Abstract
Colorectal cancer is one of the most common tumors worldwide and at least 50 % of patients with this disease develop metastases. In this setting, additional treatment options are needed for patients presenting disease progression after exhausting all standard therapies. Regorafenib is an orally administered multikinase inhibitor which has been shown to provide survival benefits to patients with metastatic colorectal cancer (mCRC). Although most adverse events (AEs) associated with regorafenib may resolve within the first 8 weeks of treatment, some of them may require dose reduction or treatment interruption. Overall, while remaining aware of the safety profile of regorafenib and how to manage the most common toxicities related to its use, this drug should be considered a new standard of care for patients with pretreated mCRC. This review addresses practical aspects of its use, such as dosing, patient monitoring, and management of the most common regorafenib-related AEs.
Keywords: AEs, mCRC, Prophylaxis, Regorafenib, Treatment
Introduction
Colorectal cancer is one of the most common tumors worldwide and over 50 % of patients with this type of tumor develop metastases. Standard treatment for metastatic colorectal cancer (mCRC) includes chemotherapy based on fluoropyrimidines, oxaliplatin and irinotecan, as well as monoclonal antibodies such as bevacizumab, cetuximab and panitumumab [1, 2] and human recombinant fusion proteins, such as aflibercept [3]. Nonetheless, additional options are needed for patients presenting disease progression after exhausting all standard therapies. Regorafenib is an orally administered multikinase inhibitor [4]. This agent targets cell-signaling pathways involved in oncogenesis and progression, including protein kinases associated with angiogenesis, such as vascular endothelial growth factor receptors 1–3 (VEGFR 1–3) and tyrosine receptor kinase 2 (TIE2). Regorafenib also has an effect in stromal signaling, which mediates the maintenance of the stroma or tumor microenvironment, by inhibiting platelet-derived growth factor receptor (PDGFR) and fibroblast growth factor receptor (FGFR). This agent also inhibits oncogenic receptor tyrosine kinases, such as KIT and RET, which leads to inhibition of neovascularization and cancer cell replication [4]. Regorafenib was successfully tested in preclinical models [4] and promising results in phase I studies were also factors in the clinical development of this agent [5, 6]. Further steps were the applications for the marketing authorization of regorafenib in the treatment of mCRC and gastrointestinal stromal tumors (GISTs) based on data of the CORRECT [7] and the GRID trials [8], respectively.
The recommended dose of regorafenib is 160 mg/daily for 3 weeks, followed by 1 week with no treatment. Although regorafenib confers survival benefits to patients with mCRC, some adverse events (AEs) commonly associated with the use of this agent may lead to dose reduction or treatment interruption thus jeopardizing treatment efficacy. Nonetheless, chemotherapy-associated AEs are cumulative, whereas most AEs associated with regorafenib may resolve within the first 8 weeks of treatment, if these are appropriately managed [9].
This paper has been developed by ten Spanish oncologists, all of them experts in the management of patients with mCRC and with a wide experience in the administration of regorafenib. The aim of this review is to address key practical aspects of regorafenib use, such as dosing, patient monitoring, and management of the most common drug-related AEs.
Efficacy and safety data of regorafenib in patients with mCRC
Regorafenib was approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of adult patients with mCRC who have previously progressed to, or are not considered candidates for, available therapies. These treatments include therapies based on fluoropyrimidines, anti-VEGF and anti-epidermal growth factor receptor (EGFR) agents in RAS wild type tumors.
CORRECT trial
CORRECT was an international, multicenter, randomized, placebo-controlled, phase III trial including 760 patients with previously treated mCRC, who were allocated to receive either regorafenib (n = 505) or placebo (n = 255), in addition to best supportive care [7]. In this study, the recruitment was completed in 11 months. Highlighting this latter the great need for new treatments in this setting. The primary endpoint of the trial, overall survival (OS), showed significant differences in favor of the regorafenib arm [6.4 vs. 5.0 months; hazard ratio (HR) 0.77; 95 % confidence interval (CI) 0.64–0.94; p = 0.0052].
Secondary end points of the trial were progression-free survival (PFS), objective response, disease control rates and safety. Differences in terms of PFS were significantly better with regorafenib (1.9 vs. 1.7 months, respectively; HR 0.49; 95 % CI 0.42–0.58; p < 0.0001). However, partial response did not differ significantly between both arms (1.0 vs. 0.4 %, respectively; p = 0.19). This may be due to the fact that regorafenib is a cytostatic, rather than a cytotoxic drug, as well as due to the fact that its activity may not be appropriately documented by the conventional Response Evaluation Criteria in Solid Tumors (RECIST). Indeed, disease control rate (i.e. partial response plus stable disease assessed at least 6 weeks after randomization) was significantly higher in patients treated with regorafenib (41 %) than in those allocated to the placebo arm (15 %) (p < 0.0001). Patients treated with regorafenib showed a trend towards benefit in all clinical subgroups in the CORRECT trial, including patients presenting KRAS mutation.
The safety profile of regorafenib in the CORRECT trial was consistent with early-phase clinical trials, as well as with other small-molecule tyrosine kinase inhibitors (TKIs). The most frequent severe AEs were hand-foot skin reaction (HFSR), fatigue, diarrhea, hypertension and rash (Table 1). Most occurred early in the course of treatment and could be managed with dose modification or reduction. In the CORRECT trial, patients’ quality-of-life (QoL) was measured according to the EORTC QLQ-C30 and EQ-5D scales. No substantial differences were found in overall change in QoL between regorafenib-treated patients and placebo recipients [10]. Both the EORTC QLQ-C30 and EQ-5D scales are validated for the measurement of QoL in patients with cancer. Although these scales do not address some of the AEs typically associated with regorafenib, the authors consider the overall results of this trial in terms of QoL are relevant.
Table 1.
Incidence of AEs in patients with mCRC in the CORRECT trial [7]
| AEs | Regorafenib + BSC, n = 500 | Placebo + BSC, n = 253 | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1–2, n (%) | Grade 3, n (%) | Grade 4, n (%) | All grades, n (%) | Grade 1–2, n (%) | Grade 3, n (%) | Grade 4, n (%) | All grades, n (%) | |
| Clinical AEs | ||||||||
| Fatigue | 189 (38) | 46 (9) | 2 (<1) | 237 (47) | 58 (23) | 12 (5) | 1 (<1) | 71 (28) |
| HFSR | 150 (30) | 83 (17) | 0 (0) | 233 (47) | 18 (7) | 1 (<1) | 0 (0) | 19 (8) |
| Diarrhea | 133 (27) | 35 (7) | 1 (<1) | 169 (34) | 19 (8) | 2 (1) | 0 (0) | 21 (8) |
| Anorexia | 136 (27) | 16 (3) | 0 (0) | 152 (30) | 32 (13) | 7 (3) | 0 (0) | 39 (15) |
| Voice changes | 146 (29) | 1 (<1) | 0 (0) | 147 (29) | 14 (6) | 0 (0) | 0 (0) | 14 (6) |
| Hypertension | 103 (20) | 36 (7) | 0 (0) | 139 (28) | 13 (5) | 2 (1) | 0 (0) | 15 (6) |
| Oral mucositis | 121 (24) | 15 (3) | 0 (0) | 136 (27) | 9 (4) | 0 (0) | 0 (0) | 9 (4) |
| Rash | 101 (20) | 29 (6) | 0 (0) | 130 (26) | 10 (4) | 0 (0) | 0 (0) | 10 (4) |
| Nausea | 70 (14) | 2 (<1) | 0 (0) | 72 (14) | 28 (11) | 0 (0) | 0 (0) | 28 (11) |
| Weight loss | 69 (14) | 0 (0) | 0 (0) | 69 (14) | 6 (2) | 0 (0) | 0 (0) | 6 (2) |
| Fever | 48 (10) | 4 (1) | 0 (0) | 52 (10) | 7 (3) | 0 (0) | 0 (0) | 7 (3) |
| Constipation | 42 (8) | 0 (0) | 0 (0) | 42 (8) | 12 (5) | 0 (0) | 0 (0) | 12 (5) |
| Dry skin | 39 (8) | 0 (0) | 0 (0) | 39 (8) | 7 (3) | 0 (0) | 0 (0) | 7 (3) |
| Alopecia | 36 (7) | 0 (0) | 0 (0) | 36 (7) | 1 (<1) | 0 (0) | 0 (0) | 1 (<1) |
| Taste alteration | 35 (7) | 0 (0) | 0 (0) | 35 (7) | 5 (2) | 0 (0) | 0 (0) | 5 (2) |
| Vomiting | 35 (7) | 3 (1) | 0 (0) | 38 (8) | 13 (5) | 0 (0) | 0 (0) | 13 (5) |
| Sensory neuropathy | 32 (6) | 2 (<1) | 0 (0) | 34 (7) | 9 (4) | 0 (0) | 0 (0) | 9 (4) |
| Nose bleed | 36 (7) | 0 (0) | 0 (0) | 36 (7) | 5 (2) | 0 (0) | 0 (0) | 5 (2) |
| Dyspnea | 27 (5) | 1 (<1) | 0 (0) | 28 (6) | 4 (2) | 0 (0) | 0 (0) | 4 (2) |
| Muscle pain | 26 (5) | 2 (<1) | 0 (0) | 28 (6) | 6 (2) | 1 (<1) | 0 (0) | 7 (3) |
| Headache | 23 (5) | 3 (1) | 0 (0) | 26 (5) | 8 (3) | 0 (0) | 0 (0) | 8 (3) |
| Pain in the abdomen | 24 (5) | 1 (<1) | 0 (0) | 25 (5) | 10 (4) | 0 (0) | 0 (0) | 10 (4) |
| Laboratory abnormalities | ||||||||
| Thrombocytopenia | 49 (10) | 13 (3) | 1 (<1) | 63 (13) | 4 (2) | 1 (<1) | 0 (0) | 5 (2) |
| Hyperbilirubinemia | 35 (7) | 10 (2) | 0 (0) | 45 (9) | 2 (1) | 2 (1) | 0 (0) | 4 (2) |
| Proteinuria | 28 (6) | 7 (1) | 0 (0) | 35 (7) | 3 (1) | 1 (<1) | 0 (0) | 4 (2) |
| Anemia | 19 (4) | 12 (2) | 2 (<1) | 33 (7) | 6 (2) | 0 (0) | 0 (0) | 6 (2) |
| Hypophosphatemia | 6 (1) | 19 (4) | 0 (0) | 25 (5) | 0 (0) | 1 (<1) | 0 (0) | 1 (<1) |
Treatment-related AEs occurring in ≥5 % of patients
AEs adverse events, BSC best supportive care, HFSR hand-foot skin reaction, mCRC metastatic colorectal cancer
Regorafenib is the first oral multikinase inhibitor with proven activity in patients with mCRC, achieving disease control rate in 41 % of treated patients and a 1.4 month absolute increase in OS, a 23 % reduction in the risk of death, and a 51 % reduction in the risk of progression or death. Regorafenib is generally well tolerated, with a toxicity profile optimally managed through early monitoring and through optimal implementation of supportive care strategies. In the light of these results, it was agreed that regorafenib should be considered a new standard of care for patients with pretreated mCRC in all clinical and molecular subgroups. Nevertheless, clinicians need guidance for regorafenib use in patients with mCRC to optimize drug tolerability and therefore keep a favorable benefit/risk profile.
CONSIGN trial
The phase IIIb CONSIGN trial (ClinicalTrials.gov Identifier: NCT01538680) is a prospective, interventional, open-label, single-arm, multicenter expanded access study. CONSIGN aims to examine the effect of providing regorafenib to patients with mCRC who have failed after standard therapy and for whom no other treatment option exists. The primary endpoint of this study is collecting additional safety data on regorafenib in this setting. Patients continued on treatment until reaching one of the main study criteria, i.e. death, unacceptable toxicity, patient’s withdrawal of consent, physician’s decision for discontinuation or substantial noncompliance with the protocol. If the disease is progressive, the patient may continue treatment at the investigator’s discretion. To date, no data have been published on the outcomes of this trial.
Incidence and management of the most common side effects of regorafenib
Fatigue/asthenia
Fatigue is a subjective symptom of exhaustion which increases gradually. Unlike weakness, fatigue can improve after a period of resting. In a phase I study conducted by Strumberg et al. [6] on 38 patients with heavily pretreated mCRC, 50 % of patients presented fatigue of any grade and 11 % of them showed grade ≥3 fatigue during their treatment with regorafenib. This symptom was one of the most common treatment-related AEs in this trial. Overall, regorafenib was permanently discontinued due to AEs in 11 (29 %) patients and in 1 of the patients treated at the 160 mg dose level, treatment discontinuation was due to fatigue. In the phase III CORRECT trial [7], fatigue was also among the most common regorafenib-related AEs, both at any grade (47 vs. 28 %), and at grade ≥3 (10 vs. 5 %) compared to placebo.
According to an analysis conducted by Grothey et al. [11] regarding the time course of regorafenib-related AEs, common grade 3 AEs occur early during the course of treatment and stabilize over time. The incidence of grade 1 and 3 fatigue was 19, 18 and 7 %, respectively, during the first cycle of treatment. The incidence of fatigue appeared to stabilize between the seventh and eighth cycles, occurring in 10 % of patients at this time point. In this analysis, the incidence of other common grade 3 AEs was also demonstrated to decrease over time.
As fatigue is a common symptom of cancer patients and its origin is often multifactorial (advanced cancer, drug toxicity, anemia, comorbidities, etc.), sometimes clinicians find difficulties in assessing to what extent the antineoplastic agent is contributing to it. Fatigue grade ≥2 has an impact in patients’ QoL, and it is important to highlight that patients should be made aware that fatigue may be affecting them prior to treatment initiation with regorafenib. As an AE of regorafenib, however, early action against fatigue plays a key role in patients treated with regorafenib. Therefore, during the first two cycles of treatment, monitoring for fatigue should be conducted every week, either by an oncologist or by a nurse. After the first two cycles, monitoring may be done less frequently.
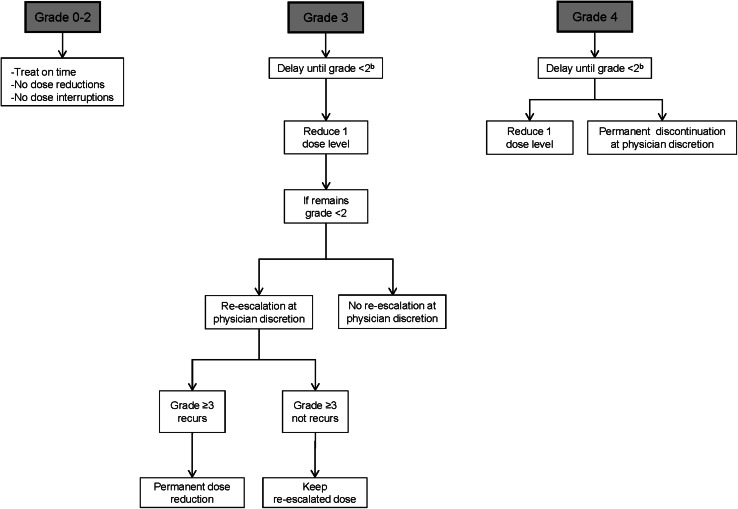
Clinicians have to bear in mind that after the first medical appointment, the effect of regorafenib on fatigue can be controlled in several ways, such as reducing the dose of regorafenib (Fig. 1), treatment interruption and/or the administration of concomitant medications to the patient. Special attention should be also paid to other potentially treatable co-existing causes of fatigue such as anemia, and appropriate treatment initiated if indicated. During the second cycle of treatment and thereafter, an improvement in the control of fatigue is frequently achieved and, thus, less frequent monitoring is required. Asthenia–anorexia syndrome can be treated with corticosteroid medication [12], or with the administration of medroxyprogesterone [13]. However, the convenience of these treatments for patients with mCRC is an aspect under discussion. Overall, initial management of fatigue includes treating common causes of fatigue, such as pain, sleep disturbance and anemia. These conditions can improve with medical treatment. Proper nutrition and prevention of malnutrition, anorexia and dehydration may also help reduce fatigue. Regular exercise is also recommended to patients with a good physical status [14]. In addition, patients with any grade of fatigue should be checked thyroid-stimulating hormone (TSH) levels [15]. In the other hand, when severe fatigue occurs, regorafenib treatment should be interrupted until the patient’s energy levels have recovered.
Fig. 1.
Dose modification/delay for toxicities related to regorafenib (except hand-foot skin reaction, hypertension and liver function test abnormalities). According to Common Terminology Criteria for Adverse Events (CTCAE) v 4.0. aExcludes alopecia, non-refractory nausea/vomiting, non-refractory hypersensitivity and asymptomatic laboratory abnormalities. bIf no recovery after a 4 week delay, treatment will be permanently discontinued
Hand-foot skin reaction
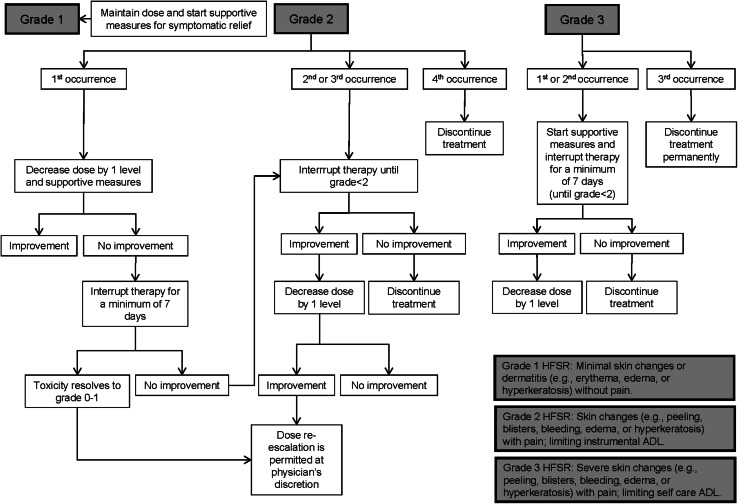
Palmar-plantar erythrodysesthesia syndrome or HFSR is a disorder characterized by redness, marked discomfort, and swelling and tingling in palms or soles. This syndrome is characterized by localized thick hyperkeratotic lesions that may be surrounded by erythematous regions within the skin, which are often painful. According to the phase III CORRECT trial [7], HFSR of any grade is very common (47 %) and grade 3 HFSR is common (17 %) in patients with mCRC treated with regorafenib. These lesions usually occur within the first 2–4 weeks of regorafenib administration and can negatively impact physical, psychological and social well-being of patients, thereby affecting their QoL. HFSR is the most common toxicity leading to dose reductions or treatment interruptions in patients receiving regorafenib and these side effects start early in patients’ treatment.
In patients treated with regorafenib, HFSR should be monitored weekly during the first two cycles and every 4 weeks thereafter. Early detection of HFSR is a key to prevent worsening of this condition (Fig. 2). Main prophylactic measures include skin examination before treatment initiation, softening and removal of calluses, protection against pressure and friction (i.e. plantar pads, adequate footwear, etc.) and the frequent use of creams and moisturizers from the start of therapy. To soften the skin of the hands and feet non-urea-based creams should be applied, whereas keratolytic creams, containing urea or salicylic acid 6 % can be used on hyperkeratotic areas. To exfoliate callused skin, patients can use alpha hydroxyl acid-based creams (5–8 %) 2 times a day [16] (Fig. 3).
Fig. 2.
Dose modification/delay of regorafenib in patients with hand-foot skin reaction. According to Common Terminology Criteria for Adverse Events (CTCAE) v 4.0. aIn case of grade 3 hand-foot skin reaction, a dose re-escalation is permitted only during first occurrence. ADL activities of daily living, HFSR hand-foot skin reaction
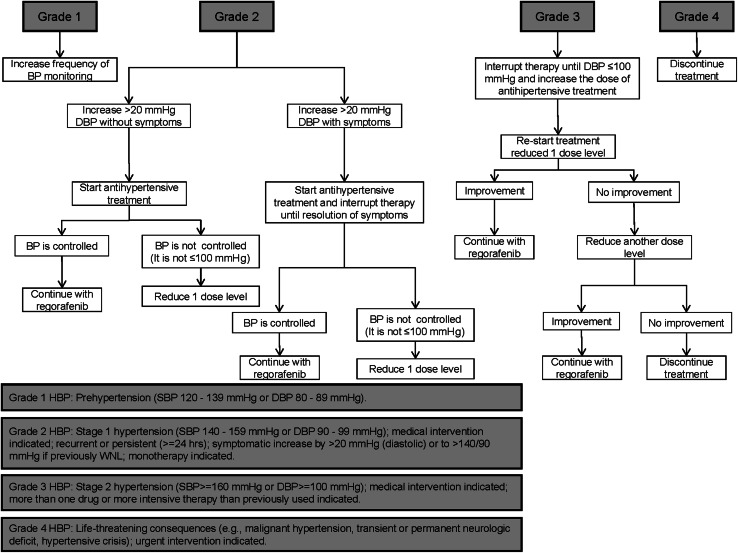
Fig. 3.
Management of treatment-emergent hypertension in patients treated with regorafenib. According to Common Terminology Criteria for Adverse Events (CTCAE) v 4.0. DBP diastolic blood pressure, HBP high blood pressure, SBP systolic blood pressure, WNL within normal limits
In cases of HFSR grade ≥2, a dermatologist consultation is recommended, and also the use of non-steroidal anti-inflammatory drugs (NSAIDS). The key objectives of HFSR management strategies for healthcare providers are to maintain or restore patient comfort and QoL, to avoid interference with patient’s daily activities and also to maintain their treatment with regorafenib for as long as possible. Accurate grading of HFSR is critical to select the appropriate management strategies. Dose reduction or treatment interruption may be required in some instances, which usually leads to the alleviation of symptoms in 1–2 weeks. Patients should be made aware of the importance of informing their healthcare team as soon as the first symptoms of HFSR are noticed. Patients must also be aware of the best practices for managing HFSR, as well as the steps that can be taken to lessen the severity of this reaction before and during their treatment with regorafenib.
Diarrhea
Diarrhea is defined as having three or more loose or liquid stools per day, or an increase in the defecatory frequency over baseline for that particular patient. Diarrhea can be accompanied by pain, defecating emergency or incontinence, and, in severe cases, may cause fluid and electrolyte depletion leading to dehydration, renal failure and even cardiovascular compromise. Therefore, adequate early intervention is key to prevent severe complications. When a patient reports diarrhea, some questions shall be formulated in order to better adjust treatment, such as the presence of fever, number of stools/day, consistency and color, symptom duration, presence of nausea and vomiting, abdominal pain, concomitant medication and type of abdominal surgery performed (whether the patient underwent a colostomy or an ileostomy). In the phase III CORRECT trial [7], diarrhea was reported in 34 % of patients with mCRC treated with regorafenib and in 7 % of them it was grade 3–4. Grade 3–4 diarrhea is the third most common side effect reported in the CORRECT trial, and the incidence of grade 3 diarrhea is constant throughout the treatment.
The incidence of grade 3–4 regorafenib-induced diarrhea seems to be higher than that observed in patients treated with drugs such as cetuximab in monotherapy (1.7 %) [17], and higher than that reported with other commonly used antineoplastic regimens in mCRC, such as FOLFIRI (10.5 %), FOLFIRI plus cetuximab (15.7 %), FOLFOX4 (9 %), FOLFOX4 plus panitumumab (18 %) and cetuximab plus irinotecan (21 %) [17–19]. Treatment of diarrhea is an important aspect in patients receiving this drug. The treatment may include anti-diarrhea diet (avoid fiber and fat), intensive oral rehydration with liquids that contain water and electrolytes, and the use of anti-diarrheal drugs such as loperamide and/or codeine every 4–8 h. Regorafenib may also be suspended to allow the patient’s recovery (i.e. when diarrhea reaches grade >2), and then treatment restarted at a lower dose (Fig. 1).
Anorexia/weight loss
Anorexia is also a frequent symptom in mCRC patients treated with regorafenib. Thirty percent of patients treated with regorafenib presented this AE in comparison with 15 % of patients in the placebo arm in the CORRECT trial [7]. However, there was no difference in the percentage of patients treated with regorafenib or placebo (3 %) who had grade 3 anorexia. With regard to weight loss, 14 vs. 2 % of patients with mCRC evaluated in this trial showed weight loss, respectively. Anorexia is a disorder frequently associated with other AEs in cancer patients. Weight loss can be easily measured; in contrast, anorexia is more subjective. Although both AEs are frequently related, patients can also present weight loss due to diarrhea, regardless of the presence of anorexia. Demetri et al. [8] conducted the GRID phase III trial that included 199 patients diagnosed with metastatic or unresectable GISTs. These patients had failed to respond to at least one previous administration of imatinib and sunitinib. In this trial, a lower percentage of patients in both study arms (21 % regorafenib arm vs. 8 % placebo arm) showed anorexia in comparison with the CORRECT trial.
Healthcare staff should ask patients at every medical appointment about their appetite and patients’ weight should be monitored in every cycle of treatment. The prophylaxis and management of the AE weight loss includes a hypercaloric diet, dietary supplements and the administration of megestrol acetate, as well as consulting an endocrinologist in some circumstances. However, as in patients presenting with fatigue, the convenience of the administration of corticoids to patients with anorexia is an aspect that is under discussion. It is important to highlight that anorexia and weight loss are frequent or very frequent AEs in patients treated with TKIs [20]. There are no data regarding the first occurrence of this AE and its outcome in patients treated with regorafenib, and there are no special requirements regarding anorexia in patients treated with this agent in this setting. In addition, clinicians have to bear in mind that patients with cancer can quickly lose 3–4 kg (grade 1 weight loss) but such weight loss does not require dose reduction. Nevertheless, grade 3 anorexia does require a dose reduction (Fig. 1).
Hypertension
Hypertension is common in patients treated with regorafenib; however, the effect of this agent on hypertension is not cumulative. The overall incidence of hypertension in the CORRECT trial was 28 and 6 % in patients treated in the regorafenib and placebo arms, respectively. The incidence of grade 3 hypertension was 7 and 1 %, respectively [7]. Nonetheless, the incidence of hypertension was higher in the GRID trial [8], as 58.5 % of patients treated with regorafenib presented this AE and in 23 % of them it was of grade 3. Several clinical trials carried out in patients treated with other TKIs have demonstrated similar or higher rates of hypertension in patients with mCRC or with GISTs treated with drugs such as sorafenib (all grades 23 %; grade 3–4 6 %), sunitinib (all grades 22 %; grade 3–4 7 %), pazopanib (all grades 36 %; grade 3–4 4 %), axitinib (all grades 40 %; grade 3–4 13 %), and regorafenib (all grades 36 %, grade 3–4 11 %) [7, 8, 21–23].
It is important to bear in mind that hypertension occurs more frequently during the first two cycles of treatment with regorafenib, but this AE is controlled in subsequent treatment cycles [11]. Measurement of blood pressure is recommended before treatment initiation and then, careful consideration shall be given to initiate or adjust antihypertensive medication if required (Fig. 3). Following treatment initiation, monitoring of blood pressure twice a week is advised during the first 2 weeks of therapy and once per week thereafter. If blood pressure is stable, the monitoring frequency may be subsequently reduced. Hypertensive patients are recommended to have their blood pressure monitored more frequently. Overall, hypertension usually occurs with mild or moderate severity in patients treated with regorafenib and after 2 weeks of treatment with this drug. Nevertheless, this AE is generally asymptomatic and rarely impairs patient’s QoL. In addition, hypertension is easily manageable with appropriate medication and, when required, regorafenib treatment can be temporarily interrupted and/or dose can be reduced.
Mucositis
Mucositis is the type of toxicity related to cancer treatment most commonly affecting QoL. The high epithelial replacement rate in mucosal tissue makes it vulnerable to damage by antineoplastic agents. Signs of this condition include atrophy of the epithelial tissue accompanied by erythema and edema, sensation of dryness and burning in the mouth, odynophagia and cotton mouth. Grade ≥2 mucositis is also accompanied by pain and interferes with oral intake, potentially leading to dehydration and weight loss. Several guidelines address the prophylaxis and management of mucositis, and some key aspects described in these guidelines are mentioned below [24–30].
Mucositis associated with biologic therapy is due to several mechanisms that differ from the mechanisms related to chemotherapy and which are not well-known. This AE affects 40 % of patients treated with standard chemotherapy, 80 % of patients treated with high-dose chemotherapy and 100 % of patients treated with radiotherapy to the head and neck. In patients treated with biologic therapies, the incidence of mucositis varies from 10 to 40 %, reaching 72 % in patients treated with afatinib. The mechanism of development of mucositis in patients treated with TKIs is not clear either; however, it seems to be related to impaired healing of microtraumas in these patients. Symptoms such as dysgeusia, dysphagia and aphthous ulcer are more frequently observed in patients treated with TKIs than in patients developing mucositis induced by conventional cytotoxic agents. The administration of a TKI together with a FOLFIRI regimen can increase the incidence and grade of mucositis. The incidence of mucositis in patients treated with TKIs fluctuates from 4 % (in patients treated with pazopanib) to 51 % (in patients treated with cabozantinib). The incidence of mucositis in the CORRECT trial was 27 and 4 % in patients in the regorafenib and placebo arms [7], respectively (3 and 0 %, respectively, showed grade 3). The mechanism for the development of mucositis in patients treated with regorafenib is still unknown, including the time point at which initiation of mucositis occurs in patients treated with this agent, whether this reaction is related to other toxicities and whether its recovery is similar to chemotherapy-related mucositis.
The risk factors involved in the development of this AE include age (the younger the patient, the greater the toxicity in the mucosal tissue), poor oral hygiene, nutritional status, tumor location (worse in tumors of head and neck) and type of treatment administered to the patient. Clinicians have to bear in mind these risk factors in order to prevent the occurrence of mucositis. In addition, the development of this AE can be accompanied by complications such as local and systemic infections, especially in patients presenting neutropenia <1,000 U/µL. Prophylaxis of mucositis includes appropriate oral hygiene, consultation with an odontologist (particularly important before treatment initiation) and the avoidance of spicy, acid, hard or hot food and drinks. With regard to treatment, recommendations include a high-protein diet and adequate hydration. In cases of severe mucositis, enteral or parenteral nutrition is advisable. Mucositis can also be treated with the use of gentle mouthwashes after meals and topical anesthetics. There are also products that cover the mucosal tissue, some of them present hyaluronic acid in their composition. This type of oral gels creates a film over the mucosa and protects it from the effects of food, liquids and saliva. Analgesics are also effective against mucositis. These agents include topical mouthwashes, opioid analgesics and anti-inflammatory drugs. Lastly, dose modifications and treatment interruptions can also alleviate the symptoms of this AE and are recommended in patients developing grade ≥3 mucositis and may be considered in case of prolonged grade 2 (Fig. 1).
Skin rash
Skin rash is characterized by the presence of macules, i.e. flat, discolored areas of skin, and papules, or solid elevations of skin. Rash symptoms include photosensitivity, erythema, dry or peeling skin, blistering and pruritus [31]. Patients must be made aware of skin toxicities, especially HFSR and rash, when they are treated with antineoplastic drugs such as regorafenib [32]. Another key aspect is that skin toxicities need to be appropriately graded. Rash is common in patients treated with regorafenib, particularly of mild and moderate severity. In the phase I study carried out by Mross et al. in patients with advanced solid tumors treated with regorafenib, rash occurred in patients who were on a dose of 120 mg/day or higher. In the CORRECT trial, 26 % of patients presented rash in the regorafenib arm and 6 % of them had grade 3 rash. Like other AEs commonly associated with regorafenib, rash occurs early, during the first and second cycle of treatment, and its incidence stabilizes over time [11]. Interestingly, this AE is more frequent in women 40–60 years old. Thus, prophylactic measures should be especially addressed for this subpopulation of patients with mCRC. Rash associated with regorafenib is defined by areas of inflamed blotching alternating with pale areas in the skin. This AE can be accompanied by fever and detriment of the general condition in patients treated with this drug.
Rash should be monitored every week during the first two cycles of treatment with regorafenib, and every 4 weeks thereafter. From the beginning of treatment, prophylaxis plays a key role. Some prophylactic measures include the regular use of emollients (those not containing alcohol), use of mild soaps and avoiding extreme temperatures and direct sun exposure. The treatment of rash includes antihistamine medication and short-time use of topical corticoids. Dermatologist consultation is advised to adopt the most appropriate treatment for every patient. Dose modification or treatment interruption of regorafenib may be also required in some patients, depending on the severity of this AE (Fig. 1).
Metabolic abnormalities
Hypothyroidism
Although no information is available regarding drug-induced hypothyroidism in the publication of the CORRECT trial [7], this information was recorded in this study and it is available in the Summary of Product Characteristics of EMA [33]. Overall, tests on TSH showed post baseline increase over upper limit of normal (ULN) in 23 % of patients in the regorafenib arm and in 13 % of patients in the placebo arm. TSH post baseline >4 times ULN was reported in 4 % of patients treated with regorafenib and in no patients treated with placebo. Concentration of free triiodothyronine (FT3) post baseline below lower limit of normal (LLN) was reported in 21 and 16 % of patients in the regorafenib and placebo arms, respectively. Concentration of free thyroxin (FT4) post baseline <LLN was reported in 8 % of patients treated with regorafenib and in 7 % of patients treated with placebo. In another trial, Demetri et al. [8] reported 18 % of hypothyroidism in patients with GISTs treated with regorafenib in the GRID trial. None of these patients presented grade ≥3 hypothyroidism. Grade ≥2 hypothyroidism requires treatment and, in some cases, the advice of an endocrinologist. This is a common class effect of TKIs but is generally mild, rarely symptomatic and easily manageable with hormone replacement therapy when appropriate.
Hypophosphatemia
Other metabolic abnormalities detected in the CORRECT trial include hypophosphatemia [7], which occurred in 5 % of patients and in 4 % of them this AE was grade 3. Hypophosphatemia symptoms include weakness, tiredness, musculoskeletal pain, and it can be caused by diarrhea or elimination by the kidney, so diagnosis requires the determination of levels of phosphate in the urine. When this AE persists, oral phosphate can be administered either through diet or dietary supplements. Moderate hypophosphatemia does not require any other treatment. However, severe hypophosphatemia requires administration of iv phosphate.
Hepatic and pancreatic enzymes
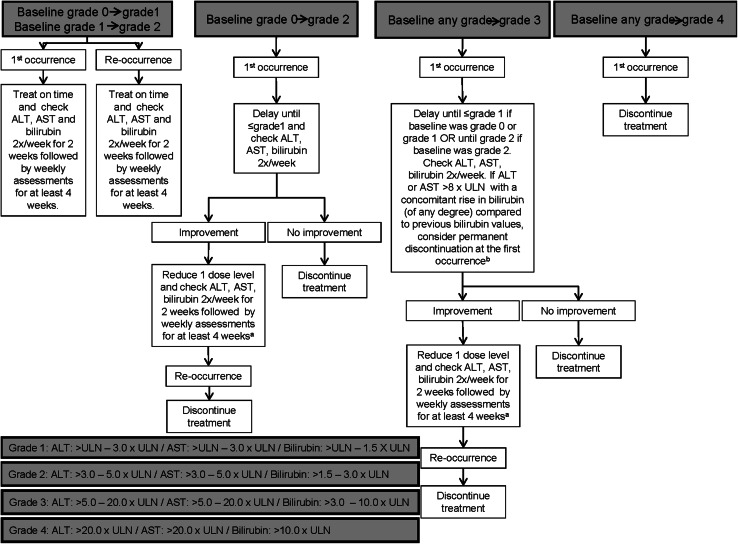
With regard to the hepatic enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST), both presented grade ≥3 increase in about 6 % of patients treated with regorafenib in the CORRECT trial [34], whereas bilirubin levels reached grade ≥3 increase in about 12 % of patients. Elevation of hepatic enzymes and bilirubin levels can be reduced by adjusting regorafenib dose. Their level should be monitored 1 week before treatment initiation, at least every 2 weeks during the first 2 months of treatment and monthly thereafter unless otherwise clinically indicated. As a general rule, regorafenib treatment should be withdrawn if the patient does not recover to baseline values after 4 weeks of treatment interruption or after 2 levels of dose reduction (Fig. 4). Patients with active viral hepatitis should be evaluated with caution, analyzing their hepatic function and maximizing monitoring, due to the fact that these patients have been excluded of the CORRECT and CONSIGN trials. Nonetheless, sorafenib is a drug with similar characteristics to regorafenib and is administered to patients presenting hepatomas and the majority of these patients have active hepatitis B or C.
Fig. 4.
Dose modification/delay for ALT and/or AST and/or bilirubin increases related to regorafenib. According to Common Terminology Criteria for Adverse Events (CTCAE) v 4.0. aIf all values remain stable for two cycles, dose re-escalation may be considered at the discretion of the investigator. After re-escalation ALT, AST and bilirubin should be checked twice a week for 2 weeks, followed by weekly assessments for at least 4 weeks. bIn case of discontinuation ALT, AST and bilirubin should be checked twice a week for 2 weeks, followed by weekly assessments until recovery to baseline. General notes: patients requiring interruption for 4 weeks must stop treatment permanently. If more than 2 dose reductions are required, treatment will be discontinued. ALT alanine aminotransferase, AST aspartate aminotransferase, ULN upper limit of normal
In the CORRECT trial, lipase elevations occurred in 6 and 1 % of patients allocated in the regorafenib and placebo arms, respectively, whereas severe lipase elevations occurred in 4 and 1 % of patients, respectively [34]. In patients presenting abdominal pain, lipase and amylase values should be analyzed. Nonetheless, patients presenting lipase and amylase elevations can be asymptomatic.
Clinicians should also warn patients about these potential side effects of regorafenib use. Nonetheless, the majority of metabolic abnormalities occur during the first two cycles of treatment.
Lastly, hematological abnormalities and especially severe hematological abnormalities, are neither frequent nor very relevant in patients treated with regorafenib. In case of occurrence, clinicians should bear in mind the recommendations of the guidelines on hematological toxicity as a side effect of chemotherapy.
Conclusions and recommendations for general practice
Regorafenib 160 mg/day orally, administered for 21 days every 4 weeks, is an active treatment, which increases survival in patients with mCRC who have failed to other standard therapies.
The selection of patients undergoing treatment with regorafenib plays a key role in this setting. Eligible patients need to fulfill four criteria: (i) ECOG = 0–1, (ii) absence of poorly-controlled hypertension, i.e. basal hypertension ≥150/90 mmHg; thus, in patients presenting with abnormal blood pressure, hypertension has to be corrected with the administration of antihypertensive therapy before treatment initiation with regorafenib, (iii) absence of severe cardiovascular disease during the last 6 months, and (iv) adequate bone marrow, liver and renal functions. Besides these four criteria, clinicians have to pay particular attention to the occurrence of warning signs such as hypertension, asthenia, HFSR, rash, mucositis or an abnormal increase in the level of hepatic enzymes during the first days or weeks of treatment with regorafenib. These signs could worsen during subsequent days or weeks if oncologists do not carry out the appropriate treatment interruptions or dose adjustments.
Regorafenib should be taken in the morning, accompanied by a low-fat breakfast. It is important to highlight that the most frequent and relevant toxicities associated with regorafenib occur during the first weeks of treatment. For this reason, patients receiving this agent should be monitored weekly during the first two cycles of treatment and monthly thereafter. The incidence of grade 3–4 AEs can be reduced with the implementation of appropriate prophylactic measures and also paying special attention to the appearance of grade 2 AEs, in order to prevent them from worsening. In this regard, clinicians should confirm that the toxicities detected during the administration of prior cycles of regorafenib present at grade ≤1 before the patient receives a new cycle of regorafenib therapy. Also, to lessen the grade of toxicity associated with regorafenib, the dose of this agent can be reduced by one dose level to 120 mg/day. This dose can be further reduced, when required, by a second dose level to 80 mg/day. However, in this regard, there is no evidence of the occurrence of accumulated toxicity in patients receiving regorafenib.
Patients must also be made aware of the toxicity profile of regorafenib, as well as about the prophylactic measures at their disposal. For example, some prophylactic measures to prevent skin toxicities include the use of mild soaps, intense hydration, comfortable clothes and shoes in order to prevent chafing in the skin and avoiding clothes that do not allow adequate ventilation of perspiration. In addition, patients using regorafenib must maintain appropriate oral hygiene to prevent, or avoid the worsening of, mucositis. Lastly, metabolic abnormalities, such as hypophosphatemia and hypothyroidism, should be monitored at least once a month and the dose of regorafenib should be modified when required. Overall, while being aware of the safety profile of regorafenib and how to manage the most common toxicities related to it, this agent should be regarded as a new standard of care in late-stage mCRC.
Acknowledgments
The authors wish to thank Ana Martín from HealthCo S.L. (Madrid, Spain) for her help in preparing the first draft of this manuscript and Bayer Spain for the financial support of the project. Bayer Spain was given the opportunity to comment on the first draft of the manuscript, but all the decisions about its content were taken by the authors. All authors have approved the final version of the submitted manuscript.
Conflict of interest
The authors declare that they do not have any conflict of interest that may inappropriately influence this work.
References
- 1.National Comprehensive Cancer Network®. NCCN clinical practice guidelines in oncology (NCCN guidelines®): colon cancer, version 3. 2014.
- 2.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 3.Ciombor KK, Berlin J. Aflibercept—a decoy VEGF receptor. Curr Oncol Rep. 2014;16:368. doi: 10.1007/s11912-013-0368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 5.Mross K, Frost A, Steinbild S, Hedbom S, Buchert M, Fasol U, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–2667. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 6.Strumberg D, Scheulen ME, Schultheis B, Richly H, Frost A, Buchert M, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106:1722–1727. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grothey A, Sobrero AF, Siena S, Falcone A, Ychou M, Humblet Y, et al. Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. ASCO Meet Abstr. 2013;31:3637. [Google Scholar]
- 10.Siena S, Grothey A, Sobrero A, Falcone A, Ychou M, Lenz HJ et al. Effects of regorafenib therapy on health-related quality of life in patients with metastatic colorectal cancer in the phase III CORRECT study. Amsterdam: European Cancer Congress ECCO; abstract 2156;2013.
- 11.Grothey A, Van Cutsem E, Sobrero AF, Siena S, Falcone A, Ychou M, et al. Time course of regorafenib-associated adverse events in the phase III CORRECT study. ASCO Meet Abstr. 2013;31:467. [Google Scholar]
- 12.Yennurajalingam S, Frisbee-Hume S, Palmer JL, Delgado-Guay MO, Bull J, Phan AT, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31:3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 13.Tassinari D, Flamini E, Poggi B, Fochessati F, Fantini M, Fabbri L, et al. Clinical benefit of palliative treatment of cancer cachexia with medroxyprogesterone acetate (MPA) ASCO Meet Abstr. 2004;22:8213. [Google Scholar]
- 14.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22:1273–1279. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 15.Grothey A, George S, van Cutsem E, Blay JY, Sobrero A, Demetri GD. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist. 2014;19:669–680. doi: 10.1634/theoncologist.2013-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayer HealthCare Pharmaceuticals Inc. Clinical study report 14387 (CORRECT). Wayne: Bayer HealthCare Pharmaceuticals Inc; 2012, pp. 1–179.
- 17.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 18.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 20.Eisen T, Sternberg CN, Robert C, Mulders P, Pyle L, Zbinden S, et al. Targeted therapies for renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst. 2012;104:93–113. doi: 10.1093/jnci/djr511. [DOI] [PubMed] [Google Scholar]
- 21.Qi WX, He AN, Shen Z, Yao Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;76:348–357. doi: 10.1111/bcp.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi WX, Lin F, Sun YJ, Tang LN, He AN, Yao Y, et al. Incidence and risk of hypertension with pazopanib in patients with cancer: a meta-analysis. Cancer Chemother Pharmacol. 2013;71:431–439. doi: 10.1007/s00280-012-2025-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 24.Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG, et al. NCCN Task Force Report. Prevention and management of mucositis in cancer care. J Natl Compr Cancer Netw. 2008;6(Suppl 1):S1–21. [PubMed] [Google Scholar]
- 25.Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010;CD001973. [DOI] [PMC free article] [PubMed]
- 26.Harris DJ, Eilers J, Harriman A, Cashavelly BJ, Maxwell C. Putting evidence into practice: evidence-based interventions for the management of oral mucositis. Clin J Oncol Nurs. 2008;12:141–152. doi: 10.1188/08.CJON.141-152. [DOI] [PubMed] [Google Scholar]
- 27.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 28.Lalla RV, Ashbury FD. The MASCC/ISOO mucositis guidelines: dissemination and clinical impact. Support Care Cancer. 2013;21:3161–3163. doi: 10.1007/s00520-013-1924-2. [DOI] [PubMed] [Google Scholar]
- 29.Peterson DE, Bensadoun RJ, Roila F. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi78–84. [DOI] [PMC free article] [PubMed]
- 30.Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2011;CD000978. [DOI] [PubMed]
- 31.Khayyam KU, Imam F, Sharma M, Pillai KK, Behera D. Pyrazinamide-induced maculopapular rash. Indian J Dermatol. 2010;55:384–386. doi: 10.4103/0019-5154.74562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 33.Stivarga®. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002573/WC500149164.pdf. Accessed 12 May 2014.
- 34.Supplement to: Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed]