Abstract
Ionotropic glutamate receptors (iGluRs) are tetrameric ligand-gated cation channels that mediate excitatory signal transmission in the central nervous system (CNS) of vertebrates. The members of the iGluR subfamily of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (AMPARs) mediate most of the fast excitatory signal transmission, and their abundance in the postsynaptic membrane is a major determinant of the strength of excitatory synapses. Therefore, regulation of AMPAR trafficking to the postsynaptic membrane is an important constituent of mechanisms involved in learning and memory formation, such as long-term potentiation (LTP) and long-term depression (LTD). Auxiliary subunits play a critical role in the facilitation and regulation of AMPAR trafficking and function. The currently identified auxiliary subunits of AMPARs are transmembrane AMPA receptor regulatory proteins (TARPs), suppressor of lurcher (SOL), cornichon homologues (CNIHs), synapse differentiation-induced gene I (SynDIG I), cysteine-knot AMPAR modulating proteins 44 (CKAMP44), and germ cell-specific gene 1-like (GSG1L) protein. In this review we summarize our current knowledge of the modulatory influence exerted by these important but still underappreciated proteins.
Keywords: AMPA, glutamate receptor, trafficking, auxiliary subunit, TARP, cornichon homologue, CNIH, shisa, SynDIG, CKAMP, GSG1L
1. Introduction
The family of iGluRs consists of 18 different subunits found in mammals which are categorized into four subfamilies based on pharmacological properties, biological function, and sequence homology. The four subunits GluA1 through GluA4 constitute the subfamily of AMPARs which assemble as homo- or heterotetramers to form functional receptors.
Synaptic plasticity is the basis of learning and memory formation. LTP and LTD—the two major mechanisms of synaptic plasticity at excitatory synapses—involve the regulation of the number of AMPA receptors in the active zone of synapses. LTP, the process of synapse strengthening, is accompanied by an increase in the number of active AMPARs at the PSD. The current knowledge about the general mechanism of LTP generation can be summarized in a three-step model: (i) exocytosis of AMPARs at the dendritic shaft or the spine lateral to the PSD; (ii) lateral diffusion of AMPARs to the PSD; and (iii) anchoring of the AMPARs at the PSD. LTD, the opposite process, leads to the endocytosis of AMPARs and thus to a reduced number of AMPARs at the PSD [1].
On their way from the ER to the PSD, AMPARs interact temporarily, either directly or indirectly, with various proteins, including chaperones, stabilizers, mediators of vesicular delivery, and anchoring proteins. These interacting proteins, which include for example GRIP1, KIF1A, KIF5, Rab8, Liprin-α, NEEP21, GRASP-1, Rab4, and Stx13, and their functions in trafficking pathways of AMPARs have recently been reviewed by Anggono and Huganir and are not described here [2]. Instead, we focus on auxiliary subunits of AMPARs: highly specialized, non-transient binding partners that—besides their role in receptor trafficking—also modulate pharmacological and electrophysiological properties of AMPARs.
1.1. The Discovery of TARPs and Their Functional Properties
The first AMPAR auxiliary subunit—stargazin—was discovered in the epileptic and ataxic stargazer mouse mutant, which lacks functional AMPARs in cerebellar granule cell synapses. In stargazer mice, the expression of the stargazin gene is disrupted by a spontaneous mutation in both its alleles. Stargazin is a four-transmembrane-domain protein showing structural and sequence similarity to the γ1 subunit of voltage-activated calcium channels, and was therefore alternatively named γ2 [3]. Eventually, several additional members of the γ subunit family, sharing sequence similarity with γ1 and γ2, were shown to also share functional similarity with γ2. These proteins defined the new family of TARPs, which was later subdivided on the basis of functional differences and sequence homologies (see below) into the type I TARPs comprising the subunits γ2, γ3, γ4, and γ8, and the type II TARPs, γ5 and γ7 (see Figure 1/Table 1). The related proteins γ1 and γ6 do not belong to the TARP family because they do not modulate AMPARs (see Table 1). TARPs are non-pore-forming integral membrane proteins with four transmembrane domains (thus resembling tetraspanins) that directly interact with AMPARs [4,5,6,7,8,9,10]. They are expressed differentially all over the brain, and γ2 in particular is expressed in every type of neuron [11]. Moreover, almost all tissues and cell types in the brain express more than one type I TARP subunit, with the exception of cerebellar granule cells, which express only one type I TARP, γ2 [11,12,13], plus one type II TARP, γ7 [14]. TARPs alter the maturation and trafficking of AMPARs; however, they do not act as classical chaperones because they are more than just temporary binding partners [10,13,15,16]. They remain bound to the receptor within the postsynaptic membrane and modulate the receptor's pharmacological and electrophysiological properties, including agonist efficacy, activation time, deactivation rate, and desensitization rate [17,18,19,20,21,22]. In doing so, they are exquisitely specific for AMPARs, not affecting other iGluRs such as kainate or NMDA receptors [23].
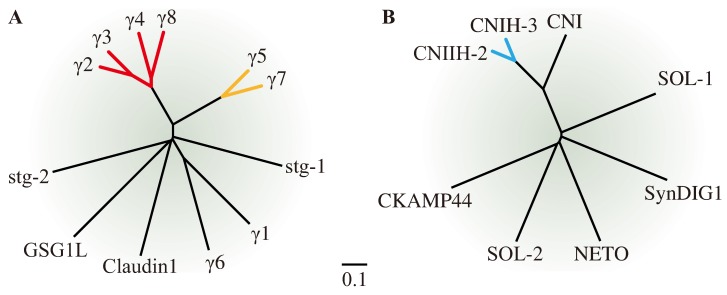
Figure 1.
(A) Phylogenetic tree of all tetraspanin-resembling four transmembrane domain-containing auxiliary subunits: type I TARPs (red), type II TARPs (orange), stg-1, stg-2, γ1, γ6, GSG1L, and claudin1 as a representative of the most closely related group of non-γ subunit proteins. (B) Phylogenetic tree of all non-tetraspanin-resembling auxiliary subunits with less than four transmembrane domains: CNIHs (blue), CNI, NETO, SOL-1, SynDIGI, SOL-2, CKAMP44. The ClustalW protein alignment was used for calculation and the Phylodendron program for the tree generation. Scale indicates number of exchanges per position along the branches connecting two proteins.
Table 1.
Modulation of AMPARs by TARPs; +, a positive modulation or interaction; −, a negative modulation; (+)/(−), possible or expected positive or negative modulation; 0, no modulation; ~, unclear/different results described; N/A, no data available. Contradictory results and observations are discussed in the text.
| Level of Influence | Interaction with AMPARs | Agonist Efficacy | Amplitude | Desensitization/Activation | ER/Golgi Export/Traffick | Maturation | Plasma Membrane Expression | Synaptic Targeting/Anchoring | ||
|---|---|---|---|---|---|---|---|---|---|---|
| γ1 | N/A | 0 A | 0 B | N/A | N/A | N/A | 0 C | N/A | ||
| γ2 | + D | + E | + F | − G | + H | + I | + J | + K | ||
| γ3 | + L | + M | + N | − O | (+) N/A P | + Q | + R | (+) N/A S | ||
| γ4 | + T | + U | + V | − W | (+) N/A X | (+) N/A Y | + Z | (+) N/A AA | ||
| γ5 | + AB | − AC | + AD | + AE | N/A | N/A | ~ AF | ~ AG | ||
| γ6 | N/A | N/A | 0 AH | N/A | N/A | N/A | N/A | N/A | ||
| γ7 | + AI | + AJ | + AK | − AL | N/A | N/A | + AM | + AN | ||
| γ8 | + AO | + AP | + AQ | − AR | (+) N/A AS | (+) N/A AT | + AU | + AV | ||
| A: [21] | Q: [54] | AG: [13,67,69,99] | ||||||||
| B: [13,21] | R: [50,51,54] | AH: [68,69] | ||||||||
| C: [21,50] | S: [9,71,75,88,94,104] | AI: [18,34,68,69,99] | ||||||||
| D: [9,10,13,55,75,88] | T: [13] | AJ: [62,68] | ||||||||
| E: [10,17,19,20,21,22,51,62,67,68,101] | U: [10,17,21,22,51,67,101,102] | AK: [34,62,69] | ||||||||
| F: [10,13,18,19,20,21,22,34,51,62,69,92,101,102,103] | V: [10,13,20,21,22,34,51,101,102,103] | AL: [14,68] | ||||||||
| G: [6,10,18,19,21,51,55,67,68,101,102] | W: [10,21,51,101,102] | AM: [14,51,68,71] | ||||||||
| H: [13,16,50,55] | X: [13,16,50,55] | AN: [14,51,68,71] | ||||||||
| I: [13,16,45,50,54] | Y: [13,16,45,50,54] | AO: [13] | ||||||||
| J: [9,19,21,23,50,54,55,67,71,88,104,105] | Z: [51] | AP: [10,17,20,21,22,34,62,67,102] | ||||||||
| K: [9,71,75,88,94,104] | AA: [9,71,75,88,94,104] | AQ: [10,13,21,22,34,51,62,65,102,103] | ||||||||
| L: [7,13] | AB: [13,18,34,68,69,99] | AR: [10,34,51,62,102] | ||||||||
| M: [10,17,21,22,51,67,102] | AC: [7,67,69] | AS: [13,16,50,55] | ||||||||
| N: [10,13,20,21,22,34,51,102,103] | AD: [13,34,68,69,99] | AT: [13,16,45,50,54] | ||||||||
| O: [10,21,51,102] | AE: [68,69,99] | AU: [34,65,71,106] | ||||||||
| P: [13,16,50,55] | AF: [13,67,69,99] | AV: [34,65,71,106] | ||||||||
The TARP γ2 is present in many different species, vertebrates as well as invertebrates. In the invertebrate Caenorhabditis elegans (C. elegans), the stargazin homologs stg-1 and stg-2 facilitate the currents of a glutamate receptor homolog that is assembled from GLR-1 subunits. The SOL-1 and SOL-2 proteins represent another group of auxiliary subunits present in C. elegans. GLR-1 has to be coexpressed with both, stg-1 and SOL-1, to achieve detectable glutamate-activated currents in heterologous expression systems [24,25,26,27]. SOL-1 and stg-1 bind directly to the GLR-1 receptor [25,28] but, in contrast to the vertebrate TARPs, they do not influence its plasma membrane expression (see Table 2) [26,27].
Table 2.
Modulation of AMPARs by auxiliary subunits other than TARPs; +, a positive modulation or interaction; −, a negative modulation; (+)/(−), possible or expected positive or negative modulation; 0, no modulation; ~, unclear/different results described; N/A, no data available. Contradictory results and observations are discussed in the text. (des. = desensitization; deac. = deactivation).
| Level of Influence | Interaction with AMPARs | Agonist Efficacy | Amplitude | Desensitization/Activation | ER/Golgi Export/Traffick. | Maturation | Plasma Membrane Expression | Synaptic Targeting/Anchoring | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stg-1 | + A | N/A | + B | − C | N/A | N/A | 0D | N/A | |||
| SOL-1 | + E | N/A | + F | − G | N/A | N/A | 0H | N/A | |||
| SOL-2 | + I | + J | + K | + L | N/A | N/A | 0M | N/A | |||
| CNIH-2 | + N | ~ ° | + P | ~ Q | + R | + S | 0 T | ~ U | |||
| CNIH-3 | + V | 0 W | + X | − Y | + Z | + AA | 0 AB | ~ AC | |||
| CKAMP44 | + AD | + AE | N/A | + des. AF − deac. |
N/A | N/A | 0 AG | (+) N/A AH | |||
| SynDIG–1 | + AI | 0 AJ | 0 AK | 0 AL | N/A | N/A | N/A | + AM | |||
| GSG1L | + AN | N/A | + AO | − AP | N/A | N/A | + AQ | N/A | |||
| A: [24] | L: [28] | W: [35] | AH: [42,44] | ||||||||
| B: [24,25] | M: [28] | X: [29,35] | AI: [40,108] | ||||||||
| C: [25] | N: [29,32,34,107] | Y: [6,29,35] | AJ: [41] | ||||||||
| D: [24,25] | O: [31,34,35,62,102] | Z: [30,58,60,61,62] | AK: [41] | ||||||||
| E: [25,26,27,28] | P: [29,31,34,35,61,62,102] | AA: [32] | AL: [41] | ||||||||
| F: [25,26,27,28] | Q: [6,31,34,35,62,102] | AB: [29] | AM: [39,40,108] | ||||||||
| G: [25] | R: [30,58,60,61,62] | AC: [29,31,34] | AN: [6,45] | ||||||||
| H: [24,25,26,27] | S: [32] | AD: [42] | AO: [6] | ||||||||
| I: [28] | T: [29,31,34,61] | AE: [42] | AP: [6,45] | ||||||||
| J: [28] | U: [6,29,31] | AF: [42,43] | AQ: [45] | ||||||||
| K: [28] | V: [29] | AG: [42,43] | |||||||||
1.2. More Recently Discovered AMPAR Auxiliary Subunits
Proteomic analyses led to the discovery of CNIHs as another class of AMPAR auxiliary subunits (see Table 2) [29]. CNIHs are small, three-transmembrane-domain proteins that were first discovered as cornichon protein (CNI) in Drosophila as participants in the Golgi secretory pathway (see Figure 2). When CNIHs are overexpressed together with AMPA receptors, they also localize in the Golgi apparatus [30,31]. CNIHs, like TARPs, directly bind to AMPARs. Reportedly, each of the AMPAR subunits GluA2, GluA3, and GluA4 has only a single site that can bind either a CNIH or a TARP, resulting in competition between CNIH and TARP binding [29,32]. Only GluA1 was suggested to have two different binding sites for CNIHs and TARPs [32]. Consequently, it was initially believed that there is a pool of AMPARs solely associated with CNIHs and another pool associated with TARPs [29,33]. There is even evidence that a majority (70%) of all AMPARs may be associated with CNIHs and not with the prototypic auxiliary subunits, the TARPs [29]. However, other investigations revealed that CNIHs predominantly alter electrophysiological properties and trafficking of GluA1 rather than of the other AMPARs [32]. The observed preferred interaction between CNIH-2 and a GluA1/γ8 complex supports these findings [34]. Other, more recent studies, suggest a different, semi-competitive model with two pairs of distinct binding sites for TARPs and CNIHs per AMPAR tetramer. At one of these pairs, γ2 and γ3 compete for binding with CNIHs, whereas the other pair can bind all type I TARPs but not CNIHs [6]. CNIH-2/3 influence the electrophysiological and pharmacological properties of AMPARs; however, they do this to a greater extent in heterologous systems (up to 10-fold increase in current amplitude) than in vivo (no significant modulation of current amplitude) [31,35]. The modulation of AMPAR trafficking by CNIHs appears to occur independently of that by TARPs [29,31,34]. Thus, it is conceivable that CNIHs and TARPs promote two different trafficking pathways for AMPARs [36,37].
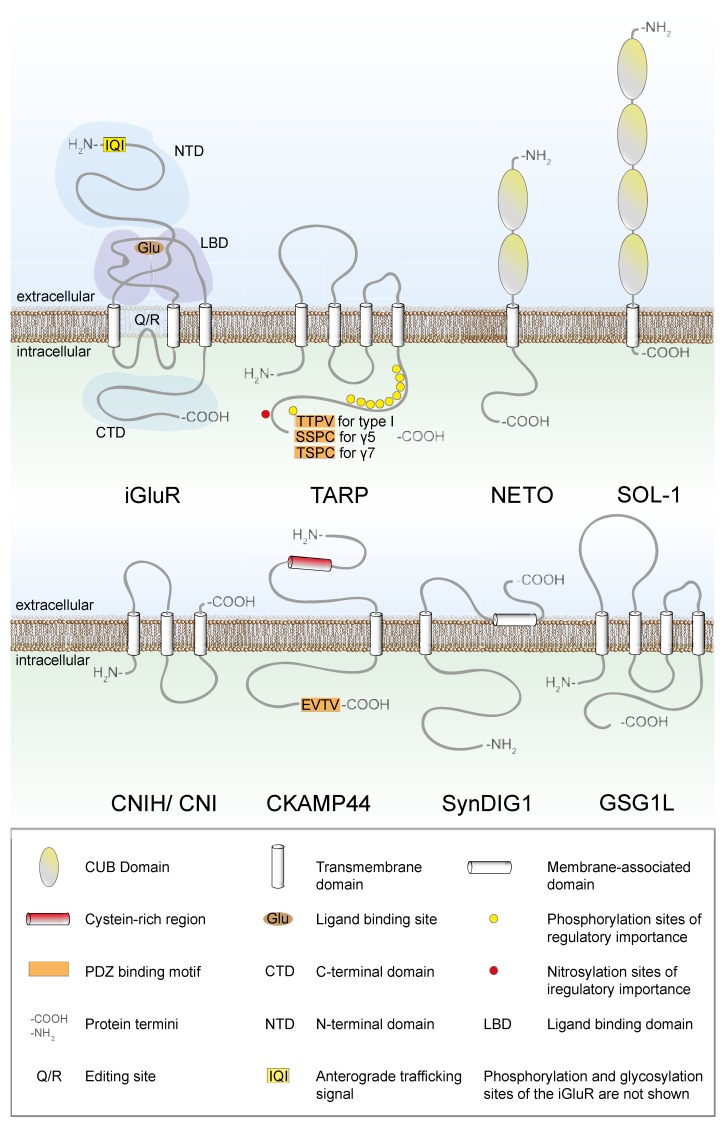
Figure 2.
Schematic structures of iGluRs, TARPs, NETO, SOL-1, CNIH/CNI, CKAMP44, SynDIG1, and GSG1L.
SynDIG1 was identified in a microarray analysis as an additional AMPAR auxiliary subunit (see Table 2) [38]. It directly interacts with and modulates AMPARs, and it additionally has an impact on NMDARs. There are several reports stating that AMPAR activity is regulated via SynDIG1, but the effect of SynDIG1 on the trafficking of AMPARs is not well understood [39,40,41]. On the one hand, the knockout of SynDIG1 down-regulates the AMPAR content in developing synapses by 50%, and SynDIG1 overexpression increases the amplitude of mEPSPs as well as their frequency up to a factor of 1.5–2 in hippocampal cells [40]. On the other hand, there is evidence from acute hippocampal brain slices that SynDIG1 does not have any influence on the surface expression level of AMPARs at all [41]. Thus, the question whether SynDIG1 should be called an auxiliary GluR subunit remains to be answered conclusively.
CKAMP44 and CKAMP52 are members of the shisa protein family, a family of signal transduction pathway-modulating proteins. CKAMP44 and CKAMP52 are auxiliary subunits that can coimmunoprecipiate with AMPARs, proving a direct AMPAR-CKAMP44 interaction (see Table 2) [42]. CKAMP44 influences the electrophysiological properties of AMPARs: Its coexpression with AMPARs in the Xenopus laevis heterologous expression system leads to a nearly complete loss of AMPAR currents and a two-fold increased deactivation rate of the remaining current [42,43,44]. Overexpression of CKAMP44 does not influence the plasma membrane expression of AMPARs in vivo or in heterologous systems [42,43].
A recently reported novel protein that influences AMPARs is GSG1L protein, a relative of the claudin tight junction proteins. It boosts the surface expression of AMPARs in heterologous systems and directly interacts with AMPARs and their complexes (see Table 2). GSG1L additionally influences the electrophysiological properties of the receptor, such as desensitization and activation [6,45].
Another family of iGluR auxiliary subunits are the NETO proteins, which were first thought to influence the NMDARs [46]. Meanwhile it has become clear that they actually modulate kainate receptor subtypes of iGluRs in vertebrates [47]. However, the Drosphila NETO protein seems to be important for the clustering of iGluRs at the neuromuscular junctions. It is therefore a good example of the versatile modulation of iGluRs by auxiliary subunits through vertebrate and invertebrate animals [48].
2. Trafficking of AMPARs between the ER and the Golgi Apparatus
2.1. TARPs and AMPAR Trafficking between the ER and the Golgi Apparatus
The four AMPAR subunits GluA1 through GluA4 have to assemble into hetero- or homotetramers to form functional receptors, a process that takes place in the ER. First, two monomers form a dimer, and then two dimers assemble to form the tetramer [49]. The TARP–receptor interaction starts within the ER and requires the tetrameric structure of the receptor [8,13]. In the ER, TARPs act as chaperones to prevent the ER export of incorrectly folded receptors [13,50]. However, whereas classical chaperones are only transient binding partners within the ER, TARPs remain bound to the receptor on its way through the Golgi apparatus to the plasma membrane and finally the active zone [15]. In this way, type I TARPs are crucial for the ER export of the receptors, which consequently become enriched in the ER when type I TARPs are lacking [30,50,51,52]. The overexpression of AMPARs alone leads to their accumulation in the cell body, and it is only the coexpression with γ2 that decreases this somatic accumulation of AMPARs [53]. However, the TARPs do not influence AMPAR maturation and trafficking indiscriminately but rather in a receptor subunit-specific way [13,50,54]. For example, in Golgi cells γ2 and γ3 can alter the composition of the AMPARs that reach the surface by promoting the surface expression exclusively of GluA2-containing AMPARs [54]. There are two possibilities of how TARPs promote the ER export of AMPARs. The first possibility is that they mask an ER retention signal of the receptor, the second is that they have their own ER export signals that trigger transport of the entire AMPAR-TARP complex [13,50,55]. Recent findings demonstrating the importance of the TARPs’ C-terminus for ER export support the second possibility, or at least a combination of the two mechanisms [56]. The AMPAR GluA1 itself has an anterograde trafficking signal (IQI) at position 7–9 in the amino acid sequence (see Figure 2), i.e., at the far amino terminal end of the protein [57]. More recent findings like the importance of the TAPR`s C-terminus for the trafficking suggest, however, that this signal may not be sufficient for successful plasma membrane delivery [56]. In addition to their function in folding and assembly, type I TARPs are suspected to influence the glycosylation state of the receptor. The AMPARs of stargazer mice show an immature ER-type glycosylation pattern, which indicates that type I TARPs are important for the correct glycosylation of AMPARs [13,50].
2.2. Other Auxiliary Subunits Impacting Trafficking between the ER and the Golgi Apparatus
The cornichon proteins are well known for their localization in the ER and their function as ER exporter proteins in Drosophila [30,58,59,60]. The CNIHs act as ER exporters just like their Drosophila homologues and are highly enriched in the ER when overexpressed [31]. CNIH-2 is involved in the COPII-mediated vesicular transport of proteins from the ER to the Golgi apparatus and cycles between these two organelles [61]. However, when CNIH-2 is accompanied by AMPARs, it forms a functional complex with them that exits this cycle to travel to the plasma membrane [61]. CNIHs enhance AMPAR export from both the ER and the Golgi apparatus [58,61]. However, the functional consequences of CNIH assembly with AMPARs in the ER, like those of TARP assembly, are more varied. CNIHs modulate protein maturation in the ER by altering the glycosylation pattern of the receptors, as observed, for example, for GluA2 and CNIH-2 [61]. Additionally, CNIH-2 competitively reduces the TARP stoichiometry in AMPAR-TARP complexes and assembles preferentially with AMPAR-TARP complexes that include γ8 [62].
AMPARs interact with a large number of proteins in the ER and Golgi apparatus. However, just a few of these AMPAR-interacting proteins interact specifically with the AMPAR—auxiliary subunit complex [2,63,64]. AP-4 is involved in the vesicular transport of AMPAR-TARP complexes, and its knockout leads to mislocalization of the complexes and their accumulation in autophagosomes. AP-4 is additionally involved in the polarized transport of AMPAR-TARP complexes to the somatodendritic domain of Purkinje cells [64]. LC2 binds to fully glycosylated GluA2 subunits in complex with γ2 on their way from the Golgi apparatus to the plasma membrane. It associates with MAP1A and is responsible for neuronal differentiation and microtubule dynamics [63]. Unlike TARPs, however, AP-4 and LC2 are not auxiliary subunits in the sense of the definition given in the introduction because they are merely transient binding partners of the AMPAR complex and do not alter receptor properties.
3. Trafficking of AMPARs to the Plasma Membrane
3.1. The Role of TARPs in Trafficking to the Plasma Membrane
Regulation of the plasma membrane expression of AMPARs is perhaps the most obvious, although not the only, characteristic property of most AMPAR auxiliary subunits [3]. As mentioned above, TARPs, CNIHs, SynDIG, and GSG1L all alter the surface expression of AMPARs. TARPs in particular are crucial for the plasma membrane delivery of AMPARs in the CNS [19,20,65,66]. Overexpression of γ2 enhances AMPAR surface expression in vivo [13,66] and in heterologous systems like Xenopus laevis oocytes by a factor of ten [23,67].
In stargazer mice, which lack the prototypical TARP γ2, the plasma membrane delivery of AMPARs in granule cells is disturbed but can be rescued by the expression of any other type I TARP [13]. In fact, the ability to restore AMPAR function in stargazer cerebellar granule cells was the criterion used to define the type I TARP subfamily. Thus, type I TARPs seem to have at least partly redundant functions. This can also be observed in several other cell types of TARP knockout mice. For example, Golgi cells express γ2 and γ3, and, consequently, a single knockout of either of these subunits does not influence the surface expression of AMPARs. It takes a double knockout of γ2 and γ3 to produce a deficit in AMPAR plasma membrane delivery in these cells [54]. This functional redundancy is the main reason why the stargazer mutant is the only single TARP knockout strain that shows a phenotype: All CNS cell types express at least two different, redundant TARPs, with the exception of cerebellar granule cells, which express only γ2 [54] out of the type I TARP subfamily. Granule cells also express the type II TARP γ7; however, this subunit cannot efficiently compensate for the lack of γ2. In addition to the basic AMPAR delivery to the surface, type I TARPs are also required for the fast cycling of AMPARs into and out of the plasma membrane, and thus underlie the mechanisms of LTP and LTD [50].
Type II TARPs differ from type I TARPs in sequence and function, but the functional differences are not as pronounced as initially thought. The type II TARP γ5 does not alter surface trafficking of AMPARs [7,13,68], its influence appears to be limited to a modulation of their electrophysiological properties [69]. However, the second type II TARP, γ7, does enhance the surface delivery of AMPARs, although its expression in stargazer granule cells rescues the surface expression levels of AMPARs only to a small extent [14,51,68,70]. The knockout of γ7 leads to a decreased number of all AMPARs in cerebellar extracts, with the biggest reduction found in Bergmann glia cells [71]. Surprisingly, the knockdown of γ7 in stargazer cerebellar granule cells increases the synaptic AMPAR density [14,70]. This shows an inhibitory function for γ7 in the synaptic delivery of AMPARs in granule cells [14,70]. Notably, γ7 distinguishes between AMPAR subunits as well as their editing variants: It enhances the surface expression of calcium-permeable AMPARs, whereas it reduces that of calcium-impermeable AMPARs [14]. This editing-dependent regulation of membrane trafficking was also described for γ2. The calcium-impermeable receptors do not need γ2 for the plasma membrane delivery whereas calcium-permeable receptors need to be accompanied by γ2 [72]. This differential trafficking of calcium-permeable and calcium-impermeable AMPARs had already been described well before the discovery of auxiliary subunits and has recently been reviewed [73]. The calcium permeability is determined by the amino acid at the narrow constriction of the ion pore-forming loop in the subunit GluA2 (see Figure 2). In the genome-encoded, unedited version, this amino acid is a glutamine, which can be altered via RNA editing to an arginine. With the arginine in place, the entire receptor complex is rendered virtually calcium-impermeable [74]. The editing state is checked in the ER, and therefore constitutes an important ER export factor by itself.
3.2. Impact of Other Auxiliary Subunits on Trafficking to the Plasma Membrane
The cornichon homologues are involved in the trafficking and surface delivery of AMPARs as well. However, their influence varies greatly between different cell types, expression systems, and AMPAR subunits [34]. In HEK cells, CNIH-2 coexpression enhances the surface delivery of GluA1 up to a factor of ten, and CNIH-2 is detectable in a complex with GluA1 on the surface [29,31,45,61]. In CA1 neurons, CNIH-2 is mandatory for the surface expression of GluA1 [32], and in stargazer granule cells, it can partially rescue extrasynaptic AMPAR expression but not synaptic receptor function [31]. In hippocampal neurons, however, the overexpression of CNIH-2 has virtually no impact on AMPAR surface expression, and CNIH-2 itself is not detectable in the plasma membrane [31].
The novel auxiliary subunit GSG1L, a structural relative of TARPs and claudins, increases the surface expression level of GluA2 in HEK cells by a factor of two, an effect comparable to that of γ2 in this specific experimental setup [45]. However, further studies in heterologous expression systems and in vivo are required to corroborate these findings.
4. Synaptic Targeting and Anchoring of AMPARs
4.1. TARPs and Synaptic Targeting and Anchoring
Besides regulating trafficking to the plasma membrane in general, auxiliary subunits are also involved in the synaptic delivery of initially extrasynaptically located AMPARs. Type I TARPs, for instance, mediate synaptic anchoring and clustering of AMPARs [51,66,75], as demonstrated by the ability of any type I TARP to rescue the lack of not only of extrasynaptic but also synaptic AMPARs in stargazer granule cells. [13,51,76,77]. Type II TARPs, on the other hand, have more varied influences on synaptic targeting. γ5 alters neither trafficking to the plasma membrane nor synaptic localization of AMPARs in stargazer granule cells, its role seems to be merely functional modulation, whereas γ7 was initially reported to rescue only extrasynaptic but not synaptic AMPARs [31,51,69]. However, more recent data indicate that γ7 in cerebellar granule cells does in fact act synergistically with γ2 in stimulating not only the surface delivery but also the synaptic targeting of AMPARs depending on their subunit composition: γ7 seems to reduce the amount of calcium-impermeable and promote that of calcium-permeable AMPARs in extrasynaptic as well as synaptic membranes [14]. Another study of various cerebellar synapses also reported an influence of γ7 on AMPAR abundance in the membrane: Knockdown of γ7 reduced the content of most AMPAR subunits in membranes, with the reduction being more pronounced at the synapse than in extrasynaptic membranes [71].
The synaptic anchoring of AMPARs is mediated via MAGUKs such as the PSD proteins PSD-95, PSD-93, and SAP-102. These scaffolding proteins are major components of the PSD and have long been known to interact indirectly with AMPARs [15,78]. The overexpression of PSD-95 in particular leads to enhanced AMPAR but not NMDAR synaptic responses in hippocampal or cortical neurons caused by a selective increase in the number and the clustering of synaptic AMPARs [66,79,80,81]. Thus, PSD-95 controls the AMPAR content at the synapse, which provides the basis for LTP generation in corticocortical synapses [66,79,81,82]. The knockdown of PSD-95 or PSD-93 silences large, non-overlapping populations of synapses, predominantly in the mature brain, whereas SAP-102 is more important for the synaptic clustering of AMPARs in the immature brain [83]. However, within mature PSD-95/-93 double knockout mice, SAP-102 is upregulated to compensate for the loss [83].
AMPARs cannot interact directly with PSD-95 because they lack a PDZ binding motif that is required for the binding to the PDZ domains of synaptic scaffolding proteins such as PSD-95 [15,84]. Type I TARPs contain such a PDZ binding motif (TTPV) at their very C-terminus that binds with the same affinity to all three PDZ domains of PSD-95 [85]. Thus, TARPs are the mediators between AMPARs and PSD-95 [75,84]. AMPARs and TARPs form a stable complex that diffuses in and out of the PSD [9,86]. Once this AMPAR-TARP complex hits a PDZ domain-containing synaptic scaffolding protein, the TARP’s PDZ binding motif binds to this protein and anchors the whole complex in the PSD. There might be a competition between various synaptic scaffolding proteins such as PSD-95/-93, MAGI-2, and other MAGUK proteins for binding to the AMPAR-TARP complex, which may serve as some sort of control mechanism, but these protein-protein interactions still need further investigation [83,87].
The efficacy of γ2 binding to PSD-95 is highly dependent on the phosphorylation of the TARP’s C-terminal domain, which contains one threonine and nine serine phosphorylation sites [45,88,89]. Phosphorylation of the threonine (321) located within the PDZ binding motif of γ2 completely abolishes the interaction between γ2 and PSD-95 [88,89]. For the serine phosphorylation sites, the opposite holds true: They need to be phosphorylated to enable binding of γ2 to PSD-95. This effect can be explained by the binding of the non-phosphorylated TARP C-terminus to the negatively charged phospholipids of the plasma membrane, which renders it inaccessible to the PDZ domain of PSD-95 [90,91]. Upon serine phosphorylation, the interaction of the TARP C-terminus with the membrane is abolished, and the PDZ binding motif becomes accessible [91]. Consequently, a mutant γ2 lacking all C-terminal serine phosphorylation sites still enhances the plasma membrane expression of AMPA receptors but is not capable of anchoring them in the PSD [92]. Likewise, γ2 with dephosphorylated C-terminal serines shows diffuse localization in the plasma membrane, whereas phosphorylated γ2 is located at the PSD [90,92,93]. These phosphorylation mechanisms play an important role in the regulation of LTP and LTD [92]. For example, the dephosphorylation of the C-terminal serines of TARPs is a common LTD pathway, and at mutant γ2 lacking all C-terminal serine phosphorylation sites prevents cerebellar and hippocampal LTP [67,92,94].
The serines of the C-terminus of γ2 are phosphorylated by CaMKII as well as PKC and are dephosphorylated by PP1 and PP2b [92]. A correlation between PKC activity and AMPAR activity had been discovered well before TARPs were recognized as auxiliary subunits [95]. CaMKII translocates to the PSD upon activation of NMDA-type glutamate receptors, phosphorylates γ2, and thus triggers diffusional trapping of AMPARs at the synapse, which means a translocation of the receptor to CaMKII-free synapses or extrasynaptic sites [96,97]. The phosphorylation at threonine 321 within the PDZ binding motif by PKA and MAPKs has the opposite effect, decreasing AMPAR clustering and anchoring at the synapse [98]. These phosphorylation and the respective dephosphorylation mechanisms as well as the proteins involved are partly different for different TARPs. For example, γ8 is dephosphorylated by PP1 and PP2A, γ2 by PP1 and PP2B [86]. The type II TARP γ5 lacks most of the phosphorylation sites as well as the classical PDZ binding motif, which are conserved only within type I TARPs. Thus, γ5 cannot bind to PSD-95 and therefore does not anchor AMPARs at the PSD [99].
The C-terminus of γ2 potentially plays a role during the entire trafficking process of the receptor because it interacts with nPIST, which is a Golgi-resident PDZ protein that cycles with the TARP-AMPAR complex between the Golgi apparatus, the plasma membrane, and the PSD [100].
The deletion of the C-terminus including the PDZ-binding motif of γ2 abolishes all synaptic trafficking but not the plasma membrane delivery for most AMPARs [75]. In keeping with this result, overexpression of γ2 lacking the C-terminal domain (γ2-∆C) or at least the terminal PDZ binding motif in stargazer hippocampal neurons increases the number of extrasynaptic AMPARs [75,92]. In non-stargazer hippocampal neurons, AMPAR activity is reduced upon overexpression of γ2-∆C, and the surface mobility of the receptor-TARP complex is increased [9]. The PDZ-binding motif is also important for γ8 function because the overexpression of γ8 without the PDZ-binding motif in hippocampal pyramidal cells decreases AMPAR activity [109].
There are additional regulatory mechanisms that impact the binding of TARPs to AMPARs and PSD-95. The C-terminus of γ2 but not γ8 can be truncated by the protease calpain. Therefore, the binding of γ2 to PSD-95 can be permanently interrupted by increased calpain activity [110]. Furthermore, a cysteine residue at position 302 within the C-terminal domain of γ2 can be nitrosylated, which augments the TARP-AMPAR interaction and ultimately leads to increased surface delivery of the receptor [111]. Palmitoylation of PSD-95 appears to be required for AMPAR anchoring at the PSD, as demonstrated by a selective loss of synaptic AMPARs following inhibition of PSD-95 palmitoylation in hippocampal neurons [112]. As suggested by heterologous expression studies in COS cells, this loss is caused by a disruption of PSD-95 clustering with TARPs and AMPARs.
4.2. The Impact of Other Auxiliary Subunits on Synaptic Targeting and Anchoring
There is no evidence that CNIH-2 influences synaptic targeting; apparently, it is only involved in extrasynaptic trafficking of AMPARs [31]. SynDIG1, on the other hand, promotes the trafficking of AMPARs to synapses and is involved in their synaptic clustering [39,108]. SynDIG1 cycles between the extrasynaptic plasma membrane, excitatory synapses, and intracellular endosomal compartments. Given its direct interaction with AMPARs, there is a good chance that SynDIG1 is accompanied by AMPARs during cycling [39,40].
CKAMP44 is most likely involved in the synaptic anchoring of AMPARs and it occurs probably the same way as for TARPs, because CKAMP44, just like TARPs, can interact with PSD-95 [44].
5. Conclusions and Outlook
For the trafficking of AMPARs to their synaptic sites of action, the presence of auxiliary subunits like TARPs or CNIHs is mandatory. TARPs and CNIHs directly interact with the AMPARs, starting immediately after translation in the ER or at least after early maturation, i.e., final modification of their glycosylation and receptor assembly. They influence receptor subunit composition and promote the export of receptor complexes from the ER and Golgi apparatus. TARPs are closely associated with AMPARs in the plasma membrane where they function as mediators between the receptor and scaffolding proteins such as PSD-95. The interaction between scaffolding proteins and TARPs is mainly regulated by the phosphorylation state of the TARP’s C-terminal domain and determines the anchoring and location of the receptor at the PSD.
While the impact of TARPs on the trafficking of AMPARs and the regulation of this process are relatively well known through a considerable number of recently published thorough studies, the modulatory influence of the other AMPAR auxiliary subunits identified to date like the CNIHs, the SynDIG protein family, the shisa protein family, and GSG1L is not yet well understood. Even though it is clear that CNIHs and GSG1L enhance the trafficking of AMPARs, the extent of their impact, the regulatory mechanisms, and the interaction sites are all still unknown. In addition, it is rather unlikely that the full complement of AMPAR auxiliary subunits has already been identified. Thus, additional proteins can be expected to come into play. Possible future players in this still growing field of AMPAR modulators include certain members of the claudin family, which are suspected to be “type III TARPs” with their own specialized modulatory effects on AMPARs [113].
An entirely open field, of which we have barely scratched the surface, is the question whether and how these multiple auxiliary subunits interact or compete with each other for AMPA receptor modulation. These questions urgently need to be addressed in future studies of these important proteins.
Acknowledgments
This work was supported by DFG grant HO 1118/11-2. SP received a PhD fellowship from the International Graduate School of Neuroscience, Ruhr University Bochum, Bochum. SCH and SP received grants from the RUB Research SchoolPlus, Ruhr University Bochum, Bochum.
Definitions
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- AMPAR
AMPA receptor
- AP-4
adaptor protein-4
- CAMKII
Ca2+/calmodulin-dependent protein kinase II
- CKAMP
cysteine-knot AMPAR modulating protein
- CNI
cornichon
- CNIH
cornichon homologue
- CNS
central nervous system
- ER
endoplasmic reticulum
- γ2-ΔC
γ2 lacking the C-terminal domain
- GLR
glutamate receptor-like protein
- GSG1L
germ cell-specific gene 1-like
- HEK
human embryonic kidney
- iGluR
ionotropic glutamate receptor
- LC2
light chain protein 2
- LTD
long-term depression
- LTP
long-term potentiation
- MAGI-2
membrane-associated guanylate kinase inverted-2
- MAGUK
membrane-associated guanylate kinase
- MAP1A
microtubule-associated protein 1A
- MAPK
mitogen-activated protein kinase
- mEPSP
miniature excitatory postsynaptic potential
- Neto
neuropilin and tolloid-like
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- nPIST
neuronal isoform of protein interacting specifically with TC10
- PKC
protein kinase C
- PDZ
PSD-95/discs large/zonula occludens-1
- PP
protein phosphatase
- PSD
postsynaptic density
- SAP
synapse-associated protein
- SOL
suppressor of lurcher
- SynDIG
synapse differentiation-induced gene I
- TARP
transmembrane AMPA receptor regulatory protein
Author Contributions
SCH wrote the main part of the manuscript and prepared all figures and tables. MH, DT, and SP contributed significantly to the writing and design of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Opazo P., Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol. Cell Neurosci. 2011;46:1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Anggono V., Huganir R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letts V.A., Felix R., Biddlecome G.H., Arikkath J., Mahaffey C.L., Valenzuela A., Bartlett F.S., II, Mori Y., Campbell K.P., Frankel W.N. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat. Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 4.Fukata Y., Tzingounis A.V., Trinidad J.C., Fukata M., Burlingame A.L., Nicoll R.A., Bredt D.S. Molecular constituents of neuronal AMPA receptors. J. Cell Biol. 2005;169:399–404. doi: 10.1083/jcb.200501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa T., Cheng Y., Ramm E., Sheng M., Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433:545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- 6.Schwenk J., Harmel N., Brechet A., Zolles G., Berkefeld H., Muller C.S., Bildl W., Baehrens D., Huber B., Kulik A., et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Tomita S., Fukata M., Nicoll R.A., Bredt D.S. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- 8.Vandenberghe W., Nicoll R.A., Bredt D.S. Stargazin is an AMPA receptor auxiliary subunit. Proc. Natl. Acad. Sci. USA. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bats C., Groc L., Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Cho C.H., St-Gelais F., Zhang W., Tomita S., Howe J.R. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Fukaya M., Yamazaki M., Sakimura K., Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci. Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Menuz K., Kerchner G.A., O’Brien J.L., Nicoll R.A. Critical role for TARPs in early development despite broad functional redundancy. Neuropharmacology. 2009;56:22–29. doi: 10.1016/j.neuropharm.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomita S., Chen L., Kawasaki Y., Petralia R.S., Wenthold R.J., Nicoll R.A., Bredt D.S. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Studniarczyk D., Coombs I., Cull-Candy S.G., Farrant M. TARP gamma-7 selectively enhances synaptic expression of calcium-permeable AMPARs. Nat. Neurosci. 2013;16:1266–1274. doi: 10.1038/nn.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita S., Nicoll R.A., Bredt D.S. PDZ protein interactions regulating glutamate receptor function and plasticity. J. Cell Biol. 2001;153:F19–F24. doi: 10.1083/jcb.153.5.F19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicoll R.A., Tomita S., Bredt D.S. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- 17.Menuz K., Stroud R.M., Nicoll R.A., Hays F.A. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- 18.Priel A., Kolleker A., Ayalon G., Gillor M., Osten P., Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J. Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turetsky D., Garringer E., Patneau D.K. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J. Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki M., Ohno-Shosaku T., Fukaya M., Kano M., Watanabe M., Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci. Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Sager C., Terhag J., Kott S., Hollmann M. C-terminal domains of transmembrane α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor regulatory proteins not only facilitate trafficking but are major modulators of AMPA receptor function. J. Biol. Chem. 2009;284:32413–32424. doi: 10.1074/jbc.M109.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kott S., Sager C., Tapken D., Werner M., Hollmann M. Comparative analysis of the pharmacology of GluR1 in complex with transmembrane AMPA receptor regulatory proteins γ2, γ3, γ4, and γ8. Neuroscience. 2009;158:78–88. doi: 10.1016/j.neuroscience.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Chen L., El-Husseini A., Tomita S., Bredt D.S., Nicoll R.A. Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol. Pharmacol. 2003;64:703–706. doi: 10.1124/mol.64.3.703. [DOI] [PubMed] [Google Scholar]
- 24.Walker C.S., Brockie P.J., Madsen D.M., Francis M.M., Zheng Y., Koduri S., Mellem J.E., Strutz-Seebohm N., Maricq A.V. Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc. Natl. Acad. Sci. USA. 2006;103:10781–10786. doi: 10.1073/pnas.0604482103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker C.S., Francis M.M., Brockie P.J., Madsen D.M., Zheng Y., Maricq A.V. Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc. Natl. Acad. Sci. USA. 2006;103:10787–10792. doi: 10.1073/pnas.0604520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y., Brockie P.J., Mellem J.E., Madsen D.M., Walker C.S., Francis M.M., Maricq A.V. SOL-1 is an auxiliary subunit that modulates the gating of GLR-1 glutamate receptors in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2006;103:1100–1105. doi: 10.1073/pnas.0504612103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y., Mellem J.E., Brockie P.J., Madsen D.M., Maricq A.V. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–457. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]
- 28.Wang R., Mellem J.E., Jensen M., Brockie P.J., Walker C.S., Hoerndli F.J., Hauth L., Madsen D.M., Maricq A.V. The SOL-2/Neto auxiliary protein modulates the function of AMPA-subtype ionotropic glutamate receptors. Neuron. 2012;75:838–850. doi: 10.1016/j.neuron.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwenk J., Harmel N., Zolles G., Bildl W., Kulik A., Heimrich B., Chisaka O., Jonas P., Schulte U., Fakler B., et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 30.Brockie P.J., Jensen M., Mellem J.E., Jensen E., Yamasaki T., Wang R., Maxfield D., Thacker C., Hoerndli F., Dunn P.J., et al. Cornichons control ER export of AMPA receptors to regulate synaptic excitability. Neuron. 2013;80:129–142. doi: 10.1016/j.neuron.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y., Suh Y.H., Milstein A.D., Isozaki K., Schmid S.M., Roche K.W., Nicoll R.A. Functional comparison of the effects of TARPs and cornichons on AMPA receptor trafficking and gating. Proc. Natl. Acad. Sci. USA. 2010;107:16315–16319. doi: 10.1073/pnas.1011706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herring B.E., Shi Y., Suh Y.H., Zheng C.Y., Blankenship S.M., Roche K.W., Nicoll R.A. Cornichon proteins determine the subunit composition of synaptic AMPA receptors. Neuron. 2013;77:1083–1096. doi: 10.1016/j.neuron.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brockie P.J., Maricq A.V. In a pickle: Is cornichon just relish or part of the main dish? Neuron. 2010;68:1017–1019. doi: 10.1016/j.neuron.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato A.S., Gill M.B., Ho M.T., Yu H., Tu Y., Siuda E.R., Wang H., Qian Y.W., Nisenbaum E.S., Tomita S., et al. Hippocampal AMPA receptor gating controlled by both TARP and cornichon proteins. Neuron. 2010;68:1082–1096. doi: 10.1016/j.neuron.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coombs I.D., Soto D., Zonouzi M., Renzi M., Shelley C., Farrant M., Cull-Candy S.G. Cornichons modify channel properties of recombinant and glial AMPA receptors. J. Neurosci. 2012;32:9796–9804. doi: 10.1523/JNEUROSCI.0345-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tigaret C., Choquet D. Neuroscience. More AMPAR garnish. Science. 2009;323:1295–1296. doi: 10.1126/science.1171519. [DOI] [PubMed] [Google Scholar]
- 37.Jackson A.C., Nicoll R.A. Neuroscience: AMPA receptors get ‘pickled’. Nature. 2009;458:585–586. doi: 10.1038/458585a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz E., Ge Y., Yang Y.H., Loh K.C., Serafini T.A., Okazaki Y., Hayashizaki Y., Speed T.P., Ngai J., Scheiffele P. Molecular analysis of gene expression in the developing pontocerebellar projection system. Neuron. 2002;36:417–434. doi: 10.1016/S0896-6273(02)01016-4. [DOI] [PubMed] [Google Scholar]
- 39.Diaz E. SynDIG1 regulation of synaptic AMPA receptor targeting. Commun. Integr. Biol. 2010;3:347–349. doi: 10.4161/cib.3.4.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalashnikova E., Lorca R.A., Kaur I., Barisone G.A., Li B., Ishimaru T., Trimmer J.S., Mohapatra D.P., Diaz E. SynDIG1: An activity-regulated, AMPA-receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron. 2010;65:80–93. doi: 10.1016/j.neuron.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovero K.L., Blankenship S.M., Shi Y., Nicoll R.A. SynDIG1 promotes excitatory synaptogenesis independent of AMPA receptor trafficking and biophysical regulation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Von Engelhardt J., Mack V., Sprengel R., Kavenstock N., Li K.W., Stern-Bach Y., Smit A.B., Seeburg P.H., Monyer H. CKAMP44: A brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. 2010;327:1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- 43.Farrant M., Cull-Candy S.G. Neuroscience. AMPA receptors—Another twist? Science. 2010;327:1463–1465. doi: 10.1126/science.1187920. [DOI] [PubMed] [Google Scholar]
- 44.Karataeva A.R., Klaassen R.V., Stroder J., Ruiperez-Alonso M., Hjorth J.J., van Nierop P., Spijker S., Mansvelder H.D., Smit A.B. C-terminal interactors of the AMPA receptor auxiliary subunit shisa9. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shanks N.F., Savas J.N., Maruo T., Cais O., Hirao A., Oe S., Ghosh A., Noda Y., Greger I.H., Yates J.R., 3rd, et al. Differences in AMPA and kainate receptor interactomes facilitate identification of AMPA receptor auxiliary subunit GSG1L. Cell Rep. 2012;1:590–598. doi: 10.1016/j.celrep.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng D., Pitcher G.M., Szilard R.K., Sertie A., Kanisek M., Clapcote S.J., Lipina T., Kalia L.V., Joo D., McKerlie C., et al. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W., St-Gelais F., Grabner C.P., Trinidad J.C., Sumioka A., Morimoto-Tomita M., Kim K.S., Straub C., Burlingame A.L., Howe J.R., et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y.J., Bao H., Bonanno L., Zhang B., Serpe M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev. 2012;26:974–987. doi: 10.1101/gad.185165.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ayalon G., Stern-Bach Y. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron. 2001;31:103–113. doi: 10.1016/S0896-6273(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 50.Vandenberghe W., Nicoll R.A., Bredt D.S. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J. Neurosci. 2005;25:1095–1102. doi: 10.1523/JNEUROSCI.3568-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milstein A.D., Zhou W., Karimzadegan S., Bredt D.S., Nicoll R.A. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall R.A., Hansen A., Andersen P.H., Soderling T.R. Surface expression of the AMPA receptor subunits GluR1, GluR2, and GluR4 in stably transfected baby hamster kidney cells. J. Neurochem. 1997;68:625–630. doi: 10.1046/j.1471-4159.1997.68020625.x. [DOI] [PubMed] [Google Scholar]
- 53.Kessels H.W., Kopec C.D., Klein M.E., Malinow R. Roles of stargazin and phosphorylation in the control of AMPA receptor subcellular distribution. Nat. Neurosci. 2009;12:888–896. doi: 10.1038/nn.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menuz K., O’Brien J.L., Karmizadegan S., Bredt D.S., Nicoll R.A. TARP redundancy is critical for maintaining AMPA receptor function. J. Neurosci. 2008;28:8740–8746. doi: 10.1523/JNEUROSCI.1319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedoukian M.A., Weeks A.M., Partin K.M. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J. Biol. Chem. 2006;281:23908–23921. doi: 10.1074/jbc.M600679200. [DOI] [PubMed] [Google Scholar]
- 56.Bedoukian M.A., Whitesell J.D., Peterson E.J., Clay C.M., Partin K.M. The stargazin C terminus encodes an intrinsic and transferable membrane sorting signal. J. Biol. Chem. 2008;283:1597–1600. doi: 10.1074/jbc.M708141200. [DOI] [PubMed] [Google Scholar]
- 57.Xia H., von Zastrow M., Malenka R.C. A novel anterograde trafficking signal present in the N-terminal extracellular domain of ionotropic glutamate receptors. J. Biol. Chem. 2002;277:47765–47769. doi: 10.1074/jbc.M207122200. [DOI] [PubMed] [Google Scholar]
- 58.Bokel C., Dass S., Wilsch-Brauninger M., Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–470. doi: 10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- 59.Roth S., Neuman-Silberberg F.S., Barcelo G., Schupbach T. Cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 60.Castillon G.A., Watanabe R., Taylor M., Schwabe T.M., Riezman H. Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic. 2009;10:186–200. doi: 10.1111/j.1600-0854.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- 61.Harmel N., Cokic B., Zolles G., Berkefeld H., Mauric V., Fakler B., Stein V., Klocker N. AMPA receptors commandeer an ancient cargo exporter for use as an auxiliary subunit for signaling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill M.B., Kato A.S., Roberts M.F., Yu H., Wang H., Tomita S., Bredt D.S. Cornichon-2 modulates AMPA receptor-transmembrane AMPA receptor regulatory protein assembly to dictate gating and pharmacology. J. Neurosci. 2011;31:6928–6938. doi: 10.1523/JNEUROSCI.6271-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ives J.H., Fung S., Tiwari P., Payne H.L., Thompson C.L. Microtubule-associated protein light chain 2 is a stargazin-AMPA receptor complex-interacting protein in vivo. J. Biol. Chem. 2004;279:31002–31009. doi: 10.1074/jbc.M402214200. [DOI] [PubMed] [Google Scholar]
- 64.Matsuda S., Miura E., Matsuda K., Kakegawa W., Kohda K., Watanabe M., Yuzaki M. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron. 2008;57:730–745. doi: 10.1016/j.neuron.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Rouach N., Byrd K., Petralia R.S., Elias G.M., Adesnik H., Tomita S., Karimzadegan S., Kealey C., Bredt D.S., Nicoll R.A. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat. Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- 66.Schnell E., Sizemore M., Karimzadegan S., Chen L., Bredt D.S., Nicoll R.A. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomita S., Adesnik H., Sekiguchi M., Zhang W., Wada K., Howe J.R., Nicoll R.A., Bredt D.S. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 68.Kato A.S., Zhou W., Milstein A.D., Knierman M.D., Siuda E.R., Dotzlaf J.E., Yu H., Hale J.E., Nisenbaum E.S., Nicoll R.A., et al. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J. Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kato A.S., Siuda E.R., Nisenbaum E.S., Bredt D.S. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59:986–996. doi: 10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 70.Bats C., Soto D., Studniarczyk D., Farrant M., Cull-Candy S.G. Channel properties reveal differential expression of TARPed and TARPless AMPARs in stargazer neurons. Nat. Neurosci. 2012;15:853–861. doi: 10.1038/nn.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamazaki M., Fukaya M., Hashimoto K., Yamasaki M., Tsujita M., Itakura M., Abe M., Natsume R., Takahashi M., Kano M., et al. TARPs gamma-2 and gamma-7 are essential for AMPA receptor expression in the cerebellum. Eur. J. Neurosci. 2010;31:2204–2220. doi: 10.1111/j.1460-9568.2010.07254.x. [DOI] [PubMed] [Google Scholar]
- 72.Zonouzi M., Renzi M., Farrant M., Cull-Candy S.G. Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nat. Neurosci. 2011;14:1430–1438. doi: 10.1038/nn.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanley J.G. Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca-permeable AMPA receptors. Semin. Cell Dev. Biol. 2013;2013:14–22. doi: 10.1016/j.semcdb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Tanaka H., Grooms S.Y., Bennett M.V., Zukin R.S. The AMPAR subunit GluR2: Still front and center-stage. Brain Res. 2000;886:190–207. doi: 10.1016/S0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- 75.Chen L., Chetkovich D.M., Petralia R.S., Sweeney N.T., Kawasaki Y., Wenthold R.J., Bredt D.S., Nicoll R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35046031. [DOI] [PubMed] [Google Scholar]
- 76.Chen L., Bao S., Qiao X., Thompson R.F. Impaired cerebellar synapse maturation in waggler, a mutant mouse with a disrupted neuronal calcium channel gamma subunit. Proc. Natl. Acad. Sci. USA. 1999;96:12132–12137. doi: 10.1073/pnas.96.21.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashimoto K., Fukaya M., Qiao X., Sakimura K., Watanabe M., Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J. Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim E., Sheng M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 79.Beique J.C., Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J. Physiol. 2003;546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ehrlich I., Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Husseini A.E., Schnell E., Chetkovich D.M., Nicoll R.A., Bredt D.S. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 82.Stein V., House D.R., Bredt D.S., Nicoll R.A. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J. Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elias G.M., Funke L., Stein V., Grant S.G., Bredt D.S., Nicoll R.A. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 84.Beique J.C., Lin D.T., Kang M.G., Aizawa H., Takamiya K., Huganir R.L. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dakoji S., Tomita S., Karimzadegan S., Nicoll R.A., Bredt D.S. Interaction of transmembrane AMPA receptor regulatory proteins with multiple membrane associated guanylate kinases. Neuropharmacology. 2003;45:849–856. doi: 10.1016/S0028-3908(03)00267-3. [DOI] [PubMed] [Google Scholar]
- 86.Inamura M., Itakura M., Okamoto H., Hoka S., Mizoguchi A., Fukazawa Y., Shigemoto R., Yamamori S., Takahashi M. Differential localization and regulation of stargazin-like protein, gamma-8 and stargazin in the plasma membrane of hippocampal and cortical neurons. Neurosci. Res. 2006;55:45–53. doi: 10.1016/j.neures.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Deng F., Price M.G., Davis C.F., Mori M., Burgess D.L. Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain. J. Neurosci. 2006;26:7875–7884. doi: 10.1523/JNEUROSCI.1851-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chetkovich D.M., Chen L., Stocker T.J., Nicoll R.A., Bredt D.S. Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J. Neurosci. 2002;22:5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi J., Ko J., Park E., Lee J.R., Yoon J., Lim S., Kim E. Phosphorylation of stargazin by protein kinase A regulates its interaction with PSD-95. J. Biol. Chem. 2002;277:12359–12363. doi: 10.1074/jbc.M200528200. [DOI] [PubMed] [Google Scholar]
- 90.Sumioka A., Yan D., Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roberts M.F., Taylor D.W., Unger V.M. Two modes of interaction between the membrane-embedded TARP stargazin’s C-terminal domain and the bilayer visualized by electron crystallography. J. Struct. Biol. 2011;174:542–551. doi: 10.1016/j.jsb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomita S., Stein V., Stocker T.J., Nicoll R.A., Bredt D.S. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 93.Tsui J., Malenka R.C. Substrate localization creates specificity in calcium/calmodulin-dependent protein kinase II signaling at synapses. J. Biol. Chem. 2006;281:13794–13804. doi: 10.1074/jbc.M600966200. [DOI] [PubMed] [Google Scholar]
- 94.Nomura T., Kakegawa W., Matsuda S., Kohda K., Nishiyama J., Takahashi T., Yuzaki M. Cerebellar long-term depression requires dephosphorylation of TARP in Purkinje cells. Eur. J. Neurosci. 2012;35:402–410. doi: 10.1111/j.1460-9568.2011.07963.x. [DOI] [PubMed] [Google Scholar]
- 95.Carroll R.C., Nicoll R.A., Malenka R.C. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J. Neurophysiol. 1998;80:2797–2800. doi: 10.1152/jn.1998.80.5.2797. [DOI] [PubMed] [Google Scholar]
- 96.Opazo P., Labrecque S., Tigaret C.M., Frouin A., Wiseman P.W., de Koninck P., Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 97.Merrill M.A., Chen Y., Strack S., Hell J.W. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol. Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Stein E.L., Chetkovich D.M. Regulation of stargazin synaptic trafficking by C-terminal PDZ ligand phosphorylation in bidirectional synaptic plasticity. J. Neurochem. 2010;113:42–53. doi: 10.1111/j.1471-4159.2009.06529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soto D., Coombs I.D., Renzi M., Zonouzi M., Farrant M., Cull-Candy S.G. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, gamma-5. Nat. Neurosci. 2009;12:277–285. doi: 10.1038/nn.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cuadra A.E., Kuo S.H., Kawasaki Y., Bredt D.S., Chetkovich D.M. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J. Neurosci. 2004;24:7491–7502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Körber C., Werner M., Kott S., Ma Z.-L., Hollmann M. The transmembrane AMPA receptor regulatory protein γ4 is a more effective modulator of AMPA receptor function than stargazin (γ2) J. Neurosci. 2007;27:8442–8447. doi: 10.1523/JNEUROSCI.0424-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gill M.B., Kato A.S., Wang H., Bredt D.S. AMPA receptor modulation by cornichon-2 dictated by transmembrane AMPA receptor regulatory protein isoform. Eur. J. Neurosci. 2012;35:182–194. doi: 10.1111/j.1460-9568.2011.07948.x. [DOI] [PubMed] [Google Scholar]
- 103.Kott S., Werner M., Körber C., Hollmann M. Electrophysiological properties of AMPA receptors are differentially modulated depending on the associated member of the TARP family. J. Neurosci. 2007;27:3780–3789. doi: 10.1523/JNEUROSCI.4185-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jackson A.C., Nicoll R.A. Stargazin (TARP gamma-2) is required for compartment-specific AMPA receptor trafficking and synaptic plasticity in cerebellar stellate cells. J. Neurosci. 2011;31:3939–3952. doi: 10.1523/JNEUROSCI.5134-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tomita S., Shenoy A., Fukata Y., Nicoll R.A., Bredt D.S. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 2007;52:87–91. doi: 10.1016/j.neuropharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 106.Fukaya M., Tsujita M., Yamazaki M., Kushiya E., Abe M., Akashi K., Natsume R., Kano M., Kamiya H., Watanabe M., et al. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur. J. Neurosci. 2006;24:2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- 107.Mauric V., Molders A., Harmel N., Heimrich B., Sergeeva O.A., Klocker N. Ontogeny repeats the phylogenetic recruitment of the cargo exporter cornichon into AMPA receptor signaling complexes. Mol. Cell Neurosci. 2013;56:10–17. doi: 10.1016/j.mcn.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 108.Diaz E. SynDIG1 regulation of excitatory synapse maturation. J. Physiol. 2012;590:33–38. doi: 10.1113/jphysiol.2011.213884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sumioka A., Brown T.E., Kato A.S., Bredt D.S., Kauer J.A., Tomita S. PDZ binding of TARPgamma-8 controls synaptic transmission but not synaptic plasticity. Nat. Neurosci. 2011;14:1410–1412. doi: 10.1038/nn.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu L., Rostamiani K., Hsu Y.T., Wang Y., Bi X., Baudry M. Calpain-mediated regulation of stargazin in adult rat brain. Neuroscience. 2011;178:13–20. doi: 10.1016/j.neuroscience.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selvakumar B., Huganir R.L., Snyder S.H. S-nitrosylation of stargazin regulates surface expression of AMPA-glutamate neurotransmitter receptors. Proc. Natl. Acad. Sci. USA. 2009;106:16440–16445. doi: 10.1073/pnas.0908949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El-Husseini Ael D., Schnell E., Dakoji S., Sweeney N., Zhou Q., Prange O., Gauthier-Campbell C., Aguilera-Moreno A., Nicoll R.A., Bredt D.S. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 113.Kösters S.C., Hollmann M. 612.26 Identification of Certain Claudins as Possible Type III TARPs; Proceedings of the Annual Meeting of the Society for Neuroscience; San Diego, CA, USA. 12 November 2013. [Google Scholar]