Abstract
Mesoporous tungsten trioxide (WO3) was prepared from tungstic acid (H2WO4) as a tungsten precursor with dodecylamine (DDA) as a template to guide porosity of the nanostructure by a solvothermal technique. The WO3 sample (denoted as WO3-DDA) prepared with DDA was moulded on an electrode to yield efficient performance for visible-light-driven photoelectrochemical (PEC) water oxidation. Powder X-ray diffraction (XRD) data of the WO3-DDA sample calcined at 400°C indicate a crystalline framework of the mesoporous structure with disordered arrangement of pores. N2 physisorption studies show a Brunauer-Emmett-Teller (BET) surface area up to 57 m2 g-1 together with type IV isotherms and uniform distribution of a nanoscale pore size in the mesopore region. Scanning electron microscopy (SEM) images exhibit well-connected tiny spherical WO3 particles with a diameter of ca. 5 to 20 nm composing the mesoporous network. The WO3-DDA electrode generated photoanodic current density of 1.1 mA cm-2 at 1.0 V versus Ag/AgCl under visible light irradiation, which is about three times higher than that of the untemplated WO3. O2 (1.49 μmol; Faraday efficiency, 65.2%) was evolved during the 1-h photoelectrolysis for the WO3-DDA electrode under the conditions employed. The mesoporous electrode turned out to work more efficiently for visible-light-driven water oxidation relative to the untemplated WO3 electrode.
Keywords: Tungsten trioxide, Mesoporous structure, Photoelectrocatalysis, Water oxidation
Background
The recent advances in nanostructured materials have expanded their potential applications in much-desired materials for efficient solar energy conversion [1-6]. Photoelectrochemical (PEC) water splitting into oxygen and hydrogen is an attractive but challenging way for the conversion of solar energy, [7] following the pioneer work on a TiO2 photoanode for water splitting by Honda and Fujishima [8]. Unfortunately, owing to its wide electronic bandgap (3.0 to 3.2 eV), TiO2 absorbs only an ultraviolet fraction of a solar spectrum (which accounts for just 4% of solar irradiation), being consequently responsible for low efficiency in utilization of solar light [2,7,9]. For solar water splitting, intensive researches have been focused on nanostructured materials with narrow bandgaps including WO3[3,4,10-19]. WO3, an n-type semiconductor, has attracted immense attention as a photoanode material for water oxidation in PEC cells because of its visible light response (bandgap, Eg = 2.6 to 2.8 eV), a valence band edge position thermodynamically possible for water oxidation (about 3 V versus the normal hydrogen electrode), and good photochemical stability under the acidic conditions [3,10-12,20-24].
Porous material design, which has been developed employing template-directed approaches using small organic compounds [25], supramolecular assembly [26], and polymer beads [27], is of great importance in many research fields because of the high porosity, large area per unit volume, and favorable design of a porous structure [25,28,29]. So far, several efforts in nanostructural and porosity controls of WO3 have been provided to increase the contact area between an electrode and an electrolyte solution and to make electron transport in WO3 films more efficient, enhancing performance of PEC water oxidation at WO3 electrodes [3,11,30-33]. For example, Santato et al. have reported that crystalline WO3 photoanodes with interconnected nanoparticulate structures improved photoelectrochemical properties [30-32]. Berger et al. have demonstrated that random porous layers of WO3 produced significantly higher photocurrent efficiency than a compact layer [33]. Our group recently demonstrated a crystalline small mesoporous network of a WO3 photoanode for high improvement in performance of PEC water oxidation [3].
Numerous methods have been employed to control the dimension, morphology, and crystal structure of WO3, e.g., vacuum evaporation [34], chemical vapor deposition [35,36], sol–gel precipitation [22,30-32], hydrothermal/solvothermal [37-40], surfactant/hard template techniques [3,41,42], and so on. Among the abundant methods, hydrothermal/solvothermal techniques can provide a cost-effective and one-step route synthesis of WO3[37-40]. Although the surfactant template techniques require a liquid tungsten precursor to utilize interaction with a surfactant in principle, we have focused on the interaction between a solid tungsten precursor and a surfactant under solvothermal conditions to yield mesoporous WO3. Herein, we report the unique and facile synthesis of mesoporous WO3 utilizing solid H2WO4 as a tungsten precursor with an organic amphiphilic molecule, dodecylamine (DDA), as a surfactant template for porosity of the nanostructure. The mesoporous WO3 exhibited high surface area and improved the performance of PEC water oxidation compared to the corresponding materials prepared without a template.
Methods
Materials
Tungstic acid (H2WO4) was purchased from Kanto Chemical Co., Inc. (Chuo-ku, Tokyo, Japan). DDA was obtained from Sigma-Aldrich (St. Louis, MO, USA). Polyethylene glycol (PEG, molecular weight = 2,000) was obtained from Wako Chemical Co. (Osaka, Japan). Marpolose (60MP-50) was purchased from Matsumoto Yushi-Seiyaku Co. (Osaka, Japan). An indium tin oxide (ITO)-coated glass substrate was obtained from Asahi Glass Co. (Tokyo, Japan). Millipore water (Merck Ltd., Tokyo, Japan) was used for all the experiments. All other chemicals unless mentioned otherwise were of analytical grade and used as received.
Synthesis of mesoporous WO3
In a typical synthesis, 1.7 g of DDA (9.0 mmol) was dissolved in 15 mL ethanol under stirring at room temperature. Tungstic acid (0.9 g; 3.6 mmol) was added to the DDA solution with stirring for 30 min to yield a suspension. It was transferred to a Teflon-lined stainless steel autoclave and then placed in an oil bath at 150°C for 24 h. After the autoclave was cooled down to room temperature, the solid product was recovered by centrifugation, then washed repeatedly by ethanol and air-dried. The solid product was calcined at 400°C with a rate of 1°C min-1 and then maintained at 400°C for 1 h in flowing N2, followed by changing to O2 flow (at 400°C) for 2 h to result in a WO3 sample (denoted as WO3-DDA). A control sample (denoted as WO3-bulk) was prepared in the same manner except for the addition of DDA.
Preparation of electrodes
The WO3 film-coated ITO electrodes (ITO/WO3) were prepared employing a doctor-blade technique. Before coating, ITO glass substrates (1.0 cm-2 area) were cleaned up by a UV-ozone treatment (photo surface processor PL16-110, Sen Lights Co., Osaka, Japan) for 15 min. In a typical procedure, WO3 powder (200 mg), PEG (100 mg), and Marpolose (20 mg) were mixed in 300 μL of water. The mixture suspension was stirred for approximately 2 to 4 h until a smooth paste was formed. The resulting paste was squeezed over an ITO glass substrate by a doctor-blade coater and dried at 80°C for 15 min. After repeating the procedure for two times, the electrodes were calcined at 400°C and maintained at 400°C in flowing N2 for 1 h, followed by changing to O2 flow (at 400°C) for 2 h.
Structural characterization
Characterization of the morphological features and the crystalline phase was conducted by field-emission scanning electron microscopy (FESEM; JSM-6500 F, JEOL Ltd., Akishima, Tokyo, Japan) and powder X-ray diffraction (XRD; MiniFlexII, Rigaku Corporation, Tokyo, Japan) using monochromated Cu Kα (λ = 1.54 Å) radiation. Nitrogen adsorption-desorption isotherms were measured using a BELSORP-miniII (BEL Japan, Inc., Osaka, Japan) at 77 K. Prior to gas adsorption, samples were degassed in vacuum for 4 h at 150°C. The Brunauer-Emmett-Teller (BET) method was utilized to calculate the surface areas. The pore size distributions were obtained from analysis of the adsorption branches of the isotherms by the Barrett-Joyner-Halenda (BJH) method. Fourier transform infrared spectra were recorded on a Jasco FT/IR-4200 spectrophotometer (Jasco Inc., Tokyo, Japan).
Photoelectrochemical measurements
Photoelectrochemical measurement was carried out in a two-compartment photoelectrochemical cell separated by a Nafion membrane using an electrochemical analyzer (HZ-3000, Hokuto Denko Co. Ltd., Tokyo, Japan). A three-electrode system has been employed by using ITO/WO3 and Ag/AgCl electrodes in one compartment as the working and reference electrodes, respectively, and a Pt wire in the other compartment as the counter electrode. An aqueous 0.1 M phosphate solution was used as an electrolyte in both compartments of the cell, which was saturated with Ar gas prior to the measurement. The cyclic voltammogram (CV) was recorded at a scan rate of 50 mV s-1 at 25°C. Light (λ > 390 nm) was irradiated from the backside of the working electrode using a 500-W xenon lamp (Optical ModuleX; Ushio Inc., Tokyo, Japan) with a UV-cut filter (L39) and liquid filter (0.2 M CuSO4) for cutting of heat ray. The output of light intensity was calibrated as 100 mW cm-2 using a spectroradiometer (USR-40; Ushio Inc., Tokyo, Japan). Photoelectrocatalysis was conducted under the potentiostatic conditions of 0.5 V versus Ag/AgCl at 25°C under illumination of light (λ > 390 nm, 100 mW cm-2) for 1 h. The amounts of H2 and O2 evolved were determined from the analysis of the gas phase (headspace volume: 87.3 mL) of counter and working electrode compartments, respectively, using gas chromatography (GC-8A with a TCD detector and molecular sieve 5A column and Ar carrier gas; Shimadzu Corporation, Kyoto, Japan).
Results and discussion
The powder XRD patterns of the WO3 samples calcined at 400°C and 500°C are shown in Figure 1. Small-angle XRD patterns (Figure 1 (a)) of WO3-DDA at 400°C showed a single diffraction peak at low 2θ, being a sign of formation of mesoporous structures, but the weak intensity and broadness of the peak are possibly due to disordered mesoporous structures. The d-spacing, calculated from the XRD peak at 2θ = 2.3° is 3.78 nm. Weakening of the intensity of the diffraction peak for WO3-DDA at 500°C (Figure 1 (b)) suggests degradation of the mesostructure at higher temperature. The wide-angle XRD patterns of both the WO3-DDA and WO3-bulk samples revealed crystallization of the framework after calcination at 400°C and higher degree of crystallization at 500°C, though crystallinity of WO3-bulk seems to be higher than that of WO3-DDA at both calcination temperatures. The d-spacings calculated from the XRD peaks of both WO3-DDA and WO3-bulk were in good agreement with phase-pure monoclinic WO3 (JCPDS number: 43–1305). Average crystallite sizes for WO3-DDA, estimated using [002] reflections were 5.7 and 11.6 nm at 400°C and 500°C, respectively, which suggests that progressive growth of the WO3 nanocrystal in the porous network is responsible for degradation of the mesostructure at 500°C.
Figure 1.

Small-angle and wide-angle X-ray diffraction patterns. (A) Small-angle and (B) wide-angle XRD patterns of WO3-DDA and WO3-bulk samples after being calcined at 400°C and 500°C. (a) WO3-DDA calcined at 400°C, (b) WO3-DDA calcined at 500°C, (c) WO3-bulk calcined at 400°C, and (d) WO3-bulk calcined at 500°C.
N2 adsorption/desorption isotherms of the WO3 samples calcined at 400°C and 500°C are shown in Figure 2. The isotherm (Figure 2 (a)) of WO3-DDA calcined at 400°C could be classified as type IV, characteristic of mesoporous materials [26,43]. In this isotherm, the adsorption amount gradually increased in a range of P/P0 = 0.4 to 0.85, which could be explained by the classical capillary condensation observed for mesopores. The H2 hysteresis loop in the isotherm (Figure 2 (a)) may be caused by roughness of the pore and particle surface [44]. The BET surface area and mesopore volume for WO3-DDA calcined at 400°C were 57 m2 g-1 and 0.08 cm3 g-1, respectively, as summarized in Table 1. The pore size distribution (Figure 2) by the BJH method shows narrow distribution with a peak pore width at 4.9 nm. Isotherm of WO3-DDA calcined at 500°C shows a predominantly type II nature, and the BET surface area was drastically reduced to 12 m2 g-1. The pore size distribution of WO3-DDA calcined at 500°C gives a wider peak at approximately 50 nm due to large interparticle pores. These results are in accordance with the degradation of the mesoporous structure of WO3-DDA due to progressive growth of WO3 nanocrystals at higher temperature of 500°C, as observed in the XRD measurement. The WO3-bulk sample synthesized without DDA exhibited typical type II isotherms, characteristic of nonporous solids. The BET surface area is 24 m2 g-1 at 400°C, which is noticeably low compared to the mesoporous WO3-DDA.
Figure 2.

N2 sorption isotherms and pore size distribution. (A) N2 sorption isotherms and (B) pore size distribution of WO3-DDA and WO3-bulk samples after being calcined at 400°C and 500°C. In N2 sorption, isotherm adsorption and desorption points are marked by filled and empty symbols, respectively. (a) WO3-DDA calcined at 400°C, (b) WO3-DDA calcined at 500°C, and (c) WO3-bulk calcined at 400°C.
Table 1.
Physicochemical properties of WO 3 samples
| Sample name | Calcination temperature (°C) | d -spacing (nm) | Surface area (m 2 g -1 ) | Pore volume (cm 3 g -1 ) | Pore size (nm) |
|---|---|---|---|---|---|
| WO3-DDA |
400 |
1.70 |
57 |
0.08 |
4.9 |
| WO3-DDA |
500 |
- |
12 |
0.09 |
48.2 |
| WO3-bulk | 400 | - | 24 | 0.19 | 52.7 |
The Fourier transform infrared (FTIR) spectra of as-made (before calcination) and calcined (400°C and 500°C) WO3-DDA samples are shown in Figure 3. C-H stretching vibration bands of the hydrocarbon chains at 2,919 cm-1 (asymmetric) and 2,844 cm-1 (symmetric) along with C-H bending vibration bands at 1,469 cm-1 of CH2 groups were clearly observed in the as-made sample. Comparing the FTIR spectra of the as-made WO3-DDA with calcined WO3-DDA samples, we could see that peaks due to C-H vibration diminished completely for the calcined samples. This indicates complete removal of DDA during calcination at 400°C and 500°C, which is very much necessary to generate high porosity for these mesoporous materials.
Figure 3.
FTIR spectra of WO3-DDA samples. (a) The as-made sample and samples after being calcined at (b) 400°C and (c) 500°C.
The scanning electron microscopy (SEM) images of the calcined WO3-DDA samples are shown in Figure 4. The SEM images of the top view (Figure 4a,b) exhibit that a mesoporous network is composed of tiny spherical WO3 particles of ca. 5 to 20 nm in diameter, being well connected to each other. In a few places, the spherical particles agglomerate to form large particles. A close look into these images suggests that the average dimension of particles increases with calcination temperature from 400°C to 500°C due to sintering of WO3 nanocrystals at higher calcination temperature. After preparation of a mesoporous WO3 film on an ITO electrode, the film thickness was measured to be ca. 12 μm from the cross-sectional SEM image (Figure 4c). This crystalline mesoporous structure of the connected WO3 particles is important to yield a large interface between the electrolyte and film as well as efficient electron transport through the film, which are consequently expected to work efficiently for PEC water oxidation since the electron and hole pairs generated by photoexcitation of WO3 would have less chance to recombine before participating in a water oxidation reaction at the WO3 surface.
Figure 4.
Scanning electron microscopic (SEM) images. Top view of WO3-DDA samples calcined at (a) 400°C and (b) 500°C. (c) Cross-sectional view of the ITO/WO3-DDA electrode after being calcined at 500°C.
The PEC properties of the ITO/WO3 electrodes were studied in a 0.1 M phosphate solution. Figure 5 shows the CVs of the ITO/WO3 electrodes. On CVs of samples calcined at 400°C for both WO3-DDA and WO3-bulk (Figure 5, left), no redox response was observed in the dark in a potential range of 0.4 ~ 1.0 V versus Ag/AgCl except for a response based on WO3/HxWO3 below 0.2 V. Upon irradiation of visible light, the anodic current (0.13 ~ 0.18 mA cm-2 at 1.0 V versus Ag/AgCl) was hardly generated for both samples. This is ascribed to insufficient crystallinity of both WO3-DDA and WO3-bulk calcined at 400°C. Crystallinity rather than porosity for samples calcined at 400°C is a dominant factor for the PEC performance of the WO3-based photoanode under the conditions employed [20,32]. On CVs of the samples calcined at 500°C (Figure 5, right), the significantly high photoanodic current due to water oxidation was observed upon visible light irradiation above an onset potential of 0.17 V versus Ag/AgCl due to higher crystallinity. The photoanodic current reached 1.1 mA cm-2 at 1.0 V for WO3-DDA, which is about three times higher compared to that for the WO3-bulk (0.36 mA cm-2 at 1.0 V) electrode in spite of the degradation of mesoporous structure for WO3-DDA calcined at 500°C. The degraded mesoporous structure for WO3-DDA might result in favorable conditions for PEC water oxidation compared with the nanoparticle structure of WO3-bulk. Otherwise, another important factor might be involved in the higher performance of the WO3-DDA electrode. In the present paper, we do not pursue interpretation of the higher performance of the WO3-DDA electrode because our attention is on the solvothermal synthesis of a mesoporous structure of WO3.
Figure 5.
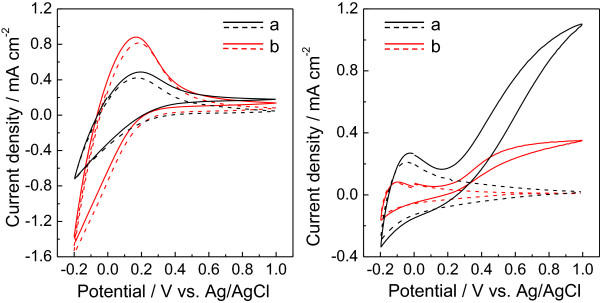
Cyclic voltammograms (CVs) for samples calcined at 400°C (left) and 500°C (right). (a) ITO/WO3-DDA and (b) ITO/WO3-bulk electrodes in a 0.1 M phosphate buffer solution with pH = 6.0. The dashed and solid lines represent CVs measured in the dark and upon irradiation of visible light (λ > 390 nm, 100 mW cm-2), respectively.
Photoelectrocatalysis over the ITO/WO3 electrodes was conducted in a 0.1 M phosphate solution (pH = 6.0) under potentiostatic conditions at 0.5 V versus Ag/AgCl for 1 h upon visible light irradiation (λ > 390 nm, 100 mW cm-2). The photocurrent-time profiles of both WO3-DDA and WO3-bulk calcined at 500°C exhibit initial spikes in the photocurrent upon illumination (related with the capacitance component at the solid–liquid interface), followed by a photocatalytic current, as shown in Figure 6. The photocurrent density of WO3-DDA at 1 min was 0.37 mA cm-2, which is 2.5 times higher than that of the WO3-bulk (0.15 mA cm-2 at 1 min) electrode. The charge amount passed during 1-h photoelectrocatalysis for WO3-DDA (0.89 C) was 3.9 times higher than that of WO3-bulk (0.23 C). As a consequence of the high charge amount, the markedly high amount (nO2 = 1.5 μmol, Faradaic efficiency (FEO2) = 65%) of O2 evolved for the WO3-DDA electrode compared to that (nO2 = 0.4 μmol, FEO2 = 58%) of the WO3-bulk electrode, as summarized in Table 2. As compared with performances of PEC water oxidation under the same conditions for WO3-based photoanodes reported earlier [3], the performance of the present mesoporous WO3-DDA is lower than that of the small mesoporous WO3 film (nO2 = 4.2 μmol, FEO2 = 79%) [3], but much higher than that of interparticle mesoporous WO3 (nO2 = 0.9 μmol, FEO2 = 61%) and bulk WO3 (nO2 = 0.4 μmol, FEO2 = 44%) [3]. The high performance of the mesoporous WO3-DDA photoanode is attributed to its high surface-to-volume ratio which offers a large number of water oxidation sites at the electrolyte-WO3 interface, and well-connected WO3 particles for efficient electron transport through the film.
Figure 6.
Photocurrent density versus time profiles during PEC water oxidation using samples calcined at 500°C. (a) ITO/WO3-DDA and (b) ITO/WO3-bulk electrodes in 0.1 M phosphate buffer solution of pH = 6.0 at 0.5 V versus Ag/AgCl with visible light irradiation (λ > 390 nm, 100 mW cm-2).
Table 2.
Summary of photoelectrocatalytic water oxidation at different ITO/WO 3 photoanodes calcined at 500°C in 0.1 M phosphate solution
| Sample name | Charge (C) | n O2 (μmol) | FE O2 a (%) | n H2 b (μmol) | FE H2 c (%) |
|---|---|---|---|---|---|
| WO3-DDA |
0.89 |
1.49 |
65.2 |
4.46 |
97.4 |
| WO3-bulk | 0.23 | 0.35 | 58.1 | 0.89 | 74.3 |
aFaradic efficiency of O2 evolution. bnH2 is the amount of H2 evolved in the Pt counter electrode compartment. cFaradic efficiency of H2 evolution.
Conclusions
We have prepared mesoporous WO3 materials by a unique and facile solvothermal method using solid H2WO4 as a tungsten precursor. DDA was used as a template for the formation of nanostructure, which generates mesoporosity after removing DDA by calcination. The present surfactant template technique is very unique in terms of use of a solid tungsten precursor in a solvothermal method, compared with a common technique using liquid tungsten precursors for interaction with surfactants in principle. The mesoporous network has a disordered arrangement of pores which is composed of well-connected tiny spherical WO3 particles with a diameter of ca. 5 to 20 nm. The DDA-templated WO3 photoanode showed three times higher photoanodic current density upon visible light irradiation and provided the efficient performance of PEC water oxidation compared to the untemplated WO3, which is promising as an efficient material for high-performance solar energy conversion.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LD prepared the samples and performed the photoelectrochemical measurements. DC carried out the analysis and optimization of the results and drafted the manuscript. KS and TY helped analyze the results. MY supervised the data analysis and interpretation of the results and helped draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Dong Li, Email: f12k503f@mail.cc.niigata-u.ac.jp.
Debraj Chandra, Email: debraj.chandra@gs.niigata-u.ac.jp.
Kenji Saito, Email: ksaito@eng.niigata-u.ac.jp.
Tatsuto Yui, Email: yui.t@eng.niigata-u.ac.jp.
Masayuki Yagi, Email: yagi@eng.niigata-u.ac.jp.
Acknowledgements
This work was partially supported by the JST PRESTO program and Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology (No. 24350028). DC thanks JSPS for providing postdoctoral fellowship.
References
- Kamat PV, Tvrdy K, Baker DR, Radich JG. Beyond photovoltaics: semiconductor nanoarchitectures for liquid-junction solar cells. Chem Rev. 2010;110:6664. doi: 10.1021/cr100243p. [DOI] [PubMed] [Google Scholar]
- Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev. 2009;38:253. doi: 10.1039/b800489g. [DOI] [PubMed] [Google Scholar]
- Chandra D, Saito K, Yui T, Yagi M. Crystallization of tungsten trioxide having small mesopores: highly efficient photoanode for visible-light-driven water oxidation. Angew Chem Int Ed. 2013;52:12606. doi: 10.1002/anie.201306004. [DOI] [PubMed] [Google Scholar]
- Kim HG, Borse PH, Jang JS, Ahn CW, Jeong ED, Lee JS. Engineered nanorod perovskite film photocatalysts to harvest visible light. Adv Mater. 2011;23:2088. doi: 10.1002/adma.201004171. [DOI] [PubMed] [Google Scholar]
- Zukalová M, Zukal A, Kavan L, Nazeeruddin MK, Liska P, Grätzel M. Organized mesoporous TiO2 films exhibiting greatly enhanced performance in dye-sensitized solar cells. Nano Lett. 2005;5:1789. doi: 10.1021/nl051401l. [DOI] [PubMed] [Google Scholar]
- Kim J, Koh JK, Kim B, Kim JH, Kim E. Nanopatterning of mesoporous inorganic oxide films for efficient light harvesting of dye-sensitized solar cells. Angew Chem Int Ed. 2012;51:6864. doi: 10.1002/anie.201202428. [DOI] [PubMed] [Google Scholar]
- Gratzel M. Photoelectrochemical cells. Nature. 2001;414:338. doi: 10.1038/35104607. [DOI] [PubMed] [Google Scholar]
- Fujishima A, Honda K. TiO2 photoelectrochemistry and photcatalysis. Nature. 1972;238:37. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Aprile C, Corma A, Garcia H. Enhancement of the photocatalytic activity of TiO2 through spatial structuring and particle size control: from subnanometric to submillimetric length scale. Phys Chem Chem Phys. 2008;10:769. doi: 10.1039/b712168g. [DOI] [PubMed] [Google Scholar]
- Ng KH, Minggu LJ, Kassim MB. Gallium-doped tungsten trioxide thin film photelectrodes for photoelectrochemical water splitting. Int J Hydrogen Energy. 2013;38:9585. doi: 10.1016/j.ijhydene.2013.02.144. [DOI] [Google Scholar]
- Kim JK, Shin K, Cho SM, Lee T-W, Park JH. Synthesis of transparent mesoporous tungsten trioxide films with enhanced photoelectrochemical response: application to unassisted solar water splitting. Energy Environ Sci. 2011;4:1465. doi: 10.1039/c0ee00469c. [DOI] [Google Scholar]
- Chatchai P, Murakami Y, Kishioka S-y, Nosaka AY, Nosaka Y. Efficient photocatalytic activity of water oxidation over WO3/BiVO4 composite under visible light irradiation. Electrochim Acta. 2009;54:1147. doi: 10.1016/j.electacta.2008.08.058. [DOI] [Google Scholar]
- Hisatomi T, Dotan H, Stefik M, Sivula K, Rothschild A, Grätzel M, Mathews N. Enhancement in the performance of ultrathin hematite photoanode for water splitting by an oxide underlayer. Adv Mater. 2012;24:2699. doi: 10.1002/adma.201104868. [DOI] [PubMed] [Google Scholar]
- Satsangi VR, Kumari S, Singh AP, Shrivastav R, Dass S. Nanostructured hematite for photoelectrochemical generation of hydrogen. Int J Hydrogen Energy. 2008;33:312. doi: 10.1016/j.ijhydene.2007.07.034. [DOI] [Google Scholar]
- Rahman G, Joo O-S. Photoelectrochemical water splitting at nanostructured α-Fe2O3 electrodes. Int J Hydrogen Energy. 2012;37:13989. doi: 10.1016/j.ijhydene.2012.07.037. [DOI] [Google Scholar]
- Li Y, Takata T, Cha D, Takanabe K, Minegishi T, Kubota J, Domen K. Vertically aligned Ta3N5 nanorod arrays for solar-driven photoelectrochemical water splitting. Adv Mater. 2013;25:125. doi: 10.1002/adma.201202582. [DOI] [PubMed] [Google Scholar]
- Maeda K, Higashi M, Lu D, Abe R, Domen K. Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst. J Am Chem Soc. 2010;132:5858. doi: 10.1021/ja1009025. [DOI] [PubMed] [Google Scholar]
- Maeda K, Domen K. Water oxidation using a particulate BaZrO3-BaTaO2N solid-solution photocatalyst that operates under a wide range of visible light. Angew Chem Int Ed. 2012;51:9865. doi: 10.1002/anie.201204635. [DOI] [PubMed] [Google Scholar]
- Abe T, Nagai K, Kabutomori S, Kaneko M, Tajiri A, Norimatsu T. An organic photoelectrode working in the water phase: visible-light-induced dioxygen evolution by a perylene derivative/cobalt phthalocyanine bilayer. Angew Chem Int Ed. 2006;45:2778. doi: 10.1002/anie.200504454. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhang Y, Drabarek E, Barnes PRF, Luca V. Enhanced photoelectrochemical activity of sol–gel tungsten trioxide films through textural control. Chem Mater. 2007;19:5664. doi: 10.1021/cm071603d. [DOI] [Google Scholar]
- Seabold JA, Choi K-S. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Chem Mater. 2011;23:1105. doi: 10.1021/cm1019469. [DOI] [Google Scholar]
- Yagi M, Maruyama S, Sone K, Nagai K, Norimatsu T. Preparation and photoelectrocatalytic activity of a nano-structured WO3 platelet film. J Solid State Chem. 2008;181:175. doi: 10.1016/j.jssc.2007.11.018. [DOI] [Google Scholar]
- Miseki Y, Kusama H, Sugihara H, Sayama K. WO3 photocatalyst showing efficient solar energy conversion for O2 production and Fe (III) ion reduction under visible light. J Phys Chem Lett. 2010;1:1196. doi: 10.1021/jz100233w. [DOI] [Google Scholar]
- Miseki Y, Fujiyoshi S, Gunji T, Sayama K. Photocatalytic water splitting under visible light utilizing I3-/I- and IO3-/I- redox mediators by Z-scheme system using surface treated PtOx/WO3 as O2 evolution photocatalyst. Catal Sci Tech. 2013;3:1750. doi: 10.1039/c3cy00055a. [DOI] [Google Scholar]
- Corma A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev. 1997;97:2373. doi: 10.1021/cr960406n. [DOI] [PubMed] [Google Scholar]
- Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710. doi: 10.1038/359710a0. [DOI] [Google Scholar]
- Chandra D, Bekki M, Nakamura M, Sonezaki S, Ohji T, Kato K, Kimura T. Dye-sensitized biosystem sensing using macroporous semiconducting metal oxide films. J Mater Chem. 2011;21:5738. doi: 10.1039/c0jm04347h. [DOI] [Google Scholar]
- Davis ME. Ordered porous materials for emerging applications. Nature. 2002;417:813. doi: 10.1038/nature00785. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Boissière C, Grosso D, Laberty C, Nicole L. Design, synthesis, and properties of inorganic and hybrid thin films having periodically organized nanoporosity. Chem Mater. 2008;20:682. doi: 10.1021/cm702100t. [DOI] [Google Scholar]
- Santato C, Ulmann M, Augustynski J. Photoelectrochemical properties of nanostructured tungsten trioxide films. J Phys Chem B. 2001;105:936. doi: 10.1021/jp002232q. [DOI] [Google Scholar]
- Santato C, Ulmann M, Augustynski J. Enhanced visible light conversion efficiency using nanocrystalline WO3 films. Adv Mater. 2001;13:511. doi: 10.1002/1521-4095(200104)13:7<511::AID-ADMA511>3.0.CO;2-W. [DOI] [Google Scholar]
- Santato C, Odziemkowski M, Ulmann M, Augustynski J. Crystallographically oriented mesoporous WO3 films: synthesis, characterization, and application. J Am Chem Soc. 2001;123:10639. doi: 10.1021/ja011315x. [DOI] [PubMed] [Google Scholar]
- Berger S, Tsuchiya H, Ghicov A, Schmuki P. High photocurrent conversion efficiency in self-organized porous WO3. Appl Phys Lett. 2006;88:203119. doi: 10.1063/1.2206696. [DOI] [Google Scholar]
- Colton RJ, Guzman AM, Rabalais JW. Electrochromism in some thin-film transition-metal oxides characterized by x-ray electron spectroscopy. J Appl Phys. 1978;49:409. doi: 10.1063/1.324349. [DOI] [Google Scholar]
- Yous B, Robin S, Donnadieu A, Dufour G, Maillot C, Roulet H, Senemaud C. Chemical vapor deposition of tungsten oxides: A comparative study by X-ray photoelectron spectroscopy, X-ray diffraction and reflection high energy electron diffraction. Mater Res Bull. 1984;19:1349. doi: 10.1016/0025-5408(84)90199-5. [DOI] [Google Scholar]
- Sivakumar R, Moses Ezhil Raj A, Subramanian B, Jayachandran M, Trivedi DC, Sanjeeviraja C. Preparation and characterization of spray deposited n-type WO3 thin films for electrochromic devices. Mater Res Bull. 2004;39:1479. doi: 10.1016/j.materresbull.2004.04.023. [DOI] [Google Scholar]
- Saha D, Jensen KMØ, Tyrsted C, Bøjesen ED, Mamakhel AH, Dippel A-C, Christensen M, Iversen BB. In situ total X-ray scattering study of WO3 nanoparticle formation under hydrothermal conditions. Angew Chem Int Ed. 2014;53:3667. doi: 10.1002/anie.201311254. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang D, Li M, Shi J, Li C. Photoelectrochemical water oxidation on photoanodes fabricated with hexagonal nanoflower and nanoblock WO3. Nanoscale. 2014;6:2061. doi: 10.1039/c3nr05601e. [DOI] [PubMed] [Google Scholar]
- Zeng W, Li Y, Zhang H. Hierarchical WO3 porous microspheres and their sensing properties. J Mater Sci: Mater Electron. 2014;25:1512. doi: 10.1007/s10854-014-1761-1. [DOI] [Google Scholar]
- Katsumata H, Inoue K, Suzuki T, Kaneco S. Facile synthesis of WO3 nanorod thin film on W substrate with enhance photocatalytic performance. Catal Lett. 2014;144:837. doi: 10.1007/s10562-014-1194-8. [DOI] [Google Scholar]
- Brezesinski T, Fattakhova Rohlfing D, Sallard S, Antonietti M, Smarsly BM. Highly crystalline WO3 thin films with ordered 3D mesoporosity and improved electrochromic performance. Small. 2006;2:1203. doi: 10.1002/smll.200600176. [DOI] [PubMed] [Google Scholar]
- Sadakane M, Sasaki K, Kunioku H, Ohtani B, Ueda W, Abe R. Preparation of nano-structured crystalline tungsten(vi) oxide and enhanced photocatalytic activity for decomposition of organic compounds under visible light irradiation. Chem Commun. 2008. p. 6552. [DOI] [PubMed]
- Chandra D, Yokoi T, Tatsumi T, Bhaumik A. Highly luminescent organic–inorganic hybrid mesoporous silicas containing tunable chemosensor inside the pore wall. Chem Mater. 2007;19:5347. doi: 10.1021/cm701918t. [DOI] [Google Scholar]
- Grosman A, Ortega C. Capillary condensation in porous materials. Hysteresis and interaction mechanism without pore blocking/percolation process. Langmuir. 2008;24:3977. doi: 10.1021/la703978v. [DOI] [PubMed] [Google Scholar]





