Abstract
Healthy aging has been found associated with less efficient response conflict solution, but the cognitive and neural mechanisms remain elusive. In a two-experiment study, we first examined the behavioural consequences of this putative age-related decline for conflicts induced by spatial stimulus–response incompatibility. We then used resting-state functional magnetic resonance imaging data from a large, independent sample of adults (n = 399; 18–85 years) to investigate age differences in functional connectivity between the nodes of a network previously found associated with incompatibility-induced response conflicts in the very same paradigm. As expected, overcoming interference from conflicting response tendencies took longer in older adults, even after accounting for potential mediator variables (general response speed and accuracy, motor speed, visuomotor coordination ability, and cognitive flexibility). Experiment 2 revealed selective age-related decreases in functional connectivity between bilateral anterior insula, pre-supplementary motor area, and right dorsolateral prefrontal cortex. Importantly, these age effects persisted after controlling for regional gray-matter atrophy assessed by voxel-based morphometry. Meta-analytic functional profiling using the BrainMap database showed these age-sensitive nodes to be more strongly linked to highly abstract cognition, as compared with the remaining network nodes, which in turn were more strongly linked to action-related processing. These findings indicate changes in interregional coupling with age among task-relevant network nodes that are not specifically associated with conflict resolution per se. Rather, our behavioural and neural data jointly suggest that healthy aging is associated with difficulties in properly activating non-dominant but relevant task schemata necessary to exert efficient cognitive control over action.
Keywords: cognitive control, adult age differences, resting-state fMRI, stimulus–response compatibility, BrainMap behavioral domains, quantitative reverse inference
1. Introduction
Healthy aging is associated with performance deterioration in several cognitive domains, among them the top-down control of action (for reviews, see Craik and Salthouse, 2008; Park and Schwarz, 2000; Proctor et al., 2005; Salthouse, 1991; but see Verhaeghen, 2011). Such cognitive control over action is required when automatic response tendencies need to be overcome. A well-established paradigm to study cognitive action control is the spatial stimulus–response compatibility (SRC) task, in which the stimuli and the speeded responses they call for either match or mismatch spatially (Fitts and Deininger, 1954). In this task, a matching (i.e. spatially compatible) stimulus– response (S-R) mapping is given if the occurrence of a lateralized stimulus requires an ipsilateral response, while a mismatching (i.e. spatially incompatible) S-R mapping is given if a lateralized stimulus requires a contralateral response. Successful performance in incompatible conditions necessitates overcoming the automatically activated ipsilateral response tendency through top-down control according to the current task set (i.e., “Respond contralaterally!”). Conceptually, this intentional “overcoming of automatic response tendencies” in SRC tasks consists of two processes: inhibiting the prepotent compatible response and initiating the nondominant incompatible one (cf. Hommel and Prinz, 1997).
In terms of performance, these top-down-controlled processes are reflected by an increase in reaction time (RT) and error rate, relative to compatible trials. This effect, in turn, was found to further increase with age (see Proctor et al., 2005, for a review). Previous studies, however, are sparse and often did not consider potential confounds. The age-related RT slowing on incompatible trials might thus not (only) be related to a selective impairment in exerting cognitive control but might rather be due to a general slowing of information processing or motor execution with age (Salthouse, 1996). Our first experiment, therefore, aimed to reproduce the increase in S-R incompatibility costs with age and, if found, examine whether the age-related increase is independent of age differences in potential confounds such as speed in compatible response selection, motor speed, speeded visuomotor coordination, and cognitive flexibility.
As for the neural underpinnings, overcoming spatial S-R incompatibility was found to activate a fronto-parieto-insular network comprising bilateral anterior insula, intraparietal sulcus (IPS), dorsal premotor cortex (dPMC), pre-supplementary motor area (pre-SMA) and adjacent midcingulate cortex, as well as right temporoparietal junction (TPJ) and right dorsolateral prefrontal cortex (DLPFC) (Cieslik et al., 2010; Matsumoto et al., 2004; Schumacher et al., 2003; Sylvester et al., 2003). With respect to age, Lee et al. (2006) reported increased incompatibility-related activity in older (vs. younger) adults in right DLPFC, anterior cingulate cortex, and left inferior parietal cortex. This age-related regional hyperactivity might have reflected compensatory processing during the solution of response conflicts in advanced age.
Besides regional activation, however, efficient communication between the nodes of the involved network is pivotal. At the neural level, this communication should be reflected by interregional functional connectivity (FC), that is, correlations among the activity time courses of task-relevant regions. Several age-related differences in FC have previously been observed (for reviews, see Ferreira and Busatto, 2013; Goh, 2011), even in the absence of changes in regional activation strength (Grady, 2005; Madden et al., 2010). As a consequence, performance differences with age might also arise from changes in FC among relevant brain regions (Chen et al., 2009; Stevens, 2009).
Two studies reported age-dependent decreases in FC within the networks involved in motor control and task switching, respectively (Madden et al., 2010; Wu et al., 2007), but to our knowledge, potential FC changes with age between brain regions related to solving response conflicts have not been examined yet. Therefore, using a large adult sample with a wide age range, our second experiment tested age-related differences in intrinsic FC between brain regions associated with responding under conditions of spatial S-R incompatibility. These regions were derived from a previous functional magnetic resonance imaging (fMRI) study that used exactly the same paradigm to uncover incompatibility-related regional brain activity (Cieslik et al., 2010). Our second experiment was thus performed in an independent sample for which no SRC-related performance measures were available. Nevertheless, basing our FC analysis on an a priori defined brain network specifically associated with the cognitive process of interest provided a strong functional-neuroanatomical link between behavioural and connectivity changes with age, as investigated in Experiments 1 and 2, respectively.
To summarize, the goals of our investigation were two-fold: (1) We aimed to examine the effect of age on overcoming incompatibility-induced response conflicts at the behavioural level, including the analysis of potential mediator variables. (2) After corroborating an age-related increase of the behavioural SRC effect in Experiment 1, we sought to investigate age effects on the intrinsic functional coupling between those brain regions that are specifically activated by such incompatibility-induced response conflicts. Juxtaposing these two complementary methodological approaches, we aimed to obtain converging evidence for the mechanisms underlying age-related differences in solving spatial incompatibility-induced response conflicts.
2. Experiment 1
Experiment 1 examined whether aging is related to difficulties in solving response conflicts induced by spatial S-R incompatibility, even when potential mediator variables (see below) are taken into account. Although previous findings are not completely consistent (Bonin-Guillaume et al., 2000; Grandjean and Collette, 2011; Lee et al., 2006; Proctor et al., 2005; Simon and Wolf, 1963; Smulders et al., 1999), we predicted that the slowing of responses under conditions of spatial S-R incompatibility would be stronger in older participants. We further expected that this effect persisted after partialling out variance explained by general response speed, response accuracy, motor speed, speeded visuomotor coordination, and cognitive flexibility.
2.1. Method
2.1.1. Sample
The sample comprised 26 young (M = 24.9, SD = 2.8, range = 20–29 years; 9 female) and 27 older (M = 59.4, SD = 6.8, range = 50–73 years; 9 female) paid volunteers, which all had normal or corrected-to-normal vision and no history of psychiatric or neurologic disorders. In order to exclude participants with dementia or clinically relevant cognitive impairments, we administered a multiple-choice vocabulary test (MWT-B; Lehrl, 2005) that assesses crystallized verbal intelligence. The test score, ranging from 0 to 37, was previously shown to decrease with increasing dementia severity (Kessler et al., 1995). All our participants fell into the normal range, with the older subsample even achieving significantly higher test scores [M = 30.8; range = 23–35) than the younger subsample (M = 25.9; range = 19–32; two-sample t-test of the subsample difference: t(51) = –4.89, p < .001]. Participants gave written informed consent to the study, which had been approved by the local ethics committee of the RWTH Aachen University Hospital.
2.1.2. Task and procedure
Participants performed a manual SRC task (Behrwind et al., 2011; Cieslik et al., 2010) requiring speeded button-press responses with the left- or right-hand index finger to lateralized visual stimuli (red dots). In trials with compatible S-R mapping, participants were to respond as fast and correctly as possible with their ipsilateral hand (e.g. with their left hand to a left-sided stimulus), while in trials with incompatible S-R mapping, participants were to respond with their contralateral hand (e.g. with their right hand to a left-sided stimulus). Stimuli were presented for 200 ms each and separated by an interstimulus interval of 1300–1700 ms (uniformly jittered).
The compatibility of the spatial S-R mapping was varied between blocks and indicated at each block’s beginning by a brief verbal instruction shown for 500 ms at the centre of the screen. Fifteen blocks of either condition were presented in pseudorandom order, separated by uniformly jittered intervals of 4.1–4.5 s, with each block containing 21–24 trials (left/right stimuli presented in random order, equally distributed across blocks).
The experiment was run in a dimly lit room using a standard PC and the software Presentation 11.3 (www.neurobs.com). Reaction time (RT) and accuracy were recorded. To make the testing more comfortable and prevent decreasing attention due to fatigue, the 30 task blocks were split into five sessions (containing 3 compatible and 3 incompatible blocks each), which were separated by resting breaks of up to 5 min. Before the experiment, participants performed a range of neuropsychological tests (see below).
2.1.3. Neuropsychological tests
2.1.3.1. Finger Tapping
To assess finger motor speed, participants tapped as rapidly as possible for 10 s using their right and left index finger, respectively. The median number of taps from 3 trials per hand (separated by short breaks to prevent muscular fatigue) was used as the test score (cf. Dafotakis et al., 2008).
2.1.3.2. Pointing Task
To assess repetitive visuomotor coordination, participants performed series of rapid horizontal pointing movements alternating between two points 30 cm apart using their right and left index finger, respectively (Defer et al., 1999). Median time needed for 10 touches per point from 3 series per hand was used as the test score.
2.1.3.3. Trail-Making Test (TMT)
Nonrepetitive visuomotor coordination ability was assessed using the TMT-A, which is a paper-and-pencil test requiring participants to connect spatially scattered numbers in ascending order by drawing lines as fast as possible (Reitan, 1955). Total execution time was used as the test score. The TMT-B additionally contains letters and requires participants to connect both item types alternatingly in ascending (numerical and alphabetical) order. Thus, the TMT-B necessitates continuous attentional switching between both item categories (numbers and letters) and poses moderate demands on working memory for maintaining the last item of one category in mind while searching for and connecting the item of the other category. The difference in time needed to complete versions B and A, respectively, is considered to reflect those aspects of cognitive flexibility (i.e. attentional switching and mnemonic updating) most validly (Sánchez-Cubillo et al., 2009) and was used as the test score (TMT-Diff).
2.1.4. Data analysis
Response speed was assessed via calculating intraindividual median RT for correct responses per condition; accuracy was assessed via calculating the intraindividual percentage of erroneous responses (error rate) per condition. Statistical group-level analysis was performed using SPSS 15.0. Initially, performance data were subjected to 2 × 2 analyses of variance (ANOVAs) with Age (young vs. old) as between-subject factor and SRC (compatible vs. incompatible) as within-subject factor. Subsequently, RT and error rate on S-R-compatible trials, finger tapping and pointing task scores, as well as TMT-A and TMT-Diff scores were entered as predictors into a linear regression analysis with the SRC effect on RT as dependent variable. For each participant, this SRC effect (ΔRT%) was calculated as difference between median RT on incompatible and compatible trials, expressed as percent RT change on compatible trials: ΔRT% = (RTincompatible – RTcompatible) / RTcompatible × 100. If the multiple regression analysis revealed that the SRC effect on RT (i.e., ΔRT%) was significantly predicted by the variables entered, we would include these variables as covariates into a between-group analysis of covariance (ANCOVA) of ΔRT% to examine whether the group differences in the SRC effect persisted when accounting for other relevant sources of variance.
2.2. Results and discussion
2.2.1. ANOVAs
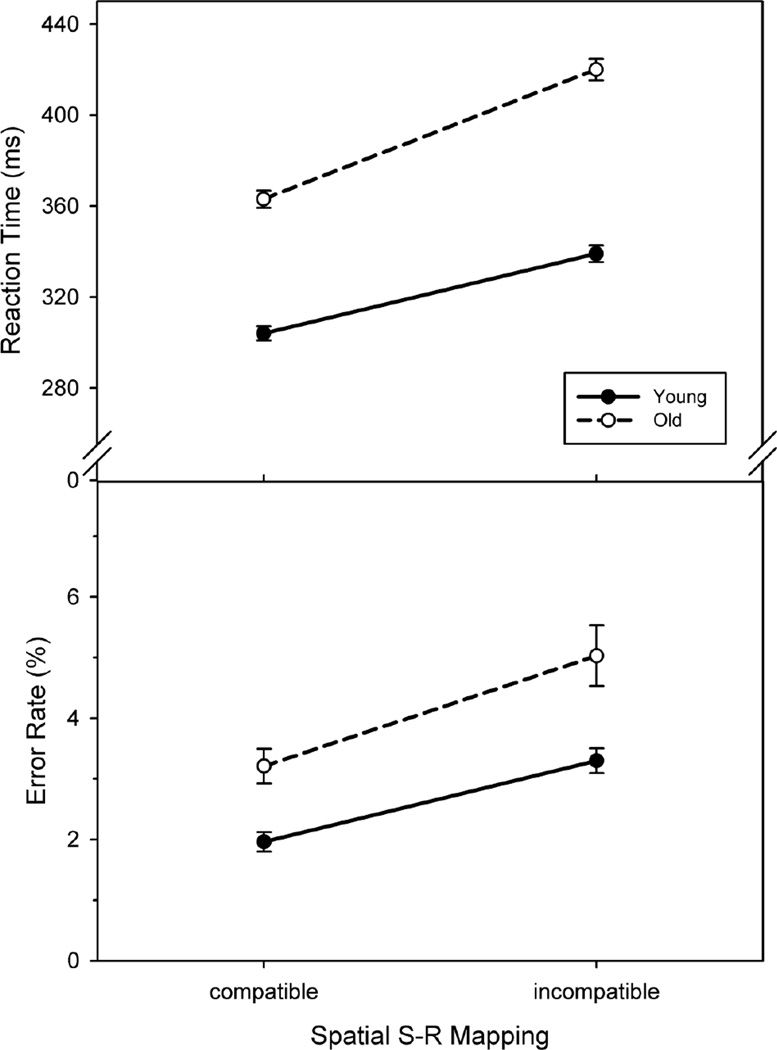
Results are shown in Fig. 1. Mean RT was significantly longer in older (vs. younger) participants [F(1, 51) = 41.1, p < .001, ηp2 = 0.45] as well as under incompatible (vs. compatible) conditions [F(1, 51) = 584.7, p < .001, ηp2 = 0.92]. Critically, the Age × SRC interaction effect was also significant [F(1, 51) = 34.6, p < .001, ηp2 = 0.40], that is, the incompatibility-related RT slowing was stronger with age. This interaction was also reflected in significantly larger ΔRT% values in the older participants (mean ΔRT% = 15.5), relative to the younger ones (mean ΔRT% = 11.2), as revealed by a supplementary two-sample t-test [t(51) = −4.6, p < .001].
Fig. 1.
Performance (group-level mean reaction time and error rate) in the spatial stimulus–response (S-R) compatibility task as a function of S-R mapping and age. Error bars represent the standard error of the mean. Connecting lines were added for illustrative purposes only.
Error rate was also significantly higher in older (vs. younger) participants [F(1, 51) = 88.4, p < .001, ηp2 = 0.63] as well as under incompatible (vs. compatible) conditions [F(1, 51) = 8.4, p = .006, ηp2 = 0.14]. There was, however, no significant Age × SRC interaction effect on error rate [F(1, 51) = 0.2], that is, the incompatibility-related accuracy decline was not stronger with age.
These results agree with previous reports (cf. Proctor et al., 2005) in showing that the impact of S-R incompatibility on speeded response selection increases with age. In their review, Proctor et al. mentioned that aging sometimes was also found to specifically affect error rate in incompatible conditions. As we did not find such an interaction, further studies are needed to assess whether an incompatibility-related accuracy decline with age is a reliable effect at all and, if so, on which specific, as-yet unknown methodological factors its presence and size depend. Given that in research on SRC effects, response speed is the dominant dependent measure, the main reason for the volatility of the age × compatibility interaction effect on accuracy might be the limited sensitivity of accuracy measures to the difficulty difference between compatible and incompatible conditions. In any case, as response speed during incompatible trials was apparently not traded off against increased accuracy in our task, the observed age × compatibility interaction effect on RT suggests that aging leads to deterioration in the efficiency of intentionally overcoming an automatically activated (dominant) response tendency in favour of another (non-dominant) response alternative. This conclusion, however, must be preliminary as long as the relevance of other variables that might account for this seemingly age-related difference has not been examined.
2.2.2. Linear regression analysis
Across the entire sample, multiple regression analysis revealed a significant linear relation (R = .41, p = .017) between the predictors entered (i.e., compatible-trial RT and error rate as well as finger tapping, pointing task, TMT-A, and TMT-Diff scores; cf. Table 1) and the relative impact of S-R incompatibility on RT (i.e., ΔRT%). This result demonstrates that a substantial part of variance (17%) in the impact of S-R incompatibility on RT is explained by these variables. Given this relationship, we included these predictors as covariates in an ANCOVA to test whether the difference in the relative impact of incompatibility between age groups persisted.
Table 1.
Means (Standard Deviations) and Difference Statistics of the Neuropsychological Test Scores in the Young and Old Subsamples of Experiment 1
| Test Score | Young | Old | t-value | p-value |
|---|---|---|---|---|
| Tap | 53.8 (6.6) | 49.7 (6.4) | 2.3 | .027 |
| Pointing | 7.0 (1.4) | 8.1 (2.4) | −2.0 | .056 |
| TMT-A | 20.0 (4.5) | 27.9 (10.0) | −3.8 | .001 |
| TMT-B | 35.6 (10.3) | 53.7 (24.6) | −3.5 | .001 |
| TMT-Diff | 15.6 (8.7) | 25.8 (22.1) | −2.2 | .033 |
Note. Tap, Finger Tapping Task; Pointing, Pointing Task; TMT-A/-B, Trail Making Test version A/B; TMT-Diff, Difference between TMT-B and TMT-A scores.
2.2.3. ANCOVA
After partialling out variance explained by the predictors included in the above regression analysis, the between-group ANCOVA still yielded a significant main effect of age on ΔRT% [F(1, 45) = 7.5, p = .009, ηp2 = 0.14]. Thus, compared with the above ANOVA and t-test results, the Age × SRC interaction effect on RT was somewhat reduced but not “explained away” by variability in response speed and accuracy on compatible trials as well as measures of motor speed, visuomotor coordination, and cognitive flexibility. Therefore, the age-related decline in the ability to overcome prepotent response tendencies appears to be specific and not mediated by factors reflected in the measures used as covariates, corroborating our preliminary conclusion.
Extending previous research (Grandjean and Collette, 2011; Proctor et al., 2005), our analysis accounted for variance in variables that partly predicted the age-specific increase of the SRC effect. Importantly, this did not remove the influence of age on dealing with spatial S-R incompatibility. Our findings thus contradict the assumption that the age-related response slowing under conditions of S-R incompatibility is simply the result of a global slowing in processing speed (cf. Bonin-Guillaume et al., 2000). Furthermore, they indicate that the observed decline in cognitive action control with age is neither mediated by a shift of the speed–accuracy trade-off towards higher accuracy, nor by differences in motor speed, visuomotor coordination abilities, or cognitive flexibility. In sum, our behavioural data suggest that aging leads to a specific deficit in exerting cognitive control over automatically activated but irrelevant action tendencies. Potential mechanisms will be considered in the General Discussion in light of the results of Experiment 2.
3. Experiment 2
In Experiment 2, we examined age-related differences in intrinsic FC (i.e., FC at “rest”) between brain regions involved in solving response conflicts that arise from S-R incompatibility. Intrinsic FC is increased between functionally and anatomically related brain regions (Fox et al., 2006; Fox and Raichle, 2007; Smith et al., 2009) and was found to predict both intraindividual trial-to-trial performance variability (Fox et al., 2007) and interindividual differences in neurological and psychiatric disease severity (see Zhang and Raichle, 2010, for a review). Thus, finding age-related variation in interregional FC strength would suggest adult age differences in neural coupling and, in turn, communication and interaction between brain regions. As we restricted our main analysis to regions specifically associated with responding under conditions of S-R incompatibility, age-related reductions in the coupling between these regions could inform us about possible and plausible neural mechanisms behind the behavioural effects observed in Experiment 1.
3.1. Method
3.1.1. Definition of incompatibility-related seed regions
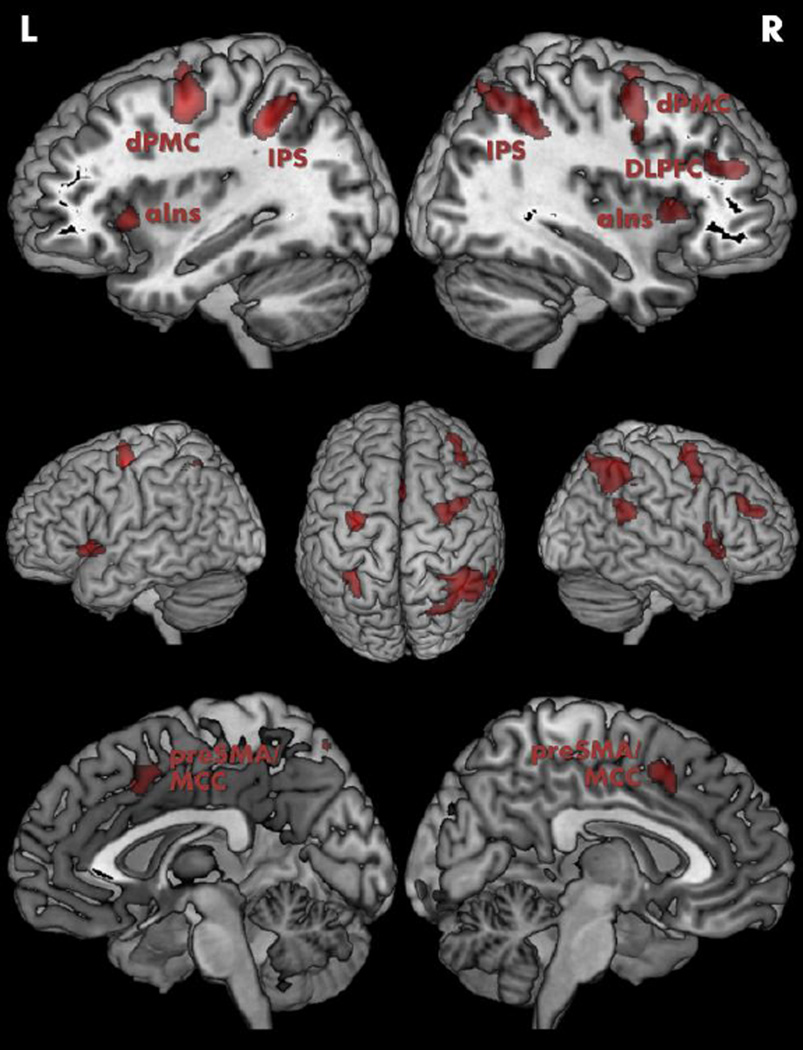
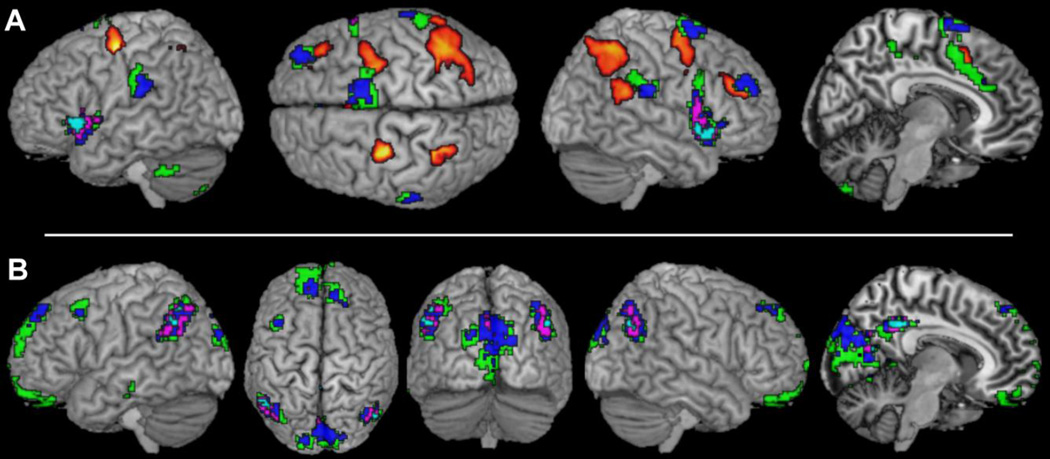
The regions of interest (“seeds”) for the present investigation had previously been identified by fMRI in a normal adult sample (n = 24; mean age = 29 yrs.; age range = 20–59 yrs.) using the very same SRC task in the scanner that was used in Experiment 1 (Cieslik et al., 2010). More specifically, the SRC-related network examined in Experiment 2 resulted from contrasting brain activity during S-R-incompatible trials with that during S-R-compatible trials, in conjunction with the two main effects (vs. resting baseline) of incompatible trials with left- and right-handed responses, respectively. This network comprised bilateral IPS (hIP3; Scheperjans et al., 2008), anterior insula, dPMC (area 6; Geyer, 2004), pre-SMA and adjacent midcingulate cortex, as well as right TPJ (area PFm; Caspers et al., 2006) and DLPFC (see Fig. 2).
Fig. 2.
Seed regions associated with solving spatial stimulus–response incompatibility as derived from Cieslik et al. (2010) for the network-based analysis of resting-state functional connectivity in Experiment 2. Abbreviations: L, left; R, right; aIns, anterior insula; DLPFC, dorsolateral prefrontal cortex; dPMC, dorsal premotor cortex; IPS, intraparietal sulcus; MCC, midcingulate cortex; preSMA, pre-supplementary motor area.
3.1.2. Sample
The analysis included resting-state fMRI data from 399 adults ranging from 18 to 85 (M = 41.8, SD = 16.8, Md = 41, IQR = 29) years of age. All participants (46% female) were without any record of neurological or psychiatric disorders and gave their written informed consent to the study. The data were contributed by four sites (see Table 2). Joint (re-)analysis of the data was approved by the local ethics committee of the Heinrich Heine University Düsseldorf.
Table 2.
Characteristics of the Sample in Experiment 2
| Contribution Site | n | Mean Age (Range) |
Sex: Male (%) |
Measurement Parametersa |
|---|---|---|---|---|
| RWTH University Hospital Aachen, Germany | 47 | 36.5 (19–59) | 46 | 3 T / 250 / 2.2 / 30 / 80° / 3.1 × 3.1 × 3.1 mm3 |
| 28 | 63.4 (55–72) | 71 | 3 T / 270 / 2.2 / 30 / 90° / 3.1 × 3.1 × 3.1 mm3 | |
| Research Centre Jülich, Germany | 51 | 28.3 (18–59) | 57 | 3 T / 250 / 2.2 / 30 / 90° / 3.1 × 3.1 × 3.1 mm3 |
| 100 | 45.1 (21–71) | 48 | 3 T / 300 / 2.2 / 30 / 90° / 3.1 × 3.1 × 3.1 mm3 | |
| ICBM, Montreal, Canadab | 41 | 40.6 (19–78) | 42 | 1.5 T / 256 / 2.0 / 50 / 90° / 4.0 × 4.0 × 4.0 mm3 |
| NKI/Rockland, Orangeburg, NY, USAb,c | 132 | 42.3 (18–85) | 59 | 3 T / 260 / 2.5 / 30 / 80° / 3.0 × 3.0 × 3.0 mm3 |
Note. ICBM, International Consortium for Brain Mapping; NKI, Nathan S. Kline Institute.
Measurement parameters: magnetic field strength of the scanner / number of acquired volumes / repetition time (in s) / echo time (in ms) / flip angle / voxel size.
These data were selected from the datasets included in Biswal et al. (2010) and made publicly available via the 1000 Functional Connectomes Project (http://fcon_1000.projects.nitrc.org).
All but the participants of the NKI/Rockland sample were instructed to keep their eyes closed during the measurement.
3.1.3. Data acquisition and preprocessing
Gradient-echo echo-planar imaging (EPI) was used to record blood oxygen level–dependent (BOLD) activity in transversal slices covering the entire cerebrum (for detailed measurement parameters of the different samples, please see Table 2). Participants lay supine in the scanner and were instructed to let their mind wander without falling asleep. All data were jointly preprocessed and analysed using SPM8 (www.fil.ion.ucl.ac.uk/spm). Four dummy scans, which preceded image acquisition to allow for magnetic field saturation, were discarded prior to further analysis. Images were first corrected for head movement by affine registration using a two-pass procedure by which images were initially realigned to the first image and subsequently to the mean of the realigned images. Each participant’s mean image was then spatially normalized to the Montreal Neurological Institute (MNI) single-subject template brain using the “unified segmentation” approach (Ashburner and Friston, 2005), and the ensuing deformation was applied to the individual EPI volumes. Hereby, volumes were resampled at 1.5 × 1.5 × 1.5 × mm3 voxel size. Images were then smoothed by a 5-mm full-width at half-maximum Gaussian kernel to meet the requirements of the general linear model and compensate for residual anatomical variation.
3.1.4. Data analysis
FC measures can be influenced by several confounds such as head movements and physiological processes (e.g., fluctuations due to cardiac and respiratory cycles; cf. Fox et al., 2009; Weissenbacher et al., 2009). In order to reduce spurious correlations, variance explained by the following nuisance variables was removed from each voxel’s BOLD signal time series (Cieslik et al., 2013b; Jakobs et al., 2012; Satterthwaite et al., 2013): (i) the six motion parameters derived from the image realignment; (ii) the first derivatives of the six motion parameters, (iii) mean gray-matter, white-matter, and cerebrospinal-fluid signal intensity per time point as obtained by averaging across voxels attributed to the respective tissue class in the SPM8 segmentation. All nuisance variables entered the regression model as first- and second-order terms, resulting in a total of 30 nuisance regressors. After confound removal, data were band-pass filtered preserving frequencies between 0.01 and 0.08 Hz, as meaningful resting-state correlations will predominantly be found in these frequencies given that the BOLD response acts as a low-pass filter (Biswal et al., 1995; Fox and Raichle, 2007; Greicius et al., 2003).
The time course of each seed region’s BOLD signal was then extracted for each participant as the first eigenvariate of activity in all gray-matter voxels located within the respective cluster (cf. Cieslik et al., 2013b; Jakobs et al., 2012). For each participant, the time-series data of each seed region were correlated with each other, and the resulting Pearson correlation coefficients were transformed into Fisher’s Z scores. The subsequent analysis of age-related changes in these interregional correlations (i.e., intrinsic FC values) was restricted to pairs of regions showing not only statistically significant (as assessed via one-sample t-tests) but substantial interconnectivity in the 100 youngest and/or 100 oldest participants. Specifically, since in rather large samples even relatively small correlations can be statistically significant, we restricted the study of age effects to those connections that showed intrinsic FC values of at least medium effect size (i.e., r ≥ .24, corresponding to Cohen’s d ≥ 0.5) in the young and/or old subgroup. For these connections, age-related changes in interregional coupling were examined by rank-correlating participants’ Fisher-Z-transformed FC values with age across the whole sample, with the influence of sex and data contribution site partialled out beforehand. The results of these Spearman correlation analyses were regarded significant if they passed a threshold of p < .05. As this study was of an exploratory nature, we report p-values uncorrected for multiple testing but mark those connections whose age-related change was large enough to survive Bonferroni correction. In addition to the correlational analyses, we performed an extreme-group comparison between the 25% youngest (18–26 yrs., n = 102) and 25% oldest (55–85 yrs., n = 108) participants using multivariate analysis of variance (MANOVA) as implemented in SPSS 15.0.
3.1.5. Quantitative functional profiling
In order to quantitatively assess the functional significance of seed regions with either age-sensitive or age-insensitive FC, we analysed the correspondence of respective network nodes (or sub-networks) with descriptors for cognitive processes as provided by the BrainMap database (www.brainmap.org; Laird et al., 2009). Along with result coordinates from thousands of neuroimaging studies, this database contains meta-data that describe the “behavioural domain” of each experimental contrast included according to a pre-specified taxonomy (Fox et al., 2005). The main categories of this taxonomy of behavioural domains include cognition, action, perception, emotion and interoception, along with their respective subcategories (for a complete list, see www.brainmap.org/scribe). By filtering this database for experiments featuring activation within a particular region and performing statistical analysis on the descriptors of the selected experiments, functional roles of individual areas may then be characterized in an unbiased manner.
We analysed the behavioural domain meta-data of BrainMap experiments associated with assessed network nodes by way of forward and reverse inference (Bzdok et al., 2013b; Cieslik et al., 2013a; Clos et al., 2013; Eickhoff et al., 2011a; Rottschy et al., 2013). For forward inference, we used binomial tests [p < .05, corrected for multiple comparisons by thresholding the false-discovery rate (FDR)] to identify behavioural domains for which the probability of activation in the respective seed region(s) was significantly above chance. That is, we tested whether the probability of finding activation in voxels of interest given a particular behavioural domain [P(Activation | Domain)] was higher than the baseline probability of finding activation in those voxels across the entire database [P(Activation)]. Reverse inference identified the most likely behavioural domains given activation in voxels of interest. This likelihood [P(Domain | Activation)] was derived from P(Activation | Domain), P(Domain) and P(Activation) using Bayes’ rule. Significance was assessed by means of chi-square tests (p < .05, FDR-corrected for multiple comparisons).
To examine the specificity of the functional profiles of seed regions with age-sensitive vs. age-insensitive connections, we performed contrast analyses, which were restricted to those experiments in BrainMap that activated either set of seeds. The results of these quantitative comparisons were thresholded at p < .05 (FDR-corrected for multiple comparisons). For differential forward inference, we compared the activation probabilities between the two seeds given a particular behavioural domain; for differential reverse inference, we compared the probabilities of a particular behavioural domain being present given activation in one or the other seed (Bzdok et al., 2013b; Cieslik et al., 2013; Clos et al., 2013; Eickhoff et al., 2011a; Rottschy et al., 2013).
3.2. Results and discussion
3.2.1. Basic FC
Substantial positive resting-state FC (i.e., r ≥ .24) was found for 19 connections (edges) between network nodes (see Table 3 and Fig. 3): (i) pre-SMA with bilateral anterior insula, bilateral dPMC, right DLPFC, and left IPS, respectively; (ii) right DLPFC with bilateral anterior insula, right dPMC, right IPS, and right TPJ, respectively; (iii) right anterior insula with right TPJ; (iv) right dPMC with bilateral IPS; (v) left dPMC with left IPS; and (vi) the interhemispheric connections between the bilateral clusters in anterior insula, dPMC, and IPS, respectively. Substantial negative FC values (i.e., r ≤ −.24) were neither found in the young nor the old subgroup.
Table 3.
Adult Age Differences in Intrinsic Functional Connectivity Among Brain Regions Involved in Solving Response Conflicts
| Pair of Regions | Mean ryoung | Mean relderly | Δr F-score | Correlation with Age (rs) |
|---|---|---|---|---|
| R DLPFC – L aIns | .37 | .21 | 23.39*** | −.25 |
| R DLPFC – R aIns | .50 | .35 | 13.27*** | −.23 |
| R DLPFC – pre-SMA/MCC | .36 | .32 | 0.90 | −.04 |
| R DLPFC – R dPMC | .33 | .32 | 0.04 | .00 |
| R DLPFC – R IPS | .45 | .45 | 0.02 | .01 |
| R DLPFC – R TPJ | .27 | .29 | 0.61 | .00 |
| L aIns – R aIns | .71 | .59 | 28.56*** | −.30 |
| L aIns – pre-SMA/MCC | .49 | .28 | 44.56*** | −.36 |
| R aIns – pre-SMA/MCC | .55 | .30 | 52.84*** | −.39 |
| R aIns – R dPMC | .28 | .13 | 15.36*** | −.24 |
| R aIns – R TPJ | .33 | .29 | 0.58 | −.12 |
| pre-SMA/MCC – L dPMC | .26 | .27 | 0.003 | .00 |
| pre-SMA/MCC – R dPMC | .35 | .33 | 0.54 | −.06 |
| pre-SMA/MCC – L IPS | .16 | .24 | 6.90** | .14 |
| pre-SMA/MCC – R IPSa | .12 | .23 | 12.57*** | .18 |
| L dPMC – R dPMC | .51 | .52 | 0.12 | .02 |
| L dPMC – L IPS | .41 | .43 | 0.13 | .05 |
| R dPMC – L IPS | .37 | .39 | 0.64 | .05 |
| R dPMC – R IPS | .36 | .41 | 1.61 | .08 |
| L IPS – R IPS | .45 | .44 | 0.08 | .00 |
Note. Mean ryoung and mean relderly denote group-averaged functional connectivity (FC) in the 100 youngest and 100 oldest participants, respectively; Δr F-score denotes the F-statistic (df = 1, 206) for the comparison of the two FC-values between both subgroups. Pairs of regions showing a significant correlation (Spearman) between their mutual FC and age are set in bold; connections whose age-related FC change survived Bonferroni correction are additionally set in italics.
R, right; L, left; DLPFC, dorsolateral prefrontal cortex; aIns, anterior insula; pre-SMA, pre-supplementary motor area; MCC, midcingulate cortex; dPMC, dorsal premotor cortex; IPS, intraparietal sulcus; TPJ, temporoparietal junction.
The intrinsic FC between this pair of regions just missed the threshold for medium effect size (r ≥ .24).
significant at p < .01;
significant at p < .001.
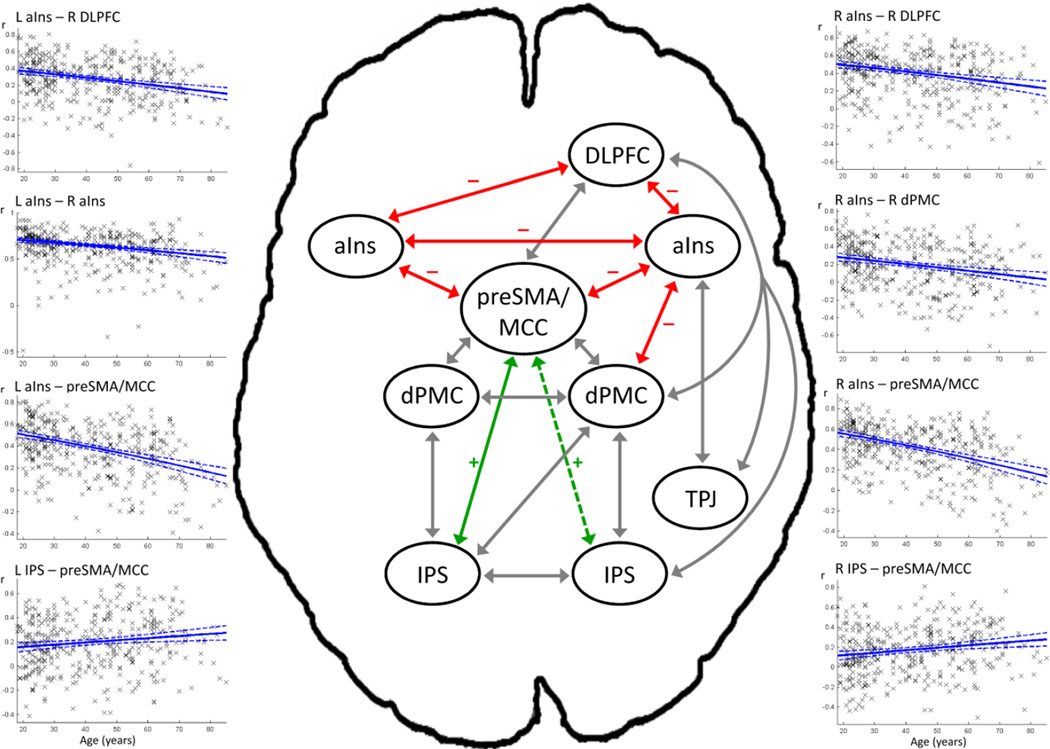
Fig. 3.
Regions of the network derived from Cieslik et al. (2010) that showed substantial (i.e. of at least medium effect size) positive functional connectivity (FC) at rest in the 100 youngest and/or oldest participants are connected with solid lines (broken lines indicate significant FC just below the effect-size cutoff). Positive correlations between FC strength and age are marked green, negative correlations are marked red; absence of significant correlation with age is denoted in gray. Insets show scatter plots with linear regression lines for connections with significant correlations between FC strength and age. Abbreviations: TPJ, temporoparietal junction; see Fig. 2 for remaining abbreviations.
3.2.2. Age effects on FC
For several of the substantial connections, resting-state FC was found to decrease significantly with age (rs range: −.23 to −.39; see Table 3 and Fig. 3). In particular, significant age effects were found for the connections between pre-SMA and right anterior insula as well as pre-SMA and left anterior insula, which both showed the largest FC decline with age. Age-related decreases in FC were furthermore found between right DLPFC and right anterior insula, right DLPFC and left anterior insula, right anterior insula and right dPMC, as well as between right and left anterior insulae. The connectivity between pre-SMA and left IPS was the only one that increased with age (rs = .14). However, a similarly small but significant age-related increase in FC at rest (rs = .18) was found for pre-SMA and right IPS, whose intrinsic interconnectivity in the older subgroup had just missed our effect-size criterion (FCelderly = .231).
The extreme-group MANOVA yielded a significant multivariate age effect [Pillai’s trace: 0.34, F(20, 187) = 4.76, p < .001] as well as significant univariate differences for exactly the same connections that showed a significant association between intrinsic FC and age (see Table 3), fully corroborating the correlational findings. As supplementary analysis, an equivalent MANOVA was performed on the FC data before partialling out sex effects, yielding neither a main effect of sex (Pillai’s trace: 0.10, p = .42), nor an interaction of sex with age (Pillai’s trace: 0.08, p = .70).
3.2.3. Quantitative functional profiling of regions with vs. without age-related FC decline
For decoding the functional significance of network nodes with mainly age-sensitive vs. age-insensitive FC in an objective and quantitative way, we determined these nodes’ significant associations with behavioural domains (and differences thereof) as provided by the BrainMap database. To recap, we conducted both forward and reverse inference analyses, with the former assessing the probability of activation in the respective region of interest given a particular behavioural domain and the latter assessing the probability of a behavioural domain given activation in a particular region of interest. Two sets of regions of interest were defined according to the results of the FC-by-age correlation analysis: The “age-sensitive set” comprised seed regions whose FC with several other seeds decreased with age (i.e., bilateral anterior insula, pre-SMA and right DLPFC), while the “age-insensitive set” comprised the remaining five seed regions (i.e., bilateral dPMC and IPS as well as right TPJ). The subsequent functional characterizations were based on all normal mapping experiments in healthy participants contained in BrainMap that reported at least one activation in each of the constituent regions of either set, with the following relaxation regarding pairs of bilateral homotopic seeds (i.e. anterior insula, dPMC, and IPS): to be included, it was sufficient for experiments to report activation only in left or right anterior insula or left or right dPMC and IPS, respectively.
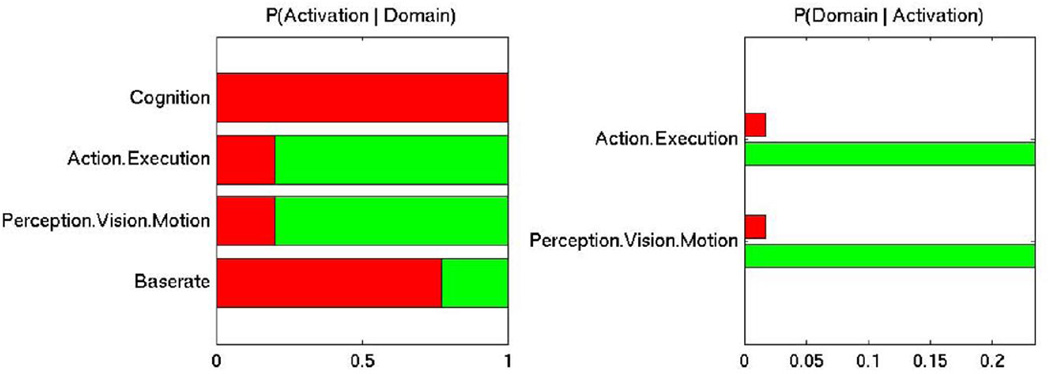
Forward and reverse inference alike indicated that both the age-sensitive and the age-insensitive sets were significantly associated with attention, while the age-insensitive set was additionally associated with visual motion perception (see Fig. S1 in Online Resource 1). The subsequent contrast analysis revealed that the age-sensitive set was more strongly associated with general cognitive processes according to forward inference, while the reverse inference analysis did not yield any significant difference (see Fig. 4). In contrast, the age-insensitive set was more strongly associated with action execution and visual motion perception, according to both forward and reverse inference (see Fig. 4).
Fig. 4.
Differential associations of the age-sensitive (red) vs. age-insensitive (green) set of seed regions with behavioural domains of the BrainMap database. BrainMap meta-data were used to perform functional forward (left panel) and reverse (right panel) inference. Forward inference determines above-chance differences in activating either set of seeds given a particular behavioural domain, while reverse inference determines above-chance differences in domain presence given activation of either set of seeds. The baserate denotes the general probability of BrainMap activation of the given seeds.
3.2.4. Supplementary analysis I: Age effects on FC in a control network
In order to collect additional evidence for the specificity of the age-related FC differences observed, we performed the very same analysis within a set of regions that were previously found to be collectively involved in the cross-modal processing of audiovisual emotional stimuli (Müller et al., 2012; see also Müller et al., 2011). This network comprised eight regions of interest: bilateral fusiform gyrus, auditory cortex, amygdala, and the posterior aspect of superior temporal sulcus (for details, see Table S1 in Online Resource 1). Between these clusters, we found three connections of substantial size (i.e., FC ≥ .24) that showed significant (albeit only uncorrected) age-related FC differences: between both fusiform gyri (rs = −.19), between both auditory cortices (rs = −.24), and between both amygdalae (rs = −.15).
3.2.5. Supplementary analysis II: Age effects on outside-network FC
A set of further analyses explored whether our seed regions showed age-related changes in FC with brain regions other than those constituting our a priori defined network. The rationale behind these exploratory whole-brain analyses was to falsify the hypothesis that age-dependent inter-network, rather than intra-network, changes in FC might drive the behavioural age effects observed in Experiment 1. To this end, we performed a set of seed-based analyses that tested for significant correlations of age with positive or negative resting-state FC between each of our nine seeds and any other voxel in the brain (for details, see Supplementary Methods in Online Resource 2). In brief, these analyses comprised tests for decreases in positive FC with age, indicating age-dependent reductions in neural coupling, and tests for increases in negative FC with age, indicating diminished neural anti-correlations in advanced age. For either case, the statistical maps obtained from each of the nine seed-based analyses were merged to identify regions that consistently showed significant age modulations of their FC with several seed regions.
With respect to declines in positive FC with age, these analyses revealed no additional regions (i.e., no regions outside the a priori task network) with significantly age-sensitive connections to more than half of the seed regions. In fact, only a few additional regions were observed with age-sensitive connections to just three or four seeds (see Fig. 5A). Importantly, these additional regions were mostly adjacent to, and partially overlapping with, one of the seeds (right DLPFC, right dPMC, right TPJ, pre-SMA). The only regions clearly outside the a priori network showing at least some consistency in age-related coupling changes were found in left supramarginal gyrus, dorsal posterior cingulate cortex, and cerebellum (Fig. 5A).
Fig. 5.
Results of whole-brain seed-based analyses of functional connectivity (FC) rendered on a standard brain. Panel A depicts the a priori defined incompatibility-related network (red-to-yellow colour scale) together with overlays of regions with age-related decreases in positive FC with 3 (green), 4 (blue), 5 (violet), or 6 (cyan) nodes of the a priori defined network. Panel B depicts overlays of regions with age-related increases in negative FC (i.e., diminished anti-correlation) with 3 (green), 4 (blue), 5 (violet), or 6 (cyan) nodes of the a priori defined incompatibility-related network.
Further, our exploratory whole-brain analysis revealed four clusters outside the target network (posterior cingulate cortex, anterior cuneus, left and right angular gyrus; see Fig. 5B) whose negative FC with six seed regions was inversely correlated with age. Beside these regions, there were several other clusters with age-sensitive anti-correlated connections to only a few seeds, in particular the dorsal and ventral medial prefrontal cortex, precuneus, medial occipital areas (V1, V2, V3), as well as left middle frontal and middle temporal gyri (Fig. 5B). Apart from the “early” visual areas in occipital cortex, all these clusters observed here were previously found to be part of the so-called “default-mode network” (Buckner et al., 2008; Greicius et al., 2003; Schilbach et al., 2012), which is typically deactivated during tasks that require sensorimotor processing such as our SRC task (Bzdok et al., 2013a, 2013b; Shulman et al., 1997; Toro et al., 2008).
3.2.6. Supplementary analysis III: Control for regional gray-matter atrophy with age
As normal aging has been found associated with regional grey-matter atrophy (Hoffstaedter et al., 2014; Salthouse, 2011), which in turn may underlie or contribute to FC changes, we performed supplementary analyses of such age-related structural changes in all our seed regions using voxel-based morphometry (VBM). This analysis was done in a subsample of 301 participants for which both resting-state fMRI data and structural T1-weighted images were available. For all seed regions that showed significant gray-matter volume changes with age, the morphological parameters obtained with VBM were subsequently used to control for the influence of regional atrophy on FC– age relationships (for details, see Supplementary Methods in Online Resource 3).
Rank correlation analyses revealed significant associations between gray-matter volume and age for 4 seed regions: bilateral anterior insula (left: rs = −.45; right: rs = −.27), pre-SMA (rs = −.30) and right dPMC (rs = −.25). Subsequent analyses demonstrated that all previously observed FC–age correlations involving these regions became smaller but, importantly, remained significant (Bonferroni-corrected) after removing the influence of grey-matter atrophy on interregional FC (correlations of age with FC between left insula – right insula: rs = −.23; left insula – pre-SMA: rs = −.29; left insula – right DLPFC: rs = −.19; right insula – pre-SMA: rs = −.36; right insula – right DLPFC: rs = −.22; right insula – right dPMC: rs = −.18). These results in the smaller subsample attest to the robustness of our main findings.
3.2.7. Discussion
Experiment 2 showed, first of all, substantial intrinsic FC between many regions associated with responding under conditions of spatial S-R incompatibility. Thus, the functionally defined, SRC task–related brain network is, to a large extent, functionally interconnected at rest as well. These findings attest to the “network status” of this set of brain regions (i.e., their intercommunication and interaction), in line with a recent study on the task-related effective connectivity among these regions (Cieslik et al., 2011). Importantly, we observed age-related decreases in intrinsic FC between several nodes of this network, with a predilection for reduced interconnectivity of the prefrontal and insular clusters. That is, FC between pre-SMA, anterior insula, and right DLPFC was particularly sensitive to age. These changes are in line with recent findings by Onoda et al. (2013), who reported similarly selective age effects on intrinsic FC within and between several resting-state networks defined via independent component analysis (i.e., significant age-related FC reductions between bilateral anterior insula and midcingulate cortex, but no FC changes within a bilateral fronto-parietal network or across both networks). Another earlier study reported decreased task-based FC with age among a set of brain regions associated with the cue-induced preparation for task switches (Madden et al., 2010). However, although several regions of this network overlapped with ours (among them right DLPFC, right TPJ, left IPS), a detailed comparison with our results is impossible, since the authors did not report FC measures for single pairs of regions but only an average score across several connections.
Our supplementary VBM analyses showed age-related grey-matter volume decreases in several seed regions, in line with previous findings (Good et al., 2001; Sowell et al., 2003). Interestingly, these morphological changes occurred mainly in those regions that also showed FC changes with age (i.e., bilateral anterior insula, pre-SMA and right dPMC). Importantly, however, these FC changes were not “explained away” by those age-related morphological changes. The selective deterioration in grey-matter volume with age thus does not appear to mediate the negative relationship between age and functional coupling observed for anterior insula, pre-SMA and right dPMC. Nevertheless, the diminishments of the FC–age relationship after accounting for regional grey-matter volume suggest that structural changes may contribute to FC decline in healthy aging.
Notably, the majority of interregional connections (12 out of 19) that showed intrinsic FC of at least medium effect size was unaffected by age, or, in one case, even increased its strength with age. This provides evidence against the notion of a general age-related decline in the functional coupling between distant brain regions. Rather, our findings argue for selective changes in the efficiency of interregional communication and interaction across the lifespan, suggesting age-related deterioration in some cognitive subprocesses but not in others. This reasoning is further supported by the results of our control analysis in a network associated with cross-modal emotional processing (Müller et al., 2012). In this network, we only found the interhemispheric connections between three pairs of homotopic seeds to be sensitive to age (Zuo et al., 2010). In Müller et al.’s analysis of task-related effective connectivity within this network, the authors observed that it was exactly these interhemispheric connections that were least important for selecting the best model. Together, this suggests that the few significant age-related changes in FC observed in our control analysis did not occur between nodes that subserve greatly different processing sub-functions. In contrast, we did find such age-dependent internodal (but no general interhemispheric) FC differences in our incompatibility-related target network, further underscoring the specificity of the changes observed therein.
The quantitative functional profiling revealed an association with attentional processing/control for both the age-sensitive and the age-insensitive parts of the task network, which is little surprising. The contrast analysis showed a stronger association of the age-sensitive sub-network with cognitive processing in general. Of note, BrainMap experiments are labelled as related to “general cognition” mostly if they do not fit into any of the more specific “cognition” subcategories, such as memory, attention, language, reasoning, social cognition, or cognition related to space or time. This argues for an association of this sub-network as a whole with some rather abstract, high-level cognitive process(es), possibly related to task-set control and implementation (cf. Dosenbach et al., 2006; Langner & Eickhoff, 2013). In contrast, the age-insensitive sub-network showed a stronger association with visual motion perception and action execution, which, together with its association with attention, points to its role in some intermediate-level, more concrete and action-oriented processing. That is, the communication between regions involved in abstract, high-level cognitive processes deteriorates in advanced age, while it remains intact (or even increases) between regions involved in more concrete sensorimotor cognition. These differential profile patterns suggest that aging affects SRC task performance via impairing high-level control processes rather than conflict resolution per se, possibly mediated – at least in part – through an age-related decline in the neural coupling between brain regions implementing task sets.
Our supplementary analysis of whole-brain positive FC of each node of the incompatibility-related network did not reveal any outside-network regions whose connections to the target network were consistently (i.e., across more than four nodes) modulated by age. We, therefore, argue that the few age-sensitive connections between target- and outside-network regions that we did observe are too inconsistent to be a major driving force behind the age-related behavioural deficits in cognitive action control observed in Experiment 1. Of note, the only two clusters with age-sensitive connections to six task-network seeds were located in left and right anterior insula (i.e., in parts of the task network), corroborating our previous network-based findings. That is, the exploratory whole-brain analysis actually provided additional evidence that the key connectivity changes are located within rather than outside the network. Conversely, the analysis of whole-brain negative FC of each task-network node yielded a set of consistently age-sensitive connections to regions of the default-mode network, associated with internally oriented cognition (e.g., mnemonic, imaginary, introspective, or social-cognitive processes; cf. Binder et al., 1999; Bzdok et al., 2012; Buckner et al., 2008; Schilbach et al., 2012). The observed age-dependent reductions in anti-correlation between these regions and several of our task-related network nodes might thus reflect difficulties in recruiting task-relevant regions selectively in advanced age, leading to “intrusions” or interference from task-irrelevant cognition (cf. Mason et al., 2007; Roski et al., 2013a; Weissman et al., 2006). Such potentially dysfunctional age-related changes in FC between functionally distinct brain networks (i.e., neural de-differentiation) have been recently reported for visual-attention and sensorimotor networks (Roski et al., 2013b) and might contribute to the behavioural deficits observed in Experiment 1 by undermining proper task-set implementation in the elderly.
In conclusion, Experiment 2 revealed selective age-related decreases in intrinsic FC predominantly among prefrontal and insular parts of a network that was previously found to be associated with solving incompatibility-induced response conflicts (cf. Cieslik et al., 2010). This result is in agreement with other reports about selective, rather than generalized, adult age differences in FC between distributed brain regions (cf. Ferreira and Busatto, 2013; Goh, 2011). Our findings in the task-related network were supplemented by the results of exploratory whole-brain FC analyses, which revealed no outside-network regions with consistent age-related reductions in their positive FC with nodes of the task network. These whole-brain analyses, however, detected some regions known to be part of the default-mode network that showed consistent age-related reductions in their functional anti-correlation (i.e., less negative FC) with several task-network nodes. Taken together, these exploratory findings corroborate our above conclusion and extend it by suggesting that the age-related behavioural deficits in speeded response conflict solution observed in Experiment 1 might partially result from a diminished de-coupling of regions subserving task-irrelevant cognition (cf. Weissman et al., 2006).
4. General discussion
4.1. Theoretical implications
In line with previous work (Grandjean and Collette, 2011; Proctor et al., 2005), we observed increased age-related costs of spatial S-R incompatibility. These costs were evident in the age-specific slowing of responses to spatially incompatible stimuli, while performance accuracy under conditions of incompatibility was not specifically reduced in advanced age. This suggests that elderly participants, although generally making more errors, manage spatial S-R incompatibility the same way young adults do but, apparently, at the expense of speed. Importantly, this age-specific slowing of overcoming incompatibility was independent of response selection speed on compatible trials, which reflects automatic (“bottom-up”) response activation and may be considered a marker of general information processing speed. The increase in age-related incompatibility costs remained also present after accounting for performance accuracy, motor speed, speeded visuomotor coordination, and cognitive flexibility. Together, these findings indicate that managing spatial S-R incompatibility is compromised in higher age, beyond a global slowing of cognitive processing and independently of potential mediator variables. Thus, extending earlier research, our results provide evidence for a selective deficit in cognitive action control in older age. The behavioural data alone, however, do not indicate which subprocesses during response conflict solution might be specifically affected by age. Next, therefore, the FC data will be discussed and examined for further clues to answer this question.
Several parts of the brain network involved in solving S-R incompatibility (Cieslik et al., 2010) showed a decrease in intrinsic FC strength with age. Besides changes in regional activity (cf. Lee et al., 2006), this intrinsic connectivity decline might contribute to the age-related drop in efficiency in response conflict resolution as observed in Experiment 1. Although our experiments do not provide direct evidence for this assumption (see section 4.2 for further discussion), it receives indirect support from the cognitive functions associated with the areas showing age-related connectivity changes: The age-related FC decrease was mainly confined to right DLPFC, bilateral anterior insula, and pre-SMA (including adjacent midcingulate cortex). In line with our functional profiling results, these heteromodal regions are involved in high-level, integrative aspects of the intentional control of attention and action (Cole and Schneider, 2007; Corbetta and Shulman, 2002; Langner et al., 2011; Posner and Petersen, 1990). More specifically, right DLPFC has been repeatedly associated with top-down (i.e. rule-based and goal-oriented) response selection according to stimulus location (Cieslik et al., 2013b; Cisek, 2006; Eickhoff et al., 2011b; Langner et al., 2013; Schumacher et al., 2003). In a recent meta-analysis (Langner and Eickhoff, 2013), the same region was also found to be consistently related to sustaining attention to simple (non-spatial) tasks over time, in line with notions of its involvement in task-set monitoring (Shallice et al., 2008) and set-contingent biasing of sensorimotor processing (Corbetta et al., 2008). However, the coupling between right DLPFC and dPMC or pre-SMA/midcingulate cortex, respectively, was unaffected by age, suggesting that the modulatory influence of DLPFC on spatial S-R mapping and motor preparation, as presumably subserved by premotor regions (cf. Cieslik et al., 2011), is not directly altered in advanced age. Rather, the coupling decreased between DLPFC and bilateral anterior insula, which in turn demonstrated a substantial reduction in FC strength with pre-SMA. Thus, age-related changes in the impact of DLPFC signalling on premotor processing appear to be more indirect, presumably mediated via anterior insula–pre-SMA circuits.
Anterior insula and pre-SMA/midcinglulate cortex are anatomically and functionally tightly connected (Augustine, 1996; Taylor et al., 2009) and appear to be jointly involved in implementing (i.e. activating and maintaining) task sets (Dosenbach et al., 2007; Dosenbach et al., 2006; Kurth et al., 2010). Task-set maintenance may include the repeated reactivation of task rules, triggered, for instance, by the occurrence of an external imperative stimulus or internal signals from performance monitoring systems (see also Langner and Eickhoff, 2013; Sridharan et al., 2008). This view accords with the functions of “energizing” (i.e. activating) task schemata and monitoring their activation level, which were ascribed to dorsomedial prefrontal cortex and inferior lateral frontal cortex/anterior insula, respectively, based on human lesion studies (Shallice et al., 2008; Stuss et al., 1995). As pre-SMA was previously found to effectively modulate primary motor cortex activity on S-R-incompatible trials, presumably via inhibiting the automatic activation of the ipsilateral response (Cieslik et al., 2011), the observed age-related decline in pre-SMA–insula coupling might lead to inefficiency in translating the instructed task set into appropriate top-down inhibitory signals and, thus, produce the specific RT slowing on incompatible trials in older age. This inefficiency might be aggravated by the additional FC decline between right anterior insula and right dPMC, which is involved in the cue-related preparation of speeded movements (Hoshi and Tanji, 2006; Langner et al., 2012; Weinrich and Wise, 1982). Finally, the reduced intrinsic coupling between anterior insula and right DLPFC with age may reflect diminished efficiency in transmitting reactive control signals from anterior insula to DLPFC. Such signalling, which may lead to adjustments of input expectations and associated biasing signals in DLPFC, has been suggested as a means by which the task-set maintenance system may counteract performance decline and (re)engage the mind in task-relevant processing with appropriate intensity (Langner and Eickhoff, 2013). In summary, the selective prefrontal and insular reductions in intrinsic FC strength with age could reflect increasingly dysfunctional levels of interaction between several task-relevant brain regions. These changes, in turn, might lead to reduced efficiency in using this network when task sets need to be implemented against prepotent response tendencies.
Taken together with the results of Experiment 1, our findings argue against an explanation purely based on a domain-general decline in cognitive efficiency with age, as suggested by the processing-speed theory of cognitive aging (Salthouse, 1996). Rather, it appears that aging might selectively affect some higher-level cognitive functions involved in task-schema activation and monitoring. The nature of these mental faculties may, in turn, contribute to the pervasiveness of age-related performance deficits across tasks that require maintaining and managing multiple task sets (e.g., dual-tasking or task switching; cf. Verhaeghen et al., 2003; Wasylyshyn et al., 2011). It might also explain the absence of consistent aging effects in tasks that “only” require attentionally selecting among different stimuli or stimulus dimensions (e.g., flanker or Stroop tasks), rather than requiring the selection of responses depending on the current (but alternating) task set (see Verhaeghen, 2011, for a review).
Since SRC tasks require the block-wise alternating implementation of two opposing task sets (i.e., responding ipsi- vs. contralaterally), they should pose greater demands on task-set maintenance and monitoring, as compared with tasks that do not involve several task sets (cf. Logan, 2007; see also Los, 1996). In the context of task-switching, such set-level mixing costs are typically referred to as global switch costs (see Kiesel et al., 2010, for a review). A recent meta-analysis demonstrated that it is those global task-switching costs that are selectively increased in advanced age, while “local” (i.e. trial-level) switch costs largely remain unaffected by age (Wasylyshyn et al., 2011). The authors concluded that “…having to maintain two task sets does involve a[n age-related] deficit over and beyond the effects of general slowing” (p. 19). This agrees well with our above interpretation and converges with our neural findings (i.e., most severe FC decreases with age between regions associated with task-set implementation and maintenance).
The predominance of task-set maintenance over switching deficits in our interpretation of age differences in overcoming automatic response tendencies is further supported by our finding that the incompatibility × age interaction effect on RT was not abolished by taking cognitive flexibility into account. Cognitive flexibility, as assessed via the TMT-B–TMT-A difference, reflects the ability to rapidly switch between two different task sets (i.e., search for numbers vs. letters; cf. Sánchez-Cubillo et al., 2009). In contrast, the block-wise performance averaging in SRC tasks rather emphasizes the ability to retrieve and maintain an instructed task set in the face of competition from a second task set regularly used within the same task. Accordingly, our findings agree well with previous suggestions that keeping competing task sets apart and suppressing the currently irrelevant one might constitute a core problem in advanced age (cf. Mayr and Liebscher, 2001; Vu and Proctor, 2008). As spatially compatible responses are highly overlearned, the S-R-compatible task set should produce substantial competition, thus making the opposing (incompatible) task set even more difficult to maintain. Coping with this biased competition might become harder with advancing age and form one of the mechanisms that contribute to the age-specific decline in managing S-R incompatibility.
Finally, although we did not investigate task-induced brain activity in the present study, we would like to comment briefly on the potential interplay between age-related changes in regional brain activation as observed previously and intrinsic interregional coupling as observed here. In fact, it has been repeatedly shown across a range of cognitive tasks that higher age is associated with the recruitment of additional (e.g. contralateral and/or prefrontal) brain regions (for reviews, see Cabeza, 2002; Dennis and Cabeza, 2008; Park and Reuter-Lorenz, 2009). Such age-related regional hyperactivity has often been interpreted as compensatory (cf. Grady, 2008; Reuter-Lorenz and Cappell, 2008). We suggest that reduced interregional coupling may be one of the neural changes with age that need to be compensated. Thus, as argued above, the observed FC changes could constitute the neural substrate of an age-related decline in the efficiency of modulatory control signalling, leading to greater functional brain responses to maintain similar levels of cognitive control (cf. Andrews-Hanna et al., 2007; Turner and Spreng, 2012). In other words, age-related reductions in FC might drive the additional recruitment of brain regions in higher age. Moreover, this interaction effect may well work both ways: age-related increases in FC, as observed here for pre-SMA–IPS connections, might also form a compensatory brain response to reduced or dedifferentiated functional brain activity. At any rate, acknowledging the possibility of compensatory changes across multiple dimensions of brain functioning should more generally serve as a caveat against a potentially premature interpretation of unidimensional brain–behaviour relationships as either compensatory or dysfunctional.
4.2. Limitations and future directions
In Experiment 1, we relied on comparisons between two age categories (i.e., younger and older adults), which maximizes the sensitivity for age effects but, without intermediate age values, precludes investigating the trajectory of age-related changes. This drawback, however, does not apply to Experiment 2, where a large sample with a continuous age distribution was studied. Furthermore, both experiments share the limitations of a cross-sectional approach, which necessarily conflates age and cohort effects. Future studies using longitudinal designs should try to overcome these limitations.
A specific limitation to Experiment 2 is the hypothesis-driven selection of regions of interest based on a previous fMRI study (Cieslik et al., 2010). This preselection might have resulted in including clusters that reflect idiosyncrasies of Cieslik et al.’s study, but, importantly, it was based on the same task as used in Experiment 1. Also, supplementary seed-based whole-brain FC analyses indicated that we most likely did not miss any consistent age-related FC changes occurring outside the a priori defined task-relevant network, such as FC changes between regions that might only become relevant for successful SRC task performance in advanced age (cf. Cabeza, 2002). Furthermore, although all participants were screened for psychiatric and neurological disorders, the sensitivity of the screenings used at the four sites could be different. Therefore, it cannot be completely excluded that some participants with sub-clinical cognitive impairments were included.
Finally, behavioural and FC changes with age were examined in two separate experiments using data from independent, non-overlapping samples. This precluded us from directly relating individual FC parameters to performance measures. However, by investigating FC in exactly the network that is associated with incompatibility-related processing in the paradigm of Experiment 1, we provide complementary evidence linked by functional neuroanatomy. In future research it would be desirable to combine performance and fMRI measurements in the same participants and to use several imaging modalities (e.g., task-related and resting-state fMRI) in order to arrive at a more comprehensive picture of neural changes with age, enabling direct tests of these changes’ (potentially compensatory) interdependencies and associations with performance (cf. Andrews-Hanna et al., 2007; Madden et al., 2010; Schulte et al., 2011). Optimally, such studies would also consider morphological parameters. This would allow for investigating the question as to what degree age-related changes in behaviour, regional brain activity, and interregional FC are mediated by structural changes, for instance due to “silent” strokes, which could occur more frequently in particular parts of the cortex.
4.3. Conclusions
Experiment 1 established that the age-related increase in S-R incompatibility costs is independent of both global slowing in cognitive processing speed with age (Salthouse, 1996) and other potential mediator variables that partially predicted the SRC effect. Experiment 2 demonstrated selective decreases in intrinsic FC between several brain regions that had previously been found to be associated with solving such incompatibility-induced response conflicts in the same task (cf. Cieslik et al., 2010). Additional analyses revealed deterioration in grey-matter volume in most of the regions showing FC decline with age, but the morphological changes did not fully explain the changes in FC. Further exploratory analyses revealed several regions of the “default mode” network whose de-coupling from the SRC-related network was consistently diminished in advanced age. The behavioural data as well as the cognitive functions of the regions whose intrinsic FC strength decreased with age jointly suggest that healthy aging is selectively associated with deterioration in maintaining/monitoring relevant task schemata, ultimately leading to less efficient cognitive control over action. Thus, our findings argue against an age-related decline in cognitive conflict resolution ability per se. They furthermore provide converging evidence against the notion of a uniform decline in the general efficiency of cognitive processes with age. On the contrary, the preserved intrinsic FC between many network nodes can be taken to indicate that the efficiency with which regions involved in top-down action control communicate with each other is, to a large degree, well maintained across the adult lifespan.
Supplementary Material
Online Resource 1 Supplementary results of the quantitative functional profiling (Fig. S1) and the connectivity analysis in an emotion-related control network (Table S1).
Online Resource 2 Supplementary methods of the exploratory whole-brain connectivity analysis.
Online Resource 3 Supplementary methods of the voxel-based morphometry analysis and the control for the influence of morphological parameters on functional connectivity.
Acknowledgments
The study was in part supported by the Human Brain Project (R01-MH074457-01A1, S.B.E.), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model, S.B.E), the Helmholtz Alliance for Mental Health in an Aging Society (HelMA, K.A.), and the German Research Foundation (DFG: EI 816/4-1, S.B.E.; and LA 3071/3-1, R.L. & S.B.E.).
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Behrwind SD, Dafotakis M, Halfter S, Hobusch K, Berthold-Losleben M, Cieslik EC, Eickhoff SB. Executive control in chronic schizophrenia: A perspective from manual stimulus-response compatibility task performance. Behav. Brain Res. 2011;223:24–29. doi: 10.1016/j.bbr.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. J. Cogn. Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bonin-Guillaume S, Possamai CA, Blin O, Hasbroucq T. Stimulus preprocessing, response selection, and motor adjustment in the elderly: An additive factor analysis. Cah. Psychol. Cogn. 2000;19:245–255. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB. Segregation of the human medial prefrontal cortex in social cognition. Front. Hum. Neurosci. 2013a;7:232. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, Eickhoff SB. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013b;81:381–392. doi: 10.1016/j.neuroimage.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Chen NK, Chou YH, Song AW, Madden DJ. Measurement of spontaneous signal fluctuations in fMRI: adult age differences in intrinsic functional connectivity. Brain Struct. Funct. 2009;213:571–585. doi: 10.1007/s00429-009-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Müller VI, Kellermann TS, Grefkes C, Halfter S, Eickhoff SB. Shifted neuronal balance during stimulus–response integration in schizophrenia: an fMRI study. Brain Struct. Funct. 2013a doi: 10.1007/s00429-013-0652-1. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb. Cortex. 2013b;23:2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Grefkes C, Eickhoff SB. Dynamic interactions in the fronto-parietal network during a manual stimulus-response compatibility task. NeuroImage. 2011;58:860–869. doi: 10.1016/j.neuroimage.2011.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Kurth F, Eickhoff SB. Dissociating bottom-up and top-down processes in a manual stimulus-response compatibility task. J. Neurophysiol. 2010;104:1472–1483. doi: 10.1152/jn.00261.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J. Neurosci. 2006;26:9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Amunts K, Laird AR, Fox PT, Eickhoff SB. Tackling the multifunctional nature of Broca’s region meta-analytically: Co-activation-based parcellation of area 44. Neuroimage. 2013;83:174–188. doi: 10.1016/j.neuroimage.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Salthouse TA. The handbook of aging and cognition. New York, NY: Psychology Press; 2008. [Google Scholar]
- Dafotakis M, Grefkes C, Eickhoff SB, Karbe H, Fink GR, Nowak DA. Effects of rTMS on grip force control following subcortical stroke. Exp. Neurol. 2008;211:407–412. doi: 10.1016/j.expneurol.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT-PD) Mov. Disord. 1999;14:572–584. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. London, UK: Lawrence Erlbaum; 2008. pp. 1–54. [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage. 2011a;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Pomjanski W, Jakobs O, Zilles K, Langner R. Neural correlates of developing and adapting behavioural biases in speeded choice reactions: An fMRI study on predictive motor coding. Cereb. Cortex. 2011b;21:1178–1191. doi: 10.1093/cercor/bhq188. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 2013;37:384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Fitts PM, Deininger RL. S-R compatibility: Correspondence among paired elements within stimulus and response codes. J. Exp. Psychol. 1954;48:483–492. doi: 10.1037/h0054967. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: description and evaluation. Hum. Brain Mapp. 2005;25:185–198. doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv. Anat. Embryol. Cell Biol. 2004;174:1–89. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- Goh JO. Functional dedifferentiation and altered connectivity in older adults: Neural accounts of cognitive aging. Aging Dis. 2011;2:30–48. [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional connectivity during memory tasks in healthy aging and dementia. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford, UK: Oxford University Press; 2005. pp. 286–308. [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann. N. Y. Acad. Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grandjean J, Collette F. Influence of response prepotency strength, general working memory resources, and specific working memory load on the ability to inhibit predominant responses: a comparison of young and elderly participants. Brain Cogn. 2011;77:237–247. doi: 10.1016/j.bandc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Roski C, Caspers S, Zilles K, Eickhoff SB. Age-related decrease of functional connectivity additional to gray matter atrophy in a network for movement initiation. Brain Struct. Funct. 2014 doi: 10.1007/s00429-013-0696-2. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Prinz W. Theoretical issues in stimulus–response compatibility. The Netherlands: North-Holland, Amsterdam; 1997. [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J. Neurophysiol. 2006;95:3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. NeuroImage. 2012;60:2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J, Fast K, Mielke R. Zur Problematik der prämorbiden Intelligenzdiagnostik mit dem MWT-B bei Patienten mit Alzheimer-Erkrankung [Premorbid intelligence diagnosis with the MWT-B (Multiple-Choice Word Test–B) in patients with Alzheimer's disease] Nervenarzt. 1995;66:696–702. [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I. Control and interference in task switching – a review. Psychol. Bull. 2010;136:849–874. doi: 10.1037/a0019842. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the Brainmap database: progress towards a probabilistic functional brain atlas. Front. Neuroinformatics. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Boers F, Sturm W, Willmes K, Eickhoff SB. Modality-specific perceptual expectations selectively modulate baseline activity in auditory, somatosensory, and visual cortices. Cereb. Cortex. 2011;21:2850–2862. doi: 10.1093/cercor/bhr083. [DOI] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Eickhoff SB, Boers F, Chatterjee A, Willmes K, Sturm W. Staying responsive to the world: Modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum. Brain Mapp. 2012;33:398–418. doi: 10.1002/hbm.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Sternkopf MA, Kellermann TS, Grefkes C, Kurth F, Schneider F, Zilles K, Eickhoff SB. Translating working memory into action: Behavioral and neural evidence for using motor representations in encoding visuo-spatial sequences. Hum. Brain Mapp. 2013 doi: 10.1002/hbm.22415. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Zhang JX, Chan CC, Yuen KS, Chu LW, Cheung RT, Chan YS, Fox PT, Gao JH. Age-related differences in response regulation as revealed by functional MRI. Brain Res. 2006;1076:171–176. doi: 10.1016/j.brainres.2005.12.124. [DOI] [PubMed] [Google Scholar]
- Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest (MWT-B) [Multiple-Choice Vocabulary Test–B] 5th ed. Germany: Spitta-Verlag, Balingen; 2005. [Google Scholar]
- Logan GD. What it costs to implement a plan: Plan-level and task-level contributions to switch costs. Mem. Cognit. 2007;35:591–602. doi: 10.3758/bf03193297. [DOI] [PubMed] [Google Scholar]
- Los SA. On the origin of mixing costs: Exploring information processing in pure and mixed blocks of trials. Acta Psychol. 1996;94:145–188. [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. NeuroImage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto E, Misaki M, Miyauchi S. Neural mechanisms of spatial stimulus-response compatibility: the effect of crossed-hand position. Exp. Brain Res. 2004;158:9–17. doi: 10.1007/s00221-004-1872-7. [DOI] [PubMed] [Google Scholar]
- Mayr U, Liebscher T. Is there an age deficit in the selection of mental sets? Eur. J Cognit. Psychol. 2001;13:47–69. [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J. Cogn. Neurosci. 2012;24:2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Schwarz N. Cognitive aging: A primer. Philadelphia, PA: Psychology Press; 2000. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Vu KP, Pick DF. Aging and response selection in spatial choice tasks. Hum. Factors. 2005;47:250–270. doi: 10.1518/0018720054679425. [DOI] [PubMed] [Google Scholar]
- Reitan RM. The relation of the trail making test to organic brain damage. J. Consult. Psychol. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008;17:177–182. [Google Scholar]
- Roski C, Caspers S, Langner R, Laird AR, Fox PT, Zilles K, Amunts K, Eickhoff SB. Adult age-dependent differences in resting-state connectivity within and between visual-attention and sensorimotor networks. Front. Aging Neurosci. 2013a;5:67. doi: 10.3389/fnagi.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roski C, Caspers S, Lux S, Hoffstaedter F, Bergs R, Amunts K, Eickhoff SB. Activation shift in elderly subjects across functional systems: an fMRI study. Brain Struct. Funct. 2013b doi: 10.1007/s00429-013-0530-x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz JB, Zilles K, Laird AR, Fox PT, Eickhoff SB. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Struct. Funct. 2013;218:1551–1567. doi: 10.1007/s00429-012-0476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Theoretical perspectives on cognitive aging. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol. Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sánchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb. Cortex. 2008;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Chanraud S, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Age-related reorganization of functional networks for successful conflict resolution: A combined functional and structural MRI study. Neurobiol. Aging. 2011;32:2075–2090. doi: 10.1016/j.neurobiolaging.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D'Esposito M. Neural evidence for representation-specific response selection. J. Cogn. Neurosci. 2003;15:1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Alexander MP, Picton TW, Derkzen D. The multiple dimensions of sustained attention. Cortex. 2008;44:794–805. doi: 10.1016/j.cortex.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks. 2. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simon JR, Wolf JD. Choice reaction-time as a function of angular stimulus-response correspondence and age. Ergonomics. 1963;6:99–105. [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders FT, Kenemans JL, Schmidt WF, Kok A. Effects of task complexity in young and old adults: Reaction time and P300 latency are not always dissociated. Psychophysiology. 1999;36:118–125. doi: 10.1017/s0048577299961590. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain Cogn. 2009;70:1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Ann. N. Y. Acad. Sci. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb. Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Executive functions and neurocognitive aging: dissociable patterns of brain activity. Neurobiol. Aging. 2012;33:826, e821–e813. doi: 10.1016/j.neurobiolaging.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. Aging and executive control: Reports of a demise greatly exaggerated. Curr. Dir. Psychol. Sci. 2011;20:174–180. doi: 10.1177/0963721411408772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: a meta-analysis. Psychol. Aging. 2003;18:443–460. doi: 10.1037/0882-7974.18.3.443. [DOI] [PubMed] [Google Scholar]
- Vu KP, Proctor RW. Age differences in response selection for pure and mixed stimulus-response mappings and tasks. Acta Psychol. 2008;129:49–60. doi: 10.1016/j.actpsy.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyshyn C, Verhaeghen P, Sliwinski MJ. Aging and task switching: a meta-analysis. Psychol. Aging. 2011;26:15–20. doi: 10.1037/a0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. J. Neurosci. 1982;2:1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. NeuroImage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Hallett M, Chen Y, Li K, Chan P. Aging influence on functional connectivity of the motor network in the resting state. Neurosci. Lett. 2007;422:164–168. doi: 10.1016/j.neulet.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain's dark energy. Nat. Rev. Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Supplementary results of the quantitative functional profiling (Fig. S1) and the connectivity analysis in an emotion-related control network (Table S1).
Online Resource 2 Supplementary methods of the exploratory whole-brain connectivity analysis.
Online Resource 3 Supplementary methods of the voxel-based morphometry analysis and the control for the influence of morphological parameters on functional connectivity.