Abstract
During the stress response to intense exercise, the sympathetic nervous system (SNS) induces rapid catabolism of energy reserves through the release of catecholamines and subsequent activation of protein kinase A (PKA). Paradoxically, chronic administration of sympathomimetic drugs (β-agonists) leads to anabolic adaptations in skeletal muscle, suggesting that sympathetic outflow also regulates myofiber remodeling. Here, we show that β-agonists or catecholamines released during intense exercise induce Creb-mediated transcriptional programs through activation of its obligate coactivators Crtc2 and Crtc3. In contrast to the catabolic activity normally associated with SNS function, activation of the Crtc/Creb transcriptional complex by conditional overexpression of Crtc2 in the skeletal muscle of transgenic mice fostered an anabolic state of energy and protein balance. Crtc2-overexpressing mice have increased myofiber cross-sectional area, greater intramuscular triglycerides and glycogen content. Moreover, maximal exercise capacity was enhanced after induction of Crtc2 expression in transgenic mice. Collectively these findings demonstrate that the SNS-adrenergic signaling cascade coordinates a transient catabolic stress response during high-intensity exercise, which is followed by transcriptional reprogramming that directs anabolic changes for recovery and that augments subsequent exercise performance.
Keywords: cAMP-response element-binding protein, Creb-regulated transcriptional coregulators, hypertrophy, skeletal muscle, sympathetic nervous system
Introduction
The sympathetic nervous system (SNS) controls the “fight or flight” response by releasing energy stores and enhancing cardiovascular dynamics during high-intensity exercise (HIE). Catecholamines released by the SNS bind and activate β-adrenergic receptors (BARs), initiating a series of molecular events that increase intracellular cAMP, which in turn liberates the catalytic subunit of protein kinase A (PKA) to induce rapid signaling pathways (Altarejos & Montminy, 2011). In skeletal muscle, PKA initiates catabolism of glycogen and intramuscular triglyceride (IMTG) energy stores in the sarcoplasm by phosphorylating of the regulatory enzymes phosphorylase kinase and hormone-sensitive lipase (Chesley et al, 1996; Jocken & Blaak, 2008). These SNS-mediated catabolic effects are rapid and transient mechanisms for countering energetic stress.
β-Adrenergic receptor agonists (β-agonists) are used clinically as sympathomimetic drugs and in research as tool compounds to determine how skeletal muscle responds to sympathetic activation. When used acutely, β-agonists parallel the ability of catecholamines to induce catabolism of energy reserves in skeletal muscle; however, prolonged administration of the same compounds induces an anabolic state and cause muscle hypertrophy (Choo et al, 1992; Mersmann, 1998; Ryall et al, 2007). It remains unclear whether the anabolic properties of β-agonists are only a pharmacological effect or whether these observations suggest that sympathetic outflow also directs skeletal muscle anabolism.
Recently studies have suggested that the transcriptional arm of β-adrenergic signaling has roles in regulating muscle hypertrophy. First, exercise or β-agonists induce expression of multiple Pgc-1α (Ppargc1a) splice variants in both skeletal muscle and cultured myocytes via activation of an alternative promoter located ˜13 kb upstream of the proximal promoter (Baar et al, 2002; Miura et al, 2008; Norrbom et al, 2011; Ruas et al, 2012). Second, muscle-specific overexpression of one of the isoforms, Pgc-1α4, causes muscle hypertrophy in mice and primary myotubes, where silencing Pgc-1α4 attenuates clenbuterol-mediated hypertrophy of primary myotubes (Ruas et al, 2012). Third, mice having loss of BAR signaling via muscle-specific Gα(s) deficiency have decreased total muscle mass and attenuated expression of total Pgc-1α (Chen et al, 2009). Finally, genetic ablation of all BARs in mice also attenuates Pgc-1α expression (Bachman et al, 2002). Thus, transcriptional responses to β-adrenergic signaling and exercise promote muscle hypertrophy, but the mechanism of transcriptional control is unknown.
β-Agonists such as clenbuterol or isoproterenol direct gene expression via the activation of the cAMP-response-element-binding protein (Creb) transcription factor and associated co-regulators, Creb-regulated transcriptional coregulators (Crtc) and Creb-binding protein (CBP) or its paralog p300 [reviewed in (Altarejos & Montminy, 2011)]. Disrupting assembly of the Creb transcriptional complex in skeletal muscle, by forced overexpression of a dominant-negative Creb molecule (A-Creb) that prevents Creb DNA binding, causes a severe dystrophic phenotype (Ahn et al, 1998; Berdeaux et al, 2007). However, neither Crtc coactivators nor Creb have been shown mediate hypertrophic responses by β-agonists or exercise.
Herein, we report that Crtc/Creb-directed transcription is initiated by β-agonists and in response to catecholamines released during exercise. Further calcium and β-adrenergic signaling is revealed to converge on the Crtc/Creb complex to increase their transcriptional output, providing a novel mechanism that integrates the level of cardiodynamic stress with the intensity of muscle contraction in working muscle. Moreover, overexpression of Ctrc2 or Crtc3 coactivators alone is shown to be sufficient to activate Creb-dependent transcription without β-adrenergic signaling. Remarkably, conditional expression of Crtc2 coactivators in the striated muscle of transgenic mice or in primary myocytes is sufficient to induce anabolic changes that drive myofiber hypertrophy, including increases in the levels of intramuscular glycogen and triglycerides. In contrast, acute PKA activation by β-agonists or HIE increases catabolism and depletes intramuscular glycogen stores in Crtc-expressing myotubes and mice. Collectively, these findings establish that the SNS governs anabolism in skeletal muscle as a mechanism of recovery and adaptation via the Crtc/Creb transcriptional complex.
Results
Enforced expression of Crtc is sufficient to activate Creb signaling
There are three mammalian Creb-regulated transcriptional coregulators – Crtc1, Crtc2 and Crtc3 – that have unique and sometimes overlapping expression patterns. The β-agonists isoproterenol (Iso) triggered the dephosphorylation and rapid translocation of Crtc2 and Crtc3 to the nucleus in cultured primary myotubes and C2C12 myotubes, while Crtc1 was not detected by immunoblotting (Fig1A–B). Absolute quantitation of transcripts of each coregulator in gastrocnemius, quadriceps and C2C12 myotubes confirmed that only Crtc2 and Crtc3 are expressed in skeletal myocytes (Supplementary Fig S1). Isoproterenol stimulation also increased phospho-Creb and phospho-Atf1 in a temporal manner, hallmarks of transcriptional activation (Fig1A; Gonzalez & Montminy, 1989).
Figure 1. Crtcs are activated by β-agonists and mimic adrenergic-dependent transcription in skeletal myocytes when overexpressed.

- Effect of β-agonist on Crtc2 and Crtc3 subcellular localization. Immunoblots of Crtc2, Crtc3, phospho-Creb/Atf1, total Creb or tubulin from cytoplasmic and nuclear extracts of cultured primary myotubes treated with 100 nM isoproterenol (Iso) for 0, 15, 30, 45 or 60 min.
- Immunofluorescence of endogenous Crtc2 (green) and nuclear staining with DAPI (DNA, blue) in murine C2C12 myotubes treated with vehicle (DMSO) or 100 nM Iso for 15 min. Scale bar, 20 μm.
- Transient transfection assays in primary mouse myocytes with a CRE-luciferase reporter gene and an expression vector for Crtc2, Crtc3 alone or with A-Creb. Myocytes were also stimulated with isoproterenol, clenbuterol (clen) or DMSO for 4 h prior to luciferase assay as indicated. Propranolol was incubated 30 min prior to stimulation, each bar is the mean ± s.e.m. of 4 wells.
- Relative fold induction of Nr4a1, Nr4a3, Irs2 and Sik1 RNA transcripts by rtPCR from primary myocytes treated with DMSO, 100 nM isoproterenol (Iso), transduced with control adenovirus or virus expressing either Crct2 or Crtc3. Values were normalized to Rpl-23. Each bar is the mean ± s.e.m. of 4 wells.
Data information: One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by *P < 0.05, **P < 0.01 and ***P < 0.001 versus DMSO.
Source data are available online for this figure.
Previous studies in other cell types have shown that overexpression of the Crtc coactivators activates Creb-dependent transcription independent of PKA signaling (Conkright et al, 2003a; Screaton et al, 2004). This occurs by overwhelming the regulatory mechanisms that retain the coactivators in the cytoplasm, where nuclear localization is sufficient for transcriptional activation (Supplementary Fig S2) (Screaton et al, 2004). We used a luciferase reporter gene regulated by cAMP response elements (CRE) and having a minimal TATA core promoter element to assess the effects of clenbuterol or isoproterenol on Creb transcriptional activity in primary myocytes, with or without Crtc2 and Crtc3 overexpression. Overexpression of either Crtc2 or Crtc3 increased luciferase activity to levels seen with either β-agonist (Fig1C). Further, propranolol, a β-adrenergic antagonist, abolished the action of the β-agonists yet did not block downstream Crtc2- or Crtc3-mediated transcription (Fig1C). Crtc2- or Crtc3-mediated transcription was Creb dependent, as the effects of Crtcs were attenuated by co-expression of the dominant-negative A-Creb molecule (Fig1C). Finally, primary myocytes stimulated with isoproterenol or transduced with adenovirus expressing Crtc2 or Crtc3 increased the relative number of transcriptions for the canonical Crtc/Creb-responsive genes Nr4a1, Nr4a3 and Sik1 (Conkright et al, 2003a; Screaton et al, 2004; Kanzleiter et al, 2010; Pearen et al, 2012), similar to those found following stimulation with isoproterenol (Fig1D). Thus, β-agonists activate the Crtc/Creb transcriptional complex and overexpression of Crtc2 or Crtc3 mimics adrenergic-dependent transcriptional activation without receptor activation.
The sympathetic nervous system activates the Crtc/Creb transcriptional complex
As β-agonists activated the Crtc/Creb transcriptional complex, we next examined whether a physiological stimulus would parallel pharmacological activation. Catecholamines released by the SNS in response to HIE bind and activate BARs. As expected, mice exercised to exhaustion had significantly higher levels of epinephrine and norepinephrine in the quadriceps muscles and plasma spillover relative to rested controls, along with a commensurate increase in intracellular levels of cAMP (Fig2A–B). HIE provoked increases in the levels of phospho-Creb and phospho-Atf1, while causing the dephosphorylation of Crtc2 and Crtc3, 30 min post-exercise (Fig2C), and led to increases in the mRNAs of the canonical Crtc/Creb-responsive genes Nr4a1, Nr4a3, Ppargc1a (Pgc1-α) and Sik1 (Herzig et al, 2001; Conkright et al, 2003a; Screaton et al, 2004; Kanzleiter et al, 2010; Pearen et al, 2012) in the quadriceps muscle (Fig2D–E). An extended kinetic analysis showed that maximal induction of these transcripts occurred between 1 and 2 h post-exercise and that transcript levels returned to resting levels after 4–8 h (Supplementary Fig S3A). Dephosphorylation and redistribution of Crtc2 followed a similar temporal pattern (Supplementary Fig S3B and C). Thus, in response to a single bout of intense exercise, the Crtc/Creb transcriptional complex activates skeletal muscle target genes in a prototypical fashion, where a burst phase is followed by an attenuation of transcription (Michael et al, 2000; Mayr & Montminy, 2001).
Figure 2. High intensity exercise activates Creb and the Crtc coactivators.
- Levels of norepinephrine and epinephrine from plasma or in quadriceps muscle normalized to total protein from mice either at rest or 30 min post-exercise. Each bar is mean ± s.e.m., n = 4 male mice at 8 weeks of age. P-value calculated by a two-tailed unpaired t-test.
- Levels of cAMP in quadriceps muscle. Values were normalized to total protein in the indicated animals rested or 30 min post-exercise. Each bar is mean ± s.e.m., n = 4 male mice at 8 weeks of age. P-value calculated by a two-tailed unpaired t-test.
- Immunoblots showing levels of phospho-Creb/Atf1, Creb, Crtc2, Crtc3 and tubulin from animal rested or 30 min post-exercise.
- Fold induction of Pgc-1α, Nr4a1 and Nr4a3 transcripts in quadriceps muscle from the indicated animals after 30 min of intense exercise. Transcript levels were measured by qRT-PCR, and values were normalized to Rpl-23 mRNA and expressed relative to those present in resting control animals; each bar is mean ± s.e.m. n = 4 male mice at 8 weeks of age. P-values calculated by a two-tailed unpaired t-test.
- Crtc3 overrides the suppression of gene expression by the adrenergic antagonist propranolol in exercised animals. Fold induction of Pgc-1α and Sik1 mRNAs was determined by qRT-PCR from the tibialis anterior (TA) muscle. Crtc3 expression was induced by electroporation of the Crtc3 plasmid into the tibialis anterior muscle. Propranolol or saline were injected i.p. 30 min before exercise. Values were normalized to levels of Rpl-23 transcripts and are expressed relative to those in control (saline/resting) TA muscle; each bar is the mean ± s.e.m. of 4 mice. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by *P < 0.05, **P < 0.01 and ***P < 0.001 versus saline.
Source data are available online for this figure.
If the Crtc/Creb transcriptional complex is indeed activated during HIE by the SNS/adrenergic signaling pathway, then administration of β-blockers should block the transcriptional activation of Crtc/Creb-dependent genes. Indeed, expression of Pgc-1α and Sik1 was also markedly induced in the tibialis anterior muscle with exercise, and this response was suppressed by the administration of the β-blocker propranolol (Fig2E). Notably, electroporation of a Crtc3 expression construct directly into the tibialis anterior muscle was sufficient to override these effects of propranolol (Fig2E). Thus, enforced Crtc expression circumvents upstream BAR signaling in vivo, and SNS release of catecholamines in response to the stress of exercise initiates Crtc/Creb-mediated gene expression.
The Crtc coactivators integrate SNS/adrenergic and cholinergic signaling
The systemic action of catecholamines that is manifest during exercise suggests that the SNS lacks the specificity needed to direct adaptive changes only to the worked muscle (Lambert et al, 2010; Roatta & Farina, 2010). However, Crtc2 functions as a coincidence regulator of cAMP and Ca2+ signaling pathways in many cell types (Screaton et al, 2004; Baxter et al, 2011; Ch'ng et al, 2012), and during HIE, cholinergic signaling is specific to the worked muscle. Here, acetylcholine depolarizes the membrane of myocytes and liberates calcium (Ca2+) stores from the endoplasmic reticulum, which can by mimicked by treatment with KCl (Macías et al, 2001). Thus, we tested whether calcium and cAMP signaling pathways converged on Crtc-dependent gene transcription by co-treating primary myocytes with isoproterenol and KCl. Isoproterenol activated Crtc/Creb-dependent expression of Nr4a1 and Nr4a3, and this response was markedly augmented by co-treatment with KCl (Fig3A). Moreover, Crtc2 or Crtc3 overexpression was sufficient to induce Nr4a1 and Nr4a3 to levels induced by co-stimulation of primary myocytes with isoproterenol and KCl (Fig3B). The dependency of Creb and the Crtc coactivators was illustrated by attenuating the transcriptional response to co-stimulation with Iso and KCl by the co-expression of A-Creb (Supplementary Fig S4). Further, increasing intracellular Ca2+ augmented the duration of beta-agonist-dependent phosphorylation of Creb and dephosphorylation Crtc2 (Fig3C), which lengthens the duration of target gene activation, prolonging the burst phase of Creb-mediated transcription (Michael et al, 2000; Mayr & Montminy, 2001). Thus, the two pathways that are simultaneously activated by HIE, the cholinergic and adrenergic-dependent signaling pathways, are integrated by Crtc coactivators in working muscle, suggesting that Crtc coactivators may function as physiological drivers of the adaptive response to exercise.
Figure 3. Adrenergic and cholinergic signaling pathways converge on the Crtc coactivators to potentiate target gene transcription.

- Fold induction of Nr4a1 and Nr4a3 RNA transcripts, quantified by qRT-PCR, in primary myotubes treated with 40 mM KCl, isoproterenol (Iso), Iso plus KCl or vehicle (DMSO) for 45 min. Values were normalized to those of Rpl-23 mRNA and were expressed relative to DMSO control; each bar is the mean ± s.e.m. of 4 wells. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by ***P < 0.001 versus vehicle (DMSO).
- Fold induction of Nr4a1 and Nr4a3 transcripts, quantified by qRT-PCR, in primary myotubes treated with 40 mM KCl plus Iso for 45 min, and in myotubes transduced with adenovirus expressing GFP, Crtc2 or Crtc3 for 24 h. One-way ANOVA was conducted, and P-values were calculated with Tukey's post hoc test and are represented by **P < 0.01 and ***P < 0.001 versus Ad-Control.
- Immunoblot analyses of levels of phospho-Creb, total Creb, Crtc2, Crtc3 and tubulin in total lysates of primary myotubes stimulated for 0, 15, 30, 60, 90 or 120 min with either 100 nM Isoproterenol or isoproterenol and 40 mM KCl.
Source data are available online for this figure.
The Crtc coactivators induce skeletal myofiber hypertrophy
We reasoned that conditional expression of the CRTC coactivators in transgenic mice could be used as a genetic tool to separate the rapid non-genomic effects of SNS/adrenergic signaling from the later transcriptional axis in a tissue-specific manner. To this end, we generated double transgenic mice (DTg) that inducibly express a constitutively active form of Crtc2, Crtc2tm, in muscle when administered doxycycline (Dox). The TRE-Crtc2tm transgenic mice contain an ORF with three point mutations (S171A, S275A and K625A) that increase the activity and stability of Crtc2 (Screaton et al, 2004; Dentin et al, 2007; Jansson et al, 2008), under the control of tetracycline-responsive elements (TRE). Skeletal muscle specificity was conferred when this transgenic line was crossed with second mouse that harbors a transgene for the reverse tetracycline transactivator (rtTA) regulated by the 1256[3Emut] MCK promoter (Grill et al, 2003).
Doxycycline (Dox) administration to DTg mice induced Crtc2 transgene expression two- to fivefold relative to endogenous levels of Crtc2 mRNA expressed in striated, but not cardiac muscle (Supplementary Fig S5A). Increased accumulation of FLAG-Crtc2 was also evident after 3–7 days of Dox administration of transgenic mice (Supplementary Fig S5B and C). In striated muscle, Crtc2 overexpression led to significant increases in the mass of the gastrocnemius and soleus muscle relative to age- and weight-matched Dox-treated wild-type (WT) littermates (Fig4A). Quantitative analysis of fiber size in histological sections of gastrocnemius muscle confirmed increased average cross-sectional area (CSA) of the fibers (Fig4B–C). The skeletal structure was similar between experimental groups as indicated by the similar tibia bone length and body weights (Fig4D–E).
Figure 4. Activation of the Crtc coactivators induces skeletal myofiber hypertrophy.
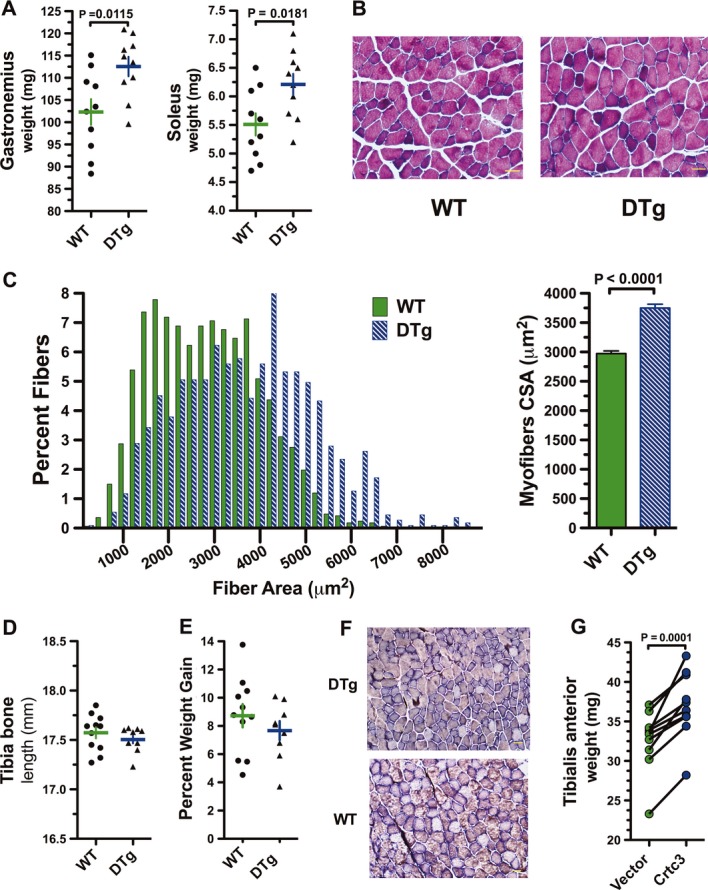
- Gastrocnemius and soleus muscle weight in wild-type (WT) and double transgenic (DTg) mice 36 days post-Dox administration. Data are represented as a scatter plot with the mean ± s.e.m. indicated, n = 10 mice. P-values calculated by a two-tailed unpaired t-test.
- Histological analysis of myofibers from gastrocnemius muscle sections with hematoxylin and eosin (H & E) staining. Scale bar: 100 μm.
- Distribution of cross-sectional areas (CSAs) of WT and DTg mice after Dox (left panel) and mean CSAs of muscle fibers (right panel). Data are represented as mean ± s.e.m. P-values calculated by a two-tailed unpaired t-test.
- Tibia bone length of WT and DTg mice after dox. Data are represented as a scatter plot with the mean ± s.e.m. indicated, n = 10 per group.
- Percent weight gain of WT and DTg mice after Dox. Data are represented as a scatter plot with the mean ± s.e.m. indicated, n = 10 per group.
- Histological analysis of myofibers from gastrocnemius muscle sections by myosin ATPase staining. Scale bar: 500 μm.
- Wet weight of TA muscles after 10 days of Crtc3 expression. The muscle was electroporated with either empty vector or with a Crtc3 expression plasmid. Each line represent the difference in mass relative to the contralateral muscle of each animal, n = 10 per group. P-values calculated by a two-tailed paired t-test.
Fiber type transitions occur primarily during development and only to a limited extent in response to chronic training (Simoneau et al, 1985; d'Albis et al, 1989; Lynch et al, 1995; Fry, 2004); however, several studies have reported that extended administration of β-agonists fosters a slow-to-fast muscle fiber transition (Maltin et al, 1986; Agbenyega & Wareham, 1990; Hayes & Williams, 1994). However, an alteration in the ratio of fiber types was not evident by myosin ATPase staining of gastrocnemius muscle of DTg mice treated with Dox (Fig4F). We therefore tested whether forced overexpression of Crtc3 would also produce fiber hypertrophy using a second approach, electric-pulse-mediated gene transfer. Notably, electric transfer of a Crtc3 expression vector provoked marked increases in wet muscle weight or the tibialis anterior muscle compared with muscle pulsed with a control vector (Fig4G). Thus, modest overexpression of either Crtc2 or Crtc3 drives hypertrophic phenotypes similar to those seen with administration of β-agonists, but without receptor activation (Shi et al, 2007; Koopman et al, 2010).
Crtc/Creb coordinates signaling circuits that drive muscle cell anabolism and hypertrophy
To investigate the mechanisms by which activation of the Crtc/Creb complex induces fiber hypertrophy, mouse primary myotubes were transduced with adenovirus expressing either Crtc2 or Crtc3. As predicted, forced overexpression of Crtc2 or Crtc3 also provoked hypertrophy in cultured myotubes (Fig5A and Supplementary Fig S6A), including elevated levels of MHC protein (Fig5B). Myotubes expressing Crtc3 typically had a greater hypertrophic response, yet Crtc2 adenovirus co-expresses GFP, which only allows examination of transduced myotube and the with use of fixatives that could affect fiber size. Finally, overexpression of either Crtc2 or Crtc3 caused a marked increases in the ratio of total protein to DNA in the myotube, establishing that hypertrophy rather that hyperplasia leads to increased fiber size (Fig5C).
Figure 5. Crtc coactivators induce signaling pathways that regulate protein degradation.

- Fluorescence microscopy of primary myotubes transduced with adenovirus expressing GFP alone (Ad-Control) or Crtc2 and GFP (Ad-Crtc2).
- Immunoblots of MHC and tubulin from primary myotubes transduced with adenovirus expressing GFP alone (Ad-Control) or Crtc2 and GFP (Ad-Crtc2).
- Total protein and DNA were isolated and quantified from control, Crtc2- or Crtc3-expressing myotubes and expressed as total protein divided by DNA. Data are represented as mean ± s.e.m. (n = 4 wells per group). One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by **P < 0.01 versus Ad Control.
- Relative fold induction of Sik1 RNA transcripts by rtPCR from primary myocytes transduced with control adenovirus or adenovirus expressing Crct2 or Crtc3. Values were normalized to Rpl-23 mRNA. Each bar is the mean ± s.e.m. of 4 wells. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by ***P < 0.001 versus Ad Control.
- Immunoblots of phospho-HDAC4, phospho-HDAC 5/7 or tubulin from extracts of primary myotubes transduced with control adenovirus or adenovirus expressing Crtc2.
- Immunoblots of phospho-HDAC4, phospho-HDAC 5/7 or tubulin from extracts of gastrocnemius muscle isolated from double transgenic mice after 0, 3 or 7 days of Dox administration.
- Immunoblots of phospho-HDAC4, phospho-HDAC 5/7, total HDAC 4, total HDAC5, Murf1, myogenin, Creb or tubulin from nuclear (left) and cytoplasmic (right) extracts of primary myotubes transduced with control adenovirus or adenovirus expressing Crtc3. Sample replicates for Ad control and Ad Crtc3 are shown.
Source data are available online for this figure.
Myotube hypertrophy triggered by forced expression of Crtc2 or Crtc3 was associated with the inhibition of the proteosomal degradation pathway. Specifically, overexpression of either Crtc coactivator in primary myocytes increased expression of Sik1 (Fig5D), which phosphorylates and inactivates the class II histone deacetylases HDAC4, HDAC5 and HDAC7 by directing their export into the cytoplasm (Takemori et al, 2009). Indeed, expression of Crtc2 and Crtc3 triggered the phosphorylation and cytoplasmic localization of HDCA4, HDAC5 and HDCA7 (Fig5E and F and Supplementary Fig S6B and C). In skeletal muscle, inactivation of HDACs attenuates protein degradative pathways by indirectly preventing the expression of myogenin and the E3 ubiquitin ligase MuRF1, which are required for the ubiquitylation step in the degradation of muscle protein (McKinsey et al, 2000). Suppression of myogenin and MuRF1 was evident by immunoblotting, demonstrating their control by Crtc/Creb signaling network (Fig5G). The currently accepted model by which β-agonists induce muscle hypertrophy is attributed to non-canonical mechanisms of GPCR signaling that are not dependent of cAMP signaling or Creb-dependent transcription (Lynch & Ryall, 2008). Specifically, β-agonists signal through the Gβγ subunits of the G-protein tetramer and have been proposed to increase PI3 kinase activity and potentiate phosphorylation and activation of Akt1 kinase (Chesley et al, 2000; Erbay & Chen, 2001; Schmidt et al, 2001; Park & Chen, 2005; Kline et al, 2007), which then increases protein synthesis in myofibers by regulating components of the mTOR signaling network (Cuenda & Cohen, 1999; Erbay & Chen, 2001; Sneddon et al, 2001; Park & Chen, 2005; Koopman et al, 2010). Our data rather indicate that the Crtc/Creb transcriptional complex stimulates muscle growth via Pgc-1α4/Igf-1/Akt1 signaling pathway. First, of the four known Pgc-1α splice variants, muscle-specific overexpression of the Pgc-1α4 isoform induced Igf1 expression and hypertrophy in primary myocytes (Ruas et al, 2012). Here, enforced expression of Crtc2 or Crtc3 mimics exercise-mediated induction of the Pgc-1α4 splice variant (Fig6A). While full-length Pgc-1α (Pgc-1α1) is regulated by p38 MAP kinase phosporylation of ATF2 in skeletal muscle (Cao et al, 2004; Akimoto et al, 2005, 2008), the Pgc-1α4 isoform is induced via activation of an alternative promoter located ˜13 kb upstream of the proximal promoter. This distal promoter contains putative cAMP-responsive elements (CREs) (Miura et al, 2008; Yoshioka et al, 2009; Ruas et al, 2012) and is regulated by exercise and by β-agonists. We cloned 500-bp fragments of the distal and proximal Pgc-1α promoters to determine whether Creb and the Crtc coactivators selectively activated either promoter. Only the distal promoter was responsive to isoproterenol stimulation (Fig6B). Further, chromatin immunoprecipitation (ChIP) analyses of the distal and proximal Pgc-1α promoters, along with the Nr4a1 and Gapdh promoters as positive and negative controls, respectively, confirmed that endogenous Creb, Crtc2 and Crtc3 are enriched at the distal Pgc-1α promoter relative to the proximal promoter following treatment of myocytes with isoproterenol (Fig6C and Supplementary Fig S7A). Overexpression of Crtc2 preferentially increased RNA transcripts originating from the distal promoter (Supplementary Fig S7B and C), consistent with previous reports stimulating myocytes with either the adenylate cyclase agonist, forskolin or clenbuterol (Miura et al, 2008; Ruas et al, 2012).
Figure 6. Crtc coactivators induce signaling pathways that regulate protein synthesis.

- Relative fold induction of Pgc1-α4 transcripts by qRT-PCR from primary myocytes transduced with control adenovirus or adenovirus expressing Crct2 or Crtc3. Values were normalized to Rpl-23 mRNA. Each bar is the mean ± s.e.m. of 4 wells. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented **P < 0.01 versus Ad Control.
- Transient transfection assays in myocytes with a −51 to +500 Pgc1α proximal or distal promoter-luciferase reporter genes. Myocytes were also stimulated with isoproterenol or DMSO vehicle control for 4 h prior to luciferase assay as indicated. Each bar is the mean fold induction ± s.e.m. of three experiments. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by *P < 0.05, **P < 0.01 and ***P < 0.001 versus Iso.
- Chromatin immunoprecipitation (ChIP) of the Pgc-1α distal, Pgc-1α proximal and Gapdh promoters with anti-Creb, anti-Phospho-Creb, anti-Crtc2 or anti-Crtc3 in isoproterenol stimulated mouse myotubes. Myotubes were stimulated with isoproterenol prior to ChIP as indicated. Pre-immunoprecipitation (Pre-IP) control DNA (left) and no antibody control (right).
- Relative fold induction of Pgc1-α4, Pdpk1, Akt1 and Igf1 transcripts by qRT-PCR from gastrocnemius muscle isolated from double transgenic mice or wild-type mice after 7 days of Dox administration. Values were normalized to Rpl-23 mRNA. Each bar is the mean ± s.e.m. of 4 wells, of two animals. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by *P < 0.05, **P < 0.01 and ***P < 0.001 versus Ad Control.
- Relative fold induction of Pdpk1, Akt1 and Igf1 RNA transcripts by qRT-PCR from primary myocytes transduced with adenovirus control or adenovirus expressing Crct2 or Crtc3. Values were normalized to Rpl-23 mRNA. Each bar is the mean ± s.e.m. of 4 wells.
- Immunoblots of Pdpk1, Akt1 and tubulin from primary myotubes expressing Crtc2 or Crtc3.
- Immunoblots of phospho-Ser 308 of AKT1/2 and tubulin from myocytes expressing Crtc2 or Crtc3.
Source data are available online for this figure.
Second, as the Pgc-1α4 isoform induces Igf1 expression, we examined the expression of Igf1, Pdpk1 (Pdk1) and Akt1 in primary myocytes and transgenic mice expressing Crtc2. Notably, there were marked increases in Igf1, Pdpk1 and Akt1 transcripts in primary myocytes overexpressing Crtc2 or Crtc3 (Fig6D) and in the gastrocnemius muscle of Dox-treated double transgenic mice (Fig6E). Increased protein levels of the Akt1 activating kinase Pdpk1 were evident, yet despite a clear and reproducible induction in Akt1 transcripts, only a very marginal change in Akt1 protein was be detected in Crtc2 or Crtc3 overexpression (Fig6F). However, Crtc3 overexpression triggered an increase in Akt phosphorylation, which is required for activation of Akt kinases (Fig6G) (Alessi et al, 1997). Thus, the Crtc/Creb complex orchestrates muscle hypertrophy by coordinately regulating both the protein degradation and muscle growth pathways.
The Crtc coactivators augment intramuscular energy storage
Repetitive exercise increases energy reserves by promoting the intramuscular storage of both neutral lipids and glycogen. Glycogen content in skeletal muscle is an excellent predictor of exercise capacity, and depletion of glycogen is a primary cause of muscle fatigue (Bergström et al, 1967; Green et al, 1995). In efforts to spare glycogen catabolism, both endurance exercise and high-intensity exercise lead to increased storage of intramuscular triglycerides (IMTGs) (Stepto et al, 2002; Hawley et al, 2011). Crtc overexpression was sufficient to provoke this anabolic response in skeletal muscle of mice, and in cultured myotubes. The gastrocnemius muscle of DTg mice contained greater IMTGs relative to wild-type mice following Dox treatment (Fig7A–B). Diacylglycerol O-acyltransferase 1 (Dgat1) catalyzes the committed step in triacylglycerol synthesis, and muscle-specific transgenic mice overexpressing Dgat-1 display increased IMTGs (Liu et al, 2009; 2007; Zhang et al, 2010). Dgat1 expression elevated in the gastrocnemius muscle of Dox-treated DTg mice relative to wild-type mice (Fig7C). Similar to the transgenic mice, myotubes overexpressing Crtc3 esterified more fatty acids to triglycerides (Fig7D–E), and there were robust increases in the levels of Dgat1 mRNA and protein in Crtc2- and Crtc3-expressing myofibers (Fig7F–G). Accordingly, myotubes ChIP analyses showed recruitment of the Crtc/Creb complex to the Dgat-1 promoter in isoproterenol-stimulated myocytes, comparable to that of canonical Creb/Crtc target such as Nr4a1 (Fig7H and Supplementary Fig S8). Thus, Crtc-mediated increases in Dgat1 are associated with the accumulation of intramuscular triglycerides in Crtc-expressing myofibers.
Figure 7. Crtc expression increases intramuscular triglycerides.
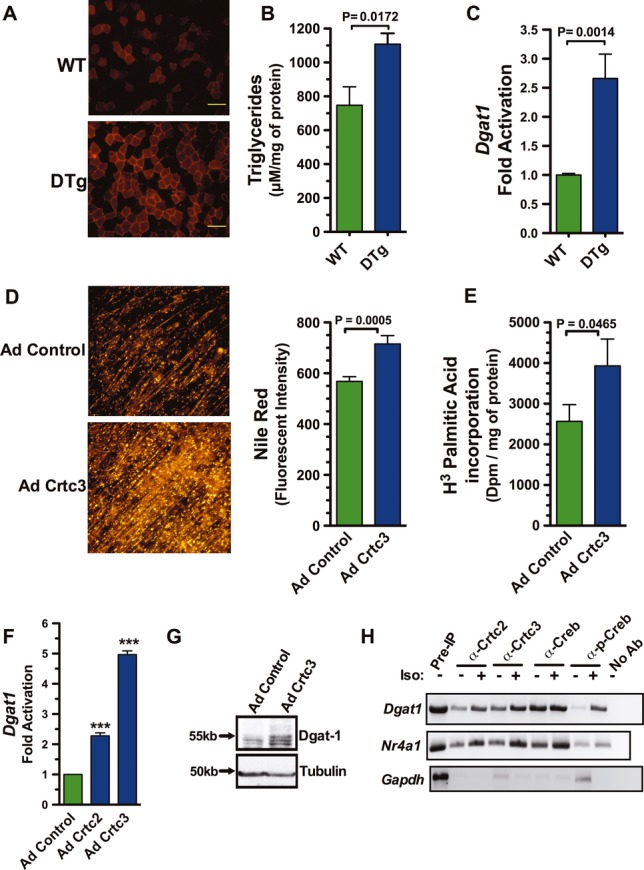
- Histological analysis of neutral lipids with Nile Red in muscle sections of WT and DTg mice after Dox treatment. Scale bar: 100 μm.
- Triglyceride levels in gastrocnemius muscle of WT and DTg mice after dox. Data are represented as a scatter plot with the mean ± s.e.m. indicated, n = 6 per group. P-value calculated by a two-tailed unpaired t-test.
- Relative fold induction of Dgat1 mRNA by qRT-PCR from gastrocnemius muscle isolated from double-transgenic or WT mice after 7 days of Dox treatment. Values were normalized to Rpl-23. Each bar is the mean ± s.e.m. of 4 wells, of two animals. P-value calculated by a two-tailed unpaired t-test.
- Neutral lipids visualized in primary myotubes expressing Crct3 by Nile Red (left) and quantified by fluorescent intensity (right). Each bar is the mean ± s.e.m. of 4 wells. P-value calculated by a two-tailed unpaired t-test.
- Incorporation of 3H- palmitatic acid in primary myotubes expressing Crct3. Each bar is the mean ± s.e.m. of 4 wells. P-value calculated by a two-tailed unpaired t-test.
- Relative fold induction of Dgat1 mRNA, quantified by qRT-PCR analyses of primary skeletal myocytes transduced with adenovirus expressing Crtc2 or Crct3 versus myocytes transduced with control adenovirus. Values were normalized to levels of Rpl-23 transcripts. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by ***P < 0.001 versus Ad Control.
- Immunoblots of Dgat1 and tubulin proteins in myotubes expressing Crtc3.
- Chromatin immunoprecipitation (ChIP) of the Dgat1, Nr4a1 and Gapdh promoters using anti-Creb, anti-Phospho-Creb, anti-Crtc2 or anti-Crtc3. Myotubes were stimulated with isoproterenol (+) or DMSO (−) for 45 min prior to ChIP. Pre-immunoprecipitation (Pre-IP) control DNA (left) and no antibody control (right).
Source data are available online for this figure.
Exercise performance is dependent on glycogen stores as an energy source, and in the short-term, PKA activation initiates glycogenesis (Galbo et al, 1975), as evidenced by the low glycogen content in the muscle of mice that were run to exhaustion (Fig8A). Glycogen content is not only replenished during rest but can increase to supraphysiological levels prior to training as a mechanism of dampening the energetic stress of future bouts of exercise (Green et al, 1995). Notably, glycogen content was dramatically increased in the muscle of DTg mice expressing Crtc2, and in the tibialis anterior (TA) muscle of mice transiently expressing Crtc3 (Fig8B and Supplementary Fig S9).
Figure 8. Crtc expression increases available intramuscular glycogen.

- Percentage of glycogen depleted in quadriceps muscle of mice post-exercise. Glycogen levels were normalized to total protein for each mouse at rest or 30 min after exercise. Each bar is the mean ± s.e.m. of n = 4 male mice at 8 weeks of age. P-value calculated by a two-tailed unpaired t-test.
- Glycogen levels in the gastrocnemius muscle of WT and DTg mice after Dox treatment. Each bar is the mean ± s.e.m. of glycogen normalized to total protein of 8 animals. P-value calculated by a two-tailed unpaired t-test.
- Glycogen levels in primary myocytes after treatment with clenbuterol or vehicle (DMSO) for 30 min. Each bar is the mean glycogen ± s.e.m. of 4 wells normalized to total protein. P-value calculated by a two-tailed unpaired t-test.
- Glycogen levels in primary myocytes transduced with adenovirus expressing Crtc2, Crtc3 or GFP control for 48 h and then stimulated with clenbuterol for 30 min. Each bar is the mean glycogen ± s.e.m. of 4 wells normalized to total protein. P-value calculated by a two-tailed unpaired t-test.
- Percentage of glycogen depleted in quadriceps muscle of Crtc2-expressing mice post-exercise. Glycogen levels were quantitated and normalized to total protein during rest or immediately after exercise or 60 min post-exercise. Each bar is the mean ± s.e.m. of n = 3 male mice at 10–12 weeks of age. One-way ANOVA was conducted and P-values were calculated with Tukey's post hoc test and are represented by ***P < 0.001 versus Rest control.
These opposing effects of the SNS/adrenergic signaling cascade suggested a model whereby rapid cAMP/PKA signaling mediates glycogen catabolism followed by the subsequent activation of Crtc/Creb transcriptional program during recovery, which mediates the adaptive anabolic response. In accord with this newly identified duality in SNS/adrenergic function, the anabolic action of Crtc2 or Crtc3 on glycogen accumulation was reversed after 30 min of clenbuterol stimulation as effectively as control adenovirus infected myotubes ex vivo (Fig8C–D). Importantly, a bout of high-intensity exercise after Crtc2 overexpression reversed the elevated glycogen levels in the skeletal muscle (Fig8E).
Crtc2 reduces metabolic stress and improves exercise capacity in skeletal muscle
Since Crtc2 overexpression fosters many of the same phenotypic changes that occur in response to the onset of a new exercise regimen, we reasoned that DTg mice would experience less stress during forced exercise. Metabolic stress and exercise capacity were tested using continuous running with an escalating velocity protocol (Fig9A). Mice were tested for exhaustion during three trials prior to induction of the Crtc2 transgene followed by an addition three trials 2 weeks after administration of Dox. Both groups displayed signs of a training or learning effect, but the DTg mice exhibited a significant increase in performance that did not occur with the wild-type cohort (Fig9B). Moreover, lower plasma lactate levels occurred after 20 min of exercise when the Crtc2 transgene was expressed, suggesting that the same amount of work induced less metabolic stress in these mice (Fig9C).
Figure 9. Crtc2 expression augments exercise performance.

- Exercise protocol for evaluating within-animal performance. Exercise capacity was assessed by determining the maximal speed achieved when the speed increased by 2 m/min every 2 min until exhaustion.
- Maximal exercise capacity before and after Dox administration. Maximal exercise capacity was determined by averaging the maximum speed achieved in three independent trails of an exercise stress test before and after Dox treatment for 2 weeks. Each symbol is the mean percent increase in the speed for each mouse, n = 5 mice per experimental group. P-values calculated by a two-tailed paired t-test.
- Blood lactate concentration in response to exercise before and after Dox administration. Each symbol is the mean blood lactate concentration of each experimental group, n = 5 mice per group. P-values calculated by a two-tailed paired t-test.
Discussion
β-Adrenergic receptor (β-AR) agonists induce skeletal muscle hypertrophy (Choo et al, 1992; Mersmann, 1998) and attenuate muscle atrophy due to sarcopenia (Ryall et al, 2007), denervation (Maltin et al, 1989; Hinkle et al, 2002) or neuromuscular-induced dystrophy (Harcourt et al, 2007; Zeman et al, 2000). These studies and others have spawned enthusiasm in the therapeutic application of β-agonists for muscle wasting (Ryall & Lynch, 2008). The precise mechanism by which β-agonists activate the Akt signaling pathway to promote muscle hypertrophy is however unclear, despite the fact that some have suggested that the Gβγ subunits of the G-protein tetramer activate PI3K to then activate Akt1 (Chesley et al, 2000; Erbay & Chen, 2001; Schmidt et al, 2001; Park & Chen, 2005; Kline et al, 2007). Rather, here we show that β-agonists or exercise activate the Crtc/Creb transcription and, using genetic approaches, establish that initiating Crtc/Creb signaling without activation of the adrenergic receptor is sufficient to induce adaptive changes, including hypertrophy and increased exercise capacity. Several upstream activators of Akt1, including Igf1 and Pdpk1 (Musaró et al, 2001; Rommel et al, 2001), are upregulated by Crtc/Creb signaling, but whether the hypertrophy observed is dependent on their function is unclear.
Activation of the Crtc/Creb transcriptional complex also inhibited the proteasomal degradation pathway, and this likely also contributes to the phenotypes manifest in Crtc2 transgenic mice. In skeletal muscle, inactivation of class II HDACs impairs protein degradative pathways via effects on the expression of myogenin and the E3 ubiquitin ligase MuRF1, which are required for the ubiquitylation of muscle protein (McKinsey et al, 2000). Crtc2 or Crtc3 overexpression appears to inactivate the histone deacetylases HDAC4, HDAC5 and HDAC7 via their induction of Sik1, which phosphorylates these HDACs and directs their export into the cytoplasm (Berdeaux et al, 2007; Takemori et al, 2009). Accordingly, myogenin and MuRF1 expression was suppressed in primary myocytes and transgenic mice expressing Crtc2. Thus, the Crtc/Creb transcriptional complex sustains muscle hypertrophy by coordinating the signaling circuits that decrease protein degradation and those that promote protein synthesis.
Despite the clear pharmacological effects of β-AR agonists, the SNS has not been previously thought to direct the anabolic adaptations fostered by exercise, largely due to the systemic effects of catecholamines. We show here that the Crtc/Creb transcriptional complex is activated in response to SNS cues, including high-intensity exercise, and that the Crtc coactivators integrate signals from both calcium and catecholamine pathways in muscle cells ex vivo and in vivo. During exercise, acetylcholine depolarizes the membrane of the worked muscle, liberating Ca2+ stores from the ER into the sarcolemmal space. Calcium not only directs contraction, but also functions as a second messenger that regulates the protein phosphatase calcineurin, which dephosphorylates NFAT transcription factors (Bassel-Duby & Olson, 2006). When catecholamines increase cAMP signaling in contracting myofibers, the two signaling pathways converge to activate the Crtc coactivators. This dual signaling integration is highly conserved, occurring in diverse tissues such as hippocampus (Ch'ng et al, 2012), cortex (Baxter et al, 2011) and islet cells (Screaton et al, 2004). The convergence of these two exercise-induced signaling pathways provides a mechanism to direct the appropriate adaptation primarily to the working muscle.
Skeletal muscle exhibits remarkable plasticity to exercise, which consists of any infinite combination of intensity, duration and resistance; however, most exercise research focuses on effects of these extremes of training regimes. This is best exemplified by comparing the differences in adaptations that result from chronic aerobic training versus resistance training (Egan & Zierath, 2013). Our data unexpectedly show adaptations that occur in both of these modalities, which are generally thought to be mutually exclusive. For example, Crtc2 expression increases myofiber cross sectional area with elevated triglyceride and glycogen stores but also stimulated the expression of genes known to induce mitochondrial biogenesis, including Pgc-1α and Nr4A3. Moreover, it is unlikely that the SNS has a major role in these classically studied modalities since catecholamine levels progressively decrease in response to the same stimulus when the absolute workload is held constant (Winder et al, 1978). If SNS/Crtc/Creb signaling coordinates an adaptive response to exercise, the question remains, in what context and time frame does this occur? The distinctive pattern of responses seen with Crtc/Creb activation reflects the unique role of catecholamines in coordinating the stress response to novel stimuli or high-intensity training. In support of this concept, several studies have shown that the onset of aerobic exercise increases myofiber hypertrophy along with mitochondrial biogenesis. For example, mice provided free wheel running access for 2 weeks have a significant increase in mitochondrial enzyme expression and generally show hypertrophy of hindlimb muscle (Allen et al, 2001, 2002; Harrison et al, 2002; Konhilas et al, 2005). Moreover, 12 weeks of cycle ergometer training induces myofiber hypertrophy in untrained young men, older men and older women (Harber et al, 2009, 2012). Thus, Crtc/Creb coordinates a unique cohort of adaptive responses tailored to the novel and intense stimuli that activate the SNS, and as training continues, the role of the SNS in directing adaptations quickly diminishes as other signaling pathways predominate.
The data presented herein suggest new functions for the SNS in coordinating both the immediate catabolic response to mobilize resources and in the anabolic adaptive changes in exercised muscle. In our model, the rapid action and transcriptional components of the SNS-adrenergic signaling cascade are regulated in a sequential, biphasic manner (Supplementary Fig S10), suggesting a revision of the current concept of the SNS as driving solely catabolism during exercise (Egan & Zierath, 2013). Specifically, although transient elevations in cAMP and PKA regulate catabolism of energy stores during exercise, following exercise activation of the Crtc/Creb transcriptional complex induces genes that direct structural and metabolic remodeling. In support of this model, elevated levels of glycogen induced by forced expression of Crtc2 or Crtc3 are reversible by acutely activating PKA with clenbuterol, or by a bout of HIE. These adaptive Crtc/Creb-driven events during rest would thus prepare the muscle for the next repetitive bout of exertion and suggest that the Crtc/Creb transcriptional complex plays a specialized role in coordinating the adaptive response to the stress or intensity of exercise to restore homeostasis. Transgenic mice showed a clear improvement in maximal exercise capacity during treadmill-based stress test after induction of the Crtc2 transgene. This is consistent with the well-known fact that athletic performance is greatly enhanced by reintroduction of high-intensity interval training and periodic changes in exercise routines (Fairbarn et al, 1991; Smith et al, 2003; Babraj et al, 2009; Richards et al, 2010). Thus, the SNS is exquisitely tailored to direct skeletal muscle adaptations that are proportional to the stress of the exercise, and our work challenges the current paradigm of SNS as primarily being mediator of rapid catabolic changes in skeletal muscle, as it clearly also controls adaptive anabolic responses via the Crtc/Creb complex.
Materials and Methods
Transgenic mice
TRE-Crtc2tm mice were generated by cloning FLAG-Crtc2 (S171A, S275A, K628R) into pTRE-Tight. Oocyte injections were conducted into pseudopregnant C57Bl6 mice at the University of Cincinnati. Skeletal muscle specificity was conferred when this transgenic line was crossed with second mouse transgenic strain that harbors a transgene for the reverse tetracycline transactivator (rtTA) that is regulated by the 1256[3Emut] MCK promoter (Grill et al, 2003). For skeletal muscle-specific expression, Crtc2tm DTg mice were treated with DOX (1.0 g/kg chow, Bio serv). Wild-type littermates were used as controls and were treated with DOX in the same manner.
Electric-pulse-mediated gene transfer
Electric-pulse-mediated gene transfer was conducted with an ECM 830 square wave using non-invasive platinum-coated tweezertrodes according to the manufacturer's guidelines. Fifty micrograms of purified plasmid DNA was injected with a 29-G needle in the tibialis anterior muscle. Immediately after the plasmid injection, four pulses of 40 mV lasting for 100 ms with an interval of 200 ms between pulses were applied. The polarity was then reversed and four additional pulses were performed.
Mouse exercise protocols
Eight-week-old male C57BL/6J mice were acclimated and pre-trained on a treadmill for 2 weeks prior to high-intensity exercise. Mice were run at 10 m/min for 15 min followed by an incremental increase of 1 m/min over the next 5 min until a speed of 20 m/min was reached. Mice were considered exhausted if they had an irregular gate or stayed on the shock grid for two continuous seconds. Mice were rested 30 min post-exercise before euthanization and tissue biopsy.
Exercise capacity was determined utilizing a treadmill-based exercise stress test that consisted of an escalating belt speed that increased by 2 m/min every 2 min at a 15-degree incline after an initial warm-up at 20 m/min for 5 min. The maximum speed achieved despite extra stimulus was recorded. Each mouse was evaluated in three independent tests separated by 1-week intervals prior to the administration of Dox, followed by three additional tests after 2 weeks of Dox administration. All animal protocols were reviewed and approved by the Institutional Animal Care and Use (IACUC) of The Scripps Research Institute, Florida.
Primary myocyte isolation and cell culture
Primary myoblasts were isolated from P1 to P3 day old C57BL/6J pups as described (Springer et al, 1997) and cultured on collagen-coated plates in expansion media consisting in 1:1 F-10/DMEM (Invitrogen) with 20% fetal calf serum and 25 μg/ml of bFGF. Sub-confluent myoblasts were differentiated into multinucleate myotubes using a 1:1 F-10/5 mM glucose DMEM supplemented with 4% heat-inactivated horse serum (Hyclone) for up to 5 days. C2C12 mouse myoblasts from American Type Collection were expanded in DMEM supplemented with 10% fetal calf serum.
Pharmacological agents
Isoproterenol 100 nM, clenbuterol 45 nM and propranolol 10 μM.
Transfections and luciferase assays
Mouse primary myoblasts were transfected with the using Lipofectamin LTX and plus reagent (Invitrogen) following the manufacturer's guidelines. Luciferase assays were carried out as described (Conkright et al, 2003b) using BrightGlo (Promega) reagent and an EnVision® Reader (PerkinElmer).
Adenoviruses
The control GFP-expressing, Crtc2-expressing and Crtc3-expressing adenoviruses have been described (Koo et al, 2005; Wu et al, 2006). For all experiments, myotubes were infected after 72 h of differentiation with 4 × 108 viral particles per ml per well for 24–48 h.
Immunoblotting and subcellular fractionation
Immunoblot analysis was performed as described (Conkright et al, 2003a). Cytoplasmic and nuclear biochemical fractionation of the myotubes was performed using the Active Motif nuclear extract kit (Active Motif) following the manufacturer's protocol. Whole-cell extracts were prepared in HEPES whole-cell lysis buffer (20 mM HEPES, pH 7.4, 1% Triton X-100, 1× phosphoSTOP complete inhibition cocktail tablets (Roche), and 1X Complete-EDTA Free protease inhibitor cocktail (Roche). Frozen muscle tissue was homogenized in lysis buffer T-PER® tissue protein extraction reagent (Thermo Scientific) by a polytron homogenizer and sonication. The protein concentration was determined using the protein assay reagent (Bio-Rad). 60–80 μg of protein was separated by SDS–PAGE and transferred to nitrocellulose membranes for immunoblotting. The antibodies used in this study were as follows: CRTC3 (C35G4)Rb, CREB (48H2)Rb, pAKT(Thr308) (244f1)Rb and pAKT(Ser473) (971s) were from Cell Signaling Technology; PGC1α ab54481 Rb and PDPK1 ab31406 Rb were from Abcam; Nur77 #2518 Rb was from Novus Biological and Epitomics; and CRTC2 #A300-638A Rb was from Bethyl Laboratories.
Tissue and cellular glycogen content
Glycogen concentration of primary myotubes was measured using an assay kit from Biovision.
Measurement of signaling molecules
Catecholamines were quantitated using the 2-CAT (Epinephrine/Norepinephrine) Research ELISA™ Kit from Rocky Mountain Diagnostic. cAMP was quantitated with a LANCE assay from PerkinElmer following the manufacturer's protocol.
RNA and qRT-PCR
Total RNA from tissue or cells was harvested using Rneasy RNA Kit (Qiagen) and cDNA prepared using Transcriptor High Fidelity cDNA Synthesis Kit (Roche). Relative abundance of cDNAs was determined a Roche LightCycler 480 and normalized the resulting data to Rpl-23 ribosomal RNA as described (Conkright et al, 2003b). Primers used were Nr4a1 (5′-ttctgctcaggcctggtact′, 5′-gattctgcagctcttccacc-3′); Nr4a3 (5′-tcagcctttttggagctgtt′, 5′-tgaagtcgatgcaggacaag-3′); Sik1 (5′-cccttattattccccctgga-3′, 5′-cttcacttgcagagaagggg-3′); Dgat1 (5′-tggttaacctggccacaatc-3′, 5′-ttggagtatgatgccagagca-3′); Pdpk1 (5′-tcacagattttggaacagcaa-3′, 5′-tgagcagctctggagaaaca-3′); and Ppargc1a isoform 1 (5′-atggcttgggacatg-3′, 5′-gttcaggaagatctgg-3′). Ppargc1a isoform 4 (5′-TCACACCAAACCCACAGAAA-3′, 5′-CTGGAAGATATGGCACAT-3′), Igf1 (5′-TTTTCGCCTCATTATCCCTG-3′, 5′-TCTCCTTTGCAGCTTCGTTT-3′).
Histology
Dissected muscle tissue was mounted in OCT medium (TissueTek) and frozen in liquid-nitrogen-cooled isopentane. Tissue sections were stained with hematoxylin and eosin for overall morphology, or ATPase activity for fast and slow fiber by standard methods. The cross-sectional area of muscle fibers was determined using ImageJ software.
Chromatin immunoprecipitation
C2C12 cells were stimulated with isoproterenol for 30 min before cross-linking in 1% formaldehyde as described (Amelio et al, 2007). We performed 28 cycles of PCR with the primers flanking the CRE sites of each promoter: Dgat1 (5′-GAACCGCAAGACGTCAGC -3′, 5′-GAGGCTGCGATGCTGCGG-3′); Nr4a1 (5′-AAGAAATAGCAGGCTGGTTGGG-3′, 5′-CAGGTAAGAGAAAGGAAGGGGTATG-3′); Gapdh (5′-GAAAAGGAGATTGCTACG-3′, 5′-GCAAGAGGCTAGGGGC-3′); and Ppargc1 distal promoter (5′-TCATTGAGCAGTGACTCCCAGG-3′, 5′-CAAACCCCTACATACCAGCAGC-3′).
Statistical analysis
Two-tailed Student's t-test was used to determine P-values between datasets assuming Gaussian distribution. Data are reported as the mean ± s.e.m., along with calculated P-values. One-way ANOVA was applied to compare means of three or more groups followed by Tukey's post hoc test for comparing pairs of means. The calculated P-values are represented by *P < 0.05, **P < 0.01 and ***P < 0.001.
Acknowledgments
The authors would like to thank Dr. Zhidan Wu for providing adenovirus expressing Crtc3. In addition, we thank Drs. John Cleveland, Andrew Butler, Tim Tellinghuisen and Dr. Patrick Griffin for their scientific review and editing during the preparation of this manuscript. This work was supported by the National Institute Health grant R01 DK081491 (MDC) and by monies to Scripps Florida from the state of Florida.
Author contributions
NB and MDC initiated the research and planned all experiments. NB performed most of the experiments and supervised the assistance of KAK, RH and MBL with cell culture and immunoblotting. NB and KAK developed and characterized the transgenic mice. ALA conducted the ChIP experiments and cloned the Pgc-1α promoter constructs. NB, NK and MDC interpreted the data. NB, KAK, KN and MDC contributed to the writing of the manuscript.
Conflict of interest
N.E. Bruno and M.D. Conkright declare a patent application related to this work. The remaining authors declare that they have no conflict of interest.
Supporting information for this article is available online: http://emboj.embopress.org
References
- Agbenyega ET, Wareham AC. Effect of clenbuterol on normal and denervated muscle growth and contractility. Muscle Nerve. 1990;13:199–203. doi: 10.1002/mus.880130305. [DOI] [PubMed] [Google Scholar]
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty D, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- Akimoto T, Li P, Yan Z. Functional interaction of regulatory factors with the Pgc-1alpha promoter in response to exercise by in vivo imaging. Am J Physiol Cell Physiol. 2008;295:C288–C292. doi: 10.1152/ajpcell.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Albis A, Couteaux R, Janmot C, Mira JC. Myosin isoform transitions in regeneration of fast and slow muscles during postnatal development of the rat. Dev Biol. 1989;135:320–325. doi: 10.1016/0012-1606(89)90182-6. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- Allen DL, Harrison BC, Leinwand LA. Molecular and genetic approaches to studying exercise performance and adaptation. Exerc Sport Sci Rev. 2002;30:99–105. doi: 10.1097/00003677-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelio AL, Miraglia LJ, Conkright JJ, Mercer BA, Batalov S, Cavett V, Orth AP, Busby J, Hogenesch JB, Conkright MD. A coactivator trap identifies NONO (p54nrb) as a component of the cAMP-signaling pathway. Proc Natl Acad Sci USA. 2007;104:20314–20319. doi: 10.1073/pnas.0707999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord. 2009;9:3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang C-Y, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Baxter PS, Martel M-A, McMahon A, Kind PC, Hardingham GE. Pituitary adenylate cyclase-activating peptide induces long-lasting neuroprotection through the induction of activity-dependent signaling via the cyclic AMP response element-binding protein-regulated transcription co-activator 1. J Neurochem. 2011;118:365–378. doi: 10.1111/j.1471-4159.2011.07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdeaux R, Goebel N, Banaszynski L, Takemori H, Wandless T, Shelton GD, Montminy M. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Feng H-Z, Gupta D, Kelleher J, Dickerson KE, Wang J, Hunt D, Jou W, Gavrilova O, Jin J-P, Weinstein LS. G(s)alpha deficiency in skeletal muscle leads to reduced muscle mass, fiber-type switching, and glucose intolerance without insulin resistance or deficiency. Am J Physiol Cell Physiol. 2009;296:C930–C940. doi: 10.1152/ajpcell.00443.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesley A, Heigenhauser GJ, Spriet LL. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am J Physiol. 1996;270:E328–E335. doi: 10.1152/ajpendo.1996.270.2.E328. [DOI] [PubMed] [Google Scholar]
- Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- Ch'ng TH, Uzgil B, Lin P, Avliyakulov NK, O'Dell TJ, Martin KC. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell. 2012;150:207–221. doi: 10.1016/j.cell.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo JJ, Horan MA, Little RA, Rothwell NJ. Anabolic effects of clenbuterol on skeletal muscle are mediated by beta 2-adrenoceptor activation. Am J Physiol. 1992;263:E50–E56. doi: 10.1152/ajpendo.1992.263.1.E50. [DOI] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, Guzmán E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol Cell. 2003a;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Conkright MD, Guzmán E, Flechner L, Su AI, Hogenesch JB, Montminy M. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol Cell. 2003b;11:1101–1108. doi: 10.1016/s1097-2765(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Erbay E, Chen J. The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J Biol Chem. 2001;276:36079–36082. doi: 10.1074/jbc.C100406200. [DOI] [PubMed] [Google Scholar]
- Fairbarn MS, Coutts KC, Pardy RL, McKenzie DC. Improved respiratory muscle endurance of highly trained cyclists and the effects on maximal exercise performance. Int J Sports Med. 1991;12:66–70. doi: 10.1055/s-2007-1024658. [DOI] [PubMed] [Google Scholar]
- Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34:663–679. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol. 1975;38:70–76. doi: 10.1152/jappl.1975.38.1.70. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Montminy M. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Green HJ, Ball-Burnett M, Symon S, Grant S, Jamieson G. Short-term training, muscle glycogen, and cycle endurance. Can J Appl Physiol. 1995;20:315–324. doi: 10.1139/h95-024. [DOI] [PubMed] [Google Scholar]
- Grill MA, Bales MA, Fought AN, Rosburg KC, Munger SJ, Antin PB. Tetracycline-inducible system for regulation of skeletal muscle-specific gene expression in transgenic mice. Transgenic Res. 2003;12:33–43. doi: 10.1023/a:1022119005836. [DOI] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1452–R1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol. 2012;113:1495–1504. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt LJ, Schertzer JD, Ryall JG, Lynch GS. Low dose formoterol administration improves muscle function in dystrophic mdx mice without increasing fatigue. Neuromuscul Disord. 2007;17:47–55. doi: 10.1016/j.nmd.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol. 2002;92:313–322. doi: 10.1152/japplphysiol.00832.2001. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Burke LM, Phillips SM, Spriet LL. Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol. 2011;110:834–845. doi: 10.1152/japplphysiol.00949.2010. [DOI] [PubMed] [Google Scholar]
- Hayes A, Williams DA. Long-term clenbuterol administration alters the isometric contractile properties of skeletal muscle from normal and dystrophin-deficient mdx mice. Clin Exp Pharmacol Physiol. 1994;21:757–765. doi: 10.1111/j.1440-1681.1994.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala U, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hinkle RT, Hodge KMB, Cody DB, Sheldon RJ, Kobilka BK, Isfort RJ. Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve. 2002;25:729–734. doi: 10.1002/mus.10092. [DOI] [PubMed] [Google Scholar]
- Jansson D, Ng AC-H, Fu A, Depatie C, Azzabi Al M, Screaton RA. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci USA. 2008;105:10161–10166. doi: 10.1073/pnas.0800796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocken JWE, Blaak EE. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav. 2008;94:219–230. doi: 10.1016/j.physbeh.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kanzleiter T, Preston E, Wilks D, Ho B, Benrick A, Reznick J, Heilbronn LK, Turner N, Cooney GJ. Overexpression of the orphan receptor Nur77 alters glucose metabolism in rat muscle cells and rat muscle in vivo. Diabetologia. 2010;53:1174–1183. doi: 10.1007/s00125-010-1703-2. [DOI] [PubMed] [Google Scholar]
- Kline WO, Panaro FJ, Yang H, Bodine SC. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol. 2007;102:740–747. doi: 10.1152/japplphysiol.00873.2006. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2005;289:H455–H465. doi: 10.1152/ajpheart.00085.2005. [DOI] [PubMed] [Google Scholar]
- Koo S-H, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Koopman R, Gehrig SM, Léger B, Trieu J, Walrand S, Murphy KT, Lynch GS. Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic {beta}-adrenoceptor stimulation in mice. J Physiol. 2010;588:4811–4823. doi: 10.1113/jphysiol.2010.196725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome–causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–172. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu Y-H. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Shi X, Choi CS, Shulman GI, Klaus K, Nair KS, Schwartz GJ, Zhang Y, Goldberg IJ, Yu Y-H. Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes. 2009;58:2516–2524. doi: 10.2337/db08-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Stephenson DG, Williams DA. Analysis of Ca2+ and Sr2+ activation characteristics in skinned muscle fibre preparations with different proportions of myofibrillar isoforms. J Muscle Res Cell Motil. 1995;16:65–78. doi: 10.1007/BF00125311. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88:729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- Macías W, Carlson R, Rajadhyaksha A, Barczak A, Konradi C. Potassium chloride depolarization mediates CREB phosphorylation in striatal neurons in an NMDA receptor-dependent manner. Brain Res. 2001;890:222–232. doi: 10.1016/s0006-8993(00)03163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltin CA, Delday MI, Reeds PJ. The effect of a growth promoting drug, clenbuterol, on fibre frequency and area in hind limb muscles from young male rats. Biosci Rep. 1986;6:293–299. doi: 10.1007/BF01115158. [DOI] [PubMed] [Google Scholar]
- Maltin CA, Hay SM, Delday MI, Lobley GE, Reeds PJ. The action of the beta-agonist clenbuterol on protein metabolism in innervated and denervated phasic muscles. Biochem J. 1989;261:965–971. doi: 10.1042/bj2610965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci USA. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann HJ. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J Anim Sci. 1998;76:160–172. doi: 10.2527/1998.761160x. [DOI] [PubMed] [Google Scholar]
- Michael L, Asahara H, Shulman A, Kraus W, Montminy M. The phosphorylation status of a cyclic AMP-responsive activator is modulated via a chromatin-dependent mechanism. Mol Cell Biol. 2000;20:1596–1603. doi: 10.1128/mcb.20.5.1596-1603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Kai Y, Kamei Y, Ezaki O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor- coactivator-1 (PGC-1) mRNA in response to 2-adrenergic receptor activation and exercise. Endocrinology. 2008;149:4527–4533. doi: 10.1210/en.2008-0466. [DOI] [PubMed] [Google Scholar]
- Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Norrbom J, Sällstedt EK, Fischer H, Sundberg CJ, Rundqvist H, Gustafsson T. Alternative splice variant PGC-1α-b is strongly induced by exercise in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E1092–E1098. doi: 10.1152/ajpendo.00119.2011. [DOI] [PubMed] [Google Scholar]
- Park I-H, Chen J. Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J Biol Chem. 2005;280:32009–32017. doi: 10.1074/jbc.M506120200. [DOI] [PubMed] [Google Scholar]
- Pearen MA, Eriksson NA, Fitzsimmons RL, Goode JM, Martel N, Andrikopoulos S, Muscat GEO. The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Mol Endocrinol. 2012;26:372–384. doi: 10.1210/me.2011-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JC, Johnson TK, Kuzma JN, Lonac MC, Schweder MM, Voyles WF, Bell C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J Physiol. 2010;588:2961–2972. doi: 10.1113/jphysiol.2010.189886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roatta S, Farina D. Sympathetic actions on the skeletal muscle. Exerc Sport Sci Rev. 2010;38:31–35. doi: 10.1097/JES.0b013e3181c5cde7. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall JG, Schertzer JD, Lynch GS. Attenuation of age-related muscle wasting and weakness in rats after formoterol treatment: therapeutic implications for sarcopenia. J Gerontol A Biol Sci Med Sci. 2007;62:813–823. doi: 10.1093/gerona/62.8.813. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Lynch GS. The potential and the pitfalls of beta-adrenoceptor agonists for the management of skeletal muscle wasting. Pharmacol Ther. 2008;120:219–232. doi: 10.1016/j.pharmthera.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Holsboer F, Spengler D. Beta(2)-adrenergic receptors potentiate glucocorticoid receptor transactivation via G protein beta gamma-subunits and the phosphoinositide 3-kinase pathway. Mol Endocrinol. 2001;15:553–564. doi: 10.1210/mend.15.4.0613. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzmán E, Niessen S, Yates JR, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Shi H, Zeng C, Ricome A, Hannon KM, Grant AL, Gerrard DE. Extracellular signal-regulated kinase pathway is differentially involved in beta-agonist-induced hypertrophy in slow and fast muscles. Am J Physiol Cell Physiol. 2007;292:C1681–C1689. doi: 10.1152/ajpcell.00466.2006. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Lortie G, Boulay MR, Marcotte M, Thibault MC, Bouchard C. Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol. 1985;54:250–253. doi: 10.1007/BF00426141. [DOI] [PubMed] [Google Scholar]
- Smith TP, Coombes JS, Geraghty DP. Optimising high-intensity treadmill training using the running speed at maximal O(2) uptake and the time for which this can be maintained. Eur J Appl Physiol. 2003;89:337–343. doi: 10.1007/s00421-003-0806-6. [DOI] [PubMed] [Google Scholar]
- Sneddon AA, Delday MI, Steven J, Maltin CA. Elevated IGF-II mRNA and phosphorylation of 4E-BP1 and p70(S6k) in muscle showing clenbuterol-induced anabolism. Am J Physiol Endocrinol Metab. 2001;281:E676–E682. doi: 10.1152/ajpendo.2001.281.4.E676. [DOI] [PubMed] [Google Scholar]
- Springer M, Rando T, Blau HM. Gene Delivery to Muscle. Curr Protoc Hum Genet. 1997;13:13.4.1–13.4.19. doi: 10.1002/0471142905.hg1304s31. [DOI] [PubMed] [Google Scholar]
- Stepto NK, Carey AL, Staudacher HM, Cummings NK, Burke LM, Hawley JA. Effect of short-term fat adaptation on high-intensity training. Med Sci Sports Exerc. 2002;34:449. doi: 10.1097/00005768-200203000-00011. [DOI] [PubMed] [Google Scholar]
- Takemori H, Katoh Hashimoto Y, Nakae J, Olson EN, Okamoto M. Inactivation of HDAC5 by SIK1 in AICAR-treated C2C12 myoblasts. Endocr J. 2009;56:121–130. doi: 10.1507/endocrj.k08e-173. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hagberg JM, Hickson RC, Ehsani AA, McLane JA. Time course of sympathoadrenal adaptation to endurance exercise training in man. J Appl Physiol. 1978;45:370–374. doi: 10.1152/jappl.1978.45.3.370. [DOI] [PubMed] [Google Scholar]
- Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen P, Bare O, Labow M, Spiegelman B, Stevenson S. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Inagaki K, Noguchi T, Sakai M, Ogawa W, Hosooka T, Iguchi H, Watanabe E, Matsuki Y, Hiramatsu R, Kasuga M. Identification and characterization of an alternative promoter of the human PGC-1alpha gene. Biochem Biophys Res Commun. 2009;381:537–543. doi: 10.1016/j.bbrc.2009.02.077. [DOI] [PubMed] [Google Scholar]
- Zeman RJ, Peng H, Danon MJ, Etlinger JD. Clenbuterol reduces degeneration of exercised or aged dystrophic (mdx) muscle. Muscle Nerve. 2000;23:521–528. doi: 10.1002/(sici)1097-4598(200004)23:4<521::aid-mus10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta. 2010;1801:1–22. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



