Abstract
BACKGROUND
Cardiovascular risk factors are associated with left ventricular hypertrophy (LVH), but little is known regarding related impact of longitudinal measures of childhood adiposity and left ventricular (LV) hemodynamics.
OBJECTIVES
The aim of this study is to examine the cumulative long-term burden and trends of excessive adiposity and elevated blood pressure (BP) during childhood on adult LVH and LV geometric remodeling patterns.
Methods
This longitudinal study consisted of 1,061 adults, aged 24 to 46 years, who had been examined 4 or more times for body mass index (BMI) and BP starting in childhood with an average follow-up of 28.0 years. The area under the curve (AUC) was calculated as a measure of long-term burden (total AUC) and trends (incremental AUC) of BMI and BP from childhood to adulthood. Four LV geometric types were defined as normal, concentric remodeling (CR), eccentric hypertrophy (EH), and concentric hypertrophy (CH), all based on LV mass indexed for body height (m2.7) and relative wall thickness.
Results
Higher values of BMI and systolic and diastolic BP of childhood and adulthood, as well as total AUC and incremental AUC were all significantly associated with higher LV mass index and LVH, adjusted for race, sex, and age. In addition, higher values of BMI and BP of childhood and adulthood, total AUC, and incremental AUC were significantly associated with EH and CH but not CR. Importantly, all these measures of BMI had a consistently and significantly greater influence on EH than measures of BP.
Conclusions
These findings indicate that the adverse influence of excessive adiposity and elevated BP levels on LVH begins in childhood.
Keywords: left ventricular hypertrophy, geometric remodeling, adiposity, blood pressure, longitudinal analysis
Left ventricular hypertrophy (LVH) independently predicts increased cardiovascular (CV) morbidity and mortality (1,2). It has been well documented that CV risk factors are strongly associated with the development of LVH. Among traditional CV risk factors, obesity and hypertension are recognized as the most important determinants of LVH in the general population (3–6). During the last few decades, evidence from epidemiologic and clinical studies indicates that measures of obesity are strong predictors of LVH, especially eccentric LVH (3). Also, elevated blood pressure (BP) plays a driving role in activating LV myocardial growth through chronic hemodynamic overload and increased central pressure (4). On the other hand, the importance of longitudinal changes of adiposity measures and BP relative to the development of LVH is not fully understood, particularly the impact on LV geometry.
We now well recognize that cardiovascular disease (CVD) begins early in life (7,8). This concept of “childhood origins” of CVD is supported by numerous publications from 4 large-scale, population-based childhood cohorts followed into adulthood (Bogalusa [Louisiana], Muscatine [Iowa], Finland, and Australia) that are now collaborating as the i3C (International Childhood Cardiovascular Cohort) Consortium (8). Previous studies, including ours, have shown that the association between CV risk factors and excessive cardiac growth occurs in children and adolescents (9–12), and early life risk factors significantly predict adult LVH and LV geometric patterns (13,14). However, information is lacking regarding the relationship between life-course burden of CV risk factors starting in childhood and adult LVH and LV geometric remodeling patterns. This study aims to examine the impact of cumulative long-term burden and trends of excessive adiposity and elevated BP measured from childhood to adulthood on the development of LVH and LV geometric patterns in a biracial cohort enrolled in the Bogalusa Heart Study.
METHODS
STUDY COHORT
The Bogalusa Heart Study is a biracial (65% white and 35% black) community-based, long-term investigation of the early natural history of CVD beginning in childhood (7). Between 1973 and 2010, 9 cross-sectional surveys of children aged 4 to 18 years and 10 cross-sectional surveys of adults aged 19 to 52 years, who had been previously examined as children, were conducted in Bogalusa, Louisiana. This panel design of repeated cross-sectional examinations has resulted in serial observations every 2 to 3 years from childhood to adulthood. In this longitudinal cohort, 1,194 adults had echocardiographic LV dimensions in adulthood captured during 2004 to 2010 and repeated measurements of CV risk factors from childhood to adulthood. After excluding subjects who were examined <4 times for CV risk factors and hypertensive patients who were under treatment, 1,061 adults (717 whites and 344 blacks; 42.6% males; age 24 to 46 years) who had been examined for LV dimensions in adulthood and CV risk factors 4 or more times (at least 2 times each in childhood and adulthood) formed the longitudinal study cohort for this report. The average follow-up period was 28.0 years.
All subjects in this study gave informed consent at each examination and, for those under 18 years of age, we obtained consent of a parent/guardian. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
GENERAL EXAMINATION
Replicate measurements of height and weight were made, and the mean values were used for analysis. Body mass index (BMI; determined by weight in kilograms divided by the square of the height in meters) was used as a measure of adiposity. BP levels were measured between 8:00 AM and 10:00 AM on the right arm of subjects in a relaxed, sitting position by 2 trained observers (3 measurements each). Systolic (SBP) and diastolic (DBP) blood pressure were recorded using a mercury sphygmomanometer. The fourth Korotkoff phase was used for DBP for children and adults because the fourth phase is more reliably measured in childhood and more predictive of adult hypertension (15). The mean values of the 6 readings were used for analysis.
ECHOCARDIOGRAPHIC LV STRUCTURE MEASUREMENTS
LV dimensions were assessed by 2-dimensional guided M-mode echocardiography with 2.25- and 3.5-MHz transducers according to American Society of Echocardiography recommendations (16). Parasternal long- and short- axis views were used for measuring LV end-diastolic and end-systolic measurements in duplicate and then averaged. LV mass was calculated from a necropsy-validated formula based on a thick-wall prolate ellipsoidal geometry (17). To take body size into account, LV mass was indexed for body height (m2.7) as LV mass index (LVMI). LV relative wall thickness (RWT) was calculated as septal wall thickness plus posterior wall thickness divided by LV end-diastolic diameter (18). The presence of LVH was defined by sex-specific cutoffs of LVMI > 46.7 g/m2.7 in women and > 49.2 g/m2.7 in men; LV geometry was considered concentric when relative wall thickness was > 0.42 (19). Four different patterns of LV geometry were defined: normal LV geometry (normal relative wall thickness with no LVH); concentric remodeling (CR, increased relative wall thickness but no LVH); eccentric hypertrophy (EH, normal relative wall thickness with LVH); and concentric hypertrophy (CH, increased relative wall thickness with LVH) (18–21).
STATISTICAL METHODS
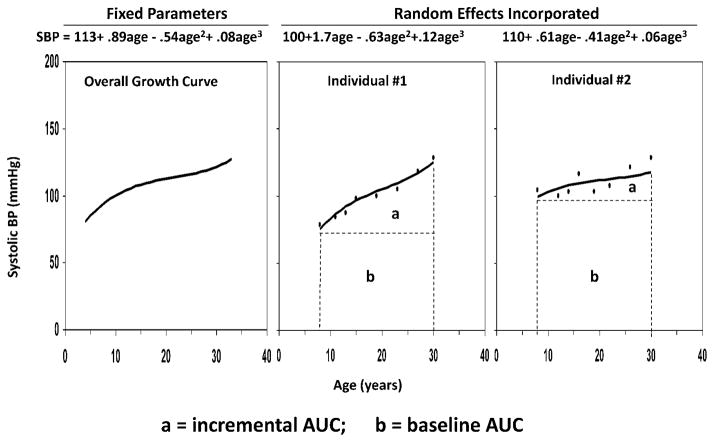
Long-term burden and trends of BMI and BP were measured as the area under the curve (AUC) calculated using the statistical models we previously developed (22–24). Growth curves of BMI and BP measured repeatedly at multiple time points from childhood to adulthood were constructed using a random effects model by SAS® PROC MIXED. A quadratic curve was fit for BMI, and a cubic curve for SBP and DBP in race-sex groups. As shown in Figure 1, using SBP as an example, the AUC was calculated as the integral of the curve parameters during the follow-up period for each individual. Since individuals had different follow-up periods, the AUC values were divided by the number of follow-up years. The AUC measures have advantages over other longitudinal analysis models in that they measure both long-term burden and trends. As seen in Figure 1, total AUC (a + b) can be considered a measure of a long-term cumulative burden; incremental AUC (a) determined by within-individual variability represents a combination of linear and nonlinear longitudinal trends as we used in our previous studies (22–24).
Figure 1. The area under the curve (AUC) of systolic blood pressure (SBP) with 2 examples.
The AUC was calculated as the integral of the curve parameters during the follow-up period for each individual. The two individuals had similar total AUCs, but different incremental AUCs. AUC = area under the curve; SBP = systolic blood pressure
Multivariable linear and logistic regression analyses were performed for LVMI and LVH, respectively, to examine the association with BMI and BP, adjusted for race, sex, and adulthood age. For LV geometry analyses, CR, EH, and CH were analyzed in separate logistic regression models using normal geometry as a control group. In the association analyses, BMI and BP were included in separate regression models in terms of childhood values (first measurement), adulthood values (last measurement), and total and incremental AUC values. Prior to regression analyses, childhood and adulthood values, as well as total and incremental AUC values, were adjusted for corresponding age by regression residual analyses and then standardized with Z-transformation (mean = 0, SD = 1) by race-sex groups in order to avoid colinearity of childhood and adulthood ages in the same model. In addition, for analyses of incremental AUC, baseline values of BMI and BP were included in the model for adjustment to control for the regression-to-the-mean bias. Interaction regression models (homogeneity-of-slopes models) were used to examine the interaction effects between BMI and BP in terms of childhood value, adulthood value, and AUC values, adjusting for covariates.
RESULTS
Table 1 summarizes the study variables in childhood and adulthood and as AUC values by race and sex. In childhood, sex and race differences in all study variables were not significant except race difference in DBP in males. In adulthood, BMI showed a significant sex difference among blacks (males<females); black females had significantly higher BMI than white females. Adulthood values of SBP and DBP showed significant race (blacks>whites) and sex (males>females) differences. Total and incremental AUC values of BMI, SBP, and DBP showed significant race and sex differences except sex difference in total AUC of BMI among blacks and race difference in incremental and total AUC of BMI among males. Blacks and males had significantly higher LV mass values than whites and females, respectively, with a borderline significance for race difference among males. LVMI showed significant race difference (blacks>whites) for both sexes and significant sex difference (males>females) among whites. Black females demonstrated significantly higher RWT than white females. Pearson correlation coefficients between BMI and SBP were 0.408, 0.396, 0.399, and 0.345 (p < 0.001 for all) for childhood, adulthood, and total and incremental AUC values, respectively; the correlation coefficients between BMI and DBP were all significant.
TABLE 1.
Characteristics (mean ± SD or %) of Study Variables by Race and Sex
| Whites
|
Blacks
|
p Value for Race Difference
|
||||
|---|---|---|---|---|---|---|
| Characteristic | Males (n = 317) | Females (n = 400) | Males (n = 135) | Females (n = 209) | Males | Females |
| Childhood (First exam) | ||||||
| Age, yrs | 9.8 (3.1) | 9.7 (3.1) | 10.1 (2.8) | 9.4 (2.8)* | 0.342 | 0.270 |
| BMI | 17.5 (3.3) | 17.5 (3.2) | 17.6 (3.7) | 17.4 (3.5) | 0.787 | 0.712 |
| SBP, mm Hg | 100.1 (9.9) | 99.3 (9.1) | 100.2(10.9) | 98.1(10.1) | 0.646 | 0.243 |
| DBP, mm Hg | 61.0 (8.2) | 61.9 (8.4) | 63.0 (8.3) | 60.7 (8.7) | 0.036 | 0.199 |
| Adulthood (Last exam) | ||||||
| Age, yrs | 37.8 (4.5) | 37.6 (4.5) | 38.4 (4.3) | 37.1 (4.8)** | 0.165 | 0.130 |
| BMI | 28.9 (5.3) | 28.0 (6.8) | 29.4 (7.4) | 31.5 (8.4)* | 0.398 | <0.001 |
| SBP, mm Hg | 118.0 (11.6) | 110.3 (11.9)** | 128.3 (18.1) | 121.1 (19.2)** | <0.001 | <0.001 |
| DBP, mm Hg | 79.5 (8.2) | 74.6 (8.8)** | 85.2 (13.2) | 80.5 (12.4)* | <0.001 | <0.001 |
| AUC measures | ||||||
| Average age, yrs | 21.8 (3.8) | 21.8 (3.8) | 21.9 (3.6) | 21.1 (3.5)* | 0.823 | 0.027 |
| Total AUC of BMI | 24.5 (4.1) | 23.5 (4.5)** | 24.6 (5.1) | 25.6 (5.6) | 0.872 | <0.001 |
| Total AUC of SBP | 112.9 (7.2) | 107.1 (6.2)** | 116.5 (8.9) | 111.0 (8.2)** | <0.001 | <0.001 |
| Total AUC of DBP | 72.1 (5.5) | 69.8 (4.6)** | 74.2 (7.1) | 71.6 (6.1)** | <0.001 | <0.001 |
| Incremental AUC of BMI | 7.0 (2.6) | 6.1 (3.5)** | 7.0 (3.2) | 8.1 (3.8)* | 0.937 | <0.001 |
| Incremental AUC of SBP | 13.0 (5.4) | 7.9 (5.8)** | 16.7 (7.2) | 13.1 (6.9)** | <0.001 | <0.001 |
| Incremental AUC of DBP | 11.4 (3.3) | 8.6 (4.1)** | 12.4 (4.3) | 10.8 (4.7)** | 0.011 | <0.001 |
| Adulthood(Last exam) | ||||||
| LV mass, g | 175.4 (50.3) | 129.8 (39.3)** | 186.9 (62.8) | 145.5 (48.7)** | 0.062 | <0.001 |
| LVMI, g/m2.7 | 37.3 (10.3) | 34.5 (10.5)* | 40.7 (13.9) | 38.7 (12.6) | 0.007 | <0.001 |
| RWT, cm | 0.35 (0.08) | 0.34 (0.06) | 0.35 (0.07) | 0.35 (0.07) | 0.839 | 0.036 |
Sex difference within racial groups:
p<0.05;
p<0.01
AUC = area under the curve; BMI = body mass index; DBP = diastolic blood pressure; LVMI = left ventricular mass index; RWT = relative wall thickness; SBP = systolic blood pressure
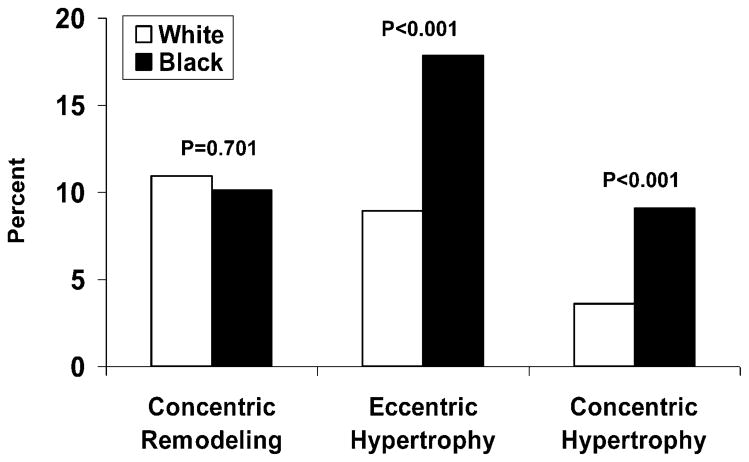
Figure 2 shows prevalence of LV geometric patterns by race. Blacks versus whites had higher prevalence of EH (17.8% vs 8.95%; p < 0.001) and CH (9.1% vs 3.6%; p < 0.001), but CR did not show a significant black-white difference (10.1% vs 10.9%; p = 0.701). In the total sample with blacks and whites combined, the prevalence was 9.1% for CR, 10.2% for EH, and 4.2% for CH.
Figure 2. Prevalence of left ventricular remodeling patterns in blacks and white.
Concentric remodeling, eccentric hypertrophy, and concentric hypertrophy were defined by cutoffs of left ventricular mass index (46.7 g/m2.7 in women and 49.2 g/m2.7 in men) and relative wall thickness (0.42).
Table 2 presents results of linear and logistic regression analyses of adult LVMI and LVH, respectively, on BMI and SBP in terms of childhood, adulthood, and AUC values in 4 separate regression models, adjusting for race, sex, and adulthood age. Adult LVMI and LVH were significantly associated with BMI and SBP in all 4 models. In linear regression models, standardized regression coefficients of BMI (0.26 to 0.42) were consistently greater than those of SBP (0.08 to 0.16) for LVMI. Similarly, in logistic regression models, odds ratios (ORs) of BMI (1.64 to 2.56) were consistently greater than those of SBP (1.26 to 1.58) for LVH. The linear and logistic regression analyses of adult LVMI and LVH, respectively, on BMI and DBP showed substantially similar patterns of the association parameters to those as shown in Table 2. The interaction effects of BMI with BP in terms of childhood, adulthood, and total and incremental AUC values on LVMI were not significant (p = 0.204 to 0.619 for BMI with SBP; p = 0.121 to 0.844 for BMI with DBP), except for BMI-DBP interaction of total AUC (p = 0.046).
TABLE 2.
Linear and Logistic Regression Analyses of LVMI and LVH on BMI and SBP
Regression coefficient different from 0: * p<0.05; ** p<0.01
| Independent Variable | Dependent Variable = LVMI (β) | Dependent Variable = LVH (OR) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Model I | Model II | Model III | Model IV | Model I | Model II | Model III | Model IV | |
| Childhood BMI* | 0.26** (0.20– 0.32) | --- | --- | --- | 1.65** (1.39– 1.97) | --- | --- | --- |
| Childhood SBP* | 0.08* (0.01– 0.14) | --- | --- | --- | 1.27* (1.04– 1.54) | --- | --- | --- |
| Adulthood BMI† | --- | 0.42** (0.37– 0.48) | --- | --- | --- | 2.53** (2.06– 3.09) | --- | --- |
| Adulthood SBP† | --- | 0.16** (0.10– 0.21) | --- | --- | --- | 1.56** (1.28– 1.90) | --- | --- |
| Total AUC of BMI‡ | --- | --- | 0.41** (0.36– 0.47) | --- | --- | --- | 2.42** (1.98– 2.95) | --- |
| Total AUC of SBP‡ | --- | --- | 0.14** (0.09– 0.20) | --- | --- | --- | 1.47** (1.20– 1.80) | --- |
| Incremental AUC of BMI§ | --- | --- | --- | 0.33** (0.28– 0.39) | --- | --- | --- | 2.09** (1.72– 2.53) |
| Incremental AUC of SBP§ | --- | --- | --- | 0.10** (0.05– 0.16) | --- | --- | --- | 1.43** (1.19– 1.72) |
| Adulthood age | 0.16** | 0.14** | 0.15** | 0.17** | 1.09** | 1.10** | 1.12** | 1.13** |
| Sex | −0.10** | −0.11** | −0.10** | −0.10** | 1.14 | 1.12 | 1.16 | 1.13 |
| Race | 0.16** | 0.17** | 0.17** | 0.17** | 2.58** | 2.97** | 2.96** | 2.80** |
OR different from 1:
p<0.05;
p<0.01
Adjusted for childhood age, and then Z-transformed (mean = 0, SD = 1)
Z-transformed (mean = 0, SD = 1)
Adjusted for average age, and then Z-transformed (mean = 0, SD = 1)
Adjusted for average age and childhood values, and then Z-transformed (mean = 0, SD = 1)
β = standardized regression coefficient (95% CI); CI = confidence interval; LVH = left ventricular hypertrophy; OR = odds ratio (95% CI); SD = standard deviation. Other abbreviations as in Table 1.
Table 3 contains logistic regression analyses of adult LV remodeling patterns on BMI and SBP in terms of childhood, adulthood, and AUC values in 4 separate regression models, adjusting for race, sex, and adulthood age. Childhood, adulthood, total AUC, and incremental AUC values of BMI and SBP were all significantly associated with EH and CH, but not with CR. Of particular interest, all 4 values of BMI had a consistently and significantly stronger association with EH compared with SBP based on nonoverlapping of the 95% confidence intervals of ORs associated with BMI and SBP; however, such patterns of differences in ORs were not noted for CH. Additionally, the logistic regression analyses of adult LV remodeling patterns on childhood, adulthood, and AUC values of BMI and DBP showed substantially similar patterns of the association parameters to those shown in Table 3.
TABLE 3.
Logistic Regression of LV Remodeling Patterns on BMI and SBP, Adjusting for Race, Sex, and Adulthood Age
| Independent Variable | Dependent Variable
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal vs. CR (811 vs. 97) | Normal vs. EH (811 vs. 108) | Normal vs. CH (811 vs. 45) | |||||||
|
| |||||||||
| OR | 95% CI Value | p | OR 95% CI Value | p | OR | 95% CI Value | p | ||
| Model I | |||||||||
| Childhood BMI* | 0.86 | 0.66–1.12 | 0.26 | 1.59 | 1.30–1.93 | <0.01 | 1.66 | 1.27–2.17 | <0.01 |
| Childhood SBP* | 1.18 | 0.93–1.50 | 0.17 | 1.22 | 0.98–1.52 | 0.07 | 1.45 | 1.03–2.03 | 0.03 |
| Model II | |||||||||
| Adulthood BMI† | 1.04 | 0.81–1.34 | 0.74 | 2.42 | 1.94–3.03 | <0.01 | 2.45 | 1.75–3.43 | <0.01 |
| Adulthood SBP† | 0.93 | 0.72–1.20 | 0.58 | 1.47 | 1.18–1.85 | <0.01 | 1.74 | 1.25–2.43 | <0.01 |
| Model III | |||||||||
| Total AUC of BMI‡ | 0.93 | 0.72–1.20 | 0.56 | 2.23 | 1.79–2.78 | <0.01 | 2.40 | 1.75–3.30 | <0.01 |
| Total AUC of SBP‡ | 1.08 | 0.86–1.37 | 0.50 | 1.41 | 1.12–1.78 | <0.01 | 1.73 | 1.22–2.44 | <0.01 |
| Model IV | |||||||||
| Incremental AUC of BMI§ | 1.05 | 0.82–1.35 | 0.68 | 2.04 | 1.65–2.52 | <0.01 | 1.99 | 1.44–2.75 | <0.01 |
| Incremental AUC of SBP§ | 0.84 | 0.66–1.07 | 0.17 | 1.28 | 1.03–1.60 | 0.03 | 1.93 | 1.40–2.66 | <0.01 |
Adjusted for childhood age, and then Z-transformed (mean = 0, SD = 1)
Z-transformed (mean = 0, SD = 1)
Adjusted for average age, and then Z-transformed (mean = 0, SD = 1)
Adjusted for average age and childhood values, and then Z-transformed (mean = 0, SD = 1)
DISCUSSION
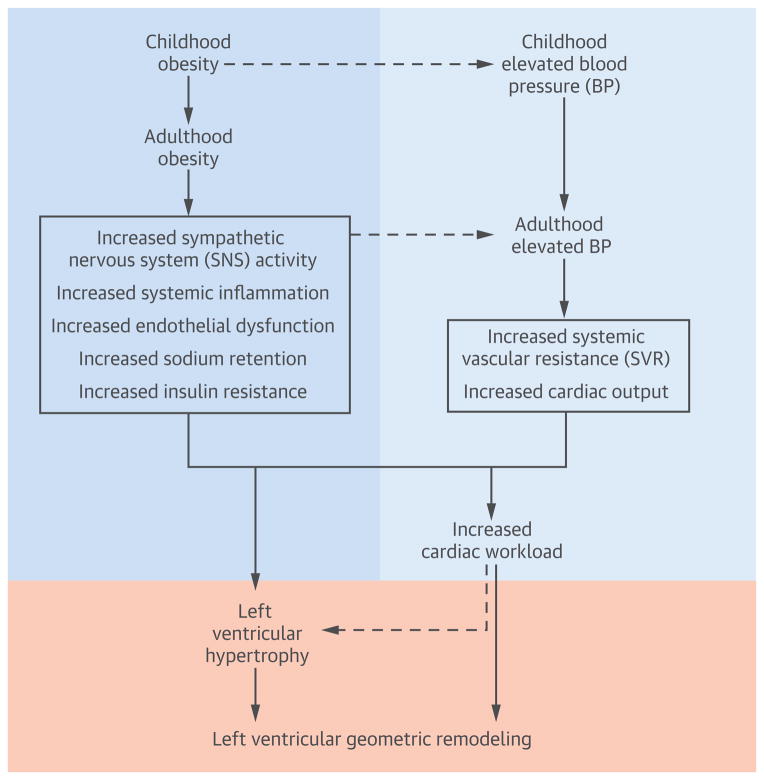
The evidence that obesity and hypertension are the most important risk factors related to LVH is almost indisputable (3,4). The association of these 2 risk factors with excessive cardiac growth even occurs in children and adolescents (9–12). The Bogalusa Heart Study has reported that childhood BMI and BP levels significantly predict adult LVH and LV geometric patterns (13,14). However, data on the impact of long-term cumulative burden of overweight/obesity and elevated BP on LV geometric remodeling patterns since early life have never been reported. Further, information regarding the relative importance of long-term measures of adiposity and BP for the development of LVH, particularly LV geometry, has not yet been available. By taking advantage of the longitudinal cohort of the Bogalusa Heart Study followed from childhood, we found that the influence of adiposity measures and BP levels began in early life. Long-term cumulative burden and trends of BMI and BP, measured as total AUC and incremental AUC from childhood, respectively, significantly predicted middle-aged adult LVH. Importantly, this study provides strong evidence that, compared with BP, BMI is more closely and consistently associated with EH using childhood, adulthood, and AUC values. These observations suggest that the process of LV enlargement and subclinical change of cardiac structure are affected by a life course of excessive adiposity and BP cumulatively and independently from early life through multiple complex pathophysiological and metabolic mechanisms, as shown in the Central Illustration.
Central Illustration. Complex Mechanisms underlying the Impact of Long-term Burden of Obesity and Elevated Blood Pressure on Left Ventricular Geometric Remodeling.
The dual burden of excessive adiposity and elevated blood pressure affects left ventricular structure and geometric remodeling cumulatively during the lifetime through multiple complex pathophysiological and metabolic mechanisms. BP = blood pressure; SNS = sympathetic nervous system; SVR = systemic vascular resistance
Echocardiography allows identification of different forms of LV geometric remodeling, including eccentric or concentric hypertrophy and disproportionate septal thickness. Although the significance of the different forms is not yet entirely defined, CH is considered to carry the highest risk for CV events (2,25) and is the predominant form in hypertensive middle-aged and elderly patients (26). According to our findings, the prevalence was 10.2% for EH and 4.2% for CH in the young adult cohort from the general population. In a review of 30 echocardiographic studies involving 37,700 hypertension patients during 2000 to 2010, EH was more frequent than CH (range 20.3% to 23.0% vs. 14.8% to 15.8%, respectively; p < 0.05) (4). In contrast, CH was found to be the predominant form in middle-aged and elderly patients with hypertension in the Framingham study cohort (26). In a review of 15 echocardiographic studies of 1,930 obese individuals with LVH, EH occurred more frequently than CH (66% vs. 34%; p < 0.01). Although EH showed a higher prevalence than CH in most previous studies, the variance in the prevalence rates of EH and CH from different studies is mainly due to age and race groups, disease status, and diagnosis criteria.
Obesity has long been an established risk factor of LVH (4,27–29). The overall odds ratio was 4.2 for the prevalence of LVH in 4,999 obese versus 6,623 nonobese individuals as reported in a review of 15 studies (3). Excess adiposity affects heart size through hemodynamic, metabolic, and inflammatory alterations, leading to LV enlargement and LVH (30–32). Our data suggest that BMI and BP were significantly correlated in terms of childhood, adulthood, and total and incremental AUC values. However, these long-term measures of BMI and BP did not show significant interactions on LVMI, except for BMI-DBP interaction of total AUC. In early studies, obesity appears to be a stronger and more consistent determinant of LVH than elevated BP levels or hypertension (4,28,33–35). In the present study, childhood and adulthood values and long-term burden and trends of BMI were all significantly associated with adult LVMI and LVH, with BMI showing a consistently greater effect on LVMI and LVH based on comparison of standardized linear regression coefficients and ORs, respectively. Furthermore, in the LV remodeling pattern analysis, we found that all 4 values of childhood, adulthood, total AUC, and incremental AUC of BMI were more strongly associated with adult EH than the 4 values of BP. These findings are consistent with previous reports emphasizing that excessive adiposity is more strongly associated with EH than with CH; the prevalence of EH (66%) was significantly higher than that of CH (34%) among obese individuals (3). Despite our consistent observations, the pathophysiological mechanisms to explain the stronger link between obesity and EH are largely unknown and need to be delineated in further studies.
LVH is a cardinal manifestation of hypertensive organ damage. Hypertension and obesity are highly correlated, associated with metabolic abnormalities, and often occur together, placing a dual burden on the left ventricle (28). During the last few decades, numerous clinical and epidemiologic studies have consistently demonstrated the role of elevated BP in LVH development (4). Also, it has been well-established that BP-lowering therapy reduces LV mass among hypertensive patients (36). Although the mechanisms underlying this process are incompletely understood, available evidence indicates that hypertensive LVH results from chronic hemodynamic overload along with the hypertrophic response modulated by genetic, ethnic, environmental, neuro-hormonal, and metabolic factors (37,38). We found that the long-term burden and trends of childhood and adulthood BP were significantly and consistently associated with adult LVH and its remodeling patterns; however, the BP-LVH association was less strong than the BMI-LVH association, particularly for the BP-EH association.
STUDY LIMITATIONS
This community-based longitudinal cohort provides a unique opportunity to examine the relationship of cumulative life-course burden of excessive adiposity and increased BP with the development of LVH and changes in cardiac structure. However, the major limitation of this study is that exclusion of individuals on antihypertensive therapy resulted in a loss of information because these patients represented a subgroup with the highest BP levels.
CONCLUSIONS
In conclusion, we demonstrated that childhood and adult values and long-term cumulative burden and trends of BMI and BP are all-powerful predictors of adult LVH and its remodeling patterns. Importantly, BMI has a consistently greater impact on LVH than BP, with the BMI-EH association stronger than BP-EH association. These results support the notion that the adverse long-term influence of BMI and BP levels on the development of LVH begins in childhood, and the dual burden of excessive adiposity and elevated BP affects LV enlargement cumulatively during the lifetime. These findings underscore the importance of undertaking preventive strategies for CVD early in life by controlling body weight and BP levels.
Perspectives.
Competency in Medical Knowledge
The adverse effects of obesity and hypertension on left ventricular structure and function begin in childhood and accumulate over time.
Translational Outlook
Further studies are needed to assess the relative impact of preventive strategies addressing each of these risk factors by controlling body weight and blood pressure early in life.
Acknowledgments
This study was supported by grants R01ES021724 from National Institute of Environmental Health Science, R01AG016592 from the National Institute on Aging and 13SDG14650068 from American Heart Association.
Abbreviations List
- AUC
area under the curve
- BMI
body mass index
- CH
concentric hypertrophy
- CR
concentric remodeling
- CV
cardiovascular
- DBP
diastolic blood pressure
- EH
eccentric hypertrophy
- LVH
left ventricular hypertrophy
- SBP
systolic blood pressure
Footnotes
Authors do not have conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 3.Cuspidi C, Rescaldani M, Sala C, Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32:16–25. doi: 10.1097/HJH.0b013e328364fb58. [DOI] [PubMed] [Google Scholar]
- 4.Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26:343–9. doi: 10.1038/jhh.2011.104. [DOI] [PubMed] [Google Scholar]
- 5.Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1992;19:130–4. doi: 10.1016/0735-1097(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 6.Gardin JM, Brunner D, Schreiner PJ, et al. Demographics and correlates of five-year change in echocardiographic left ventricular mass in young black and white adult men and women: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 2002;40:529–35. doi: 10.1016/s0735-1097(02)01973-3. [DOI] [PubMed] [Google Scholar]
- 7.Berenson GS, McMahan CA, Voors AW, et al. In: Cardiovascular risk factors in children: the early natural history of atherosclerosis and essential hypertension. Andrews C, Hester HE, editors. New York, NY: Oxford University Press; 1980. pp. 47–123. [Google Scholar]
- 8.Dwyer T, Sun C, Magnussen CG, et al. Cohort Profile: the international childhood cardiovascular cohort (i3C) consortium. Int J Epidemiol. 2013;42:86–96. doi: 10.1093/ije/dys004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanevold C, Waller J, Daniels S, Portman R, Sorof J. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–33. doi: 10.1542/peds.113.2.328. [DOI] [PubMed] [Google Scholar]
- 10.Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97:1907–11. doi: 10.1161/01.cir.97.19.1907. [DOI] [PubMed] [Google Scholar]
- 11.Dhuper S, Abdullah RA, Weichbrod L, Mahdi E, Cohen HW. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity (Silver Spring) 2011;19:128–33. doi: 10.1038/oby.2010.134. [DOI] [PubMed] [Google Scholar]
- 12.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995;91:2400–6. doi: 10.1161/01.cir.91.9.2400. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Li S, Ulusoy E, Chen W, Srinivasan SR, Berenson GS. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation. 2004;110:3488–92. doi: 10.1161/01.CIR.0000149713.48317.27. [DOI] [PubMed] [Google Scholar]
- 14.Toprak A, Wang H, Chen W, Paul T, Srinivasan S, Berenson G. Relation of childhood risk factors to left ventricular hypertrophy (eccentric or concentric) in relatively young adulthood (from the Bogalusa Heart Study) Am J Cardiol. 2008;101:1621–5. doi: 10.1016/j.amjcard.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Elkasabany AM, Urbina EM, Daniels SR, Berenson GS. Prediction of adult hypertension by K4 and K5 diastolic blood pressure in children: the Bogalusa Heart Study. J Pediatr. 1998;132:687–92. doi: 10.1016/s0022-3476(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 16.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 18.Foppa M, Duncan BB, Rohde LEP. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound. 2005;3:17. doi: 10.1186/1476-7120-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Simone G, Kitzman DW, Chinali M, et al. Left ventricular concentric geometry is associated with impaired relaxation in hypertension: the HyperGEN study. Eur Heart J. 2005;26:1039–45. doi: 10.1093/eurheartj/ehi019. [DOI] [PubMed] [Google Scholar]
- 20.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–8. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 21.Toprak A, Reddy J, Chen W, Srinivasan S, Berenson G. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the Bogalusa Heart Study) Am J Cardiol. 2009;103:978–84. doi: 10.1016/j.amjcard.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Li S, Cook NR, et al. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: The Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:462–9. doi: 10.1038/sj.ijo.0802610. [DOI] [PubMed] [Google Scholar]
- 23.Cook NR, Rosner BA, Chen W, Srinivasan SR, Berenson GS. Using the area under the curve to reduce measurement error in predicting young adult blood pressure from childhood measures. Stat Med. 2004;23:3421–35. doi: 10.1002/sim.1921. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Li S, Srinivasan SR, Boerwinkle E, Berenson GS. Autosomal genome scan for loci linked to blood pressure levels and trends since childhood: the Bogalusa Heart Study. Hypertension. 2005;45:954–9. doi: 10.1161/01.HYP.0000161881.02361.11. [DOI] [PubMed] [Google Scholar]
- 25.Verdecchia P, Angeli F, Achilli P, et al. Echocardiographic left ventricular hypertrophy in hypertension: marker for future events or mediator of events? Curr Opin Cardiol. 2007;22:329–34. doi: 10.1097/HCO.0b013e3280ebb413. [DOI] [PubMed] [Google Scholar]
- 26.Savage DD, Garrison RJ, Kannel WB, et al. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham Study. Circulation. 1987;75:I26–33. [PubMed] [Google Scholar]
- 27.Chirinos JA, Segers P, De Buyzere ML, et al. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–8. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavie CJ, Ventura HO, Messerli FH. Left ventricular hypertrophy. Its relationship to obesity and hypertension. Postgrad Med. 1992;91:131–2. 135–8, 141–3. doi: 10.1080/00325481.1992.11701350. [DOI] [PubMed] [Google Scholar]
- 29.Kuch B, Hense HW, Gneiting B, et al. Body composition and prevalence of left ventricular hypertrophy. Circulation. 2000;102:405–10. doi: 10.1161/01.cir.102.4.405. [DOI] [PubMed] [Google Scholar]
- 30.Alpert MAAJ. Cardiac morphology and obesity in man. In: Alpert MAAJ, editor. The heart and lung in obesity. Armonk, NY: Futura Publishing; 1998. pp. 25–44. [Google Scholar]
- 31.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–76. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 32.De Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation. 1981;64:477–82. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 33.Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43:1182–8. doi: 10.1161/01.HYP.0000128738.94190.9f. [DOI] [PubMed] [Google Scholar]
- 34.Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African-American and white young adults: the CARDIA study. J Am Coll Cardiol. 2003;41:955–60. doi: 10.1016/s0735-1097(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 35.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–7. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 36.Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension. 2009;54:1084–91. doi: 10.1161/HYPERTENSIONAHA.109.136655. [DOI] [PubMed] [Google Scholar]
- 37.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–35. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmieder RE. The role of non-haemodynamic factors of the genesis of LVH. Nephrol Dial Transplant. 2005;20:2610–2. doi: 10.1093/ndt/gfi190. [DOI] [PubMed] [Google Scholar]