Abstract
Purpose
Fenretinide (4-HPR) is a cytotoxic retinoid with minimal systemic toxicity that has shown clinical activity against recurrent high-risk neuroblastoma. To identify possible synergistic drug combinations for future clinical trials, we determined if ABT-737, a small-molecule BH3-mimetic that inhibits most proteins of the anti-apoptotic Bcl-2 family, could enhance 4-HPR activity in neuroblastoma.
Experimental Design
Eleven neuroblastoma cell lines were tested for the cytotoxic activity of 4-HPR and ABT-737 as single agents and in combination using the DIMSCAN fluorescence digital imaging cytotoxicity assay. The effect of these agents alone and in combination on mitochondrial membrane depolarization and apoptosis (by flow cytometry), cytochrome c release, caspases, Bax-α, t-Bid, and Bak activation, and subcutaneous xenografts in nu/nu mice was also determined.
Results
Multilog synergistic cytotoxicity was observed for the drug combination in all of the eleven neuroblastoma cell lines tested, including multi-drug resistant lines and those insensitive to either drug as single agents. 4-HPR + ABT-737 induced greater mitochondrial membrane depolarization and mitochondrial cytochrome c release, greater activation of caspases, Bax-α, t-Bid, and Bak, and a higher level of apoptosis than either drug alone. In vivo, 4-HPR + ABT-737 increased the event-free survival (EFS) of the multidrug-resistant human neuroblastoma line CHLA-119 implanted subcutaneously in nu/nu mice (194.5 days for the combination vs. 68 days for ABT-737 and 99 days for 4-HPR).
Conclusion
Thus, the combination of 4-HPR with a BH3-mimetic drug warrants clinical trials in recurrent neuroblastoma.
Keywords: Fenretinide, ABT-737, drug resistance, Bcl-2, neuroblastoma, apoptosis
Introduction
Neuroblastoma (NB) is an aggressive childhood tumor of the sympathetic nervous system accounting for 8–10% of all childhood cancers and approximately 15% of cancer deaths in children (1). Treatment of high-risk NB (stage 4 patients > 1-year-old at diagnosis and stage 3 disease with MYCN amplification or unfavorable histopathology) with, multiagent chemotherapy, radiotherapy, and myeloablative chemotherapy supported stem cell transplant followed by treatment of minimal residual disease by 13-cis-retinoic acid (13-cis-RA) (2, 3), and more recently anti-GD2 antibody, cytokines, and 13-cis-RA has significantly improved the outcome for these patients (4, 5). However, as many high-risk patients still ultimately die from tumor that is refractory to initial therapy or recurrent, resistant disease, novel therapies effective against multidrug-resistant NB are needed.
4-HPR, is a synthetic derivative of retinoic acid that has a broad-spectrum of cytotoxic activity against primary tumor cells, cell lines, and/or xenografts of various cancers (including neuroblastoma) (6–9) and has been tested in early phase clinical trials in recurrent NB (10–12). In contrast to retinoids such as all-trans-retinoic acid (ATRA) and 13-cis-retinoic acid (13-cis-RA) that cause arrest of cell growth and morphological differentiation of human NB cell lines, 4-HPR induces cell death in NB cells via both apoptotic and non-apoptotic mechanisms (6–8). The observation that 4-HPR remains cytotoxic in NB cell lines that are resistant to all-trans-retinoic acid, 13-cis-RA, alkylating agents, and etoposide suggests that it may be active in high-risk NB patients resistant to standard therapy (7, 9, 13). Indeed, a recent phase I trial in refractory/recurrent neuroblastoma patients of a novel oral powder 4-HPR formulation (4-HPR LXS oral powder) that improves 4-HPR exposures documented 4 complete responses (11).
Overexpression of anti-apoptotic Bcl-2 family proteins is a common mechanism by which cells become resistant to conventional chemotherapy, providing attractive therapeutic targets (14, 15). NB cell lines obtained from patients with recurrent disease after treatment exhibit increased levels of Bcl-2 expression, which may be responsible for their drug resistance (16). Strong Bcl-2 immunoreactivity was also detected in islets of residual NB cells in MYCN non-amplified primary tumors of treated patients (17). These observations suggest that an increased expression of anti-apoptotic Bcl-2 family of proteins may be one mechanism for the resistance of neuroblastoma cells to cytotoxic agents, including 4-HPR.
ABT-737 is a small molecule that mimics the direct binding of the BH3-only protein Bad to anti-apoptotic Bcl-2 family proteins like Bcl-2, Bcl-XL, and Bcl-w and therefore displaces BH-3-only death-activating proteins (Bim, Bid) (14) . By binding to proteins of the Bcl-2 family, ABT-737 prevents proteins like Bid and Bim (direct activators of Bax and Bak) from forming heterodimers with anti-apoptotic Bcl-2 family proteins, thereby promoting Bax and Bak activation via oligomerization (18). ABT-737 has been reported to be cytotoxic as a single agent, and to sensitize a wide variety of cancer cells to several chemotherapeutic agents in vitro and in vivo (14, 19–23). We have previously shown that ABT-737 synergistically enhances 4-HPR cytotoxicity in acute lymphoblastic leukemia cells, but that 4-HPR + ABT-737 was not toxic for normal non-proliferating lymphocytes (24).
We therefore hypothesized that the combination of an inhibitor of proteins of the Bcl-2 family (such as ABT-737) with 4-HPR would be synergistic against NB with minimal systemic toxicities. To test this hypothesis, we evaluated the activity of ABT-737 and 4-HPR (alone and in combination) on a panel of human NB cell lines and in a xenograft model of recurrent multidrug-resistant human NB.
Materials and Methods
Cell Culture
We used a panel of eleven human NB cell lines obtained from patients at various stages of disease: two cell lines established at diagnosis prior to any therapy (CHLA-15, SMS-KAN); seven cell lines obtained at the time of progressive disease during induction therapy (SK-N-BE(2), SK-N-RA, CHLA-119, LA-N-6, CHLA-20, SMS-KCNR, CHLA-140); and two cell lines established at relapse after myeloablative therapy and bone marrow transplantation (CHLA-79, CHLA-136). All cell lines were established in the senior author’s lab, except SK-N-RA and SK-N-BE(2) which were a gift of Dr. L Helson; characterization of these NB cell lines has been previously reported (25, 26). Cell line identity was confirmed at time of the experiments using a 15 loci short tandem repeat (STR) assay + amelin for sex determination (27), with the genetic signature compared to the Children’s Oncology Group STR database (www.COGcell.org). We also tested the human normal fibroblast cell line CRL-2076 obtained from the American Type Culture Collection (ATCC, Manassas, VA).
SMS-KAN, SK-N-BE(2), SK-N-RA, LA-N-6, SMS-KCNR and CRL-2076 cells were cultured in RPMI-1640 medium (Irvine Scientific, Santa Ana, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gemini Bio-Products, Inc., Calabasas, CA). CHLA-15, CHLA-119, CHLA-20, CHLA-140, CHLA-79 and CHLA-136 were cultured in Iscove’s modified Dulbecco’s medium (IMDM; Bio Whittaker, Walkersville, MD) containing 20% heat-inactivated FBS and supplemented with 3 mM L-glutamine (Gemini Bio-Products, Inc., Calabasas, CA), insulin, and transferrin (5 μg/ml each) and selenium (5 ng/ml) (ITS Culture Supplement, CollaborativeBiomedical Products, Bedford, MA). All cell lines were continuously culturedat 37°C in a humidified incubator containing 95% air + 5% CO2 without antibiotics. Experiments were carried out using NB cell lines at passage15–35. Cells were detached from culture plates or flasks with the use of a modified Puck’s Solution A plus EDTA (Puck’s EDTA), containing 140 mM NaCl, 5 mM KCl, 5.5mM glucose, 4 mM NaHCO3, 0.8 mM EDTA, 13 μM phenol red,and 9 mM HEPES buffer (pH 7.3) (28).
Drugs and Reagents
ABT-737 was kindly provided by Abbott Laboratories (Abbott Park, IL). 4-HPR was obtained from the Developmental Therapeutics Programof the National Cancer Institute (Bethesda, MD). Fenretinide was formulated as LYM-X-SORBTM oral powder (3% 4-HPR by weight, 4-HPR LXS) by Avanti Polar Lipids, Inc, Alabaster, AL (29), and was kindly provided to the investigators by Barry J. Maurer, MD PhD. Eosin Y was purchased from Sigma Chemical Co.(St. Louis, MO) and fluorescein diacetate (FDA) was obtained from Eastman Kodak Co. (Rochester, NY). Mitochondrial membrane potential probe JC-1 (5,5', 6,6’-tetrachloro-1, 1’, 3,3’-tetraethylbenzimidazolyl-carbocyanine iodide) was obtained from Molecular Probes (Eugene, OR); the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) kit and the caspase-8 enzyme inhibitor, Z-IETD-FMK were obtained from BD Biosciences (APO-DIRECTTM, San Diego, CA). The pan-caspase enzyme inhibitor, Boc-d-fmk, was purchasedfrom MP Biomedicals, LLC, (Solon, OH). Stock solutions of ABT-737 (5 mM), 4-HPR (10 mM), FDA (1 mg/ml), JC-1 (2 mg/ml), Z-IETD-FMK (10mM), and Boc-d-fmk (20 mM) were dissolved in dimethyl sulfoxide (DMSO) except 4-HPR which was dissolved in 95% ethanol. All reagents were stored at −20°C.
Cytotoxicity Assay
The cytotoxicity of ABT-737, 4-HPR, and their combination (at a 1:1 molarratio) was determined in 96-well plates using the semiautomatic fluorescence-based Digital Imaging Microscopy System (DIMSCAN) (30, 31). DIMSCAN uses digital imaging microscopy to quantify viable cells, which selectively accumulate fluorescein diacetate (FDA). DIMSCAN is capable of measuring cytotoxicity over a four log dynamic range by quantifying total fluorescence per well (which is proportional to the number of viable cells) after elimination of the background fluorescence by digital thresholding and eosin Y quenching. Cells were seeded into 96-well plates in 100 μl of complete medium at 5,000 to 10,000 cells per well, depending on cell line growth rate. After overnight incubation, ABT-737, 4-HPR, or their combination was added to each well at various concentrations in 50μL of culture medium. We used the following drug concentrations: 0, 1.25, 2.5, 5, 10 μM or 0, 2.5, 5, 7.5, 10 μM in replicates of 12 wells for each experimental condition. After incubation with the drugs for 96 hours at 37°C, FDA (final concentration 10 μg/ml) and eosin Y (final concentration 0.1% [w/v]) were added to each well and the cells were incubated for an additional 20 minutes at 37°C. Total fluorescence per well was then measured using DIMSCAN, and the results were expressed as the ratio of the fluorescence in treated wells to the fluorescence in untreated wells (survival fraction).
Assessment of Apoptosis by Flow Cytometry
Apoptosis in cells was examined by flow cytometry using a commercial TUNEL kit (APO-DIRECTTM, BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Cells treated with ABT-737, 4-HPR or their combination in the presence or absence of the pancaspase inhibitor Boc-d-fmk (40 μM for 1 hour) were collected, washed, centrifuged and resuspended in 1% (w/v) paraformaldehyde in phosphate buffered saline (PBS) (pH 7.4). They were then kept on ice for 60 minutes, washed in PBS, centrifuged, and fixed in 70% (v/v) ice-cold ethanol at -20°C for 12-18 hours, before being stained with terminal deoxynucleotidyltransferase (TdT) and FITC-labeled deoxyuridine triphosphates (FITC-dUTP) for 2 hours at 37°C. After washing with PBS, cells were resuspended in 0.5 ml of propidium iodide (PI) and RNase containing buffer (5 μg/ml PI, 200 μg/ml RNase). Cells were then incubated in the dark for 30 minutes at room temperature prior to analysis by flow cytometry. The percentage of TdT-mediated fluorescent cells was measured by flow cytometry using band pass filters of 525 ± 25 nm for FITC and 610 ± 25 nm for PI (7, 32) in a BD LSR II system (BD Biosciences, San Jose, CA) equipped with the DiVA software (version 4.1.2; BD Biosciences).
Determination of Mitochondrial Membrane Potential (Δ ψm) Transition
Cells were treated with ABT-737, 4-HPR, or the combination, collected in 5 mL polystyrene tubes, centrifuged at 300 g for 5 minutes, and resuspended in 1 ml of medium containing 10 μg/ml of JC-1, incubated at 37°C for 10 minutes and analyzed by flow cytometry. The detection of a fluorescence emission shift from red (610 ± 10 nm) to green (525 ± 10 nm) was an indication of mitochondrial membrane depolarization (33).
Western Blot Analysis
Cells were lysed in radioimmunoprecipitation (RIPA) lysis buffer(Upstate,Lake Placid, NY), containing 15 μl/ml of phenylmethanesulphonylfluoride (PMSF),and 40 μl/ml of Protease Inhibitor Cocktail (Sigma, St Louis, MD). The lysates were left on ice for 15 minutes, briefly sonicated, and centrifuged at 12,000 g for 15 minutes. Protein concentration in the supernatants was determined using the BCAprotein assay kit (Pierce Biotechnology, Rockford, IL), and 20 μg of protein in each sample was resolved by electrophoresis in a 10–20% gradient acrylamide gel containing 0.1% SDS (Invitrogen, Carlsbad, CA). After electrophoresis, the gels were transferred to a protein nitrocellulose transfer membrane (Whatman GmbH, Germany). The membrane was hybridized with primary antibodies followed by horseradish peroxidase (HRP)-conjugated secondary antibodies, and immunocomplexes detected by chemiluminescence (Pierce Biotechnology, Rockford, IL) and visualized on autoradiography film (Denville Scientific, Inc, Metuchen, NJ). Quantification was obtained by scanning the immunoblots using an Epson Expression 1680 system (Epson, Long Beach, CA). Antibodies used were: anti-Bax rabbit polyclonal antibody (#554104) from BD Biosciences, San Diego, CA; anti-β-actin goat polycolonal antibody, anti-GAPDH mouse monoclonal antibody, and HRP-conjugated secondary anti-mouse, anti-goat, and anti-rabbit antibodies rom Santa Cruz Biotechnology, Santa Cruz, CA; anti-caspase-9 rabbit polyclonal antibody (#9502), anti-caspase-3 rabbit polyclonal antibody (#9662), anti-Bid rabbit polyclonal antibody (#2002), anti-Bcl-2 rabbit polyclonal antibody (#2870), anti-Bcl-XL rabbit polyclonal antibody (#2764), anti-Bcl-w rabbit polyclonal antibody (#2724), anti-Mcl-1 rabbit polyclonal antibody (#5453), and anti-Bak rabbit polyclonal antibody (#3814) from Cell Signaling Technology, Danvers,MA; anti-Bak mouse monoclonal antibody (Ab-1) which recognizes only conformationally active Bak was from Calbiochem, La Jolla, CA; anti-cytochrome c rabbit polyclonal antibody and anti-OxPhos Complex IV (COX IV) mouse monoclonal antibody were from Clontech, Mountain View, CA. Densitometric analysis was performed using Image J digital imaging software (National Institute of Health, USA).
Detection of Cytochrome c Release from Mitochondria
After treatment with ABT-737, 4-HPR, or the combination for 6 or 24 hours, cells were subjected to a digitonin-based subcellular fractionation (34) to separate the cytosol (supernatant) from intact mitochondria (pellet). The pellets were then lysed in RIPA lysis buffer and western blot analysis conducted as described above.
Caspase-8 Activation
A caspase-8/FLICE colorimetic assay (Invitrogen, Carlsbad, CA) was used according to the manufacturer's instructions to detect the activation of caspase-8.
RNA interference
Validated small interfering RNAs (siRNAs) specific for Bax (5’-GCUCUGAGCAGAUCAUGAATT-3’) and Bak (5’-GCGAAGUCUUUGCCUUCUCTT-3’) were purchased from Qiagen (Valencia, CA). A non-specific non-silencing siRNA (AllStars Negative Control siRNA; QIAGEN) was used as negative control. Neuroblastoma cells were transfected with 100 nM of Bax or Bak siRNA or control siRNA using Lipofectamine iMax transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Human Neuroblastoma Xenograft Model
The CHLA-119 cell line, established from a neuroblastoma patient at time of disease progression (26), was injected at 17 x 106 cells subcutaneously (s.c.) between the shoulder blades of 4 to 6-week-old female athymic (nu/nu) mice. Once palpable and progressing tumors of 100 to 200 mm3 had developed, mice were treated with a maximal-tolerated dose of 4-HPR LXS oral powder (240 mg/kg/day) and ABT-737 (50 mg/kg/day) for 5 days per week, alone or in combination or with the vehicle as control. When given alone, the dose of ABT-737 was increased to 100 mg/kg/day. 4-HPR LXS was prepared as slurry in sterile water and given orally by gavage while ABT-737 was administered by intraperitoneal (i.p.) injection. Tumor volume was determined from measurements taken twice weekly using the formula 0.5 × height × width × length (35). When tumor volumes reached 1,500 mm3, the mice were sacrificed
Statistical Analysis
For in vitro experiments, synergistic drug interactions were determined by fixed ratio dose-response assays of the drugs alone and in combination (4-HPR: ABT-737 = 1:1). The IC90 value (the drug concentration that is cytotoxic or growth inhibitory for 90% of a cell population) and the combination index (CIN) value were obtained by DIMSCAN analysis and calculated using Calcusyn software (Biosoft, Cambridge, United Kingdom). Calculation of a CIN is a method to numerically quantify drug synergism based on the multiple drug–effect equation of Chou-Talalay derived from enzyme kinetic models (36, 37). With this method, a CIN lower than 0.9 indicates synergism; a CIN of 0.9 to 1.10 indicates additive activity; and a CIN greater than 1.10 indicates antagonism.
In vivo data were analyzed using the software of Graphpad Prism (GraphPad Software, Inc., version 4.03, La Jolla, CA). Event-free-survival (EFS) curves were compared by Kaplan-Meier log-rank test and quantified as the time taken from the initiation of treatment until an event, with events defined as tumor volume reaching 1,500 mm3 or when mice died or were sacrificed because of treatment-related toxicity. An EFS T/C value was defined as the ratio of the median time to event of the treatment group and the median time to event of the respective control group (38). For the EFS T/C measure, agents are considered highly active if they met three criteria: (a) an EFS T/C > 2; (b) a significant difference (P≤0.05) in EFS distribution between treated and control (or single agent) and (c) a net reduction in median tumor volume for animals in the treated group at the end of treatment as compared to treatment initiation. Agents meeting the first two criteria, but not having a net reduction in median tumor volume for treated animals at the end of the study, are considered to have intermediate activity. Agents with an EFS T/C < 2 are considered to have low levels of activity.
Relative tumor volumes (RTV) for control (C) and treatment (T) mice (Tumor volume T/C values) were calculated on Day 21 (38). The mean RTVs for control and treatment mice for each study were then calculated and the T/C value was the mean RTV for the treatment group divided by the mean RTV for the control group. For the tumor volume T/C response measure, agents producing a T/C of ≤ 15% are considered highly active, those with a mean tumor volume T/C of ≤ 45% but > 15% are considered to have intermediate activity, and those with mean T/C values >45% are considered to have low levels of activity.
Results with a P value less than 0.05 were regarded as significant (log-rank for EFS by Graphpad Prism or unpaired two-sided Student’s t test by Microsoft Excel 2000 for other data).
Results
The combination ABT-737 plus 4-HPR was synergistically cytotoxic against NB cell lines in vitro
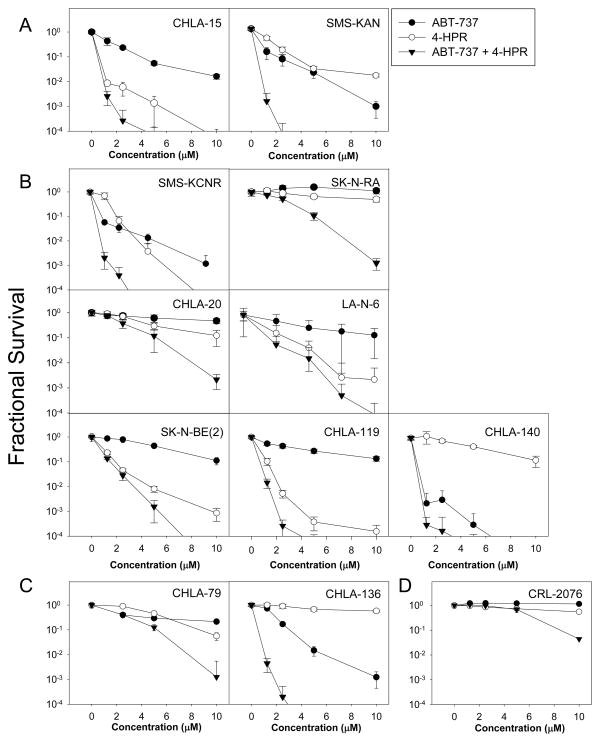
We first determined the cytotoxicity of ABT-737, 4-HPR, and ABT-737 plus 4-HPR (0 to 10 μM for each drug) at 1:1 molar ratio in eleven NB cell lines using the DIMSCAN cytotoxicity assay. Representative drug cytotoxicity dose-response curves are shown in Fig. 1 and CIN values calculated at the fixed-ratio drug concentrations tested for the cytotoxicity assays and IC90 values of both agents are in Table 1. 4-HPR + ABT-737 showed synergistic activity in all eleven cell lines (CIN < 0.9). In eight of the eleven cell lines, including those resistant to 4-HPR alone (CHLA-136), ABT-737 alone (SK-N-BE(2), CHLA-119) or both (SK-N-RA, CHLA-20), 4-HPR + ABT-737 showed very strong to good synergistic activity. Only in three cell lines (LA-N-6, CHLA-79, and CHLA-140) did 4-HPR + ABT-737 show only moderate or slight synergistic activity. There was no apparent association between sensitivity to single agents and synergy of the drugs in combination. ABT-737 and 4-HPR as single agents or in combination were minimally toxic for CRL-2076 fibroblasts at concentrations up to up to 5 μM and the combination showed only modest toxicity at 10 μM for each drug (Fig. 1D).
Figure 1. The combination ABT-737 plus 4-HPR is synergistically cytotoxic against NB cell lines but not normal fibroblasts cell line in vitro.
Dose response curves of NB cell lines treated with ABT-737 (●), 4-HPR (○), and the combination (▼) as measured by DIMSCAN. The concentrations applied for the cell lines were 1.25 to 10 μM for ABT-737 and/or 4-HPR (except for LA-N-6 and CHLA-79, from 2.5 to 10 μM). Each condition had 12 replicates, and error bars represent standard deviations. (A-C) Eleven tested NB cell lines were divided into three different groups according to therapy received by the patients prior to the line being established: (A) cell lines established prior any therapy (CHLA-15, SMS-KAN), (B) cell lines obtained at the time of progression disease during induction therapy (SMS-KCNR, SK-N-RA, CHLA-20, LA-N-6, SK-N-BE(2), CHLA-119, CHLA-140), (C) cell lines established at relapse after myeloablative therapy (CHLA-79 and CHLA-136). (D) The human normal fibroblast cell line CRL-2076.
Table 1.
IC90 of 4-HPR, ABT-737 and CIN values of ABT-737 at concentrations in combination with 4-HPR
| Group | Cell line | Phase of therapy | IC90 (μM)
|
CIN at each concentration (μM)
|
||||
|---|---|---|---|---|---|---|---|---|
| 4-HPR | ABT-737 | 1.25 | 2.5 | 5 | 10 | |||
| A. Prior to any therapy | CHLA-15 | DX | 3.7 | 0.5 | 0.486 | 0.349 | 0.416 | 0.001 |
| SMS-KAN | DX | 2.4 | 1.4 | 0.152 | 0.086 | 0.073 | 0.063 | |
|
| ||||||||
| B. At progressive disease during or after therapy | SMS-KCNR | PD | 2.3 | 1 | 0.342 | 0.414 | 0.321 | 0.382 |
| SK-N-RA | PD | 12.3 | >20 | 0.255 | 0.402 | 0.474 | 0.324 | |
| CHLA-20 | PD | 10.3 | >20 | 1.458 | 0.648 | 0.56 | 0.161 | |
| LA-N-6 | PD | 3.2 | 13.7 | - | 0.794 | 1.016 | 0.425 | |
| SK-N-BE(2) | PD | 2.2 | 12.3 | 0.827 | 0.543 | 0.16 | 0.058 | |
| CHLA-119 | PD* | 1.1 | 15.8 | 0.619 | 0.353 | 0.496 | 0.109 | |
| CHLA-140 | PD* | 2.2 | 0.4 | 0.521 | 0.922 | 0.785 | 0.86 | |
|
| ||||||||
| C. At relapse after myeloablative therapy and bone marrow transplantation | CHLA-79 | PD-BMT | 8.6 | >20 | - | 1.481 | 0.817 | 0.332 |
| CHLA-136 | PD-BMT | 11.3 | 2.9 | 0.237 | 0.218 | 0.209 | 0.238 | |
Lines in the panel included those established at diagnosis before therapy (DX), at time of progressive disease during or after non-myeloablative therapy (PD), after therapy with 13-cis-RA (PD*), after myeloablative therapy and bone marrow transplantation (PD-BMT).
A CIN less than 0.9 indicates synergism; 0.1, very strong synergism; 0.1 to 0.3, strong synergism; 0.3 to 0.7, good synergism; 0.7 to 0.85, moderate synergism; 0.85 to 0.9, slight synergism; 0.9 to 1.1, addictive; and more than 1.1, antagonism. - Indicates not applicable.
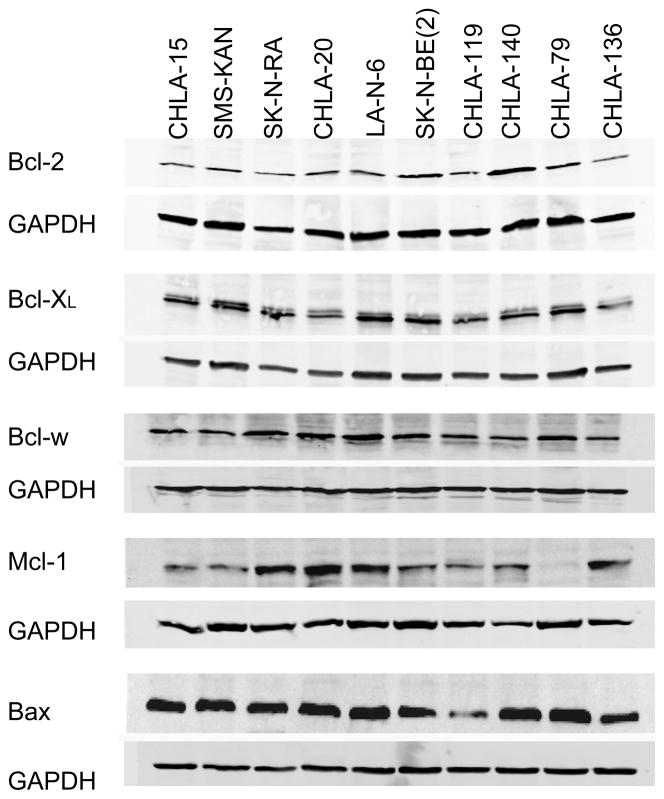
The basal levels of pro-apoptotic and anti-apoptotic proteins in 10 of these cell lines was examined by western blot analysis (Fig. 2). There was no apparent association between protein basal levels of Bcl-2, Bcl-XL, Bcl-w, or Mcl-1 and response to 4-HPR, ABT-737, or the two drugs in combination (Fig. 1).
Figure 2. The basal expression of Bcl-2 family members in NB cell lines.
(A) Western blot analysis of basal expression of Bcl-2 family proteins in ten NB cell lines. The data shown are representative of two independent experiments showing similar results.
The combination of ABT-737 and 4-HPR induced caspase-dependent apoptosis through mitochondrial membrane depolarization and cytochrome c release
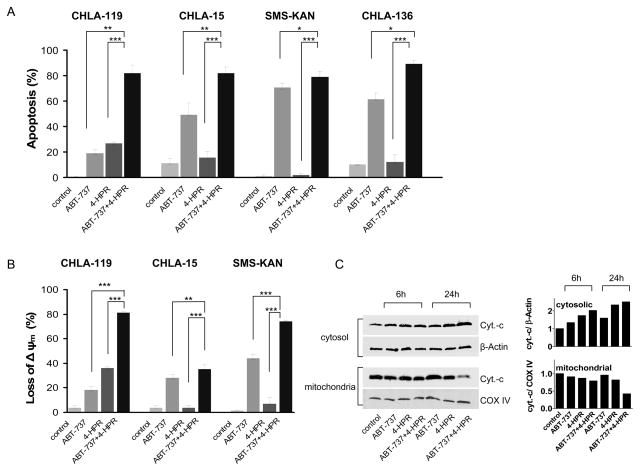
Because apoptosis is a major mechanism of action for both 4-HPR and ABT-737, we examined the effect of single reagents and their combination on apoptosis. In all four cell lines tested (CHLA-119, CHLA-15, SMS-KAN and CHLA-136), we observed a higher level of apoptosis when the two drugs were combined in comparison with each drug alone (Fig. 3A). For example, in CHLA-119 cells, the percentage of apoptotic cells at 24 hours was 0.6 ± 0.4% (control), 19.2 ± 2.9% in the presence of 2.5 μM ABT-737, 26.9 ± 0.9% in the presence of 2.5 μM 4-HPR, and 82.1 ± 6.0% in the presence of both drugs (P < 0.005 and P < 0.001 relative to ABT-737 and 4-HPR as single agents, respectively). By contrast, 4-HPR did not cause a significant increase of apoptosis in the other cell lines (CHLA-15, SMS-KAN, and CHLA-136), but the combination of 4-HPR and ABT-737 significantly increased apoptosis compared with ABT-737 alone. ABT-737 and 4-HPR (either alone or in combination) did not induce apoptosis in CRL-2076 normal fibroblasts (P > 0.05) (supplementary Fig. 1).
Figure 3. The combination of ABT-737 and 4-HPR induced caspase-dependent apoptosis through mitochondrial membrane depolarization and cytochrome c release.
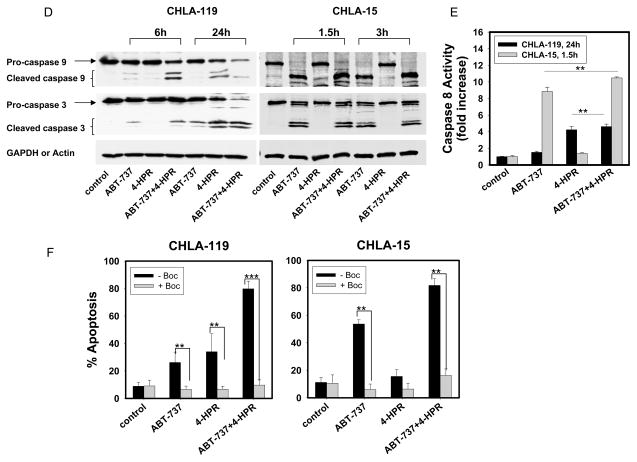
(A and B) CHLA-119, CHLA-15, SMS-KAN or CHLA-136 NB cells were treated with ABT-737, 4-HPR, or the combination for 24 hours (for CHLA-15, 3 hours). Drug concentrations used were 2.5 μM for CHLA-119 and CHLA 15, and 5 μM for SMS-KAN and CHLA-136. (A) Cells were then analyzed for apoptosis (TUNEL assay) by flow cytometry. Bars show the percentages of TUNEL-positive cells, defined as apoptotic. (B) CHLA-119, CHLA-15, and SMS-KAN cells were incubated with the JC-1 mitochondrial probe and analyzed by flow cytometry. Bars show the percentages of mitochondrial membrane-depolarized cells. (C) Cytostolic and mitochondrial extracts from CHLA-119 cells incubated for 6 or 24 hours with ABT-737, 4-HPR or the combination were prepared and immunoblotted with an anti-cytochrome c (cyt.-c) antibody. β-Actin and COX IV were used as the loading control for cytosolic and mitochondrial fractions, respectively. (D - F) CHLA-119 and CHLA-15 cells were incubated with ABT-737, 4-HPR, or the combination for 1.5, 3 hours (CHLA-15) or 6, 24 hours (CHLA-119). (D) Cell lysates were then examined by western blot analysis for caspase-9 and caspase-3. (E) Cell lysates from CHLA-119 (24h) and CHLA-15 (1.5h) were incubated with caspase-8 substrate using a colorimetric assay. Bars show the mean fold increase of casapse-8 activity. (F) CHLA-119 and CHLA-15 cells were pre-treated with Boc-d-fmk (40 μM) for 1 hour before being exposed to 2.5 μM ABT-737, 4-HPR, or the combination for 3 or 24 hours. After treatment, apoptotic cells were measured by flow cytometric TUNEL assay. Bars show the percentage of TUNEL-positive cells, defined as apoptotic. Data shown are representative of two independent experiments. In A, B, E, and F, data represent mean + standard deviations (SD) of triplicate samples. ** p < 0.005, *** p <0.001.
We next investigated the effect of 4-HPR and ABT-737 on the loss of mitochondrial membrane potential (Δψm) in CHLA-119, CHLA-15, and SMS-KAN cells. Treatment with ABT-737 and 4-HPR resulted in a greater than additive loss of Δψm when compared with the sum of the losses observed with each single agent (Fig. 3B). ABT-737 or 4-HPR alone, and especially the combination, caused release of cytochrome c from the mitochondria into the cytosol, and a decrease of cytochrome c in the mitochondrial fraction in CHLA-119 cells (Fig. 3C).
Caspase-9 is a key intermediate in the mitochondrial or intrinsic apoptotic pathway, and caspase-3 is the main final "effector caspase" inducing cell death. We therefore examined the effects of ABT-737, 4-HPR and their combination on the expression and activation of these two caspases (Fig. 3D). The data indicated that both caspase-9 and caspase-3 were cleaved and activated upon treatment with ABT-737 alone or in combination with 4-HPR. To determine whether the effect of 4-HPR and ABT-737 solely involved activation of the intrinsic pathway, we also examine their effect on the activation of caspase-8, the major caspase involved in the extrinsic apoptosis (Figure 3E). Caspase-8 activity was significantly higher in CHLA-119 and CHLA-15 cells treated with the 4-HPR + ABT-737 than in untreated cells or in cells treated with either single agent. These data suggest that ABT-737 plus 4-HPR induced both extrinsic and intrinsic apoptotic pathways.
To confirm that apoptosis induced by 4-HPR, ABT-737, or the combination was caspase-dependent, we pretreated the cells with a pan-caspase inhibitor Boc-d-fmk (40 μM) 1 hour before drug exposure and examined cells for apoptosis by TUNEL assay (Fig. 3F). In all cases (single drug and combination), Boc-d-fmk Boc-d-fmk returned the levels of apoptosis to levels observed in the absence of any drug (control), indicating that that the apoptotic effects of ABT-737, 4-HPR, and the combination were caspase-dependent.
The combination of ABT-737 and 4-HPR increased expression of members of the pro-apoptotic Bcl-2 family of proteins
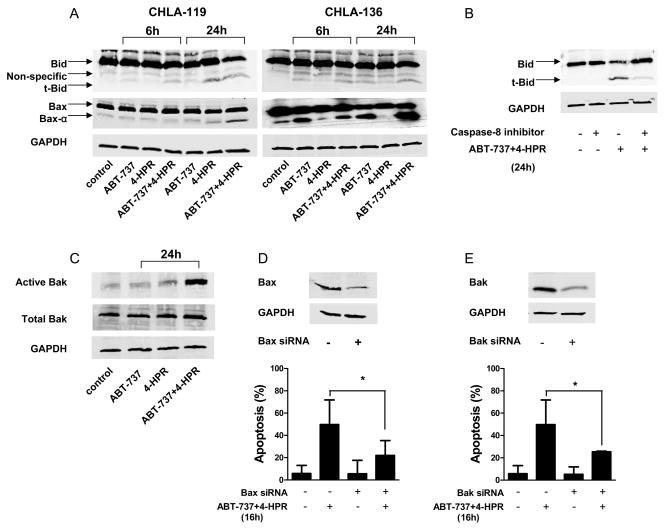
To further explore the mechanisms of apoptosis induced by ABT-737 and 4-HPR, we determined the effects of ABT-737, 4-HPR, and the combination on the expression of pro-apoptotic proteins Bid and Bax by western blot analysis (Fig. 4A). The activation of Bid was shown by the presence of the 15 kDa truncated form of Bid (t-Bid), and the activation of Bax by the presence of Bax-α (21 kDa), the most active form of Bax that plays a key role in cytochrome c release (14). In CHLA-136 cells (sensitive to ABT-737 and resistant to 4-HPR), ABT-737 activated Bid as indicated by the presence of t-Bid and Bax-α, and a greater effect was seen with the combination whereas 4-HPR alone had little effect. In CHLA-119 cells, which are relatively sensitive to 4-HPR but resistant to ABT-737, treatment with 4-HPR induced the truncation of Bid in atime-dependent manner, and a maximum activation was observed in the presence of 4-HPR + ABT-737. Bax activation occurred earlierin CHLA-136 than CHLA-119, perhaps due to differences intheir relative sensitivity to ABT-737 as asingle agent (Fig. 1).
Figure 4. Effects of ABT-737 plus 4-HPR on the activation of pro-apoptotic proteins.
CHLA-119 or CHLA-136 cells were incubated with ABT-737 or 4-HPR (2.5, 5 μM, respectively) or the combination for 6 or 24 hours. (A) Cell lysates were then examined for the presence of pro-apoptotic Bid and Bax. (B) CHLA-119 cells were pretreated with Z-IETD-FMK (20 μM) for 1 hour before being exposed to ABT-737+4-HPR (2.5 μM each) for 24 hours. Cell lysates were then examined for Bid cleavage into t-Bid by western blot analysis. (C) CHLA-119 cells were incubated with ABT-737 or 4-HPR (2.5 μM each) or the combination for 24 hours, and the presence of conformationally active Bak and total Bak were detected by western blot. (D and E) CHLA-119 cells were transfected with siRNA against Bax or Bak or control siRNA as indicated in materials and methods. Knockdown of Bax (D) and Bak (E) protein expression was assessed by western blot (upper panels). 48 hours after transfection, cells were treated with ABT-737+ 4-HPR (2.5 μM each) for another 16 hours and apoptosis was determined by flow cytometric TUNEL assay (lower panels). The bar graph shows the mean percentage of apoptotic cells (± SD) of triplicate samples. All the data shown are representative of two independent experiments showing similar results. * P < 0.05.
The cleavage of Bid into tBid further supported the contribution of the extrinsic apoptotic pathway to the apoptosis induced by 4-HPR and ABT-737 since Bid is a substrate for caspase-8 (39). To confirm this possibility, we tested whether the activation of Bid into tBid in cells treated with ABT-737 and 4-HPR could be prevented by the caspase-8 inhibitor Z-VAD-FMK (20 μM, 1 hour pre-exposure). As shown in Fig. 4B, Z-VAD-FMK markedly decreased Bid cleavage into tBid in CHLA-119 cells treated with 4-HPR and ABT-737.
In addition to Bax and Bid activation upon treatment with 4-HPR and ABT-737 (Fig. 4A), activation of another proapoptotic Bcl-2 family protein, Bak, was detected by western blot using an antibody which specifically recognizes activated Bak (Fig. 4C). To determine whether the activation of Bax and Bak is involved in the apoptosis induced by ABT-737 + 4-HPR, we knocked down Bax or Bak by siRNA and examined apoptosis by TUNEL assay induced by ABT-37 + 4-HPR compared to control siRNA. Knockdown of Bax (Fig. 4D) or Bak (Fig. 4-E) significantly decreased apoptosis induced in CHLA-119 cells by ABT-737 + 4-HPR.
Increased in vivo activity of ABT-737 and 4-HPR combination to recurrent NB xenografts of CHLA-119
To determine whether ABT-737 and 4-HPR would have a synergistic activity in vivo, we selected CHLA-119, a cell line derived from a patient whose disease progressed during intensive multiagent chemotherapy (26) and that is sensitive to 4-HPR but resistant to ABT-737 and for which strong synergism was observed in vitro (Table 1).
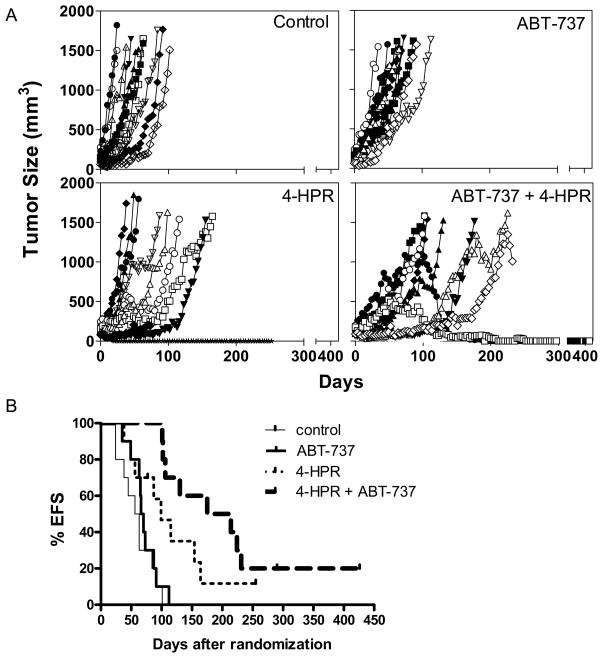
The tumor growth rate over time was recorded (Fig. 5A) and tumor volume T/C on day 21 was calculated to evaluate the capability of inhibiting tumor growth (Supplementary Table 1). We observed that ABT-737 only showed a low activity (tumor volume T/C = 66.8%), but both 4-HPR as a single agent and 4-HPR + ABT-737 showed intermediate activity with tumor volume T/C = 45.9 % and 30.9 % respectively, which was consistent with CHLA-119 cells being resistant to ABT-737 but sensitive to 4-HPR alone and the combination in vitro. Median EFS and EFS T/C values were also calculated to evaluate prolonged survival by the log-rank analysis (Fig. 5B and Supplementary Table 1) and the results were consistent with tumor growth rate analysis. Only 4-HPR (EFS T/C=1.7) and 4-HPR + ABT-737 (EFS T/C = 3.3) statistically increased EFS (P = 0.0002) relative to controls, whereas ABT-737 had no significant effect (EFS T/C = 1.1, P > 0.05). ABT-737 + 4-HPR also increased EFS relative to either single drug alone (P < 0.001).
Figure 5. In vivo antitumor activity of ABT-737 combined with 4-HPR against human neuroblastoma cells.
Athymic (nu/nu) mice carrying CHLA-119 NB subcutaneous xenografts were treated with vehicle control (thin lines), ABT-737 (bold lines), 4-HPR (thin dotted lines) or 4-HPR + ABT-737 (bold dotted lines). 4-HPR/LYM-X-SORB oral powder in water (240 mg/kg) was given by oral gavage five days/week. ABT-737 (100 mg/kg) was administered by i.p. injection 5 days/week. When used in combination, ABT-737 was administered at a dose of 50 mg/kg/day of ABT-737 (half of the dose used in single agent treatment). (A) Tumor volumes were measured twice per week and tumor volumes calculated as described in materials and methods. Each line represents tumor growth in a mouse. Mice were sacrificed when tumors reached 1,500 mm3. (B) The event-free-survival (EFS) of mice was calculated from the time of tumor injection to the time the tumor volume reached 1,500 m3 or the mice had to be sacrificed due to treatment-related toxicity. Each line represents the proportion of mice remaining event-free over time.
Discussion
The Bcl-2 family of proteins provides one mechanism by which malignant cells can survive various cytotoxic drugs and inhibition of Bcl-2 family anti-apoptotic proteins by drugs like ABT-737 is a promising approach for the treatment of cancer . ABT-737 is a small-molecule inhibitor of Bcl-2, Bcl-XL, and Bcl-w, that has been reported to be active against lymphoid cancers (19-23), and to have minimal systemic toxicity in animal preclinical models (18). A related orally bioavailable compound, ABT-263, is currently undergoing early Phase adult clinical trials (40, 41) . We have recently shown that it synergizes 4-HPR in lymphoid malignancies in vitro (24). 4-HPR is a well-tolerated drug that has shown activity against recurrent NB in the laboratory (6, 7) and in patients (10, 11). Here we showed that ABT-737 is active against most NB cell lines in vitro and has a synergistic anti-tumor effect when combined with 4-HPR at a concentration range that is active and tolerable in mouse xenograft models (18). Concentrations of 4-HPR used in this study were within the range achieved in children in clinical trials (10) with little hematopoietic toxicity observed.
In most of the NB cell lines and drug concentrations tested in this study, we found that 4-HPR alone had a modest effect on apoptosis, whereas ABT-737 alone readily induced apoptosis in sensitive cell lines. In some cell lines, like CHLA-119, the cells were found relatively resistant to ABT-737. However, despite of this resistance, in all cell lines the combination of the two drugs was synergistic in vitro. We also found that ABT-737 enhanced the activity of 4-HPR for CHLA-119 in vivo. Interestingly, we observed that the combination of these two agents had minimal toxic effect on normal human fibroblasts, which is consistent with similar observations made in normal resting lymphocytes (24), and suggests that the combination should have minimal toxicity for non-neoplastic cells in vivo.
The combination of 4-HPR and ABT-737 induced the loss of ΔΨm and the release of cytochrome c to the cytoplasm (Fig. 3B and C), indicating that this drug combination acts via the mitochondrial death pathway. Activation of caspase-9 and caspase-3 by ABT-737 in the presence or absence of 4-HPR further confirmed that ABT-737 acts via the mitochondria-dependent apoptotic pathway (Fig. 3D). In addition, the observation that caspase-8 was activated by these two agents (Fig. 3E), and that ABT-737 + 4-HPR-induced cleavage of Bid into tBid was inhibited by a caspase-8 inhibitor, indicate the involvement of the extrinsic apoptotic pathway as well (Fig. 4B). Furthermore, the majority of apoptosis induced by ABT-737 alone and by the combination of ABT-737 + 4-HPR occurred largely via caspase-dependent pathways as indicated by the fact that the pancaspase inhibitor Bok-d-fmk inhibited the effect of ABT-737 and 4-HPR on apoptosis (Fig. 3B).
The mitochondrial apoptotic pathway is controlled by a balance between the pro-apoptotic protein members (i.e. the multi-domain pro-apoptotic Bax, Bak, and BH-3 only pro-apoptotic Bid, Bim, Bad, Bik, Noxa, Puma, Bmf, Hrk) and anti-apoptotic protein members (i.e. the multi-domain anti-apoptotic Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl/A1) of the Bcl-2 family. Those multi-domain Bcl-2 proteins are functionally regulated by the BH-3 only proteins (42, 43). Bid is localized in the cytosolic fraction of cells as an inactive precursor (44) and truncated Bid (tBid), the active form of Bid, is generated upon proteolytic cleavage by caspase-8 (45, 46).
We showed that ABT-737 together with 4-HPR increased the release of sequestered tBid from Bcl-2 and Bcl-XL, and the induction of Bax and Bak oligomerization (Fig. 4). The observation that Bid was cleaved into tBid in cells treated with ABT-737 alone or in combination with 4-HPR is similar to the previously reported activity of ABT-737 on leukemic and human pancreatic cancer cells (20, 21). The fact that Bax or Bak knockdown significantly impaired the apoptosis induced by ABT-737 + 4-HPR further confirms the importance of Bax and/or Bak activation in the synergistic effect observed with ABT-737 and 4-HPR. Another anti-apoptotic Bcl-1 family protein Mcl-1 has been shown to be overexpressed in cells resistant to ABT-737 (22, 47-50) and a recent study in ALL demonstrated that the combination of ABT-737 and 4-HPR was associated with Mcl-1 inactivation by 4-HPR (24). However, ABT-737 + 4-HPR did not alter Mcl-1 levels in NB cells (supplementary Fig. 2).
We extended our in vitro cytotxicity studies by showing that 4-HPR and ABT-737 enhanced EFS when compared to either drug alone in CHLA-119 tumor-bearing mice, a mouse xenograft model of recurrent, TP53-mutated, p53-non-functional, multidrug-resistant NB (Fig. 5). Thus, we have demonstrated a positive synergistic interaction between 4-HPR and the BH3 mimetic agent ABT-737 in NB cell lines and consistent with the in vitro activity we showed anti-NB activity of this novel combination in a NB mouse xenograft model. These data support clinical trials combining 4-HPR with BH3 mimetic drugs in children with recurrent NB.
Supplementary Material
Supplementary Figure 1. The combination ABT-737 plus 4-HPR is not cytotoxic against normal fibroblast cells in vitro.
CRL-2076 fibroblasts were incubated with ABT-737 (5 μM), 4-HPR (5 μM) or the combination for 24 hours. Apoptosis was measured by TUNEL and flow cytometry. The bar graph shows the percentage of apoptotic cells and the values represent means (± SD) of triplicate samples.
Supplementary Figure 2. Effects of ABT-737 plus 4-HPR on Mcl-1. CHLA-119 cells were incubated with ABT-737 or 4-HPR (2.5 μM) or the combination for 6 or 24 hours. Cell lysates were then examined for the presence of Mcl-1.
Translational Relevance.
Fenretinide (4-HPR) is a synthetic retinoid that has broad-spectrum in vitro cytotoxicity against various cancers and clinical activity against recurrent high-risk neuroblastoma. Cell lines established from recurrent neuroblastoma patients exhibit increased levels of Bcl-2 expression which may confer drug resistance. We determined if ABT-737, a BH3 mimetic small-molecule inhibitor of Bcl-2, Bcl-XL, and Bcl-w, could enhance activity of 4-HPR against laboratory models of neuroblastoma. We demonstrate a positive synergistic interaction between 4-HPR and ABT-737 in neuroblastoma cell lines through their inhibitory activity against caspase-dependent apoptosis involving both intrinsic and extrinsic pathways. Consistent with their synergistic in vitro activity, we show a similar anti-neuroblastoma activity in a mouse xenograft model of recurrent, multidrug-resistant neuroblastoma. These data support clinical trials combining 4-HPR with BH3 mimetic drugs in children with recurrent neuroblastoma.
Acknowledgments
Supported by National Cancer Institute grants CA81403 (C.P.R.), CA82830 (C.P.R.), CA129377 (Y.A.D), and also by Cancer Prevention & Research Institute of Texas grant RP10072 (C.P.R). H.F was supported by The Saban Research Institute of CHLA as a recipient of Pre-doctoral Award. The authors thank Abbott Laboratories for providing ABT-737. 4-HPR LXS was kindly provided Dr. B J Maurer and was produced via a NCI Rapid Access to Intervention Discovery (RAID) grant.
Footnotes
Authors' contributions
H.F., T.M.H., Y.A.D., and C.P.R. designed the research and analyzed the data; H.F., T.M.H., O.K., and V.M. performed the research and analyzed the data. H.F. wrote the paper; Y.A.D. and C.P.R. edited the manuscript.
Reference List
- 1.Maris JM, Matthay KK. Molecular biology of neuroblastoma. Journal of Clinical Oncology. 1999;17:2264–79. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 2.Matthay K, Villablanca JG, Seeger RC, Stram DO, Harris R, Ramsay NK, et al. Treatment of high risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol. 2009;27:1007–13. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New England Journal of Medicine. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. Ca-A Cancer Journal for Clinicians. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Divinci A, Geido E, Infusini E, Giaretti W. Neuroblastoma cell apoptosis induced by the synthetic retinoid N-(4-hydroxyphenyl)retinamide. International Journal of Cancer. 1994;59:422–6. doi: 10.1002/ijc.2910590322. [DOI] [PubMed] [Google Scholar]
- 7.Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. Journal of the National Cancer Institute. 1999;91:1138–46. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 8.Ponzoni M, Bocca P, Chiesa V, Decensi A, Pistoia V, Raffaghello L, et al. Differential-effects of N-(4-hydroxyphenyl)retinamide and retinoic acid on neuroblastoma-cells - apoptosis versus differentiation. Cancer Research. 1995;55:853–61. [PubMed] [Google Scholar]
- 9.Reynolds CP, Wang Y, Melton LJ, Einhorn PA, Slamon DJ, Maurer BJ. Retinoic-acid-resistant neuroblastoma cell lines show altered MYC regulation and high sensitivity to fenretinide. Medical & Pediatric Oncology. 2000;35:597–602. doi: 10.1002/1096-911x(20001201)35:6<597::aid-mpo23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds PC. Phase I trial of oral fenretinide in children with high-risk solid tumors: A report from the Children's Oncology Group (CCG 09709) Journal of Clinical Oncology. 2006;24:3423–30. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- 11.Marachelian A, Kang MH, Hwang K, Villablanca JG, Groshen S, Matthay KK, et al. Phase I study of fenretinide (4-HPR) oral powder in patients with recurrent or resistant neuroblastoma: New Approaches to Neuroblastoma Therapy (NANT) Consortium trial. Journal of Clinical Oncology. 2009:27. [Google Scholar]
- 12.Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, et al. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clinical Cancer Research. 2003;9:2032–9. [PubMed] [Google Scholar]
- 13.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003 Jul 18;197:185–92. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 14.Kang MH, Reynolds CP. Bcl-2 Inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clinical Cancer Research. 2009;15:1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nature Reviews Drug Discovery. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 16.Lasorella A, Iavarone A, Israel MA. Differentiation of neuroblastoma enhances Bcl-2 expression and induces alterations of apoptosis and drug-resistance. Cancer Res. 1995;55:4711–6. [PubMed] [Google Scholar]
- 17.Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius VM, Niskanen E, et al. Reduced expression of proapoptotic gene Bax Is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995;55:4471–8. [PubMed] [Google Scholar]
- 18.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. A novel Bcl-2/Bcl-X-L/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26:2374–80. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 20.Huang SB, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering bim and bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–51. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang MH, Kang YH, Szymanska B, Wilczynska-Kalak U, Sheard MA, Harned TM, et al. Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood. 2007;110:2057–66. doi: 10.1182/blood-2007-03-080325. [DOI] [PubMed] [Google Scholar]
- 22.Tahir SK, Yang XF, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 23.Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB, et al. The Bcl-2/Bcl-X-L family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clinical Cancer Research. 2007;13:7191–8. doi: 10.1158/1078-0432.CCR-07-0362. [DOI] [PubMed] [Google Scholar]
- 24.Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. Journal of the National Cancer Institute. 2008;100:580–95. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- 25.Keshelava N, Seeger RC, Groshen S, Reynolds CP. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58:5396–405. [PubMed] [Google Scholar]
- 26.Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–93. [PubMed] [Google Scholar]
- 27.Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A. 2001;98:8012–7. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds CP, Biedler JL, Spengler BA, Reynolds DA, Ross RA, Frenkel EP, et al. Characterization of human neuroblastoma cell lines established before and after therapy. J Natl Cancer Inst. 1986;76:375–87. [PubMed] [Google Scholar]
- 29.Maurer BJ, Kalous O, Yesair DW, Wu X, Janeba J, Maldonado V, et al. Improved oral delivery of N-(4-hydroxyphenyl)retinamide with a novel LYM-X-SORB organized lipid complex. Clin Cancer Res. 2007;13:3079–86. doi: 10.1158/1078-0432.CCR-06-1889. [DOI] [PubMed] [Google Scholar]
- 30.Frgala T, Kalous O, Proffitt RT, Reynolds CP. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Molecular Cancer Therapeutics. 2007;6:886–97. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 31.Keshelava N, Frgala T, Krejsa J, Kalous O, Reynolds CP. DIMSCAN: a microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy. Methods Mol Med. 2005;110:139–53. doi: 10.1385/1-59259-869-2:139. [DOI] [PubMed] [Google Scholar]
- 32.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–9. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 33.Mancini M, Anderson BO, Caldwell E, Sedghinasab M, Paty PB, Hockenbery DM. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. Journal of Cell Biology. 1997;138:449–69. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz GD, Li QJ, Dashwood RH. Caspase-8 and apoptosis-inducing factor mediate a cytochrome c-independent pathway of apoptosis in human colon cancer cells induced by the dietary phytochemical chlorophyllin. Cancer Res. 2003;63:1254–61. [PubMed] [Google Scholar]
- 35.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemotherapy and Pharmacology. 1989;24:148–54. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 36.Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with 2 or more mutually exclusive and non-exclusive inhibitors. European Journal of Biochemistry. 1981;115:207–16. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 37.Chou TC, Talalay P. Quantitative-analysis of dose-effect relationships - the combined effects of multiple-drugs or enzyme-Inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 38.Houghton PJ, Maris JM, Friedman HS, Keir ST, Lock RB, Gorlick R, et al. Pediatric preclinical testing program (PPTP) evaluation of the KSP inhibitor ispinesib (SB-715992) Ejc Supplements. 2006;4:98. [Google Scholar]
- 39.Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813:558–63. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi L, Camidge DR, Ribeiro de OM, Bonomi P, Gandara D, Khaira D, et al. Phase I Study of Navitoclax (ABT-263), a Novel Bcl-2 Family Inhibitor, in Patients With Small-Cell Lung Cancer and Other Solid Tumors. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–59. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 43.Letai A. BCL-2: found bound and drugged! Trends in Molecular Medicine. 2005;11:442–4. doi: 10.1016/j.molmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Luo X, Budihardjo I, Zou H, Slaughter C, Wang XD. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 45.Gross A, Yin XM, Wang K, Wei MC, Jockel J, Millman C, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-X-L prevents this release but not tumor necrosis factor-R1/Fas death. Journal of Biological Chemistry. 1999;274:1156–63. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 46.Yin XM, Wang K, Gross A, Zhao YG, Zinkel S, Klocke B, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–91. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing bak activation and bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 48.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, et al. 'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-X-L inhibitor ABT-737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 49.Moore VD, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. Journal of Clinical Investigation. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wesarg E, Hoffarth S, Wiewrodt R, Kroell M, Biesterfeld S, Huber C, et al. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. International Journal of Cancer. 2007;121:2387–94. doi: 10.1002/ijc.22977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The combination ABT-737 plus 4-HPR is not cytotoxic against normal fibroblast cells in vitro.
CRL-2076 fibroblasts were incubated with ABT-737 (5 μM), 4-HPR (5 μM) or the combination for 24 hours. Apoptosis was measured by TUNEL and flow cytometry. The bar graph shows the percentage of apoptotic cells and the values represent means (± SD) of triplicate samples.
Supplementary Figure 2. Effects of ABT-737 plus 4-HPR on Mcl-1. CHLA-119 cells were incubated with ABT-737 or 4-HPR (2.5 μM) or the combination for 6 or 24 hours. Cell lysates were then examined for the presence of Mcl-1.