Abstract
NAADP is a potent Ca2+ mobilizing messenger in a variety of cells but its molecular mechanism of action is incompletely understood. Accumulating evidence indicates that the poorly characterized two-pore channels (TPCs) in animals are NAADP sensitive Ca2+-permeable channels. TPCs localize to the endo-lysosomal system but are functionally coupled to the better characterized endoplasmic reticulum Ca2+ channels to generate physiologically relevant complex Ca2+ signals. Whether TPCs directly bind NAADP is not clear. Here we discuss the idea based on recent studies that TPCs are the pore-forming subunits of a protein complex that includes tightly associated, low molecular weight NAADP-binding proteins.
Keywords: Ca2+ Signaling, NAADP, Two-Pore Channels, Endosomes, Lysosomes, Photoaffinity Labelling
INTRODUCTION
Changes in cytosolic Ca2+ signal a multitude of cellular events spanning from birth to death and likely everything in between (Berridge et al., 2000). Unsurprisingly, these changes are remarkably complex displaying marked temporal and spatial heterogeneity (Diambra and Marchant, 2009). Deregulated Ca2+ signals underlie many pathologies including neurodegeneration and heart failure (Sammels et al., 2010). This underscores not only the physiological importance of Ca2+ but the need to define precisely the mechanism of Ca2+ homeostasis. There exist a diverse range Ca2+-permeable channels on the plasma membrane which open to mediate Ca2+ influx from the essentially infinite reservoir of Ca2+ in the extracellular fluid (Berridge et al., 2000). However Ca2+ is also dynamically regulated by a much more limited number of intracellular Ca2+ release channels on intracellular Ca2+ stores (Berridge et al., 2000). These stores include the well characterized endoplasmic reticulum (ER) (Berridge, 2002) and also a morphologically eclectic variety of acidic organelles such as lysosomes which comprise the acidic Ca2+ stores (Patel and Docampo, 2010; Patel and Muallem, 2011). Their associated channels are activated by Ca2+ mobilizing messengers produced in response to cellular stimulation by a wide spectrum of extracellular cues that include hormones and neurotransmitters. Recruitment of intracellular Ca2+ channels then represents a key point of convergence in mediating Ca2+-dependent output.
Inositol trisphosphate (IP3) and cyclic ADP-ribose (cADPR) are Ca2+ mobilizing messengers that activate well-defined Ca2+ channels (IP3 receptors and ryanodine receptors) on ER Ca2+ stores (Patel et al., 1999; Foskett et al., 2007; Fill and Copello, 2002; Hamilton and Serysheva, 2009). Nicotinic acid adenine dinucleotide phosphate (NAADP) is the most recent addition to the Ca2+ mobilizing messenger family (Lee, 2011). Like its counterparts, NAADP is produced in response cell surface stimulation (Galione et al., 2010) to regulate a variety of Ca2+ dependent cellular processes including fertilization (Churchill et al., 2003), contraction (Boittin et al., 2003), growth (Brailoiu et al., 2005) and differentiation (Brailoiu et al., 2006) (reviewed in (Hooper and Patel, 2011)). Definition of NAADP’s molecular mechanism of action however has proven difficult (Guse, 2009). Much evidence now indicates that NAADP activates the two-pore channels (TPCs) to mediate Ca2+ release from acidic organelles (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009) (reviewed in (Patel et al., 2010; Zhu et al., 2010). Here, were relate the properties of endogenous NAADP sensitive Ca2+ channels and NAADP binding sites to those of the TPCs. Based on recent photo-affinity labeling studies (Lin-Moshier et al., 2011; Walseth et al., 2011), we highlight the possibility that TPCs may not bind NAADP directly but rather associate with low molecular weight NAADP binding proteins to trigger Ca2+ release. Finally, we discuss the consequences of such a model in relation to established features of NAADP action.
NAADP-MEDIATED Ca2+ SIGNALING
Homogenates of sea urchin eggs have proven invaluable in the study of Ca2+ mobilizing messengers (Morgan and Galione, 2008). Large quantities of eggs can be readily obtained and disrupted using simple homogenization procedures to yield cell free organellar preparations that are amenable to deep-freezing without apparent loss of Ca2+ sequestration and Ca2+ release activity. It was using this preparation that Lee and colleagues began study of the mechanisms regulating Ca2+ release from intracellular Ca2+ stores, reporting in 1985 (Clapper and Lee, 1985) the Ca2+ mobilizing properties of IP3—then a nascent messenger (Streb et al., 1983). It was also the same preparation that this team noted the Ca2+ mobilizing ability of NAD and NADP (Clapper et al., 1987). The slow kinetics of Ca2+ release by NAD were ascribed to conversion of NAD by an unknown enzyme to a product named “E-NAD”. From a series of beautiful follow-up studies, we now know this enzyme to be a member of the multifunctional ADP-ribosyl cyclase family, and E-NAD as cADPR (Lee et al., 1989; Lee and Aarhus, 1991). Although the effects of NADP were more rapid in onset than NAD they were subsequently ascribed not to NADP itself but rather a structurally related minor contaminant (NAADP) (Lee and Aarhus, 1995). It is always worth stressing that the findings reported in a single paper (Clapper et al., 1987) are the origins of modern day research into not one but two Ca2+ mobilizing messengers.
Many of the established properties concerning the NAADP signaling stem from early work in the egg which we briefly review here. From the outset, it was clear that the Ca2+ mobilizing effects of NAADP were distinct from those of IP3 and cADPR (Clapper et al., 1987). Thus, the outcome of simple cross desensitization experiments indicated that all three messengers appeared to target distinct Ca2+ release channels (Clapper et al., 1987). This was confirmed by the lack of effect IP3and ryanodine receptor antagonists on Ca2+ release by NAADP (Chini et al., 1995). Rather NAADP appeared to be selectively blocked by L-type Ca2+ channel antagonists (Genazzani et al., 1996). Further distinguishing features included the lack of regulation of NAADP by cytosolic Ca2+ concentration (Chini and Dousa, 1996). This sharply contrast to the biphasic effects of Ca2+ on both IP3 and cADPR/ryanodine receptor mediated Ca2+ signals—a form of regulation that no doubt contributes to the complex spatial and temporal nature of the Ca2+ signals mediated by these messengers (Foskett et al., 2007; Fill and Copello, 2002). Additionally, NAADP-sensitive channels showed a marked propensity to inactivate such that prior treatment with sub-threshold concentrations of NAADP (<1 nM) were able to completely abolish subsequent release by a normally maximally effective concentration both in homogenates and intact eggs (Aarhus et al., 1996; Genazzani et al., 1996). On a spatial level, localized photolysis of NAADP could clearly shape Ca2+ signals upon global challenge with NAADP (Churchill and Galione, 2001b)—an elegant proof of principle that NAADP might regulate patterning events during development if generated endogenously. Finally, NAADP appeared to release Ca2+ not from the ER but rather from a distinct Ca2+ store. Thus, fractionation of egg homogenates on density gradients yielded a broad distribution of NAADP-responsive vesicles that contrasted to the distribution of IP3/cADPR-sensitive vesicles that co-migrated with markers for the ER (Clapper et al., 1987). Indeed, NAADP-evoked Ca2+ release persisted upon depletion of ER Ca2+ with the Ca2+ pump blocker, thapsigargin (Genazzani and Galione, 1996). Separation of NAADP-sensitive Ca2+ stores from those associated with ER could also be demonstrated in the intact egg. Gentle centrifugation of eggs on sucrose cushions results in stratification of organelles within the cytoplasm. Subsequent global release of messengers showed that whereas IP3 and cADPR release Ca2+ from the ER-rich nuclear pole, NAADP releases Ca2+ from the opposite pole of the cell (Lee and Aarhus, 2000). The identity of store came from the work by Churchill et al., who provided several lines of evidence in support of the idea that NAADP mediates Ca2+ release from the reserve granule—a lysosome-like organelle (Churchill et al., 2002). Again, such findings further enforce the non-canonical nature of NAADP. The humble sea urchin egg thus defined three key features of the NAADP pathway namely
that NAADP activates a Ca2+ channel with distinct properties to ER Ca2+ channels
that this channel displays unusual inactivation properties and
that it is functionally expressed in a lysosome-like organelle.
NAADP has since been shown to activate Ca2+ release in a multitude of different cells including many mammalian cell types (Cancela et al., 1999; Lee, 2005). In general the properties of the channel match those in the urchin. Notably, NAADP appears to mobilize Ca2+ from acidic organelles such as secretory granules (Mitchell et al., 2003), lysosomes (Yamasaki et al., 2004) and endosomes (Menteyne et al., 2006). One key difference that emerged is the mode by which NAADP inactivates in mammalian cells. This was recognized by Cancela et al. who showed that whereas nanomolar levels of NAADP were capable of evoking Ca2+ signals in pancreatic acinar cells (inferred through changes in Ca2+-dependent ion conductances), micromolar concentrations were paradoxically ineffective (Cancela et al., 1999). Thus, the concentration response curve for NAADP is “bell shaped” (Berg et al., 2000). Like in the sea urchin eggs then, NAADP undergoes an unusual form of “self” inactivation. However, in mammalian cells this inactivation occurs at supramaximal concentrations as opposed to sub-threshold levels. It should be noted that most studies in mammalian systems have been performed using intact cells and it is rather sad that an in vitro system as robust as the sea urchin egg homogenate has yet to be found. But it was the use of more physiological preparations that led to the final key property of the NAADP pathway namely the ability of NAADP to generate complex Ca2+ signals through the recruitment of other Ca2+ channels. Again, it was the work of Cancela et al. using the pancreatic acinar cell that established this paradigm (Cancela et al., 1999). They noted that NAADP responses were blocked by both IP3 and cADPR antagonists. In contrast, inactivating NAADP-sensitive channels (with self-inactivating concentrations of NAADP) did not alter the response to IP3 and cADPR. These data were rationalized in the “trigger” hypothesis where NAADP activates an NAADP-sensitive Ca2+ channel to generate a small release of Ca2+ which is subsequently amplified by Ca2+ channels on the ER through the process of Ca2+-induced Ca2+ release (Cancela et al., 1999). Indeed, that NAADP-sensitive Ca2+ channels are not themselves regulated by cytosolic Ca2+ (see above) would favour this linear sequence of events—a sequence that is presumably interrupted upon cellular disruption. This model has received widespread support (reviewed in (Patel and Brailoiu, 2012)). Returning to the sea urchin, a variation of this model is the so called “two pool model” whereby communication between channels is mediated by Ca2+ uptake into the ER resulting in the priming of ER Ca2+ channels (Churchill and Galione, 2001a)—a mechanism also likely operative in mammalian heart (Macgregor et al., 2007). Indeed, in intact sea urchin eggs, the two forms of “chatter” (Patel et al., 2001) co-exist whereby Ca2+-induced Ca2+ release locally amplifies NAADP-responses (Churchill and Galione, 2000) whereas Ca2+ uptake in to the ER likely drives long term Ca2+ oscillations (Churchill and Galione, 2001a).
NAADP BINDING SITES
With the identity of the target protein unknown and the consequent lack of molecular tools such as antibodies, early studies relied upon radioligand binding techniques to gain insight into the NAADP receptor. The characteristics of NAADP binding have been well characterized in sea urchin egg homogenates. Original studies identified a high “affinity” binding site for NAADP using enzymatically synthesized NAADP (Aarhus et al., 1996). This binding site was selective for NAADP as a range of other nucleotides (including NADP) were much less ineffective in competition assays (Table I). NAADP binding was insensitive to cytoplasmic Ca2+, pH and L-type Ca2+ channel blockers (consistent with Ca2+ release measurements, see Section 2) but inhibited by high ionic strength (Patel et al., 2000a; Genazzani et al., 1997). Unusually, NAADP appeared to bind in a non-reversible manner (Aarhus et al., 1996; Patel et al., 2000a; Billington and Genazzani, 2000). Thus, unlabeled NAADP was able to effectively compete with radiolabelled NAADP in the sub-nanomolar range when the two ligands were incubated with homogenate simultaneously (Table I). However, it was unable to displace already bound radiolabel as evidenced by the lack of effect of unlabeled NAADP once binding of radioligand had been initiated (Aarhus et al., 1996; Patel et al., 2000a; Billington and Genazzani, 2000). It is tempting to speculate that this binding site correlates with an inactivated conformation of the target protein (see Section 2) which is perhaps distinct from a lower affinity site associated with Ca2+ release. Indeed, this two site model (Patel, 2004) for NAADP action is supported by recent pharmacological studies. Rosen et al. have identified a methyl ester analogue of the NAADP antagonist NED19 (NED19.4) that competes with NAADP in binding assays, prevents inactivation of Ca2+ release by sub threshold concentrations of NAADP but has little effect on NAADP-evoked Ca2+ release (Rosen et al., 2009). Conversely, a second fluorinated analogue (NED20), has no effect on NAADP binding but prevents NAADP-evoked Ca2+ release (Rosen et al., 2009). These data are consistent with NED19.4 binding to the inactivation site whereas NED20 binds to the activation sites. The most unusual kinetics of dissociation (or lack thereof) of NAADP binding to the putative inactivation site in the egg appear to be regulated by K+. Thus, whereas binding is for all intents and purposes irreversible in a physiological intracellular-like medium, simple omission of K+ or its replacement with Na+ uncovers reversible components of binding (Dickinson and Patel, 2003). The physiological relevance of this regulation is not clear however this bizarre feature has proven a useful diagnostic in the molecular identification of NAADP targets (see Section 5).
Table I. NAADP binding sites.
Shown are the IC50 values for competition of either unlabeled NAADP or NADP with radiolabelled NAADP, and the associated selectivity ratio in the indicated cell type/tissue. Asterisked values represent Kd measurements.
| Cell type/tissue | IC50NAADP | IC50NADP | Selectivity (NAADP:NADP) | Reference |
|---|---|---|---|---|
| Sea urchin eggs | 0.54±0.07 nM | 639±64 nM | 1183 | (Patel et al., 2000a) |
| 0.19±0.04 nMa | 107 nM | 554 | (Billington and Genazzani, 2000) | |
| Mouse brain | 200±17 nM | — | — | (Patel et al., 2000b) |
| Rabbit heart | 4.2±0.71 nM | 206±24.5 nM | 49 | (Bak et al., 2001) |
| MIN6 cells | 130±31 nMa | — | — | (Masgrau et al., 2003) |
| 12±0.6 µMa | ||||
| Rat basophilic cells | 125±20 nM | 932±24 nM | 7 | (Billington et al., 2006) |
| Rat peritubular smooth muscle cells | 1.79±0.24 nM | 45.8±9.5 nM | 26 | (Gambara et al., 2008) |
| Mouse liver | 6.6±3.5 nM | 4.5±2.3 µM | 681 | (Calcraft et al., 2009) |
| 7.2±0.8 µM |
Two lines of evidence suggest that the identified binding site for NAADP relates to Ca2+ releasing machinery. First, the rank order of potency of NAADP analogues in competing with NAADP in binding assays (Patel et al., 2000b) is similar to that in evoking Ca2+ release (Lee and Aarhus, 1997). Second, although egg homogenates represent a robust system for characterization of Ca2+ release by Ca2+ mobilizing messengers, certain preparations are encountered occasionally that do not release Ca2+ in response to NAADP despite retaining full sensitivity to IP3 and cADPR. The reasons for this selective loss in sensitivity is not known but such preparations are also incapable of binding NAADP (Patel et al., 2000a) thus further correlating identified binding sites with Ca2+ release.
NAADP binding sites have been successfully solubilized using non-ionic and zwitterionic detergents. Soluble preparations bind NAADP with similar potency and selectivity as membranes (Berridge et al., 2002). Substantial enrichment can be obtained using affinity columns based on triazine dyes which act as NAADP agonists (Billington et al., 2004). The non-dissociating nature of NAADP binding allowed convenient tracking during several biochemical fractionation procedures and definition of key properties of the ligand-bound target. The labeled proteins migrated as a single species with an isoelectric point of ~6 (Berridge et al., 2002). Curiously, NAADP binding sites displayed anomalous migration during gel filtration and sucrose density gradient centrifugation. Using these two techniques, the apparent molecular weight of the tagged protein was estimated as ~400–500 kDa and ~120 kDa, respectively (Berridge et al., 2002). The latter technique relies upon buoyant molar mass. Possible association of the target protein with phospholipids which are required for solubilized receptors to optimally bind NAADP (Churamani et al., 2005) might have underestimated the native molecular weight since the partial specific volume of lipid is greater than protein.
A further peculiarity of the soluble NAADP binding protein is its stabilisation upon binding its ligand. Thus, membrane preparations that are incubated with their ligand for extended periods show less dissociation during subsequent solubilisation and fractionation by gel filtration than preparations labeled for shorter periods (Churamani et al., 2006). The kinetics of stabilization are much slower than the kinetics of NAADP binding perhaps reflecting time-dependent conformational changes in the target protein that in essence form a rudimentary “memory” of the time for which receptors had been exposed to their ligand (Churamani et al., 2006). Again it is tempting to correlate this feature with inactivation of NAADP-induced Ca2+ release by sub threshold concentrations of NAADP which are also slow to develop (Aarhus et al., 1996; Genazzani et al., 1996), However, stabilisation is abolished at low temperatures (Churamani et al., 2006) whereas inactivation of Ca2+ release by NAADP is not (Genazzani et al., 1996).
Binding sites for NAADP have also been identified in several mammalian preparations (Table I) but less is known concerning their properties compared with NAADP-binding sites in the sea urchin egg homogenate. For example, in the brain, autoradiographical analysis reveals that NAADP binding sites are widespread and have a distribution distinct from IP3 and ryanodine receptors (Patel et al., 2000b). The half-maximal inhibitory concentration (IC50) for NAADP competition is much higher and analysis of the Hill coefficients suggest that multiple NAADP binding sites are likely present (Patel et al., 2000b). This is also consistent with high and low affinity binding sites characterized in MIN6 cells (Masgrau et al., 2003) and mouse liver (Calcraft et al., 2009). Despite differing affinities, the rank order of potency of NAADP analogues in competing with radiolabeled NAADP was similar to that in the urchin eggs again suggestive of tight linking between identified binding sites and NAADP-evoked Ca2+ release (Patel et al., 2000b). A clear distinguishing feature of binding in the brain (Patel et al., 2000b) and also heart (Bak et al., 2001) compared to sea urchin eggs is that in the former cases it is fully reversible. Moreover, in general, binding affinities appear to be lower in mammalian cells and thus correlate better with the concentration ranges over which NAADP activates Ca2+ release (Table I). Consequently, NAADP may preferentially label high affinity inactivation sites in the egg and intermediate affinity activation sites in mammalian tissues. Given the very different modes of inactivation of NAADP-induced Ca2+ release in mammalian versus egg preparations (see Section 2), it is possible that inactivation sites in the former evade detection due to their presumed lower micromolar affinities.
TWO-PORE CHANNELS AS NAADP-SENSITIVE Ca2+ CHANNELS
At odds with the notion that NAADP activates a novel channel located on acidic organelles, several reports concluded instead that NAADP mobilized Ca2+ from the ER through direct activation of ryanodine receptors (Hohenegger et al., 2002; Gerasimenko et al., 2003; Steen et al., 2007). An effect of NAADP on plasma membrane TRPM2 channels was also reported (Beck et al., 2006). Consistent with an action of NAADP on acidic organelles are studies by Zhang et al. implicating TRP mucolipin 1 as an NAADP target (Zhang et al., 2011). This TRP channel family member localizes to lysosomes and when mutated results in mucolipidosis IV (Bargal et al., 2000). However, several of the above findings have been challenged (Copello et al., 2001; Yamaguchi et al., 2011) such that there is no clear consensus. In 2009, three independent reports were published in rapid succession which provided evidence that the two-pore channels (TPCs) are the long sort NAADP targets (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009).
Two-pore channels were cloned in 2000 from the rat (Ishibashi et al., 2000) and Arabidopsis (Furuichi et al., 2001). TPCs show sequence similarity to voltage-gated Na+/Ca2+ channels. Whilst the latter possess four shaker-like domains each comprising six trans-membrane regions and a pore loop, TPCsare unusual in that they possess only two homologous domains (Yu et al., 2005). This led to the suggestion that TPCs may represent an evolutionary intermediate between one repeat (such as K+ channels) and four repeat voltage-gated ion channels (Ishibashi et al., 2000). Functional characterization of TPCs was initially limited to plants (Hedrich and Marten, 2011). Notwith-standing a potential role of rice TPC in mediating Ca2+ influx across the plasma membrane (Hamada et al., 2012), the study of Peiter et al. (Peiter et al., 2005) localized Arabidopsis TPC to the vacuole and provided compelling evidence that it mediates the slow-vacuolar current thought to underlie Ca2+ release from this organelle (Ward and Schroeder, 1994). The likely localization of plant TPCs to an acidic Ca2+ store and their Ca2+ permeability raised the possibility that animal TPCs maybe localized to the analogous lysosome and thus serve as NAADP-sensitive Ca2+ channels.
The TPC gene possesses a rather distinctive phylogenomic profile in the animal kingdom (Brailoiu et al., 2009; Calcraft et al., 2009). Although TPCs are not well represented in protosomes (including their notable absence in flies and worms) most deuterostomes including the basal sea urchins possess three isoforms (TPC1-3) (Brailoiu et al., 2010a). However, there is striking lineage-specific degeneration of TPC3 in mammals. Thus, TPC3 is a pseudogene in humans (Brailoiu et al., 2010a) but is likely intact in other closely related primates such as New World Monkeys and prosimians (Brailoiu et al., 2010a; Cai and Patel, 2010). Additionally, TPC3 appears to be completely lost in rodents but present in closely related rabbits.
Over-expression of both sea urchin and mammalian TPC isoforms in SKBR3 cells and HEK cells is generally associated with a marked potentiation of NAADP-induced Ca2+ signals (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009; Brailoiu et al., 2010a; Brailoiu et al., 2010b; Ruas et al., 2010; Ogunbayo et al., 2011). These data provided the first evidence that TPCs are NAADP targets. Similar potentiating effects have been observed upon overexpression of TPCs in a megakaryocyte cell line (Dionisio et al., 2011) and a primary cell type (astrocytes) (Pereira et al., 2011). Importantly, mutation of a conserved residue within the putative pore region of TPC1 (Brailoiu et al., 2009) and TPC2 (Brailoiu et al., 2010b) prevents such potentiation and reveals dominant negative activity (Brailoiu et al., 2009; Pereira et al., 2011) providing evidence that endogenous TPCs mediate NAADP action. Consistent with this, knockdown of TPC1 using shRNA (Brailoiu et al., 2009; Dionisio et al., 2011) or TPC2 (using gene knock out) (Calcraft et al., 2009) abolishes the effects of NAADP although in the latter case direct measurements of Ca2+ were lacking. Biophysical analyses of TPCs using a variety of techniques including a novel planar patch clamp method (Schieder et al., 2010a) have confirmed that human TPCs are NAADP-gated Ca2+-permeable channels (Brailoiu et al., 2010b; Schieder et al., 2010b; Pitt et al., 2010; Rybalchenko et al., 2012). For TPC2, micromolar concentrations of NAADP were shown to be less effective than nanomolar levels in stimulating channel activity (Schieder et al., 2010b; Pitt et al., 2010) consistent with inactivation of endogenous Ca2+ release by relatively high concentrations of NAADP (see Section 2). Electrophysiological analyses allow ready access to the luminal face of TPCs which has uncovered a stimulatory effect of luminal Ca2+ on channel activity (Pitt et al., 2010; Rybalchenko et al., 2012). One can envisage then that as TPC channels open, the resulting drop in luminal Ca2+ may serve as a feedback mechanism to limit channel activity. Such regulation could conceivably drive Ca2+ oscillations (Rybalchenko et al., 2012). Thus, nature appears not to have totally abandoned regulation of NAADP responses by Ca2+ but rather flipped its site of action across the membrane. Perhaps such a mechanism safeguards against Ca2+ depletion of acidic organelles which are more discrete and smaller entities relative to the interconnected and much larger ER network.
In accord with the vacuolar localization of plant TPC, animal TPCs localize to the endo-lysosomal system (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009; Brailoiu et al., 2010a; Ruas et al., 2010; Pereira et al., 2011). Intriguingly, TPC2 localized to late endosomes/lysosomes whereas TPC1 showed a broader distribution localizing to both endosomes and lysosomes (Brailoiu et al., 2009; Calcraft et al., 2009). The exact sub-cellular distribution of TPC3 is not known. Many TPCs possess a dileucine motif in their N-termini (Brailoiu et al., 2010b). This motif targets integral membrane proteins to the endo-lysosomal system (Bonifacino and Traub, 2003). Accordingly, deletion or mutation of this site re-directs human TPC2 from its normal lysosomal location to the plasma membrane (Brailoiu et al., 2010b). Similar findings were recently reported for Arabidopsis TPC (Larisch et al., 2012). Consistent with an endo-lysosomal localization, TPC-mediated Ca2+ signals were abolished by interfering with acidic organelles with agents such as bafilomycin A1 (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009; Brailoiu et al., 2010a; Brailoiu et al., 2010b; Ruas et al., 2010; Ogunbayo et al., 2011; Dionisio et al., 2011; Pereira et al., 2011).
Despite the discrete localization of TPCs to the endolysosomal system, NAADP-evoked Ca2+ signals comprise a substantial ER component. These findings are entirely consistent with the trigger hypothesis (Section 2). For example, in SKBR3 cells overexpressing human TPCs, NAADP-induced Ca2+ signals were almost completely blocked by inhibiting ryanodine receptors with ryanodine (Brailoiu et al., 2009; Brailoiu et al., 2010b). Similarly in HEK cells overexpressing TPC2, NAADP-evoked Ca2+ signals were markedly inhibited by depletion of ER Ca2+ stores with thapsigargin or blocking IP3 receptors with heparin (Calcraft et al., 2009). Importantly, in cells expressing plasma membrane-targeted TPC2, NAADP was still able to evoke Ca2+ signals but these signals were insensitive to blockade of intracellular Ca2+ stores (Brailoiu et al., 2010b). Thus, in this case, TPCs are effectively uncoupled from ER Ca2+ release. These data underscore the need to maintain precise geometry of Ca2+ channels in order to effect functional coupling. This in turns perhaps points to specialized membrane contact sites between the ER and acidic organelles (Patel and Brailoiu, 2012) akin to those between the ER and mitochondria where both Ca2+ and lipid dialogue has been established (Toulmay and Prinz, 2011). They also argue against a direct effect of NAADP on ER Ca2+ release channels.
Efforts are now focusing on the basic structure and regulation of the TPCs. Using a combination of techniques including fluorescence protease protection assays (Lorenz et al., 2006), the overall topology of TPCs has been defined (Hooper et al., 2011). Such analyses place both the N- and C-termini within the cytosol indicative of an even number of trans-membrane regions (likely 12) organized in two homologous domains (Hooper et al., 2011). Luminal regions preceding the pore of each domain have also been mapped (Hooper et al., 2011). Notably mutation of N-glycosylation sites prior to pore region II enhances NAADP-evoked Ca2+ release suggesting a role for N-glycosylation in regulating activity (Hooper et al., 2011). Each of the domains is capable of independent insertion in to the membrane likely though a sequential translation of each hydrophobic regions (Churamani et al., 2012). Individual domains and also pairs of transmembrane regions are capable of forming oligomers (Churamani et al., 2012) similar to full length channels (Zong et al., 2009). Interestingly, crosslinking studies show that trans-membrane regions 5 and 6 (which encompass the pore loop) form tetramers consistent with a dimeric assembly of full length subunits in the native channel (Churamani et al., 2012). However, individual domains encompassing all 6 trans-membrane regions form only dimers (Churamani et al., 2012). These data are suggestive of complex homo and heteromeric interactions between the two domains in the assembly of the native channel (Rietdorf et al., 2011).
It is of course notoriously difficult for two or more scientists to agree on all details! It is therefore notable that the above findings were amassed by several independent groups who reached the same conclusion that TPCs are likely NAADP-sensitive and Ca2+ permeable. Inevitably however some differences were noted between studies. For example the exact sensitivity of the different isoforms to NAADP is not entirely clear perhaps most notable for mouse TPC1 and sea urchin TPC3 which in appear active in some studies (Brailoiu et al., 2009; Brailoiu et al., 2010a) but not others (Zong et al., 2009; Ruas et al., 2010). Certainly more work is required to resolve such discrepancies which might well provide further insight in to the regulation of TPCs. Overall however, a consensus was reached that TPCs are NAADP targets.
ARE TWO-PORE CHANNELS NAADP RECEPTORS?
Given the features of NAADP binding described in Section 3, what do we know about the structural basis of NAADP interaction with the TPCs? The answer is currently little. Overexpression of human TPC2 in HEK cells is associated with enhanced NAADP binding to isolated membranes (Calcraft et al., 2009). Additionally, immuno-precipitation of sea urchin TPCs using isoform-specific antibodies recovered NAADP binding sites (Ruas et al., 2010). Importantly, dissociation of NAADP in the latter case showed the unusual sensitivity to K+ as reported for endogenous binding sites in egg homogenates (Dickinson and Patel, 2003). Moreover, recombinant TPCs show discrepant migration during gel filtration and sucrose density gradients (Churamani et al., 2012) again in analogy with endogenous NAADP binding sites (Berridge et al., 2002). These findings are consistent with the most parsimonious conclusion that TPCs bind NAADP. However, substantial TPC2 overexpression only modestly enhances NAADP binding (Calcraft et al., 2009) and neither the purity of TPC immunoprecipitates nor the extent of binding recovery from egg homogenates was reported (Ruas et al., 2010). Additionally, whether knockdown of TPCs abolishes NAADP binding is not known. The binding site(s) for NAADP on the TPC channel has therefore not been identified.
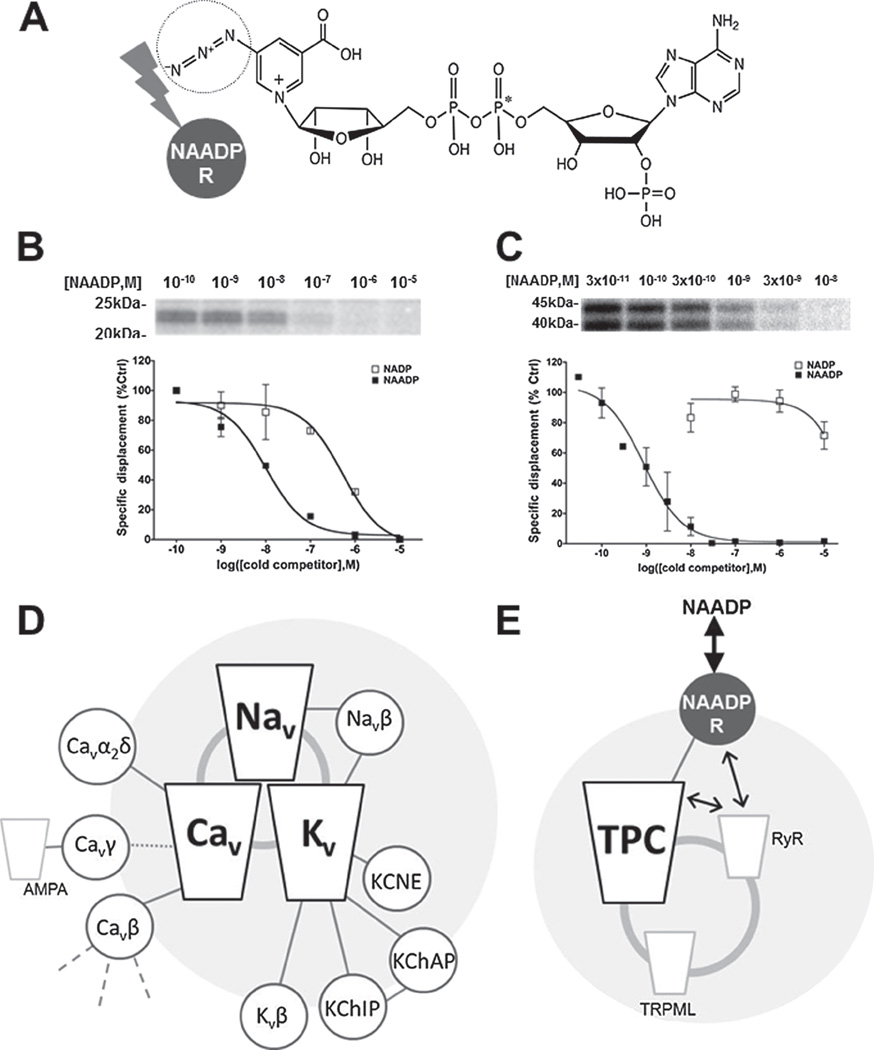
Recent data has raised the possibility that NAADP may not directly interact with TPCs, but bind to distinct accessory protein(s) within a larger TPC complex that regulate TPC activity indirectly (Lin-Moshier et al., 2011; Walseth et al., 2011). This new evidence is based upon photolabelling data. The 5-position of the nicotinic ring of NAADP is tolerant to substitution (Jain et al., 2010), such that a photolabile azido group can be incorporated while preserving high affinity binding and Ca2+ release activity (Lin-Moshier et al., 2011; Walseth et al., 2011). Using such a derivitized, radioactive probe (32P-5-azido-nicotinic acid adenine dinucleotide 2′-phosphate, 32P-5N3-NAADP), NAADP-binding sites were resolved by following the 32P signal after photoactivation of the probe to effect crosslinking to endogenous NAADP-binding proteins (Fig. 1(A)). In essence, this approach allows identification of the NAADP interactome in any preparation of choice—binding partners, and ideally binding sites (Tomizawa et al., 2007; Tanabe et al., 1999) can then be identified by resolving what proteins/residues are labeled by the photoprobe. Application of the photo-labelling approach in the sea urchin and mammalian system revealed three key findings.
In both preparations, 32P-5N3-NAADP specifically labeled low molecular weight protein doublets that displayed a diagnostic pharmacology of NAADP-evoked Ca2+ release and considerable selectivity for NAADP over NADP (Figs. 1(B and C)). The intensity of photolabelling of these lower molecular weight NAADP-binding proteins correlated with NAADP responsiveness, trending from a low proportion in mammalian cell lines to higher levels in primary pancreatic tissue and near exclusive labeling in sea urchin egg homogenate, the ‘gold-standard’ system for studying NAADP-evoked Ca2+ signaling. Further, as expected in the urchin system, photolabeling of the low molecular weight candidates exhibited irreversibility in high K+ as observed in 32P-NAADP binding studies (Dickinson and Patel, 2003) (Section 3). Finally, the extent of photolabelling paralleled 32P-NAADP binding activity in different homogenate preparations, and the observed affinities of specific NAADP binding sites measured either by photolabelling densitometry or conventional radioligand binding were identical (Walseth et al., 2011).
Unexpectedly, overexpression of TPC isoforms failed to change the photolabeling profile, while labeling of the lower molecular weight candidates was preserved in tissue samples from a TPC1 and TPC2 knockout mouse, implying a molecular identity independent from TPC proteins (Lin-Moshier et al., 2011).
In sea urchin egg homogenates, immunoprecipitation with TPC antibodies recovered a fraction of the low molecular weight photolabelled proteins, thereby evidencing an association of these NAADP-binding proteins with sea urchin TPCs (Walseth et al., 2011).
Figure 1. Photolabeling the NAADP receptor.
(A) Structure of the radioactive photoprobe used to label NAADP-binding sites. The radioactive moiety (*) and photolabile nitrene group (circle) are indicated. Exposure to UV light effects crosslinking to the NAADP receptor(s), shown as grey circle. (B and C) Top, Photolabeling data in (B) membrane fractions from SKBR3 cells (P100 fraction) and (C) sea urchin egg homogenates showing protection of photolabeling by increasing concentrations of NAADP. Bottom, representative densitomery from such experiments employing NAADP (solid squares) and NADP (open squares) to illustrate selectivity of the low molecular weight proteins for NAADP over NADP. (D) Accessory subunit interactome of major voltage-gated ion channels (Nav, Cav, Kv). Discrete accessory proteins are shown (small circles) linked to the core channel complex. Many accessory subunits have multiple targets (e.g., shown just for Cavβ), which often encompass other ion channels (e.g., shown for AMPA-Rs with Cavγ and Kv4.2 with Navβ1). Channel subunits are depicted as integral membrane (within large grey circle), or peripheral membrane proteins (circumference of large grey circle). Clustering between the core ion channel families themselves is represented by the dark grey circle. (E) Proposal depicting a TPC accessory subunit as a discrete NAADP receptor (NAADP-R, solid circle). Binding of NAADP to this subunit is required to activate the TPC channel. This NAADP-R subunit, as with many other ion channel accessory subunits, may be promiscuous and serve to regulate other cellular Ca2+ channels (e.g., ryanodine receptors; RyR). Indeed, NAADP-R may coordinate interaction between TPCs and other ion channels. Association of TPCs with other organellar Ca2+ channels is shown (e.g., TRPML).
Collectively, these data suggest that the TPCs act as NAADP-activated Ca2+ release channels but not as NAADP ‘receptors’. How outlandish is this proposal that NAADP-binding and channel forming subunits comprise discrete molecular entities? A starting point for discussion is consideration of the homology between TPCs and voltage-gated ion channels in terms of their overall sequence identity and domain organization. Sequence identity is particularly notable between the fifth and sixth trans-membrane regions encompassing the pore in voltagegated ion channels (Brailoiu et al., 2009). This likely explains block of TPCs by L-type Ca2+ channel antagonists (Genazzani et al., 1996). There is also sequence identify within the first four TM regions which constitute the voltage sensor of voltage-gated ion channels (Patel et al., 2011). Indeed, there is partial conservation of positively charged arginine residues within the fourth trans-membrane region of TPCs which likely contribute to voltage gating of plant TPC and the recently reported voltage sensitivity of human TPC1 to activation by NAADP (Rybalchenko et al., 2012). A further key similarity with voltage-gated channels would be the architectural assembly of TPCs as multiprotein complexes. Voltage-gated ion channel interactomes can be surprisingly large, for example approximately 200 proteins-constitute the nanoenvironment of Cav2 channels (Muller et al., 2010). Within the core channel complex, each major class of voltage-operated channels incorporates accessory subunits (Fig. 1(D)) that interact with the pore-forming subunits as either integral membrane (e.g., KCNE, Navβ, Cavα2δ) and/or cytoplasmically-associated proteins (e.g., Kvβ, KChAP, KChIP, Cavβ). TPCs also contain sequence motifs that resemble known accessory subunit interaction domains (e.g., the alpha interaction domains of Cav channels (Hooper and Patel, 2011)). These accessory subunits act to modify expression levels and/or functional properties of the associated channel (Li et al., 2006; Hidalgo and Neely, 2007; Goldfarb, 2012).
While it is well appreciated that the intrinsic subunit composition of channels can confer unique agonist sensitivity (GABAAγ2 subunit and benzodiazepines (Mohler, 2006), GluN1/N3 subunits and glycine (Pachernegg et al., 2012)), it is also clear that accessory subunits of Ca2+ channels confer ligand binding sites that serve to indirectly regulate channel activity. Examples include binding of gabapentinoids (Davies et al., 2007; Hendrich et al., 2008) to Cavα2γ subunits, binding of FK506/rapamycin to FKBPs associated with ER Ca2+ release channels (MacMillan and McCarron, 2009), charybdotoxin binding to residues within the β subunit of BKCa channels (Knaus et al., 1994) and the association of K+ openers/blockers to the sulfonylurea receptor subunits (SURx) of KATP channels (Hibino et al., 2010). The most familiar of these examples pertain to exogenous ligands and indeed targeting accessory subunits and accessory subunit binding interfaces is receiving increasing attention for a novel therapeutic strategy (Araud et al., 2010; Cook et al., 2012; Brittain et al., 2011; Young et al., 1998). However, individual examples worth underscoring are the separation of Ca2+ channel and ‘receptor’ functions in polycystin heterodimer complexes (PKD1/PKD2, (Hanaoka et al., 2000); PKD1L3/PKD2L1, (Ishimaru et al., 2006)), as well as regulation of the auxillary SURx subunits of KATP channels by endogenous nucleotide diphoshophates (Nichols, 2006). Therefore, the proposal of an endogenous agonist-regulated subunit while more unusual, is by no means unique, especially as NAADP is a novel second messenger in terms of potency and the unusual properties of NAADP binding and action (Sections 2 and 3). Perhaps a non-canonical molecular mechanism underpins such phenomenology and there is merit in considering the consequences of this new model.
Most obviously, the partitioning of agonist binding and channel forming subunits into discrete molecular entities raises the possibility that NAADP may act as a promiscuous messenger (Fig. 1(E)), with the lower molecular NAADP-receptors conferring NAADP-sensitivity to multiple, different families of intracellular Ca2+ channels (Guse, 2012). There is evidence in some cell types that NAADP appears to activate ryanodine receptors independently of Ca2+-induced Ca2+ release suggesting a more direct effect of NAADP on other Ca2+ channels. In support NAADP, at nanomolar concentrations gates “purified” ryanodine receptors incorporated into lipid bilayers (Hohenegger et al., 2002) and modestly stimulates ryanodine binding (Dammermann et al., 2009). Might these effects be mediated by tight association of NAADP binding subunits? Such promiscuity could endow considerable flexibility to the cellular customization of NAADP-evoked Ca2+ transients and harmonize conflicting data concerning the identity of cellular Ca2+ channels targeted by NAADP. Again, this proposal finds established precedent. Both integral and peripheral accessory subunits of voltage-operated ion channels can be found as components of different ion channel complexes. For example, the transmembrane Navβ1 accessory subunit of voltage-gated Na+ channels associates with and regulates K+ channel (Kv4.2) excitability (Marionneau et al., 2012). Accessory subunits of K+ and Ca2+ channels have been shown to associate with other cellular partners (Hidalgo and Neely, 2007; Bunse et al., 2009). FKBPs regulate both IP3 receptors and ryanodine receptors (MacMillan and McCarron, 2009). Such associations may not be exclusive: perhaps the low-molecular weight NAADP-binding proteins could physically coordinate assembly of macromolecular complexes containing multiple ion channels. The association of TPCs with TRPMLs (Yamaguchi et al., 2011), and possibly ryanodine receptors (Patel et al., 2010) are noteworthy in this regard. Could the NAADP receptor complex contain multiple types of Ca2+ channel? One notes the local environment of voltage-gated Ca2+ channels comprises multiple interacting ion channels and subunits (Muller et al., 2010). Finally, the potential for a reversible association of the NAADP-receptor with the TPC channel core is immediately suggestive of a mechanism for regulating cellular NAADP responsiveness and NAADP-induced channel inactivation (through uncoupling of the receptor protein from the channel). One iteration of this idea of ligand induced uncoupling of accessory proteins is seen with the immunophilins. Dissociation of FKBPs from IP3 receptors and/or ryanodine receptors changes the intrinsic activity of the underlying Ca2+ channels and the dissociated complex is endowed with new binding activity for specific cellular targets (calcineurin, mTOR) depending on the identity of the bound ligand (FK506, rapamycin). The former mechanism has been proposed (Tang et al., 2002; Noguchi et al., 1997; Teggatz et al., 2005; Zheng et al., 2010), although not consistently supported (Bai et al., 2005), for cADPR-mediated activation of ryanodine receptors.
OUTLOOK
As discussed, there is now much evidence supporting the notion that TPCs are NAADP targets responsible for mobilization of acidic Ca2+ stores. Their identification has stimulated renewed interest in what has always been an atypical Ca2+ signaling pathway. Prior to their identification, the lack of molecular tools meant probing the physiological roles of NAADP represented somewhat of a challenge. It is thus satisfying that TPC knockdown studies are beginning to confirm roles for NAADP (Boittin et al., 2003; Brailoiu et al., 2006; Brailoiu et al., 2010c) in processes such as smooth muscle contraction (Tugba Durlu-Kandilci et al., 2010), differentiation (Aley et al., 2010) and endothelial cell activation (Esposito et al., 2011). And it is exciting that molecular approaches are revealing unanticipated roles for NAADP in trafficking (Ruas et al., 2010) and autophagy (Pereira et al., 2011). Of significance are recent findings implicating NAADP/TPCs in deregulated autophagy mediated by the Parkinson’s disease-related protein LRRK2 (Gomez-Suaga et al., 2011). Clearly then molecular approaches targeting the TPCs are providing pathophysiological insight into the NAADP pathway. Consequently, the recent photoaffinity labeling studies suggesting that NAADP may not bind the TPCs directly urgently requires molecular identification of the low molecular weight NAADP binding proteins, and resolution of the molecular basis of their interaction with the TPCs. This is placed in the more general context of defining the TPC interactome. Identification of the candidates must also explain differences in the properties of the candidates (doublet size and sub-cellular distribution) between the invertebrate and mammalian systems employed in these studies. These differential properties may be explained by molecular diversity (different gene products, isoforms or post-translational modifications), or differential usage of molecules to assemble high affinity inactivation sites in the sea urchin and lower affinity activation sites in mammalian systems (Section 3). The recent photolabelling data revealed that multiple proteins in mammalian systems bind NAADP (Lin-Moshier et al., 2011), although only the low molecular weight doublet bound NAADP with high affinity and selectivity over NADP., This target represented <10% of total labeling, a consideration when evaluating NAADP binding data obtained using conventional methods, and Ca2+ responses from mammalian systems compared with data from sea urchin egg homogenates. In the latter, the high affinity and highly selective NAADP binding site(s) predominate (~90% of total labeling). The egg is an extremely specialized cell type, and a unique molecular solution that tailors high NAADP sensitivity to the cell biology of the urchin egg is not an unreasonable proposition. Equally, the observation of multiple low affinity NAADP binding sites by both conventional radioligand binding (Section 3) and photolabeling methods in mammalian systems, should turn attention to the broader NAADP interactome likely encompassing synthetic and degradative enzymes, as well as novel NAADP targets. Further molecular analyses are thus sure to follow.
Acknowledgments
This work was supported by grants from the BBSRC (BB/G013721/1 to SP) and the NIH (GM088790 to JSM). YPL was supported by a Doctoral Dissertation Fellowship from the University of Minnesota Graduate School. SP thanks Chi Li and Martin Stocker for useful discussion.
REFERENCES
- Aarhus R, Dickey DM, Graeff R, Gee KR, Walseth TF, Lee HC. Activation and inactivation of Ca2+ release by NAADP+ J. Biol. Chem. 1996;271:8513–8516. doi: 10.1074/jbc.271.15.8513. [DOI] [PubMed] [Google Scholar]
- Aley PK, Mikolajczyk AM, Munz B, Churchill GC, Galione A, Berger F. Nicotinic acid adenine dinucleotide phosphate regulates skeletal muscle differentiation via action at two-pore channels. Proc. Natl. Acad. Sci. USA. 2010;107:19927–19932. doi: 10.1073/pnas.1007381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araud T, Wonnacott S, Bertrand D. Associated proteins: The universal toolbox controlling ligand gated ion channel function. Biochem. Pharmacol. 2010;80:160–169. doi: 10.1016/j.bcp.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Bai N, Lee HC, Laher I. Emerging role of cyclic ADP-ribose (cADPR) in smooth muscle. Pharmacol. Ther. 2005;105:189–207. doi: 10.1016/j.pharmthera.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bak J, Billington RA, Timar G, Dutton AC, Genazzani AA. NAADP receptors are present and functional in the heart. Curr. Biol. 2001;11:987–990. doi: 10.1016/s0960-9822(01)00269-x. [DOI] [PubMed] [Google Scholar]
- Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G. Identification of the gene causing mucolipidosis type IV. Nat. Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- Beck A, Kolisek M, Bagley LA, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J. 2006;20:962–964. doi: 10.1096/fj.05-5538fje. [DOI] [PubMed] [Google Scholar]
- Berg I, Potter VL, Mayr GW, Guse AH. Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+ signaling. J. Cell Biol. 2000;150:581–588. doi: 10.1083/jcb.150.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge G, Dickinson G, Parrington J, Galione A, Patel S. Solubilization of receptors for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate. J. Biol. Chem. 2002;277:43717–43723. doi: 10.1074/jbc.M203224200. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Billington RA, Bak J, Martinez-Coscolla A, Debidda M, Genazzani AA. Triazine dyes are agonists of the NAADP receptor. Br. J. Pharmacol. 2004;142:1241–1246. doi: 10.1038/sj.bjp.0705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington RA, Bellomo EA, Floriddia EM, Erriquez J, Distasi C, Genazzani AA. A transport mechanism for NAADP in a rat basophilic cell line. FASEB J. 2006;20:521–523. doi: 10.1096/fj.05-5058fje. [DOI] [PubMed] [Google Scholar]
- Billington RA, Genazzani AA. Charcterisation of NAADP+ binding in sea urchin eggs. Biochem. Biophys. Res. Commun. 2000;276:112–116. doi: 10.1006/bbrc.2000.3444. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Galione A, Evans AM. Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ. Res. 2003;91:1168–1175. doi: 10.1161/01.res.0000047507.22487.85. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Pandey V, Brailoiu GC, Tuluc F, Patel S, Dun NJ. Messenger-specific role for NAADP in neuronal differentiation. J. Biol. Chem. 2006;281:15923–15928. doi: 10.1074/jbc.M602249200. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Hoard JL, Filipeanu CM, Brailoiu GC, Dun SL, Patel S, Dun NJ. NAADP potentiates neurite outgrowth. J. Biol. Chem. 2005;280:5646–5650. doi: 10.1074/jbc.M408746200. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S. An ancestral deuterostome family of two-pore channels mediate nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J. Biol. Chem. 2010a;285:2897–2901. doi: 10.1074/jbc.C109.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 2010b;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu GC, Gurzu B, Gao X, Parkesh R, Aley PK, Trifa DI, Galione A, Dun NJ, Madesh M, Patel S, Churchill GC, Brailoiu E. Acidic NAADP-sensitive calcium stores in the endothelium: agonist-specific recruitment and role in regulating blood pressure. J. Biol. Chem. 2010c;285:37133–37137. doi: 10.1074/jbc.C110.169763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat. Med. 2011;17:822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunse S, Locovei S, Schmidt M, Qiu F, Zoidl G, Dahl G, Dermietzel R. The potassium channel subunit Kvbeta3 interacts with pannexin 1 and attenuates its sensitivity to changes in redox potentials. FEBS J. 2009;276:6258–6270. doi: 10.1111/j.1742-4658.2009.07334.x. [DOI] [PubMed] [Google Scholar]
- Cai X, Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Chini EN, Beers KW, Dousa TP. Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J. Biol. Chem. 1995;270:3216–3223. doi: 10.1074/jbc.270.7.3216. [DOI] [PubMed] [Google Scholar]
- Chini EN, Dousa TP. Nicotinate-adenine dinucleotide phosphate-induced Ca2+ release does not behave as a Ca2+-induced Ca2+-release system. Biochem. J. 1996;316:709–711. doi: 10.1042/bj3160709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churamani D, Dickinson GD, Patel S. NAADP binding to its target protein in sea urchin eggs requires phospholipids. Biochem. J. 2005;386:497–504. doi: 10.1042/BJ20041990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churamani D, Dickinson GD, Ziegler M, Patel S. Time sensing by NAADP receptors. Biochem. J. 2006;397:313–320. doi: 10.1042/BJ20060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churamani D, Hooper R, Brailoiu E, Patel S. Domain assembly of NAADP-gated two-pore channels. Biochem. J. 2012;441:317–323. doi: 10.1042/BJ20111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Galione A. Spatial control of Ca2+ signalling by nicotinic acid adenine dinucleotide phosphate diffusion and gradients. J. Biol. Chem. 2000;275:38687–38692. doi: 10.1074/jbc.M005827200. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPr-sensitive Ca2+ stores. EMBO. J. 2001a;20:1–6. doi: 10.1093/emboj/20.11.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Galione A. Prolonged inactivation of NAADP-induced Ca2+ release mediates a spatiotemporal memory. J. Biol. Chem. 2001b;276:11223–11225. doi: 10.1074/jbc.M009335200. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Churchill GC, O‘Neil JS, Masgrau R, Patel S, Thomas JM, Genazzani AA, Galione A. Sperm deliver a new messenger: NAADP. Curr. Biol. 2003;13:125–128. doi: 10.1016/s0960-9822(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Clapper DL, Lee HC. Inositol trisphosphate induces calcium release from nonmitochondrial stores in sea urchin egg homogenates. J. Biol. Chem. 1985;260:13947–13954. [PubMed] [Google Scholar]
- Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- Copello JA, Qi Y, Jeyakumar LH, Ogunbunmi E, Fleischer S. Lack of effect of cADP-ribose and NAADP on the activity of skeletal muscle and heart ryanodine receptors. Cell Calcium. 2001;30:269–284. doi: 10.1054/ceca.2001.0235. [DOI] [PubMed] [Google Scholar]
- Dammermann W, Zhang B, Nebel M, Cordiglieri C, Odoardi F, Kirchberger T, Kawakami N, Dowden J, Schmid F, Dornmair K, Hohenegger M, Flugel A, Guse AH, Potter BV. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc. Natl. Acad. Sci. USA. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Diambra L, Marchant JS. Localization and socialization: experimental insights into the functional architecture of IP3 receptors. Chaos. 2009;19:037103. doi: 10.1063/1.3147425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson GD, Patel S. Modulation of NAADP receptors by K+ ions: Evidence for multiple NAADP receptor conformations. Biochem. J. 2003;375:805–812. doi: 10.1042/BJ20030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio N, Albarran L, Lopez JJ, Berna-Erro A, Salido GM, Bobe R, Rosado JA. Acidic NAADP-releasable Ca(2+) compartments in the megakaryoblastic cell line MEG01. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbamcr.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Esposito B, Gambara G, Lewis AM, Palombi F, D’Alessio A, Taylor LX, Genazzani AA, Ziparo E, Galione A, Churchill GC, Filippini A. NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood. 2011;117:4968–4977. doi: 10.1182/blood-2010-02-266338. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T, Cunningham KW, Muto S. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 2001;42:900–905. doi: 10.1093/pcp/pce145. [DOI] [PubMed] [Google Scholar]
- Galione A, Morgan AJ, Arredouani A, Davis LC, Rietdorf K, Ruas M, Parrington J. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem. Soc. Trans. 2010;38:1424–1431. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- Gambara G, Billington RA, Debidda M, D’Alessio A, Palombi F, Ziparo E, Genazzani AA, Filippini A. NAADP-induced Ca2+ signaling in response to endothelin is via the receptor subtype B and requires the integrity of lipid rafts/caveolae. J. Cell Physiol. 2008;216:396–404. doi: 10.1002/jcp.21407. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Empson RM, Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ release. J. Biol. Chem. 1996;271:11599–11602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Galione A. Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem. J. 1996;315:721–725. doi: 10.1042/bj3150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AA, Mezna M, Dickey DM, Michelangeli F, Walseth TF, Galione A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br. J. Pharmacol. 1997;121:1489–1495. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Maruyama Y, Yano K, Dolman N, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J. Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. Voltage-gated sodium channel-associated proteins and alternative mechanisms of inactivation and block. Cell Mol. Life Sci. 2012;69:1067–1076. doi: 10.1007/s00018-011-0832-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suaga P, Luzon-Toro B, Churamani D, Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC, Hilfiker S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum. Mol. Genet. 2011;21:511–525. doi: 10.1093/hmg/ddr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse AH. Second messenger signaling: multiple receptors for NAADP. Curr. Biol. 2009;19:R521–R523. doi: 10.1016/j.cub.2009.05.045. [DOI] [PubMed] [Google Scholar]
- Guse AH. Linking NAADP to Ion Channel Activity: A Unifying Hypothesis. Sci. Signal. 2012;5:e18. doi: 10.1126/scisignal.2002890. [DOI] [PubMed] [Google Scholar]
- Hamada H, Kurusu T, Okuma E, Nokajima H, Kiyoduka M, Koyano T, Sugiyama Y, Okada K, Koga J, Saji H, Miyao A, Hirochika H, Yamane H, Murata Y, Kuchitsu K. Regulation of a proteinaceous elicitor-induced Ca2+ influx and production of phytoalexins by a putative voltage-gated cation channel, OsTPC1, in cultured rice cells. J. Biol. Chem. 2012;287:9931–9939. doi: 10.1074/jbc.M111.337659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SL, Serysheva II. Ryanodine receptor structure: Progress and challenges. J. Biol. Chem. 2009;284:4047–4051. doi: 10.1074/jbc.R800054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Marten I. TPC1-SV channels gain shape. Mol. Plant. 2011;4:428–441. doi: 10.1093/mp/ssr017. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Hidalgo P, Neely A. Multiplicity of protein interactions and functions of the voltage-gated calcium channel beta-subunit. Cell Calcium. 2007;42:389–396. doi: 10.1016/j.ceca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Hohenegger M, Suko J, Gscheidlinger R, Drobny H, Zidar A. Nicotinic acid-adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem. J. 2002;367:423–431. doi: 10.1042/BJ20020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J. Biol. Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Patel S. In: NAADP on Target, Calcium Signaling. Islam MS, editor. 2011. [Google Scholar]
- Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Slama JT, Perez-Haddock LA, Walseth TF. Nicotinic acid adenine dinucleotide phosphate analogues containing substituted nicotinic acid: Effect of modification on Ca(2+) release. J. Med. Chem. 2010;53:7599–7612. doi: 10.1021/jm1007209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca(2+)-activated K+ channel from smooth muscle. J. Biol. Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- Larisch N, Schulze C, Galione A, Dietrich P. An N-Terminal Dileucine Motif Directs Two-Pore Channels to the Tonoplast of Plant Cells. Traffic. 2012 doi: 10.1111/j.1600-0854.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- Lee HC. NAADP-mediated calcium signaling. J. Biol. Chem. 2005;280:33693–33696. doi: 10.1074/jbc.R500012200. [DOI] [PubMed] [Google Scholar]
- Lee HC. Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling. Sci. China Life Sci. 2011;54:699–711. doi: 10.1007/s11427-011-4197-3. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. ADP-ribosyl cyclase: An enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;2:203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. Structural determinants of nicotinic acid adenine dinucleotide phosphate important for Its calcium-mobilizing activity. J. Biol. Chem. 1997;272:20378–20383. doi: 10.1074/jbc.272.33.20378. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. Functional visualisation of the separate but interacting calcium stores sensitive to NAADP and cyclic ADP-ribose. J. Cell Sci. 2000;113:4413–4420. doi: 10.1242/jcs.113.24.4413. [DOI] [PubMed] [Google Scholar]
- Lee HC, Walseth TF, Bratt GT, Hayers R, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 1989;264:1608–1615. [PubMed] [Google Scholar]
- Li Y, Um SY, McDonald TV. Voltage-gated potassium channels: Regulation by accessory subunits. Neuroscientist. 2006;12:199–210. doi: 10.1177/1073858406287717. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz H, Hailey DW, Wunder C, Lippincott-Schwartz J. The fluorescence protease protection (FPP) assay to determine protein localization and membrane topology. Nat. Protoc. 2006;1:276–279. doi: 10.1038/nprot.2006.42. [DOI] [PubMed] [Google Scholar]
- Macgregor A, Yamasaki M, Rakovic S, Sanders L, Parkesh R, Churchill GC, Galione A, Terrar DA. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J. Biol. Chem. 2007;282:15302–15311. doi: 10.1074/jbc.M611167200. [DOI] [PubMed] [Google Scholar]
- MacMillan D, McCarron JG. Regulation by FK506 and rapamycin of Ca2+ release from the sarcoplasmic reticulum in vascular smooth muscle: The role of FK506 binding proteins and mTOR. Br. J. Pharmacol. 2009;158:1112–1120. doi: 10.1111/j.1476-5381.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marionneau C, Carrasquillo Y, Norris AJ, Townsend RR, Isom LL, Link AJ, Nerbonne JM. The sodium channel accessory subunit navbeta1 regulates neuronal excitability through modulation of repolarizing voltage-gated K+ channels. J. Neurosci. 2012;32:5716–5727. doi: 10.1523/JNEUROSCI.6450-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJH, Galione A. NAADP: A new second messenger for glucose-induced Ca2+ responses in clonal pancreatic b-cells. Curr. Biol. 2003;13:247–251. doi: 10.1016/s0960-9822(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela JM. Generation of specific Ca2+ signals from Ca2+ stores and endocytosis by differential coupling to messengers. Curr. Biol. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Lai FA, Rutter GA. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate (NAADP) receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic b cells (MIN6) J. Biol. Chem. 2003;278:11057–11064. doi: 10.1074/jbc.M210257200. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. Investigating cADPR and NAADP in intact and broken cell preparations. Methods. 2008;46:194–203. doi: 10.1016/j.ymeth.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Muller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, Schulte U. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc. Natl. Acad. Sci. USA. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J. Biol. Chem. 1997;272:3133–3136. doi: 10.1074/jbc.272.6.3133. [DOI] [PubMed] [Google Scholar]
- Ogunbayo OA, Zhu Y, Rossi D, Sorrentino V, Ma J, Zhu MX, Evans AM. cADPR activates ryanodine receptors while NAADP activates two pore domain channels. J. Biol. Chem. 2011;286:9136–9140. doi: 10.1074/jbc.M110.202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachernegg S, Strutz-Seebohm N, Hollmann M. GluN3 subunit-containing NMDA receptors: Not just one-trick ponies. Trends Neurosci. 2012;35:240–249. doi: 10.1016/j.tins.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Patel S. NAADP-induced Ca2+ release—a new signaling pathway. Biol. Cell. 2004;96:19–28. doi: 10.1016/j.biolcel.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Patel S, Brailoiu E. Triggering of Ca2+ signals by NAADP-gated two-pore channels. A role for membrane contact sites? Biochem. Soc. Trans. 2012 doi: 10.1042/BST20110693. [DOI] [PubMed] [Google Scholar]
- Patel S, Churchill GC, Galione A. Unique kinetics of nicotinic acid-adenine dinucleotide phosphate (NAADP) binding enhance the sensitivity of NAADP receptors for their ligand. Biochem. J. 2000a;352:725–729. [PMC free article] [PubMed] [Google Scholar]
- Patel S, Churchill GC, Galione A. Coordination of Ca2+ signalling by NAADP. Trends Biochem. Sci. 2001;26:482–489. doi: 10.1016/s0968-0004(01)01896-5. [DOI] [PubMed] [Google Scholar]
- Patel S, Churchill GC, Sharp T, Galione A. Widespread distribution of binding sites for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate, in the brain. J. Biol. Chem. 2000b;275:36495–36497. doi: 10.1074/jbc.C000458200. [DOI] [PubMed] [Google Scholar]
- Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- Patel S, Marchant JS, Brailoiu E. Two-pore channels: Regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium. 2010;47:480–490. doi: 10.1016/j.ceca.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Muallem S. Acidic Ca2+ stores come to the fore. Cell Calcium. 2011;50:109–112. doi: 10.1016/j.ceca.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Patel S, Ramakrishnan L, Rahman T, Hamdoun A, Marchant JS, Taylor CW, Brailoiu E. The endo-lysosomal system as an NAADP-sensitive acidic Ca2+ store: Role for the two-pore channels. Cell Calcium. 2011;50:157–167. doi: 10.1016/j.ceca.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- Pereira GJ, Hirata H, Fimia GM, do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, Piacentini M, Patel S, Smaili SS. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt SJ, Funnell T, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, Parrington J, Galione A, Sitsapesan R. TPC2 is a novel NAADP-sensitive Ca2+-release channel, operating as a dual sensor of luminal pH and Ca2+ J. Biol. Chem. 2010;285:24925–24932. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdorf K, Funnell TM, Ruas M, Heinemann J, Parrington J, Galione A. Two-pore channels form homo- and heterodimers. J. Biol. Chem. 2011;286:37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D, Lewis AM, Mizote A, Thomas JM, Aley PK, Vasudevan SR, Parkesh R, Galione A, Izumi M, Ganesan A, Churchill GC. Analogues of the nicotinic acid adenine dinucleotide phosphate (NAADP) antagonist Ned-19 indicate two binding sites on the NAADP receptor. J. Biol. Chem. 2009;284:34930–34934. doi: 10.1074/jbc.M109.016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, Churchill GC, Zhu MX, Platt FM, Wessel GM, Parrington J, Galione A. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ Signaling and endolysosomal trafficking. Curr. Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S. Membrane potential regulates NAADP dependence of the pH and Ca2+ sensitive organellar two-pore channel TPC1. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.359612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammels E, Parys JB, Missiaen L, De SH, Bultynck G. Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium. 2010;47:297–314. doi: 10.1016/j.ceca.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott C. Planar patch clamp approach to characterize ionic currents from intact lysosomes. Sci. Signal. 2010a;3:l3. doi: 10.1126/scisignal.3151pl3. [DOI] [PubMed] [Google Scholar]
- Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott CA. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J. Biol. Chem. 2010b;285:21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M, Kirchberger T, Guse AH. NAADP mobilizes calcium from the endoplasmic reticular Ca(2+) store in T-lymphocytes. J. Biol. Chem. 2007;282:18864–18871. doi: 10.1074/jbc.M610925200. [DOI] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a non-mitochondrial store of pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Tucker SJ, Matsuo M, Proks P, Ashcroft FM, Seino S, Amachi T, Ueda K. Direct photoaffinity labeling of the Kir6.2 subunit of the ATP-sensitive K+ channel by 8-azido-ATP. J. Biol. Chem. 1999;274:3931–3933. doi: 10.1074/jbc.274.7.3931. [DOI] [PubMed] [Google Scholar]
- Tang WX, Chen YF, Zou AP, Campbell WB, Li PL. Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am. J. Physiol Heart Circ. Physiol. 2002;282:H1304–H1310. doi: 10.1152/ajpheart.00843.2001. [DOI] [PubMed] [Google Scholar]
- Teggatz EG, Zhang G, Zhang AY, Yi F, Li N, Zou AP, Li PL. Role of cyclic ADP-ribose in Ca2+-induced Ca2+ release and vasoconstriction in small renal arteries. Microvasc. Res. 2005;70:65–75. doi: 10.1016/j.mvr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Maltby D, Medzihradszky KF, Zhang N, Durkin KA, Presley J, Talley TT, Taylor P, Burlingame AL, Casida JE. Defining nicotinic agonist binding surfaces through photoaffinity labeling. Biochemistry. 2007;46:8798–8806. doi: 10.1021/bi700667v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: The tip of the iceberg. Curr. Opin. Cell Biol. 2011 doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugba Durlu-Kandilci N, Ruas M, Chuang KT, Brading A, Parrington J, Galione A. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J. Biol. Chem. 2010;285:24925–24932. doi: 10.1074/jbc.M110.129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) proteins in sea urchin egg. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Jha Y, Li Q, Soyombo AA, Dickinson GD, Churamani D, Brailoiu E, Patel S, Muallem S. Transient receptor potential mucolipin 1 (TRPML1) and two-pore channels are functionally independent organellar ion channels. J. Biol. Chem. 2011;286:22934–22942. doi: 10.1074/jbc.M110.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJH, Galione A. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J. Biol. Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- Young K, Lin S, Sun L, Lee E, Modi M, Hellings S, Husbands M, Ozenberger B, Franco R. Identification of a calcium channel modulator using a high throughput yeast two-hybrid screen. Nat. Biotechnol. 1998;16:946–950. doi: 10.1038/nbt1098-946. [DOI] [PubMed] [Google Scholar]
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol. Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xu M, Han WQ, Li PL. Reconstitution of lysosomal NAADP-TRP-ML1 signaling pathway and its function in TRP-ML1(−/−) cells. Am. J. Physiol Cell Physiol. 2011;301:C421–C430. doi: 10.1152/ajpcell.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wenzhi B, Miao L, Hao Y, Zhang X, Yin W, Pan J, Yuan Z, Song B, Ji G. Ca(2+) release induced by cADP-ribose is mediated by FKBP12.6 proteins in mouse bladder smooth muscle. Cell Calcium. 2010;47:449–457. doi: 10.1016/j.ceca.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Zhu MX, Ma J, Parrington J, Galione A, Evans AM. TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS Lett. 2010;584:1966–1974. doi: 10.1016/j.febslet.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]