Abstract
Background: With the aging of the population, primary care physicians are increasingly expected to manage patients with Alzheimer's disease. For patients with this disease to obtain the best outcomes over the long term, early diagnosis and effective treatment are critical. Currently, cholinesterase inhibitors are the only drugs approved in the United States for the treatment of mild-to-moderate Alzheimer's disease.
Method: Relevant clinical studies were identified through a search of the MEDLINE database using the terms Alzheimer's disease and donepezil, galantamine, or rivastigmine, using the limits of English language and publication dates of 1995 to 2003. Published studies were selected that provided information on the long-term use, defined as 1 year or longer, of second-generation cholinesterase inhibitors approved by the U.S. Food and Drug Administration for the treatment of mild-to-moderate Alzheimer's disease.
Results: 590 references were identified. Of these, 11 studies met the final study criteria, and 10 were selected (1 of the 11 was an interim analysis).
Conclusions: The benefits of sustained long-term treatment with cholinesterase inhibitors have been demonstrated over the last several years. By reducing cognitive and functional decline over time, long-term cholinesterase inhibitor therapy enables patients to stay at home longer and may decrease the burdens faced by patients, caregivers, and society.
As the population of America ages, primary care physicians will be faced with diagnosing, treating, and managing more patients with dementia than in previous decades. The number of patients with Alzheimer's disease in the United States is projected to reach 14 million by the year 2050.1 Alzheimer's disease is marked by its insidious onset and unrelenting cognitive and functional decline, with difficult behaviors frequently becoming problematic as the disease progresses. A chronic, debilitating condition, Alzheimer's disease produces a significant burden on patients, caregivers, and society.
Although the etiology of Alzheimer's disease remains uncertain, the well-accepted cholinergic hypothesis postulates that a deficiency in acetylcholine (ACh) levels in the cerebral cortex contributes to the cognitive, and possibly behavioral, disturbances associated with the disease.2 Cholinesterase (ChE) inhibitors are the only drug class approved by the U.S. Food and Drug Administration (FDA) for the treatment of mild-to-moderate Alzheimer's disease. The efficacy of the ChE inhibitors is attributed to inhibition of the enzyme responsible for ACh hydrolysis, acetylcholinesterase, and the resultant increase in ACh levels in neuronal synapses of the central nervous system.
Prior to the approval of ChE inhibitors for the treatment of mild-to-moderate Alzheimer's disease, patient care management approaches and psychiatric drugs for behavioral disturbances were the only available treatment options. In 1993, tacrine was the first drug approved by the FDA for the treatment of symptoms of mild-to-moderate Alzheimer's disease. Its 4-times-daily dosing schedule and hepatotoxicity have led to its infrequent use today in favor of the second-generation ChE inhibitors. Donepezil was approved in 1997, followed by rivastigmine in 2000 and galantamine in 2001. These second-generation ChE inhibitors have the benefit of less frequent dosing and a better safety profile than tacrine. Although not a cure for Alzheimer's disease, the ChE inhibitors are the first drugs with demonstrated benefits in slowing the decline in cognition and global functioning in patients. In the 2001 guidelines published by the American Association of Neurology for the treatment of mild-to-moderate Alzheimer's disease, the use of ChE inhibitors was supported as standard therapy, and vitamin E (1000 IU p.o. b.i.d.) was considered an option; the use of other agents was not supported.3 Nonpharmacologic approaches for managing patients with dementia were also evaluated; evidence supported the use of behavior modification and graded assistance to enhance functional performance, interventions such as music and light exercise for behavioral problems, and education and support for caregivers.3
For many families, the goal of treatment is to keep their loved ones at home for as long as possible, despite the hardships of caregiving.4 When cognition is maintained, patients can remember family member names and engage in conversation; when functioning is preserved, patients are able to help care for themselves; and when behavioral problems are minimized, the stress of coping with behaviors is lessened. These symptomatic benefits translate into reducing the burden on caregivers.5,6 Ultimately, such treatment benefits may delay patient nursing home placement, which often occurs when functional and behavioral problems become overwhelming.7 Caregivers view this delay as preserving the patient's quality of life.4
In clinical trials, assessment scales are used to monitor drug effects. Cognitive functioning is typically assessed with the Alzheimer's Disease Assessment Scale–cognitive subscale (ADAS-cog) and the Mini-Mental State Examination (MMSE), while functioning is assessed with a variety of measurements of basic (eating, toileting) and instrumental (shopping, preparing food) activities of daily living, and behavior is assessed with the Neuropsychiatric Inventory (NPI). Ultimately, the goal of delaying nursing home placement can be measured only by studies that assess the outcome of patients receiving treatment versus those receiving no active drug treatment. To date, few studies have assessed this outcome.8–10
In short-term (≤ 6 months), double-blind, placebo-controlled clinical trials, the ChE inhibitors have been shown to benefit the cognitive,11–14 functional,12–14 and behavioral14,15 symptoms associated with Alzheimer's disease. However, successful management of the disease requires treating patients over the long term. Although Alzheimer's disease remains incurable, long-term ChE inhibitor treatment can provide symptomatic relief by attenuating or delaying the inevitable decline, thereby conferring meaningful benefits on patients and their families (e.g., reducing caregiver burden and disturbing behaviors, delaying nursing home placement). Positive treatment outcomes in Alzheimer's disease can be defined as improvement, stabilization, or less-than-expected decline in cognitive, functional, and behavioral symptoms. Emerging data illustrate the value and effectiveness of long-term ChE inhibitor therapies and suggest their utility in treating Alzheimer's disease in the primary care setting. By understanding the implications of the data reported from trials lasting 1 year or more, physicians will be better able to educate families about the long-term benefits of managing this devastating disease.
METHOD
A MEDLINE search was conducted using the terms Alzheimer's disease and donepezil, galantamine, or rivastigmine, with the limits of English language and publication dates of 1995 to 2003. Selected studies met the criteria of being at least 1 year in duration and being large (at least 100 participants) clinical studies for the treatment of mild-to-moderate Alzheimer's disease with cognitive or functional primary efficacy measures.
RESULTS
Five hundred ninety citations were returned. Of these, 11 met all the study selection criteria, and 10 were selected (1 of the 11 was an interim analysis).
Long-Term Efficacy of the ChE Inhibitors
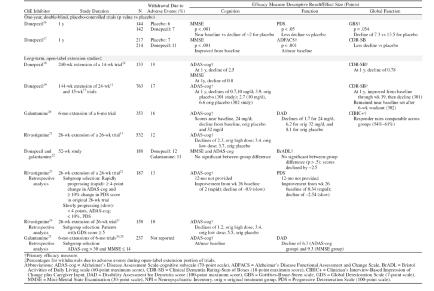
Of the 10 studies identified (Table 1), 7 report findings from the full study cohort. Two were double-blind, placebo-controlled studies,16,17 and the rest included open-label extension studies of short-term, double-blind, placebo-controlled trials18–21 and an open-label comparative study.22 Because large-scale clinical investigations of donepezil have been underway longer than those for rivastigmine or galantamine, there are more extensive published clinical trial data for this drug. Although retrospective analyses have been published for some approved ChE inhibitors,23–25 prospective studies provide the most rigorous approach to understanding long-term drug effects.
Table 1.
Long-Term Clinical Trials for the Treatment of Alzheimer's Disease With Cholinesterase Inhibitors
In the 2 double-blind, placebo-controlled clinical studies,16,17 long-term donepezil treatment was shown to attenuate cognitive and functional decline. In the first 1-year study,16 286 patients were enrolled in a double-blind, placebo-controlled study of donepezil. Cognition, as measured by the MMSE, showed no significant decline in donepezil-treated patients over the study period, while patients receiving placebo declined approximately 2 points (p < .001). Similarly, activities of daily living (ADL), as measured by the Progressive Deterioration Scale, were significantly better with donepezil treatment than placebo. Scores on a comprehensive global assessment scale, the Gottfries-Brane-Steen scale (GBS), showed that donepezil-treated patients deteriorated approximately half as much as placebo-treated patients (7.3 ± 2.1 vs. 13.5 ± 2.1 GBS units ± SD).16 Additionally, 431 patients were randomly assigned to donepezil or placebo in a second double-blind 1-year study17 designed to assess drug effects on functional decline (assessed with the Alzheimer's Disease Functional Assessment and Change Scale [ADFACS]). Among donepezil-treated patients, 51% showed no clinically evident decline in ADL, while 35% of placebo-treated patients showed no decline. The authors concluded that there was a 38% risk reduction for decline among donepezil-treated patients, and that patient functioning was maintained 72% longer in donepezil-treated patients than placebo-treated patients.17
Through October 2003, 1-year placebo-controlled studies have not been reported for the other available ChE inhibitors. However, in a 52-week open-label study of rivastigmine, treatment improved ADAS-cog scores compared with scores projected for placebo.21 Cognition and daily functioning were maintained at or near baseline for up to 52 weeks in an open-label study of galantamine.20 In a year-long open-label study comparing the efficacy of galantamine and donepezil, no significant between-group differences were reported on the primary outcome of function or the secondary outcomes of cognition and behavior.22 At 52 weeks, scores on functional and cognitive assessments declined from baseline. However, in long-term extension studies of donepezil, ADAS-cog scores were maintained at or above baseline for 51 weeks19 and decline in cognition and global functioning was attenuated for up to 4.9 years.18
Treatment effects cannot be directly compared across trials. Furthermore, the disparate characteristics of the studies and the complexity within extension studies further preclude comparison. For instance, an open-label extension study of donepezil19 included patients from 2 trials with differing designs: study 301 included patients receiving 12 weeks of treatment and 3 weeks of washout,27 while study 302 consisted of 24 weeks of treatment followed by 6 weeks of washout.11 Similarly, during open-label extension studies, patients who had received placebo or low-dose or high-dose ChE inhibitors all received active drug during the open-label extension. Due to this stratification, many studies analyze effects based on patients' original drug/placebo assignment. In addition, retrospective analyses defined patient populations based on a variety of intents, including the desire to examine drug effects on patients who deteriorated at fast versus slow rates23 or patients with more advanced moderate disease.24,25 Despite these limitations, the descriptive analyses and treatment effects provided in Table 1 demonstrate that ChE inhibitor treatment provides benefit, generally delaying decline or maintaining patients near baseline at 1 year of treatment. It is important to note that in untreated patients with Alzheimer's disease, a decline of 9 to 11 points on the ADAS-cog29 or 2 to 4 points on the MMSE30 is expected per year. In studies reporting treatment effects on these measures, substantially less decline is reported.
Optimizing Long-Term Therapy Benefits
By appreciating the issues involved in long-term management of Alzheimer's disease, physicians will be better positioned to provide the best possible treatment strategies for their patients. Long-term care of patients with Alzheimer's disease involves managing the symptomatic dimensions of the disease, treating comorbidities that may be masked by dementia,31 ensuring effective therapy, and providing care for the caregiver (Table 2).
Table 2.
Managing Patients With Alzheimer's Disease in Primary Care: Long-Term Considerations
Early diagnosis and treatment.
In the primary care setting, nearly half of dementia patients go undiagnosed, and the diagnosis is frequently delayed for as much as 2 years following the first appearance of symptoms.32 However, with the diagnosis of Alzheimer's disease facilitated through the use of accurate and readily applied criteria, primary care physicians are increasingly able to offer patients therapy early in their disease course. Data from open-label extension studies highlight the importance of early therapy. Results from studies with donepezil, rivastigmine, and galantamine show that patients receiving placebo for 3 months, 6 months, or 26 weeks (respectively) prior to starting ChE inhibitor treatment failed to achieve the same level of cognitive functioning as patients who received drug treatment throughout the trials.19–21 These data argue strongly for early diagnosis, which allows early initiation of therapy and the opportunity for patients to maintain the highest possible levels of cognitive and functional ability. Because drug treatment benefits are diminished when therapy is delayed,19–21 it is also important for patients to receive an optimally effective dose as early as possible following initiation of therapy. Dosing titration to reach effective doses raises the possibility that patients will sustain functional decline while the ChE inhibitor dose is titrated upward, especially if titration is delayed or overlooked.
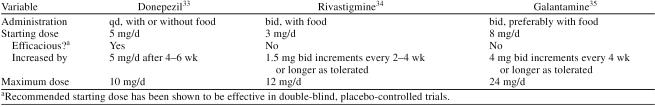
Recommended dosing initiation schemes for donepezil, rivastigmine, and galantamine are illustrated in Table 3. The literature provides very little additional guidance on dose selection, but the pivotal trials data suggest that, since a higher proportion of patients respond to higher doses of ChE inhibitors, all patients should be titrated to the highest dose they can tolerate well.11–14,20,21
Table 3.
Cholinesterase Inhibitor Dosing Information
Continuous treatment.
An open-label extension study of donepezil19 illustrates the importance of sustained long-term treatment. In this study, long-term cognitive and functional benefits were realized with uninterrupted donepezil treatment; however, a 6-week interruption of therapy resulted in a complete loss of treatment benefits. These benefits were not recovered when medication was restarted.
Safety and tolerability.
Since clinical trials suggest that optimal treatment benefit occurs with early and continuous ChE inhibitor treatment, it is important that therapies are safe and well tolerated in frail patients with Alzheimer's disease over the long term. Additionally, agents that are easy to use over both the short term and the long term are optimal from the perspective of both the patient and the prescriber.
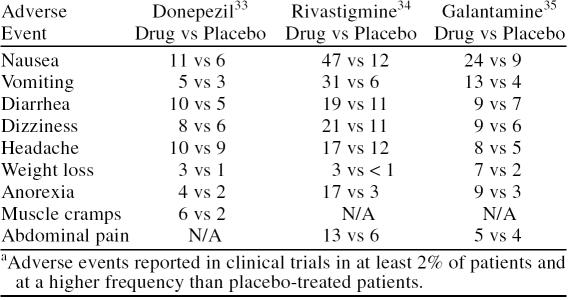
Adverse events associated with ChE inhibitors are typically transient, mild, and gastrointestinal in nature (Table 4). The most frequent incidence of adverse events occurs during therapy initiation and titration to higher doses. To minimize the incidence of adverse events, administration instructions, which stipulate the need for food coadministration and time of day, should be followed (Table 3). Slow dose-titration schedules (i.e., at least 4 weeks between dose titration steps) have also been reported to reduce the incidence of adverse events. Antiemetics may be useful in helping alleviate nausea and vomiting.36
Table 4.
Adverse Events Associated With Cholinesterase Inhibitorsa
Results from long-term open-label extensions of double-blind trials show that donepezil, rivastigmine, and galantamine are generally well tolerated, with a low incidence of discontinuation due to adverse events in the open-label phases (Table 1).19–21 In the double-blind, placebo-controlled portions of the open-label extension studies, forced dose titration led to high discontinuation rates in some trials. However, the lower incidence during the long-term portion of the trials reflects the observation that the incidence of adverse events decreased once the maintenance phase was established. Furthermore, in a 1-year, double-blind, placebo-controlled study of donepezil,16 adverse events led to the withdrawal of only 7% of donepezil patients compared with 6% of placebo patients. Results from a 12-week open-label trial of donepezil and rivastigmine37 showed that while both drugs were similarly efficacious, donepezil patients were much less likely to discontinue therapy because of an adverse event (donepezil, 11%; rivastigmine, 22%). In the 52-week open-label study of galantamine and donepezil, discontinuations were similar (Table 1), but the rates of adverse events were higher in galantamine-treated than in donepezil-treated patients.22
The mean age of patient cohorts in ChE inhibitor clinical trials has typically been in the 70s. However, as the general population ages, more patients with Alzheimer's disease will be reaching their ninth decade and beyond (the so-called “oldest old”), and these patients present with a high level of comorbidities and concomitant medication usage. In a 6-month double-blind study of very elderly patients with Alzheimer's disease residing in nursing homes (mean age, 85.7 years) with a high prevalence of comorbidities and concomitant medication usage, donepezil was shown to be generally safe and well tolerated.38 Study discontinuation due to adverse events occurred in 11% of donepezil-treated patients compared with 18% of placebo-treated patients. This study represents the first double-blind placebo-controlled trial demonstrating the safety and tolerability of a ChE inhibitor in a population specifically selected to include more elderly and frail patients with Alzheimer's disease.
Weight loss is a critical issue in older patients with Alzheimer's disease and has been indicated as a predictor of mortality.39 Clinically significant weight loss (≥ 7% of body weight at baseline) has been observed with galantamine (11% galantamine vs. 4% placebo)14 and rivastigmine treatment (21% rivastigmine vs. 2% placebo).13 The incidence of clinically significant weight loss was similar between donepezil- and placebo-treated patients in trials conducted in elderly nursing home patients with Alzheimer's disease (9% donepezil vs. 6% placebo)38 and in moderate-to-severe Alzheimer's disease (7% donepezil vs. 8% placebo).15
Disease stages and settings.
As the disease progresses and patients with Alzheimer's disease require increasingly more assistance, settings of care that are needed typically advance from community dwelling to assisted living to nursing home facilities. While some physicians may cease ChE inhibitor treatment of patients at admission to nursing homes, it is important to recognize that sustained treatment may continue to provide benefit to those with more advanced Alzheimer's disease. Indeed, treatment efficacy has been demonstrated in a clinical trial in institutionalized patients with Alzheimer's disease, with significant drug-placebo differences favoring donepezil in the measures of cognition (MMSE) and overall dementia severity (Clinical Dementia Rating-Sum of Boxes [CDR-SB]).38 Furthermore, significant improvements in cognition (MMSE), function (Disability Assessment for Dementia score [DAD]), behavior (NPI), and global functioning (Clinician's Interview-Based Impression of Change plus Caregiver Input [CIBIC+]) have been demonstrated for donepezil versus placebo treatment in more severely impaired patients with Alzheimer's disease.15 As of October 2003, no double-blind placebo-controlled trials had been published regarding the efficacy or tolerability of rivastigmine or galantamine in nursing home settings.
Treatment expectations.
Physicians, patients, and caregivers must have reasonable expectations of the long-term benefits of ChE inhibitor treatment. Since curative or restorative therapies are not yet available for Alzheimer's disease, caregivers should receive information on the value of delaying symptomatic decline in the face of the progressive degeneration characteristic of the disease. It is therefore important to convey to patients and their caregivers the spectrum of likely outcomes and reinforce the idea that attenuating or delaying decline provides meaningful benefits. Realistic expectations are especially crucial since patients and their families will make decisions to continue treatment based on whether they perceive benefits.
The Value of Long-Term ChE Inhibitor Treatment
A growing number of long-term, and numerous short-term, clinical trials show that symptomatic benefits of ChE inhibitor treatment translate into clinically meaningful outcomes as determined using measures of global functioning. Improvements in global functioning may provide valuable benefits to both patients and their caregivers.
Keeping patients at home.
For many caregivers, a primary goal of treatment is keeping their loved ones at home in a familiar environment for as long as possible. By improving cognitive and functional abilities, long-term ChE inhibitor treatment may allow patients to retain a greater degree of independence, improve feelings of self-worth, and allow patients to remain at home longer than might be possible without therapy. A retrospective study of clinical trials8 showed that, after 2 years, patients treated with high doses (> 80 mg/day) of tacrine were 2.8 times less likely to be placed in nursing homes than patients treated with low doses (≤ 80 mg/day) of tacrine. In a long-term follow-up study9 of 135 patients with Alzheimer's disease treated with ChE inhibitors (96% donepezil, 2% rivastigmine, 2% tacrine) and a matched group of patients not treated with ChE inhibitors, patients in the treatment group were one third as likely to be placed in nursing homes as patients not receiving treatment.
In an unrelated study,40 donepezil treatment (≥ 5 mg/day, 9–12 months) was associated with delays of 21.4 months in first nursing home placement for dementia-related reasons compared with minimal or no donepezil exposure.
As the disease progresses, the associated declines in cognition and functioning and difficulties associated with managing behavioral disturbances frequently force patients into long-term care facilities. Improving behavioral symptoms may allow patients to stay in their homes longer or improve a patient's manageability in a nursing facility. In a 5-month trial, galantamine treatment maintained behavioral symptoms (measured by total NPI scores) near baseline.14,15 In a 6-month study of donepezil, treatment improved behavioral symptoms compared with placebo, with significant donepezil-placebo differences for anxiety, apathy, and depression/dysphoria.15,41 While behavioral disturbances in dementia are often treated with psychotropic medications, patients treated with ChE inhibitors exhibit fewer disturbances42,43 and receive fewer sedatives than patients who go untreated.43
Managing caregiver burden.
Managing the caregiver is a particularly critical element in the successful long-term management of Alzheimer's disease, especially since 50% of caregivers experience clinical depression.44 Being attentive to caregiver concerns, stress, and depression is important, as a caregiver's well-being can impact the quality of care they are able to provide for their loved ones. Since distressed caregivers can hinder the process of providing patients with Alzheimer's disease with optimal health care, providers should routinely refer patients and their caregivers to support and education programs such as those offered by the Alzheimer's Association (www.alz.org) or other agencies (e.g., Area Councils on Aging).
Long-term ChE inhibitor treatment of patients with Alzheimer's disease also confers significant benefits to caregivers, who sustain substantial emotional and financial burdens. Improvements in Alzheimer's disease symptoms may help decrease caregiver stress and depression. A long-term study with donepezil reported a trend toward decreased time spent caring for patients with Alzheimer's disease and a decrease in health care utilization by caregivers and their patients.45 Galantamine has also been reported to reduce caregiver time spent providing assistance to individuals with mild-to-moderate Alzheimer's disease.46 The direct costs (e.g., nursing home and day care, hospital and physician services, medication) and indirect costs (unpaid caregiver hours, reduced work hours) associated with caring for a patient with Alzheimer's disease are substantial, with direct costs escalating dramatically upon institutionalization. The high costs associated with nursing home care can be postponed when effective treatments enable families to continue caring for their loved ones at home.
DISCUSSION
The optimal method for managing patients with Alzheimer's disease over the long term involves a team approach. Besides primary care physicians, social workers, nurses, therapists, adult day care workers, and other health care professionals all play a role in addressing the emotional, physical, and social needs of patients and families. By initiating ChE inhibitor therapy early in the disease course and continuing treatment persistently, implementing nonpharmacologic strategies to reduce behavioral disturbances or using psychotropic agents as necessary, setting realistic expectations for treatment, addressing caregiver needs, and referring families to support services, physicians can help families cope with the demands of this devastating disease.
As patients progress to more advanced stages of the disease, it is noteworthy that although approved for mild-to-moderate Alzheimer's disease, ChE inhibitors may continue to benefit patients with more severe Alzheimer's disease.15,47 At this point in the disease course, continued treatment may benefit patient functioning and behavior—both meaningful outcomes for caregivers. There are, however, no guidelines for knowing when to stop the ChE inhibitors, and the timing and monitoring of treatment discontinuation remain in the domain of clinical judgment. The prescriber generally must individualize the decision to stop therapy, keeping the needs and desires of the patient and family in mind. The likelihood of clinically meaningful benefit seems low, however, once a patient no longer recognizes his or her spouse and children. Once the ChE inhibitor is withdrawn, the patient should be monitored for the rapid development of meaningful functional losses, such as cessation of walking, impaired feeding, or worsened toileting/incontinence. Should there be a clearly identified functional loss within a few weeks of discontinuation, data suggest that therapy should be resumed as early as possible to prevent an irrecoverable decrement in ability.19
It is important to consider the safety profiles of the drugs before initial treatment in order to maximize the chance that patients can continue therapy. In addition, patients should be carefully assessed for characteristics important in selecting treatment (e.g., galantamine is not recommended in patients with severe hepatic impairment). In practice, flexible dosing allows physicians to tailor the drug dose to the individual—optimizing benefits while minimizing adverse effects. If ChE inhibitor therapy is interrupted for more than a few days, treatment should always be restarted at the lowest possible dose and titrated back to the maximally effective and tolerated dose as quickly as feasible. Long-term clinical studies demonstrate that ChE inhibitor treatment is safe, with a decrease in the incidence of adverse events with long-term use. Data suggesting that ChE inhibitor treatment benefits behavior15,48 may decrease the need to use multiple antipsychotics. However, increasingly problematic behaviors may necessitate the use of antidepressants and antipsychotics when nonpharmacologic methods are insufficient.49 Medications for behavior should be slowly withdrawn and reevaluated periodically to assess the need for them. Special care must be taken to avoid drugs with anticholinergic activity, especially the older conventional antipsychotics and heterocyclic antidepressants.
Treatment effect may be difficult to discern due to the symptomatic nature of ChE inhibitor treatment and the preconceived expectations of many patients, families, and physicians. Therapy that slows decline is considered successful in Alzheimer's disease, but how is this gauged? In clinical practice, treatment success may be accepted when a patient shows improvement or no change. There is no single comprehensive measure of success of ChE inhibitor therapy. Generally, the instruments used for clinical trials assessment are not feasible in practice settings. The clinician must therefore rely on a combination of caregiver reports of daily functioning and behavior, which can be supplemented by using a brief cognitive scale like the MMSE. Cognition, or MMSE scores, should not be overemphasized as an outcome, however, because functional and behavioral benefits may be present, even at a point where cognition has declined.15,16,50 Nonetheless, unrelenting decline is characteristic of this progressive debilitating disorder and must be expected even in the face of drug therapy. Since slowed decline is also a favorable outcome, it is important not to withdraw treatment prematurely. In a novel study50 designed to assess the question of perceived benefit, patients who were subjectively judged by their physician to show clinically meaningful cognitive decline during donepezil therapy were randomly assigned to placebo or continuation on donepezil therapy. Patients who continued on therapy obtained cognitive (ADAS-cog), functional (DAD), and behavioral benefits (NPI) compared with patients who were switched to placebo. Thus, even when treatment effect was questioned, patients were benefiting from treatment.
As the prevalence of Alzheimer's disease rises in the next few decades, primary care physicians will be increasingly faced with caring for patients with Alzheimer's disease over the long term. To prepare for this inevitability, it is critical that physicians recognize the value of diagnosing Alzheimer's disease early and have an appreciation of the impact of long-term drug treatment. The long-term (≥ 1 year) efficacy of ChE inhibitors is supported by both placebo-controlled and open-label extension studies. These studies show that long-term treatment benefits patient cognition, functioning (ADLs), and behavior. Results from long-term, double-blind, and open-label studies show ChE inhibitor treatment to be effective and safe. Cholinesterase inhibitor therapy provides meaningful benefits to patients with Alzheimer's disease across the disease continuum. The evidence-based practice parameter of the American Academy of Neurology for the management of dementia now considers ChE inhibitor therapy standard care for mild-to-moderate Alzheimer's disease.3
As more patients with Alzheimer's disease are treated and managed in the primary care setting, opportunities to optimize treatment outcomes must be considered. By employing early and continuous ChE inhibitor therapy, communicating realistic expectations of long-term treatment benefits, and addressing caregiver issues that arise over the course of the disease, physicians can provide the best possible care for their patients with Alzheimer's disease.
Drug names: donepezil (Aricept), galantamine (Reminyl), rivastigmine (Exelon), tacrine (Cognex).
Footnotes
Dr. Geldmacher has received research funding from Janssen and Novartis and consulting honoraria, research support, and speaker's fees from Eisai and Pfizer. The development of this manuscript was supported by Eisai and Pfizer; the final content was determined solely by Dr. Geldmacher.
REFERENCES
- Katzman R, Fox PJ. The world-wide impact of dementia: projections of prevalence and costs. In: Mayeux R, Christen Y, eds. Epidemiology of Alzheimer's Disease: From Gene to Prevention. Heidelberg, Germany: Springer-Verlag. 1999 1–17. [Google Scholar]
- Francis PT, Palmer AM, and Snape M. et al. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999 66:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Stevens JC, and Beck C. et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 56:1154–1166. [DOI] [PubMed] [Google Scholar]
- Karlawish JH, Klocinski JL, and Merz J. et al. Caregivers' preferences for the treatment of patients with Alzheimer's disease. Neurology. 2000 55:1008–1014. [DOI] [PubMed] [Google Scholar]
- Feldman H, Gauthier S, and Hecker J. et al. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer's disease and the effect on caregiver burden. J Am Geriatr Soc. 2003 51:737–744. [DOI] [PubMed] [Google Scholar]
- Farcnik K, Persyko MS. Assessment, measures and approaches to easing caregiver burden in Alzheimer's disease. Drugs Aging. 2002;19:203–215. doi: 10.2165/00002512-200219030-00004. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Fox P, and Newcomer R. et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002 287:2090–2097. [DOI] [PubMed] [Google Scholar]
- Knopman D, Schneider L, and Davis K. et al. Long-term tacrine (Cognex) treatment: effects on nursing home placement and mortality, Tacrine Study Group. Neurology. 1996 47:166–177. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, and Wisniewski S. et al. Cholinesterase inhibitor treatment alters the natural history of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002 72:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher DS, Provenzano G, and McRae T. et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer's disease. J Am Geriatr Soc. 2003 51:937–944. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, and Doody RS. et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease; Donepezil Study Group. Neurology. 1998 50:136–145. [DOI] [PubMed] [Google Scholar]
- Burns A, Rossor M, and Hecker J. et al. The effects of donepezil in Alzheimer's disease: results from a multinational trial. Dement Geriatr Cogn Disord. 1999 10:237–244. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol. 1998;1:55–65. [Google Scholar]
- Tariot PN, Solomon PR, and Morris JC. et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD; The Galantamine USA-10 Study Group. Neurology. 2000 54:2269–2276. [DOI] [PubMed] [Google Scholar]
- Feldman H, Gauthier S, and Hecker J. et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer's disease. Neurology. 2001 57:613–620. [DOI] [PubMed] [Google Scholar]
- Winblad B, Engedal K, and Soininen H. et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001 57:489–495. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Doody RS, and Morris JC. et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001 57:481–488. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Doody RS, and Pratt RD. et al. Long-term efficacy and safety of donepezil in the treatment of Alzheimer's disease: final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol. 2000 10:195–203. [DOI] [PubMed] [Google Scholar]
- Doody RS, Geldmacher DS, and Gordon B. et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer's disease. Arch Neurol. 2001 58:427–433. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, and Wessel T. et al. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension; The Galantamine USA-1 Study Group. Neurology. 2000 54:2261–2268. [DOI] [PubMed] [Google Scholar]
- Farlow M, Anand R, and Messina J Jr. et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer's disease. Eur Neurol. 2000 44:236–241. [DOI] [PubMed] [Google Scholar]
- Wilcock G, Howe I, and Coles H. et al. A long-term comparison of galantamine and donepezil in the treatment of Alzheimer's disease. Drugs Aging. 2003 20:777–789. [DOI] [PubMed] [Google Scholar]
- Farlow M, Hake A, and Messina J. et al. Response of patients with Alzheimer disease to rivastigmine treatment is predicted by the rate of disease progression. Arch Neurol. 2001 58:417–422. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Krishnan KR, and Anand R. et al. Long-term effects of rivastigmine in moderately severe Alzheimer's disease: does early initiation of therapy offer sustained benefits? Prog Neuropsychopharmacol Biol Psychiatry. 2002 26:705–712. [DOI] [PubMed] [Google Scholar]
- Blesa R, Davidson M, and Kurz A. et al. Galantamine provides sustained benefits in patients with ‘advanced moderate’ Alzheimer's disease for at least 12 months. Dement Geriatr Cogn Disord. 2003 2:79–87. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial; The Donepezil Study Group. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Doody RS, and Mohs RC. et al. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study; The Donepezil Study Group. Arch Intern Med. 1998 158:1021–1031. [DOI] [PubMed] [Google Scholar]
- Wessel T, Gaens E. The long-term cognitive benefits of galantamine treatment in patients with Alzheimer's disease. Presented at the 125th Annual Meeting of the American Neurological Association. 15–18October2000 Boston, Mass. [Google Scholar]
- Stern R, Mohs R, and Davidson M. et al. A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994 151:390–396. [DOI] [PubMed] [Google Scholar]
- Galasko D, Edland SD, and Morris JC. et al. The consortium to establish a registry for Alzheimer's disease (CERAD), pt 11: clinical milestones in patients with Alzheimer's disease followed over 3 years. Neurology. 1995 45:1451–1455. [DOI] [PubMed] [Google Scholar]
- McCormick WC, Kukull WA, and van Belle G. et al. Symptom patterns and comorbidity in the early stages of Alzheimer's disease. J Am Geriatr Soc. 1994 42:517–521. [DOI] [PubMed] [Google Scholar]
- Knopman D, Donohue JA, Gutterman EM. Patterns of care in the early stages of Alzheimer's disease: impediments to timely diagnosis. J Am Geriatr Soc. 2000;48:300–304. doi: 10.1111/j.1532-5415.2000.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Aricept [package insert]. Teaneck, NJ: Eisai Inc. 2001. [Google Scholar]
- Exelon [package insert]. East Hanover, NJ: Novartis. 2001. [Google Scholar]
- Reminyl [package insert]. Titusville, NJ: Janssen Pharmaceutica. 2002. [Google Scholar]
- Jhee SS, Shiovitz T, and Hartman RD. et al. Centrally acting antiemetics mitigate nausea and vomiting in patients with Alzheimer's disease who receive rivastigmine [letter]. Clin Neuropharmacol. 2002 25:122–123. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Passmore P, and Bullock R. et al. A multinational, randomized 12-week comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer's disease. Int J Clin Pract. 2002 56:441–446. [PubMed] [Google Scholar]
- Tariot PN, Cummings JL, and Katz IR. et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. J Am Geriatr Soc. 2001 49:1590–1599. [PubMed] [Google Scholar]
- White H, Pieper C, Schmader K. The association of weight change in Alzheimer's disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- Geldmacher D, Provenzano G, and McRae T. et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer's disease. J Am Geriatr Soc. 2003 51:937–944. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Feldman H, and Hecker J. et al. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer's disease. Int Psychogeriatr. 2002 14:389–404. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Nadel A, and Masterman D. et al. Efficacy of metrifonate in improving the psychiatric and behavioral disturbance of patients with Alzheimer's disease. J Geriatr Psychiatry Neurol. 2001 14:101–108. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Donohue JA, Brooks RL. The relationship between donepezil and behavioral disturbances in patients with Alzheimer's disease. Am J Geriatr Psychiatry. 2000;8:134–140. [PubMed] [Google Scholar]
- Schulz R, O'Brien AT, and Bookwala J. et al. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995 35:771–791. [DOI] [PubMed] [Google Scholar]
- Wimo A, Winblad B, and Engedal K. et al. An economic evaluation of donepezil in mild to moderate Alzheimer's disease: results of a 1-year, double-blind, randomized trial. Dement Geriatr Cogn Disord. 2003 15:44–54. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Sadik K. Galantamine reduces caregiver time spent providing assistance to individuals with mild-to-moderate Alzheimer's disease. Am J Geriatr Psychiatry. 2002;10(2 suppl 1):84–85. [Google Scholar]
- Rabinowicz A, Koumaras B, and Cummings J. et al. Effects of rivastigmine treatment on the psychiatric and behavioral disturbances of nursing home residents with moderate to severe Alzheimer's disease (final results). Presented at the 11th Annual Congress of the International Psychiatric Association. 18–22August2003 Chicago, Ill. [Google Scholar]
- Trinh NH, Hoblyn J, and Mohanty S. et al. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA. 2003 289:210–216. [DOI] [PubMed] [Google Scholar]
- Desai A, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3:93–109. doi: 10.4088/pcc.v03n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen P, Barcikowska M, and Heun R. et al. Donepezil-treated Alzheimer's disease patients with apparent initial cognitive decline demonstrate significant benefits when therapy is continued: results from a randomized, placebo-controlled trial [abstract]. J Neurol. 2003 250suppl 2. 126. [Google Scholar]