44.Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003 Jul;19(3):1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 45.Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011 Jul;134(Pt 7):2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardini S, Venneri A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain research bulletin. 2012 Feb 10;87(2–3):205–211. doi: 10.1016/j.brainresbull.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Lane RD, Quinlan DM, Schwartz GE, Walker PA, Zeitlin SB. The Levels of Emotional Awareness Scale: a cognitive-developmental measure of emotion. J Pers Assess. 1990 Fall;55(1–2):124–134. doi: 10.1080/00223891.1990.9674052. [DOI] [PubMed] [Google Scholar]
- 48.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainsville, FL: University of Florida; 2008. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- 49.Hogarth L, Chase HW, Baess K. Impaired goal-directed behavioural control in human impulsivity. Quarterly journal of experimental psychology (2006) 2012;65(2):305–316. doi: 10.1080/17470218.2010.518242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klossek UM, Russell J, Dickinson A. The control of instrumental action following outcome devaluation in young children aged between 1 and 4 years. Journal of experimental psychology. General. 2008 Feb;137(1):39–51. doi: 10.1037/0096-3445.137.1.39. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka SC, Balleine BW, O’Doherty JP. Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci. 2008 Jun 25;28(26):6750–6755. doi: 10.1523/JNEUROSCI.1808-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000 Jun;10(6):552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- 53.Moeller SJ, Honorio J, Tomasi D, et al. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb Cortex. doi: 10.1093/cercor/bhs345. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JH, Garwood M, Menon R, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995 Sep;34(3):308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 55.Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001 Jul;46(1):6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- 56.Caparelli E, Tomasi D. K-space spatial low-pass filters can increase signal loss artifacts in Echo-Planar Imaging. Biomedical Signal Processing and Control. 2008;3(1):107–114. doi: 10.1016/j.bspc.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into image registration. Neuroimage. 1997 Nov;6(4):344–352. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- 58.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 59.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 60.Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source for visual shapes. Brain Res Cogn Brain Res. 2003;17(1):75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 61.Tardif CL, Collins DL, Pike GB. Sensitivity of voxel-based morphometry analysis to choice of imaging protocol at 3 T. Neuroimage. 2009 Feb 1;44(3):827–838. doi: 10.1016/j.neuroimage.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 62.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000 Jun;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 63.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005 Jul 1;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 64.Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005 Dec;24(12):1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- 65.Alia-Klein N, Parvaz MA, Woicik PA, et al. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011 Mar;68(3):283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanabe J, Tregellas JR, Dalwani M, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65(2):160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makris N, Oscar-Berman M, Jaffin SK, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008 Aug 1;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konova AB, Moeller SJ, Tomasi D, et al. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. Eur J Neurosci. doi: 10.1111/j.1460-9568.2012.08211.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frewen P, Lane RD, Neufeld RW, Densmore M, Stevens T, Lanius R. Neural correlates of levels of emotional awareness during trauma script-imagery in posttraumatic stress disorder. Psychosomatic medicine. 2008 Jan;70(1):27–31. doi: 10.1097/PSY.0b013e31815f66d4. [DOI] [PubMed] [Google Scholar]
- 70.Lane RD, Sechrest L, Riedel R. Sociodemographic correlates of alexithymia. Comprehensive psychiatry. 1998 Nov-Dec;39(6):377–385. doi: 10.1016/s0010-440x(98)90051-7. [DOI] [PubMed] [Google Scholar]
- 71.Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012 Feb 23;73(4):842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004 Nov;23(3):967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 73.Sturm VE, Sollberger M, Seeley WW, et al. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Social cognitive and affective neuroscience. 2012 Mar 20; doi: 10.1093/scan/nss023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shin LM, Dougherty DD, Orr SP, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry. 2000 Jul 1;48(1):43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 75.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006 Apr;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 76.Gilbert SJ, Spengler S, Simons JS, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006 Jun;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 77.Bolla K, Ernst M, Kiehl K, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004 Fall;16(4):456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franklin T, Wang Z, Suh JJ, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011 May;68(5):516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Culbertson CS, Bramen J, Cohen MS, et al. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. 2011 May;68(5):505–515. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011 Jan;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amiez C, Sallet J, Procyk E, Petrides M. Modulation of feedback related activity in the rostral anterior cingulate cortex during trial and error exploration. Neuroimage. 2012 Nov 15;63(3):1078–1090. doi: 10.1016/j.neuroimage.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Konova AB, Moeller SJ, Tomasi D, et al. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. Eur J Neurosci. 2012 Oct;36(7):2979–2988. doi: 10.1111/j.1460-9568.2012.08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holman BL, Carvalho PA, Mendelson J, et al. Brain perfusion is abnormal in cocaine-dependent polydrug users: a study using technetium-99m-HMPAO and ASPECT. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1991 Jun;32(6):1206–1210. [PubMed] [Google Scholar]
- 84.Levin JM, Holman BL, Mendelson JH, et al. Gender differences in cerebral perfusion in cocaine abuse: technetium-99m-HMPAO SPECT study of drug-abusing women. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1994 Dec;35(12):1902–1909. [PubMed] [Google Scholar]
- 85.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009 Dec;12(12):1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008 Jul;33(8):1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chua HF, Liberzon I, Welsh RC, Strecher VJ. Neural correlates of message tailoring and self-relatedness in smoking cessation programming. Biological psychiatry. 2009 Jan 15;65(2):165–168. doi: 10.1016/j.biopsych.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chua HF, Ho SS, Jasinska AJ, et al. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nature neuroscience. 2011 Apr;14(4):426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008 Apr;14(2):203–222. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- 90.Wilkinson G. The Wide-Range Achievement Test 3- Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]
- 91.Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 92.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 93.Logan GD, Crump MJ. Cognitive illusions of authorship reveal hierarchical error detection in skilled typists. Science. 2010 Oct 29;330(6004):683–686. doi: 10.1126/science.1190483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.