Abstract
Topical 5-fluorouracil (5-FU) is approved for the treatment of superficial basal cell carcinoma and actinic keratosis. However, 5-FU suffers from poor skin permeation. Microneedles have been successfully applied to improve the skin permeability of small and large molecules, and even nanoparticles, by creating micron-sized pores in the stratum corneum layer of the skin. In this report, the feasibility of using microneedles to increase the skin permeability of 5-FU was tested. Using full thickness mouse skin mounted on Franz diffusion apparatus, it was shown that the flux of 5-FU through the skin was increased by up to 4.5-fold when the skin was pretreated with microneedles (500 μm in length, 50 μm in base diameter). In a mouse model with B16-F10 mouse melanoma cells implanted in the subcutaneous space, the antitumor activity of a commercially available 5-FU topical cream (5%) was significantly enhanced when the cream was applied on a skin area that was pretreated with microneedles, as compared to when the cream was simply applied on a skin area, underneath which the tumor cells were implanted, and without pretreatment of the skin with microneedles. Fluorouracil is not approved for melanoma therapy, but the clinical efficacy of topical 5-FU against tumors such as basal cell carcinoma may be improved by integrating microneedle technology into the therapy.
KEY WORDS: Microneedles, 5-Fluorouracil, Cytotoxicity, Melanoma, Immunohistochemistry, Flux, Transdermal, Antitumor activity, Skin permeability
Graphical abstract
Pretreatment of mouse skin with microneedles significantly increased the skin permeability of 5-fluorouracil (5-FU) in vitro (left), and enhanced the antitumor activity of topically applied 5-FU against B16-F10 mouse melanoma cells implanted subcutaneously in mice (right).
1. Introduction
Fluorouracil (5-FU) is an antimetabolite that is used in the treatment of various types of cancers, including breast, head and neck, and colorectal cancer1,2. The fluorinated pyrimidine analog is available in topical formulations, which were approved by the United States Food and Drug Administration (US FDA) to treat actinic keratosis (non-cancerous) and superficial basal cell carcinoma (BCC)3,4. Approved topical products include 5-FU solutions (e.g., Fluoroplex 1% 5-FU solution, Allergan, Inc., Irvine, CA, USA), creams (e.g., Efudex®, Valeant Pharmaceuticals, Bridgewater, NJ, USA), and a 0.5% microsphere-based cream (Carac®, Valeant Pharmaceuticals). Other reported clinical applications of topical 5-FU include the treatment of nail psoriasis3,5, cholesteatoma6, lentigo maligna7 and some premalignant ophthalmic conditions8. Topical treatment with 5-FU, when applicable, is usually more preferred than surgical removal of affected lesions for cosmetic reasons, especially for multiple lesions and/or facial lesions9. Unfortunately, the skin permeability of topically applied 5-FU is poor10–12, likely due to its hydrophilic nature (LogP=−0.89)13. The use of topical 5-FU in BCC therapy is only limited to superficial BCC, and it is not recommended for invasive forms of BCC4,14. This is based on a study by Mohs et al.14, who reported that topical application of 5-FU in invasive BCCs can mislead clinicians by showing superficial improvement, while the deeper parts of the cancerous lesions continue to grow unnoticed.
Several approaches to improve the skin permeation of topical 5-FU have been evaluated with different degrees of success. For example, Paolino et al.11 described the formulation of 5-FU-loaded bola-surfactant-based niosomes to improve the percutaneous permeation and antitumor activity of 5-FU. The proposed niosomes exhibited an 8-fold increase in the percutaneous permeation of 5-FU through human skin, as compared to 5-FU in an aqueous solution. The 5-FU niosomes were significantly more cytotoxic against SKMEL-28 human melanoma cells in culture than 5-FU solution, which was attributed to the improved cellular uptake of 5-FU in the niosomes11. Other researchers reported the use of penetration enhancers such as azone, isopropyl myristate and lauryl alcohol12, or the use of pharmaceutical formulation technologies (e.g., microemulsions10 and nanogels13), to increase the percutaneous permeability of 5-FU. Physical methods to increase the permeability of 5-FU have also been evaluated. For example, Fang et al.15 studied the effect of a series of physical methods, namely iontophoresis, electroporation, erbium:YAG (erbium:yttrium–aluminum–garnet) laser and their combination, on the permeability of 5-FU. Both iontophoresis and electroporation significantly enhanced the percutaneous permeability of 5-FU, but the controlled removal of the stratum corneum by erbium:YAG laser was most effective15, confirming that it is the stratum corneum that limits the skin permeability of 5-FU. However, Meidan et al.16 found that ultrasound unexpectedly lowered the permeability of 5-FU through whole rat skin; an effect that was attributed to the back-diffusion of 5-FU to the ultrasonic coupling gel filled in the donor compartment.
Microneedle technology had been successfully applied to enhance the skin permeability of small molecules, macromolecules and even nanoparticles by creating an array of micro-sized holes in the stratum corneum of skin13,17–23. However, it remains unclear whether it is feasible to increase the skin permeability of 5-FU by pretreating skin with microneedles. In the present study, the feasibility of using microneedles to improve the skin permeability of 5-FU was tested in vitro using mouse skin mounted on a Franz diffusion apparatus. In addition, the feasibility of using microneedles to improve the in vivo antitumor activity of 5-FU was tested by comparing the ability of an FDA-approved topical 5-FU cream in inhibiting the growth of subcutaneously implanted B16-F10 tumors in mice. The 5-FU cream was applied on the mouse skin area where the tumor cells were implanted with or without pretreatment (of the skin area) with a microneedle roller. Topical 5-FU is not approved for melanoma treatment, but 5-FU was reported to be effective against melanoma cells in culture and in animal models19,20. The B16-F10 tumor cells are implanted subcutaneously in mice, which allows indirect evaluation of the in vivo permeation of 5-FU across mouse skin as well.
2. Materials and methods
2.1. Materials
The Dermaroller® microneedle roller was kindly provided by Cynergy, LLC (Carson City, NV, USA). There are 192 needles (500 µm in length, 50 µm in base diameter) on the roller. The topical 5-FU cream (5%) was from Taro Pharmaceuticals USA, Inc. (Hawthorne, NY, USA). Phosphate buffered saline (PBS, pH 7.4), cell culture medium and antibiotics were from Invitrogen (Life Technologies, Carlsbad, CA, USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and 5-FU were from Sigma-Aldrich (St. Louis, MO, USA).
2.2. In vitro cytotoxicity assay
B16-F10 murine melanoma cells were from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco's modified eagle medium (DMEM) with 10% (v/v) fetal bovine serum, 10 U/mL of penicillin and 100 μg/mL of streptomycin. Cells (2000/well) were seeded in 96-well plates and incubated overnight at 37 °C, 5% CO2. Cells were then incubated in the presence of various concentrations of 5-FU in PBS solution (pH 7.4) for 24 h or 48 h. Cell viability was determined using an MTT assay following the manufacturer's instruction. The formed formazan crystals were dissolved in 100 μL of dimethyl sulfoxide, and the absorbance of the resultant solution was measured at 570 nm and 630 nm using a BioTek Synergy HT Multi-Mode Microplate Reader (Winooski, VT, USA).
2.3. In vitro permeation of 5-FU in solution through mouse skin
In vitro permeation assay was completed as previously described19. Full thickness dorsal skin from C57BL/6 mice was used in the permeation study. Hair was carefully trimmed using an electric clipper 24 h before the collection of the skin. The harvested skins were stored at −20 °C and used within one month. On the day when the permeability study was performed, the skin was also treated with the Veet® hair removal cream (Reckitt Benckiser, Inc., UK) for 5 min and washed three times with water. The skin was then mounted onto the Franz diffusion cells (PermeGear, Inc., Hellertown, PA, USA) with the epidermis side facing upward. The receiver compartment contained 5 mL of PBS (pH 7.4, 10 mmol/L) and was maintained at 37 °C (Haake SC 100 Water Circulator, ThermoScientific, Wellington, NH, USA). The diffusion area of the skin was 0.64 cm2. The donor compartment was loaded with 400 μg of 5-FU in 400 µL of PBS (pH 7.4, 10 mmol/L) and covered with parafilm to prevent evaporation. To test the effect of treatment with microneedles on the permeation of 5-FU through the skin, the skin samples were also treated with a Dermaroller® microneedle roller as previously described before it was mounted onto the Franz diffusion cells21. Briefly, the skin sample was placed onto the flat surface of a balance, and the microneedle roller was rolled in 4 perpendicular lines over the skin surface, 5 times each for a total of 20 times, with an applying pressure of 600–800 g, which was constantly measured using the balance. At pre-determined time points (1, 2, 3, 6 and 18 h), samples (100 µL) were withdrawn from the receiver compartment and immediately replenished with fresh medium. Samples were then analyzed using HPLC. Chromatography was carried out with an Agilent Technologies 1260 Infinity HPLC workstation with an Agilent ZORBAX Eclipse Plus C18 column (150 mm×4.6 mm, 5 μm) using a potassium phosphate buffer (40 mmol/L, pH 7.0) as the mobile phase. The flow rate was 0.5 mL/min. The detector wavelength was 260 nm.
2.4. Animal study
Animal study was carried out following the US National Research Council guide for the care and use of laboratory animals. The animal protocol was approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin. Female C57BL/6 mice (8–10 weeks) were from Charles River Laboratories (Wilmington, MA, USA). Hair in the lower dorsal skin of anesthetized mice was trimmed using an electric clipper. B16-F10 murine melanoma cells (100,000 cells per mouse) in 100 μL DMEM were injected subcutaneously in the hair-trimmed area on day 0. On day 9, mice were randomly grouped (5–6 mice per group) and treated as follows: mice in the 5-FU cream group were treated with the 5% 5-FU cream (100 mg of cream per mouse) on the area (about 1 cm×2 cm) where the tumor cells were injected using a spatula, once daily for 8 consecutive days; mice in the MN+cream group were treated similarly, except that the application area was pretreated with the microneedle roller (MN); mice in the i.v. 5-FU group (positive control) were injected with 5-FU in sterile PBS intravenously via the tail vein (50 mg/kg body weight) on days 9 and 15. Negative control groups include tumor-bearing mice that were left untreated or tumor-bearing mice that were treated with the microneedle roller in the skin area where the tumor cells were injected, but without further treatment with 5-FU (i.e., MN group). To treat the mice with the microneedle roller, mice were placed onto the flat surface of a balance, and the microneedle roller was rolled over the skin area where the tumor cells were injected, 10 times parallel to the mouse length, with an applying pressure of around 400 g, which was measured using the balance. Tumor growth was monitored, and tumor size measured using a digital caliper. Tumor volume was calculated based on the following equation: tumor volume (mm3)=(length×width×width)/2. On the last day of the study (day 17), mice were euthanized, and tumor tissues were collected and weighed.
2.5. Histology and immunohistochemistry
Tumor tissues were fixed with a buffered formalin (10%) solution for 48 h and then transferred to 70% ethanol until sections were prepared, following paraffin embedding. The sections were stained using hematoxylin–eosin (H & E) or an anti-CD31 antibody (an angiogenesis marker, Abcam, Cambridge, MA, USA) in the Histology and Tissue Processing Facility in the Dell Pediatric Research Institute at the University of Texas at Austin. Slides were scanned, and images were taken using the ScanScope XT (Aperio Technologies, Vista, CA, USA).
2.6. Statistical analyses
Statistical analyses were completed by performing analysis of variance followed by Fisher's protected least significant difference procedure. A P-value of≤0.05 (two-tail) was considered significant.
3. Results and discussion
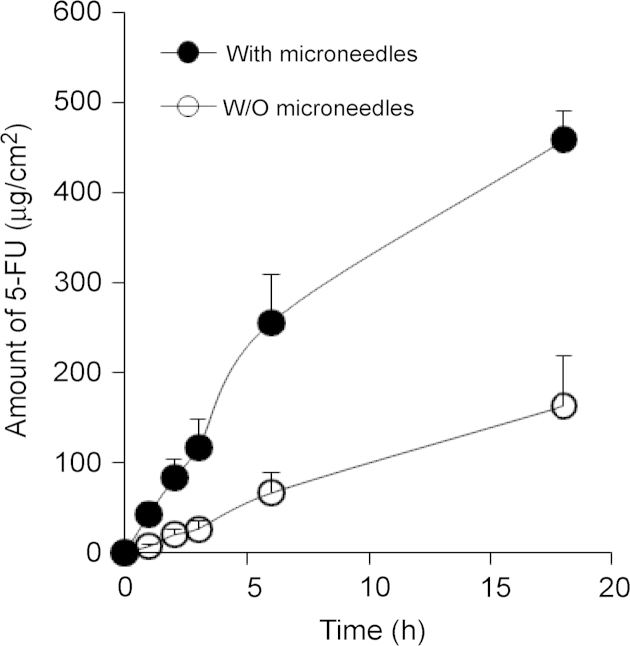
Topical 5-FU products are available on the market for the treatment of keratosis and superficial BCC. The poor skin permeation of 5-FU limits its application against various other cancerous and non-cancerous conditions. Moreover, increasing the skin permeability of 5-FU may also improve the clinical outcomes of topical 5-FU in BCC therapy. Microneedles have been successfully applied to increase the skin permeability of large and small molecules, and even nanoparticles, by creating micro-sized holes in the stratum corneum of skin17–21. The feasibility of using microneedles to improve the skin permeability of 5-FU was tested in the present study by pretreating skin with a microneedle roller that has solid microneedles (500 μm in length, 50 μm in base diameter) on it. The effect of pretreatment of mouse skin with microneedles on the diffusion of 5-FU (in an aqueous solution) through full thickness mouse skin (C57BL/6 mice) was initially evaluated in vitro using a Franz diffusion apparatus. As expected, the permeation of 5-FU through intact full thickness mouse skin was limited, with a flux of 8.93±4.55 μg/cm2/h (Fig. 1). Previously, Fang et al.15 reported a flux value of 1.5–2 μg/cm2/h (at pH 5.0) for the diffusion of 5-FU across full thickness nude mouse skin. However, the permeability of 5-FU through mouse skin that was pretreated with microneedles was significantly higher, with a flux of 39.75±18.50 μg/cm2/h, which is 4.5-fold larger than the flux of 5-FU through mouse skin that was not pretreated with microneedles (P<0.05). Clearly, pretreatment of mouse skin with solid microneedles significantly increased the permeability of 5-FU through the skin.
Figure 1.
The amount of 5-FU in an aqueous solution diffused through full thickness mouse skin treated (•) or not treated (○) with microneedles as a function of time. Data shown are means±S.E.M (n=3). At all the time points tested, with the exception of 0 h, the values between the microneedle-treated and -untreated groups are significantly different (P<0.05).
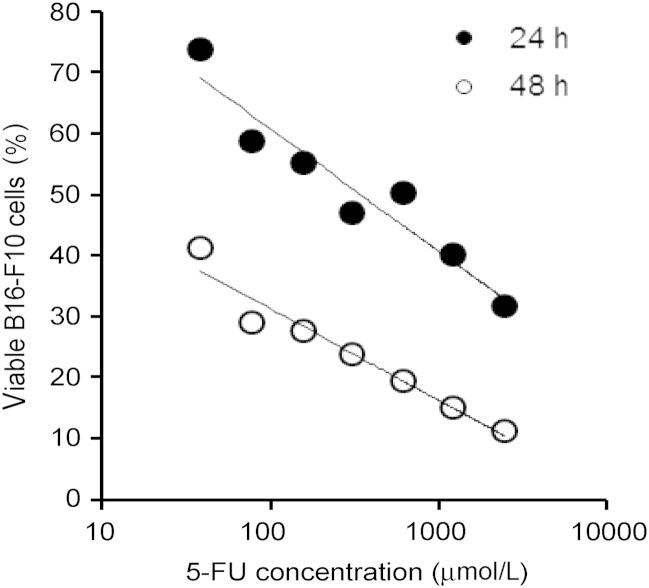
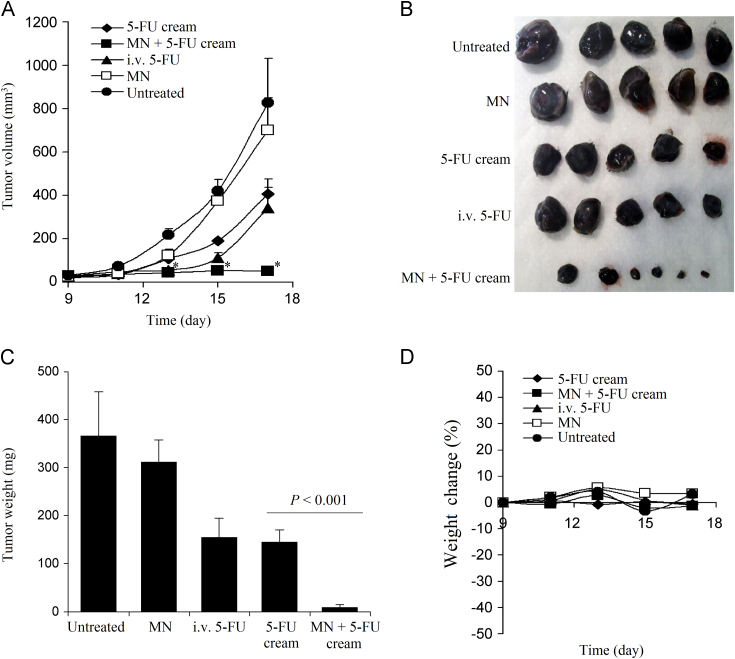
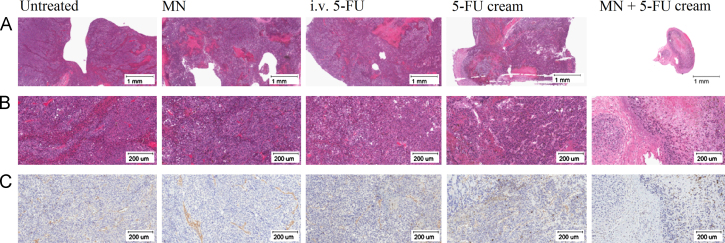
To test whether pretreatment of mouse skin with microneedles can improve the antitumor activity of topical 5-FU by increasing its skin permeability, the ability of a topical 5-FU cream to inhibit the growth of mouse B16-F10 melanoma cells implanted subcutaneously in the application area in mice was evaluated; the area where the 5-FU cream was applied was pretreated with the microneedle roller every time before the application of the 5-FU cream. Although 5-FU is not approved for melanoma therapy, it was previously shown to be cytotoxic against human and murine melanoma cells13,22,23. Our own data also confirmed that 5-FU inhibited the growth of the B16-F10 mouse melanoma cells in culture (Fig. 2). B16-F10 mouse melanoma cells were subcutaneously implanted in the rear dorsal area of female C57BL/6 mice. When tumor sizes reached 20–40 mm3 (9 days following implantation), mice were treated with the 5-FU cream (5%) topically on the skin area where the tumor cells were implanted. As shown in Fig. 3A, B16-F10 tumors grew aggressively if left untreated. Topical treatment with 5-FU cream (without pretreatment of the skin area with microneedles) significantly inhibited the tumor growth (Fig. 3A). However, the 5-FU cream became significantly more effective in inhibiting the tumor growth when the skin area where the 5-FU cream was applied was pretreated with the microneedle roller (Fig. 3A). At the end of the study, mice were euthanized to collect tumor tissues (Fig. 3B). The average weight of tumors in mice that were topically treated with the 5-FU cream without pretreatment with microneedles was significantly smaller than in mice that were left untreated, but was significantly larger than in mice that were treated with microneedles before the application of the 5-FU cream (Fig. 3C). In fact, the growth of tumors in mice that were treated with the topical 5-FU cream following pretreatment of the skin area with the microneedle roller was completely inhibited (Fig. 3A–C). The experiment was repeated with twice daily applications of 5-FU cream as well, and similar results were obtained (data not shown). The antitumor activity of the topical 5-FU following pretreatment (of the skin area) with microneedles was not simply due to the mechanical activity of applying the microneedle roller daily on the mouse skin area where the tumor cells were subcutaneously injected, because treatment with the microneedle roller alone without subsequent application of the 5-FU cream did not show any significant effect on the growth of the B16-F10 tumors (Fig. 3A–C). The body weights of the mice were also monitored during the treatment period, and it was found that the weights of mice that were treated with the 5-FU cream following pretreatment with microneedles, once daily for 8 consecutive days, did not significantly change (Fig. 3D), suggesting that the practice of applying 5-FU cream on a skin area pretreated with microneedles is potentially safe. Finally, the tumor tissues were also collected from mice and examined microscopically. H & E staining revealed that except in mice that were treated with microneedles prior to the application of the 5-FU cream (i.e., the MN+5-FU cream group), tumors in all other groups were large (Fig. 4A), with densely packed cells and scattered necrotic areas (Fig. 4B). On the contrary, tumors in mice that were treated with 5-FU cream after pretreatment with microneedles were smaller (Fig. 4A), and the tumor cells were loosely distributed with large intercellular spaces (Fig. 4B). Anti-CD31 staining (a maker for angiogenesis) also revealed that the extent of CD31-positive staining was significantly less in tumors in mice in the MN+5-FU cream group than in tumors in mice from other groups (Fig. 4C).
Figure 2.
In vitro cytotoxicity of 5-FU against B16-F10 mouse melanoma cells. Cells were incubated with various concentrations of 5-FU for 24 h (•) or 48 h (○), and cell viability was determined using an MTT assay. Data shown are means (n=6). Standard deviations are not shown for clarity.
Figure 3.
(A) The growth curves of B16-F10 tumors in C57BL/6 mice. (B) Digital photograph of tumors at the end of the study. (C) The weights of tumors at the end of the study. (D) The changes in the body weight of B16-F10 tumor-bearing mice. C57BL/6 mice were subcutaneously injected with B16-F10 tumor cells on day 0. Starting on day 9, mice were grouped (n=5–6) and topically treated with 5-FU cream (5%) daily for 8 consecutive days on the skin area where the tumor cells were injected. The application area was pretreated (MN+5-FU cream) or not (5-FU cream) with a microneedle roller before the application of the cream. As a positive control, mice were intravenously injected with a 5-FU solution (i.v. 5-FU) on day 9 and 15. Other controls included tumor-bearing mice left untreated, or tumor-bearing mice treated with a microneedle roller in the skin area where the tumor cells were injected (i.e., MN). The asterisks (⁎) in A indicates that the values of the 5-FU cream group and the MN+5-FU cream group are different on day 13, 15, and 17 (P<0.05). Data shown in A and C are mean±S.E.M (n=5–6). Standard deviations are not included in D for clarity (n=5–6).
Figure 4.
Representative images of tumor tissues after H&E staining (A: 2×, bar=1 mm, B: 10×, bar=200 μm) or anti-CD31 staining (C: 10×, bar=200 μm).
Taken together, it is clear that the 5-FU cream was more effective in inhibiting the growth of the B16-F10 tumor cells subcutaneously implanted underneath the skin when it was applied on a mouse skin area pretreated with microneedles than without microneedle pretreatment (Fig. 3), likely because pretreatment with microneedles enabled more 5-FU to permeate through the skin and reach the tumor cells in the subcutaneous space. Data in Fig. 1 clearly demonstrated that pretreatment of mouse skin with microneedles significantly increased the permeability of 5-FU through the skin. As mentioned earlier, topical 5-FU is approved by the US FDA for the treatment of superficial BCC. BCC is developed from basal cells in basement membrane layer in the skin epidermis 24. The data generated in the present study with the B16-F10 melanoma cells established in the subcutaneous space underneath the skin dermis layer may not accurately predict the effect of topical 5-FU applied on a skin area pretreated with microneedles on the growth of spontaneously developed BCC. However, in both cases, the 5-FU applied topically onto the skin needs to ‘travel’ across the stratum corneum layer before it can reach the live epidermis layer underneath the stratum corneum or the subcutaneous space. Data from previous studies showed that the stratum corneum layer is rate-limiting in the percutaneous absorption of 5-FU15. In addition, microneedles' ability to increase the skin permeability of molecules is mainly attributed to the micro-pores or holes created by them in the stratum corneum19,20. Therefore, the finding that pretreatment of a skin area with microneedles significantly enhanced the antitumor activity of topical 5-FU against the subcutaneously implanted mouse B16-F10 tumor cells may be exploited to improve the efficacy of topical 5-FU against human BCC in clinics, especially considering that microneedle rollers, including the one that was used in the present study, are already used by humans for cosmetic purposes and dermatology therapy to stimulate collagen and elastin production20,21,25. Moreover, since the dermal permeability of 5-FU can be significantly increased by pretreatment of the skin with microneedles, it may become possible to evaluate this treatment strategy for transdermal systemic delivery of 5-FU to treat invasive forms of BCC or other cancers (e.g., breast, colorectal, pancreatic and gastric cancers), against which intravenous 5-FU is currently approved. Of course, comprehensive safety tests will have to be completed to evaluate whether the microneedle technology can be safely integrated into topical 5-FU therapy clinically. For example, although microneedles such as the microneedle roller used in the present study are already used by humans for cosmetic purposes and dermatology therapy20,21,25, and topical 5-FU is approved by the US FDA for the treatment of keratosis and superficial BCC, it remains unclear whether frequent applications of 5-FU on a (human) skin area pretreated with microneedles will increase the local and systemic toxicity of topical 5-FU. In addition, although microneedles may not reach tumor cells when applied onto the skin, it is unknown whether breaching the skin, or even the stratum corneum alone, using the microneedles will negatively affect the development of the tumors in the application area.
4. Conclusions
In the present study, it was shown that the in vitro skin permeability and in vivo antitumor activity of topical 5-FU were significantly enhanced when the application area was pretreated with microneedles. Microneedle technology may be integrated into topical 5-FU therapy to improve its efficacy.
Acknowledgments
This work was supported in part by the University of Texas at Austin, College of Pharmacy and a grant from the National Institutes of Health (CA135274) to Z. Cui. Youssef Naguib was supported by a doctoral scholarship from the Egyptian Ministry of Higher Education. The authors acknowledge Cynergy, LLC (Carson City, NV) for generously providing Dermaroller® microneedle rollers, free of charge.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Chu E. Clinical colorectal cancer: ode to 5-fluorouracil. Clin Colorect Cancer. 2007;6:609. [Google Scholar]
- 2.Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 3.Moore A.Y. Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J Dermatol Treat. 2009;20:328–335. doi: 10.3109/09546630902789326. [DOI] [PubMed] [Google Scholar]
- 4.Ceilley R.I. Mechanisms of action of topical 5-fluorouracil: review and implications for the treatment of dermatological disorders. J Dermatol Treat. 2012;23:83–89. doi: 10.3109/09546634.2010.507704. [DOI] [PubMed] [Google Scholar]
- 5.de Vries A.C., Bogaards N.A., Hooft L., Velema M., Pasch M., Lebwohl M. Interventions for nail psoriasis. Cochrane Database Syst Rev. 2013;1:CD007633. doi: 10.1002/14651858.CD007633.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi H., Funabiki K., Hasebe S., Fukuda-Yamamoto T., Kaieda S., Iwanaga T. Clinical efficacy of 5-fluorouracil (5-FU) topical cream for treatment of cholesteatoma. Auris Nasus Larynx. 2005;32:353–357. doi: 10.1016/j.anl.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Litwin M.S., Krementz E.T., Mansell P.W., Reed R.J. Topical chemotherapy of lentigo maligna with 5-fluorouracil. Cancer. 1975;35:721–733. doi: 10.1002/1097-0142(197503)35:3<721::aid-cncr2820350327>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.de Keizer R.J., de Wolff-Rouendaal D., van Delft J.L. Topical application of 5-fluorouracil in premalignant lesions of cornea, conjunctiva and eyelid. Doc Ophthalmol. 1986;64:31–42. doi: 10.1007/BF00166683. [DOI] [PubMed] [Google Scholar]
- 9.Gross K., Kircik L., Kricorian G. 5% 5-fluorouracil cream for the treatment of small superficial basal cell carcinoma: efficacy, tolerability, cosmetic outcome, and patient satisfaction. Dermatol Surg. 2007;33:433–439. doi: 10.1111/j.1524-4725.2007.33090.x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R.R., Jain S.K., Varshney M. AOT water-in-oil microemulsions as a penetration enhancer in transdermal drug delivery of 5-fluorouracil. Colloids Surf B Biointerfaces. 2005;41:25–32. doi: 10.1016/j.colsurfb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Paolino D., Cosco D., Muzzalupo R., Trapasso E., Picci N., Fresta M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int J Pharm. 2008;353:233–242. doi: 10.1016/j.ijpharm.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Singh B.N., Singh R.B., Singh J. Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. Int J Pharm. 2005;298:98–107. doi: 10.1016/j.ijpharm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Sabitha M., Sanoj R.N., Nair A., Lakshmanan V.K., Nair S.V., Jayakumar R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr Polym. 2013;91:48–57. doi: 10.1016/j.carbpol.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 14.Mohs F.E., Jones D.L., Bloom R.F. Tendency of fluorouracil to conceal deep foci of invasive basal cell carcinoma. Arch Dermatol. 1978;114:1021–1022. [PubMed] [Google Scholar]
- 15.Fang J.Y., Hung C.F., Fang Y.P., Chan T.F. Transdermal iontophoresis of 5-fluorouracil combined with electroporation and laser treatment. Int J Pharm. 2004;270:241–249. doi: 10.1016/j.ijpharm.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Meidan V.M., Walmsley A.D., Docker M.F., Irwin W.J. Ultrasound-enhanced diffusion into coupling gel during phonophoresis of 5-fluorouracil. Int J Pharm. 1999;185:205–213. doi: 10.1016/s0378-5173(99)00168-4. [DOI] [PubMed] [Google Scholar]
- 17.Park J.H., Choi S.O., Seo S., Choy Y.B., Prausnitz M.R. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010;76:282–289. doi: 10.1016/j.ejpb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Prausnitz M.R. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A., Wonganan P., Sandoval M.A., Li X., Zhu S., Cui Z. Microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic PLGA nanoparticles. J Control Release. 2012;163:230–239. doi: 10.1016/j.jconrel.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A., Li X., Sandoval M.A., Rodriguez B.L., Sloat B.R., Cui Z. Permeation of antigen protein-conjugated nanoparticles and live bacteria through microneedle-treated mouse skin. Int J Nanomed. 2011;6:1253–1264. doi: 10.2147/IJN.S20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W., Li X., Wong Y.S., Zheng W., Zhang Y., Cao W. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano. 2012;6:6578–6591. doi: 10.1021/nn202452c. [DOI] [PubMed] [Google Scholar]
- 23.Qu X., Felder M.A., Perez H.Z., Sondel P.M., Rakhmilevich A.L. Antitumor effects of anti-CD40/CpG immunotherapy combined with gemcitabine or 5-fluorouracil chemotherapy in the B16 melanoma model. Int Immunopharmacol. 2013;17:1141–1147. doi: 10.1016/j.intimp.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bath-Hextall F.J., Perkins W., Bong J., Williams H.C. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2007;1:CD003412. doi: 10.1002/14651858.CD003412.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Bariya S.H., Gohel M.C., Mehta T.A., Sharma O.P. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11–29. doi: 10.1111/j.2042-7158.2011.01369.x. [DOI] [PubMed] [Google Scholar]