Abstract
In this study, we apply the concept of “ambiguity,” as developed in the decision theory literature, to an analysis of potential psychological consequences of uncertainty about cancer prevention recommendations. We used Health Information National Trends Survey (HINTS) 2003 data to examine how perceived ambiguity about cancer prevention recommendations relates to three other cognitive variables known to influence cancer-protective behavior: perceived cancer preventability, perceived cancer risk, and cancer-related worry. Using logistic regression analyses, we tested several predictions derived from a review of literature on the effects of ambiguity perceptions on decision making, cognitions, and emotions. We found perceived ambiguity to have a strong negative relationship with perceived cancer preventability, consistent with “ambiguity aversion”—a pessimistic bias in the interpretation of ambiguity. Cancer worry moderated this relationship; ambiguity aversion increased with higher levels of worry. At the same time, perceived ambiguity was positively related to both perceived cancer risk and cancer worry. Furthermore, perceived risk partially mediated the relationship between perceived ambiguity and worry. These findings suggest that perceived ambiguity about cancer prevention recommendations may have broad and important effects on other health cognitions. We discuss ethical implications of these findings for health communication efforts, and propose a tentative causal model to guide future research.
What is at issue might be called the ambiguity of this information, a quality depending on the amount, type, reliability, and “unanimity” of information, and giving rise to one’s degree of “confidence” in an estimate of relative likelihood …. “Ambiguity” may be high … even where there is ample quantity of information, when there are questions of reliability and relevance of information, and particularly where there is conflicting opinion and evidence.
In his seminal paper that engendered an important body of research in decision theory, Ellsberg (1961) employed the term “ambiguity” to describe an under-studied factor influencing judgment and decision making about risky alternatives: uncertainty about the risk information at hand. This was a phenomenon distinct from the basic uncertainty inherent to all decisions involving risk and arising from the indeterminacy of future outcomes. “Ambiguity” represented a different type of uncertainty, not about decisional outcomes, but about the “reliability, credibility, or adequacy” of one’s information concerning the likelihood of these outcomes. This notion of ambiguity has since been interpreted by others as “uncertainty about uncertainty” (Einhorn & Hogarth, 1985, 1986; Kahn & Sarin, 1988) and “epistemic unreliability” (Gardenfors & Sahlin, 1983). Ambiguity is high when risk evidence is unreliable, conflicting, or incomplete or when expert knowledge is contested (Curley & Yates, 1989; Einhorn & Hogarth, 1986; Ellsberg, 1961; Kunreuther, Meszaros, Hogarth, & Spranca, 1995), and represents an added dimension of uncertainty that influences people’s judgments of the risks and desirability of different choice outcomes. Subsequent work has confirmed these effects in a variety of decision-making settings (Camerer & Weber, 1992).
In perhaps no other contemporary setting of decision making is ambiguity more conspicuous than in the realm of cancer prevention. In recent years, scientific controversies regarding various cancer prevention and screening measures (e.g., antioxidant vitamins, tamoxifen, prostate-specific antigen [PSA] screening, mammography in women aged 40–49) have received a great deal of media attention. While such publicity may have various consequences, many experts contend that it has increased public confusion and skepticism about health recommendations (Angell & Kassirer, 1994; Dobias, Moyer, McAchran, Katz, & Sonnad, 2001; Holmes-Rovner & Charles, 2003; Schwartz & Woloshin, 2002; Taubes, 1995; Wald, 2000; Yamey & Wilkes, 2002). As sociologist Renée Fox (1980) observed, mass media has contributed to a rising consciousness of medical uncertainty in modern society, and recent coverage of debates over cancer prevention and screening recommendations may manifest this trend.
At the same time, health professionals have been increasingly encouraged to disclose to patients the ambiguities surrounding the benefits and risks of controversial cancer-protective interventions. In recent years the concepts of informed and shared decision making have been widely promoted as normative ideals for health care in general (Bekker et al., 1999; Charles, Gafni, & Whelan, 1997; Emanuel & Emanuel, 1992; Whitney, 2003) and cancer screening in particular (Briss et al., 2004; Rimer, Briss, Zeller, Chan, & Woolf, 2004). Proponents of these ideals argue that interventions such as mammography in younger women and PSA screening epitomize situations in which informed and shared decision making are critical: where evidence on the balance of benefits and harms is unclear and more than one reasonable course of action exists. In these circumstances physicians have an ethical obligation to increase patients’ awareness of scientific uncertainties and the range of available decisional options (Briss et al., 2004; Kaplan, 2004; Rimer Briss, Zeller, Chan, & Woolf, 2004). Promoting patients’ awareness of ambiguity concerning controversial cancer-protective interventions has thus become an instrumental goal in the process of informing and involving patients in decision making.
To the extent that increasing awareness of ambiguity about cancer prevention recommendations represents not only a sociohistorical trend but an ethical norm for health care, it is important to fully understand its consequences. For example, an often-cited concern among clinicians and public health advocates is that communicating ambiguity about cancer prevention and screening measures may discourage people from pursuing these services (Angela & Raffle, 2001; Austoker, 1999; Briss et al., 2004; Rimer, 1994). Supporting this concern, some researchers have demonstrated in studies that educating people about uncertainties surrounding controversial cancer screening measures decreases their interest in screening (Frosch, Kaplan, & Felitti, 2001; Jepson, Forbes, Sowden, & Lewis, 2001; Volk, Spann, Cass, & Hawley, 2003; Wolf, Nasser, & Schorling, 1996). Other studies have shown, however, that perceptions of scientific uncertainty do not influence people’s screening intentions or behaviors, and that other factors such as beliefs, attitudes, and physician recommendations play a more critical role (Farrell, Murphy, & Schneider, 2002; Nekhlyudov, Ross-Degnan, & Fletcher, 2003; Taplin, Urban, Taylor, & Savarino, 1997).
Despite questions about the influence of ambiguity perceptions upon behaviors and psychological factors related to cancer prevention and screening, very little research has been devoted to this problem. Furthermore, the construct of ambiguity concerning health recommendations has not been incorporated into existing theories of health behavior, although some theories allude to its importance (Cameron, 1997; Leventhal, Brissette, & Leventhal, 2003). Thus we also lack conceptual models to guide further research.
The 2003 HINTS provided an opportunity to explore the phenomenon of ambiguity about cancer prevention recommendations. HINTS respondents were asked their level of agreement with the statement, “There are so many different recommendations about preventing cancer, it’s hard to know which ones to follow.” Respondents also were surveyed on other cognitive variables related to cancer-protective behaviors. The objective of our study was to use HINTS data to explore the relationships between perceived ambiguity about cancer prevention recommendations and 3 other cognitive variables known to influence cancer protective behavior: perceived cancer preventability, perceived cancer risk, and cancer-related worry. Because the cross-sectional design of HINTS limits inferences about causality, our goal was to generate hypotheses and preliminary theoretical models to guide future research. Our analytic bias was to treat ambiguity as an independent variable affecting other cancer-related cognitions, and we derived predictions by reviewing research from various theoretical perspectives, which we present below. Although health behavior theories do not incorporate ambiguity as a discrete construct, a large body of research in decision psychology sheds light on the potential relationships between perceived ambiguity about cancer prevention recommendations and other health cognitions.
Ambiguity and Perceptions of Cancer Preventability and Cancer Risk
How might ambiguity about cancer prevention recommendations influence people’s perceptions of the preventability of cancer and of their personal cancer risk? Although this question has not been examined before, previous work in decision theory provides indirect but strong evidence that perceived ambiguity may decrease perceptions of cancer preventability while increasing perceptions of cancer risk.
Ellsberg’s original contribution was his discovery of “ambiguity aversion”—the propensity of decisionmakers to choose against options having estimated risks of uncertain reliability, accuracy, or precision, mathematical probabilities being otherwise equal (Ellsberg, 1961). Ambiguity aversion was demonstrated by the “Ellsberg paradox,” a classic thought experiment in which subjects were asked their preferences for betting between two events involving equal likelihoods of winning: (1) drawing a red (or black) ball from an urn containing exactly 50 red and 50 black balls, or (2) drawing a red (or black) ball from an urn containing 100 balls in an unknown proportion of red and black. Although the likelihood of drawing a given colored ball in each case is equivalent (P = .5), most people have been shown to prefer betting on the first event involving a certain rather than an uncertain probability. This finding was important because it represented a violation of rational decision-making axioms (specifically, Savage’s “sure-thing principle”) of subjective expected utility theory. Ellsberg’s paradox showed that people base risky decisions upon judgments about not only the probability and utility of alternative outcomes, but also about a “third dimension”: the “nature of one’s information” concerning the likelihood of these outcomes (Ellsberg, 1961). When information is ambiguous, decision-makers behave as though the likelihood associated with the ambiguous option is lower than it really is, and they favor the unambiguous option. Ambiguity aversion thus amounts to a pessimistic bias influencing people’s likelihood judgments.
Subsequent work has confirmed Ellsberg’s findings and clarified the nature of ambiguity aversion (Camerer & Weber, 1992). Refined definitions of ambiguity have been proposed, as by Frisch and Baron (1988): “Ambiguity is uncertainty about probability, created by missing information that is relevant and could be known.” They have argued that ambiguity aversion manifests decisionmakers’ use of a “missing information” heuristic that biases people against making decisions or taking action when information is incomplete. Viscusi (1997) has further characterized ambiguity aversion as an “alarmist response” to risk information, a systematic tendency of decisionmakers to “devote excessive attention to the worst case scenarios” contained in ambiguous risk information.
Ambiguity aversion has been shown to be robust, persisting even when odds favor the ambiguous option (Curley & Yates, 1985, 1989; Keren & Gerritsen, 1999), although at lower probabilities of the unambiguous option decisionmakers become indifferent to ambiguity and may even seek it (Curley & Yates, 1989; Einhorn & Hogarth, 1985; Lauriola & Levin, 2001). Ambiguity aversion also may depend on whether gains or losses are at stake (Camerer & Weber, 1992; Einhorn & Hogarth, 1986; Kahn & Sarin, 1988). With potential gains (e.g., winning money) people are ambiguity averse, while with potential losses (e.g., losing money) people are ambiguity seeking. This finding has not been consistently obtained (Keren & Gerritsen, 1999), however, and ambiguity aversion itself varies considerably among individuals. In experimental manipulations of the Ellsberg paradox, for example, a substantial minority of decisionmakers—more than 30% in some studies—exhibit either ambiguity indifference or ambiguity seeking (Camerer & Weber, 1992; Einhorn & Hogarth, 1986).
The psychological causes of ambiguity aversion are not completely clear, although several explanations have been proposed. Ambiguity aversion has been hypothesized to result from decisionmakers’ beliefs that decision outcomes are unfairly biased against them, their concerns about how decisions will be evaluated by others (Curley, Yates, & Abrams, 1986), and their preferences against betting in situations where they feel incompetent or uninformed (Heath & Tversky, 1991). Although it is unclear whether any of these theories really account for why people consider an ambiguous option as unsafe in the first place (Keren & Gerritsen, 1999; Yates & Zukowski, 1976), they help explain the nature of ambiguity aversion as a general decision-making heuristic.
Ellsberg (1961) distinguished between objective ambiguity—an inherent characteristic of the risk information at hand—and subjective perceptions of ambiguity, which vary among individuals. Perceived ambiguity has been shown to have important effects on judgment and decision making. It causes people to be less willing to take action (Curley Yates & Abrams, 1986; Frisch & Baron, 1988), and to alter their estimates of risks associated with decisional options, possibly through an “anchoring-and-adjustment” process in which decisionmakers use an initial risk estimate as an reference point, and make adjustments for ambiguity (Einhorn & Hogarth, 1985, 1986). The magnitude of adjustment is proportional to the magnitude of perceived ambiguity, and the direction—downward or upward—depends upon individual differences in attitudes toward ambiguity. Ambiguity-averse individuals adjust risk estimates downward, reflecting a pessimistic bias, while ambiguity-seeking individuals adjust upward, reflecting an optimistic bias (Camerer & Weber, 1992; Einhorn & Hogarth, 1985, 1986; Kahn & Sarin, 1988; Lauriola & Levin, 2001).
A few studies have examined the influence of perceived ambiguity in health decisions. Two studies (Ritov & Baron, 1990; Meszaros et al., 1996) showed that increasing people’s awareness of ambiguity about a vaccine’s safety made them reluctant to receive vaccination. The “ambiguity” manipulated in these studies consisted of missing information about whether subjects belonged to a “risk group” for adverse outcomes (Ritov & Baron, 1990) and scientific disagreement about adverse effects of vaccination (Meszaros et al., 1996). Viscusi and colleagues (1991, 1999) examined the influence of ambiguous information on people’s perceptions of health risks. In studies involving hypothetical risks for nerve disease and cancer, increasing the ambiguity of risk information—by altering the numeric range of estimates presented to subjects—led people to perceive greater disease risks (Viscusi, Magat, & Huber, 1991, 1999).
This limited review of the decision theory literature suggests several predictions for how perceived ambiguity about cancer recommendations might influence other cancer-related cognitions and behaviors. First, past research suggests that most people will be averse to ambiguity about cancer prevention recommendations, and may manifest this ambiguity aversion through pessimistic interpretations about the preventability of cancer—that is, lower preventability beliefs.
Second, past research also supports the prediction that ambiguity about cancer prevention recommendations will increase people’s perceived risk of cancer. Previous studies linking ambiguity and “alarmist” health risk perceptions have examined ambiguity concerning only experts’ health risk estimates—not their risk-reduction recommendations. It is conceivable, however, that ambiguity concerning cancer prevention recommendations also might lead to heightened cancer risk perceptions. This might be a direct effect to the extent that such ambiguity causes people to avoid, suspend, or postpone taking action against cancer threats—thus potentially increasing their risk. It could also represent an indirect effect of ambiguity about cancer prevention recommendations first diminishing cancer preventability beliefs—which would then logically lead to heightened cancer risk perceptions. The latter situation would predict a mediational effect of perceived cancer preventability in the relationship between perceived ambiguity and perceived cancer risk.
Ambiguity and Cancer-related Worry
How might ambiguity about cancer prevention recommendations relate to worry about cancer? A vast body of research on the psychological effects of ambiguity provides indirect evidence that perceived ambiguity will increase cancer-related worry, and that worry will also moderate people’s aversion to ambiguity. This varied work has conceptualized “ambiguity” more broadly—referring to situational uncertainty of various causes—than in the decision theory literature. Nevertheless, its findings are applicable to understanding the effects of “ambiguity” more narrowly defined.
Research on “tolerance of ambiguity” (Furnham & Ribchester, 1995), “uncertainty orientation” (Sorrentino & Roney, 2000), and “need for closure” (Kruglanski & Webster, 1996) represent separate but conceptually related areas of work that each postulate a fundamental relationship between uncertainty (including ambiguity) and affect, and highlight individual differences in people’s reactions to uncertainty. These independent lines of research similarly conceive of ambiguity as a stress-inducing phenomenon, provoking coping and affective responses that differ according to individuals’ ambiguity tolerance. Individuals with high ambiguity tolerance experience less negative affect and pursue rational adaptive measures to resolve ambiguity, while individuals with low ambiguity tolerance experience greater negative affect and avoid ambiguity. Individual differences in ambiguity tolerance or orientation have been shown to predict numerous psychological outcomes (Furnham & Ribchester, 1995; Kruglanski & Webster, 1996). Brouwers and Sorrentino (1993) studied uncertainty orientation in the context of a health threat, and found that it predicted not only individuals’ information-seeking behavior, but also their reliance upon religious coping and their adoption of fatalistic attitudes toward the threat.
Ambiguity, therefore, is thought to have predictable psychological consequences, inducing affective and coping responses that vary according to individual differences. Extrapolating these findings to the influence of perceived ambiguity about cancer recommendations upon people’s worry about cancer, one would expect ambiguity to increase cancer worry directly, proportionate to individual differences in ambiguity tolerance. Ambiguity might also increase worry indirectly, through its predicted effect of increasing perceived cancer risk (discussed previously). This mechanism would be consistent with past evidence demonstrating that perceived risk influences worry while also mediating worry-inducing effects of other cognitions (Cunningham et al., 1998; Lipkus, Klein, Skinner, & Rimer, 2005).
At the same time, worry may itself influence how people respond to ambiguous information. Related mood states such as anxiety have been shown to lead to cognitive processing biases in the interpretation of ambiguous information (Beck, Emery, & Greenberg, 1986; Lawson & MacLeod, 1999; MacLeod & Cohen, 1993). These biases are both attentional and interpretive; individuals with both trait and state anxiety attend selectively to threat-related information, while also imposing threatening or pessimistic interpretations on ambiguous information (Calvo & Castillo, 1997; Mathews & MacLeod, 1994; Hazlett-Stevens & Borkovec, 2004; MacLeod & Cohen, 1993). Negative mood states also have been shown to bias risk-related perceptions and judgments in situations that are ambiguous with respect to their certainty or controllability; fear leads to pessimistic risk estimates and risk-averse choices, while anger leads to optimistic risk estimates and risk-seeking choices (Lerner & Keltner, 2000, 2001).
Mood states also have been shown to influence individuals’ reliance upon systematic versus heuristical processing of persuasive but ambiguous communications (Bohner, Chaiken, & Hunyadi, 1994). In one study, cancer-related fear inhibited systematic processing of ambiguous information aimed at persuading individuals to undergo cancer-screening “check-ups” (Jepson & Chaiken, 1990). High-fear subjects processed these messages less carefully—being less able to detect logical errors planted within these messages—and were also more persuaded by the message than were low-fear subjects. Sad mood also has been shown to decrease systematic while increasing heuristic processing of ambiguous messages (Bohner et al., 1994).
These diverse lines of research support a common prediction that cancer worry will moderate ambiguity aversion, predisposing people to more pessimistic interpretations of ambiguity concerning cancer prevention recommendations—that is, lower preventability beliefs.
Perceived Ambiguity About Cancer Prevention Recommendations: Predictions
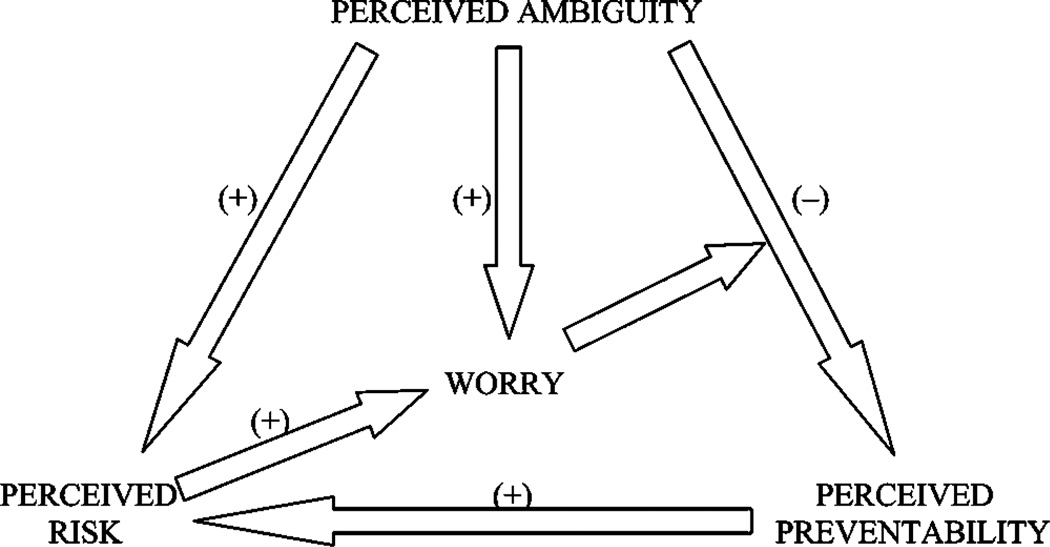
In summary, the diverse research relating to ambiguity suggests several predictions, which are outlined in Figure 1:
Perceived ambiguity about cancer prevention recommendations will be negatively associated with perceived cancer preventability and positively associated with perceived cancer risk, manifesting “ambiguity aversion.”
Cancer preventability beliefs will mediate the relationship between perceived ambiguity and perceived cancer risk.
Perceived ambiguity will be positively associated with cancer-related worry, reflecting intolerance of ambiguity.
Perceived cancer risk will mediate the relationship between perceived ambiguity and cancer-related worry.
Cancer-related worry will moderate the relationship between perceived ambiguity and perceived cancer preventability, predisposing people to lower preventability beliefs.
We used these predictions to guide our analysis of the HINTS, and explored the extent to which they were supported by the data.
Figure 1.
Predicted relationships between perceived ambiguity, perceived cancer preventability, perceived cancer risk, and cancer worry.
Methods
Data Source and Study Population
HINTS surveys a nationally representative sample of U.S. adults aged 18 and older, with oversampling of African American and Hispanic households (Nelson et al., 2004). For the 2002–2003 HINTS, interviews were completed with a total of 6,149 adults aged 18 and older; the response rate for the household screener was 55%, and the response rate for the extended interview was 62.8%. Further details about the study methods, survey design, and variables are published elsewhere (Nelson et al., 2004). We limited our analysis to the subpopulation of individuals aged 40 and older with no prior history of cancer (N = 3,375), with the rationale that it is this group for which cancer prevention and screening recommendations are most salient and potentially controversial.
Data Collection
Primary Outcome Variables
The outcome variables for our analyses were assessed by three survey items coded on Likert scales. Perceived preventability of cancer was assessed by the statement, “There is not much people can do to lower their chances of getting cancer.” Response options were “strongly agree,” “somewhat agree,” “somewhat disagree,” “strongly disagree,” and “no opinion.” Perceived cancer risk was assessed by the question, “How likely do you think it is that you will develop cancer in the future? Would you say your chance of getting cancer is…?” Response options were “very low,” “somewhat low,” “moderate,” “somewhat high,” and “very high.” Cancer-related worry was assessed by the question, “How often do you worry about cancer? Would you say…?” Response options were “rarely or never,” “sometimes,” “often,” and “all the time.”
Perceived Ambiguity
The primary independent variable for all analyses was perceived ambiguity about cancer prevention recommendations. This construct was assessed by the statement, “There are so many different recommendations about preventing cancer, it’s hard to know which ones to follow.” Likert scale responses were “strongly agree,” “some-what agree,” “somewhat disagree,” “strongly disagree,” and “no opinion.”
Sociodemographic Variables
Sociodemographic factors were treated as covariates in all analyses, and included age, gender, race, and education level. These factors have been shown to be associated with both health cognitions—e.g., perceived risk and preventability beliefs—as well as health-protective behaviors such as cancer screening; the potential for confounding effects justified controlling for these variables (Demark-Wahnefried et al., 1995; Nijs, Essink-Bot, DeKoning, Kirkels, & Schroder, 2000; Wolf et al., 1996; Wolf, Philbrick, & Schorling, 1997). Because income was previously found to be highly correlated with education level, it was not included in subsequent analyses in order to avoid potential problems with multicollinearity.
Data Analysis
To adjust for the complex sampling design of the HINTS (Nelson et al., 2004), we used the statistical program SUDAAN in all analyses (Shah, Barnwell, & Bieler, 1997).
We excluded individuals with “not ascertained,” “no opinion,” “don’t know,” or “refused” responses to any of the main survey items examined; this amounted to 271 (7.9%), 313 (9.3%), 271 (8.0%), and 9 (0.2%) respondents to the perceived ambiguity, perceived cancer preventability, perceived cancer risk, and cancer-related worry items, respectively.
Logistic regression was performed to examine the relationships between perceived ambiguity and each of the primary outcome variables. Outcome variables having four-category response scales (perceived cancer preventability, cancer worry) were dichotomized, with the two lower and two higher response categories, respectively, comprising the “low” and “high” values of the dichotomized variable; binary logistic regression then was used to analyze the association of these variables with perceived ambiguity, which was similarly dichotomized. The perceived risk variable used a five-category response scale and was therefore not dichotomized, but was analyzed using ordinal logistic regression. All analyses were adjusted for sociodemographic variables. We explored predicted interactions and mediating relationships between perceived ambiguity and the primary outcome variables.
Results
Distributions and U.S. population-weighted percentages for the sociodemographic and cognitive variables examined in this study are shown in Table 1. Most respondents were less than age 60, White, and reported high school or greater education. Seventy-nine percent of respondents reported a high level of perceived ambiguity about cancer recommendations. Nevertheless, 70% of respondents still reported high cancer preventability beliefs. The vast majority of respondents reported low perceptions of vulnerability to cancer; 16% reported “somewhat high” or “very high” estimates of personal cancer risk, and only 8% reported worrying about cancer “often” or “all the time.”
Table 1.
Distribution and weighted percentages of characteristics of HINTS respondents aged 40 and older with no personal history of cancer (2002–2003 HINTS)
| N* | %† | |
|---|---|---|
| Sociodemographic variables | ||
| Age | 3350 | |
| 40–49 | 1190 | 39.0 |
| 50–59 | 917 | 27.1 |
| 60–69 | 627 | 18.1 |
| 70+ | 616 | 15.8 |
| Education level | 3255 | |
| Less than high school | 409 | 17.0 |
| High school graduate | 995 | 33.0 |
| Some college | 843 | 24.5 |
| College graduate | 1008 | 25.5 |
| Race | 3115 | |
| White | 2539 | 82.9 |
| Black | 394 | 10.9 |
| Other | 182 | 6.2 |
| Gender | 3375 | |
| Female | 2053 | 51.6 |
| Male | 1322 | 48.4 |
| Cognitive variables | ||
| Perceived ambiguity‡ | 3104 | |
| Low | 686 | 21.2 |
| High | 2418 | 78.8 |
| Perceived preventability‡ | 3062 | |
| Low | 929 | 30.1 |
| High | 2133 | 69.9 |
| Perceived cancer risk | 3104 | |
| Very low | 715 | 22.5 |
| Somewhat low | 777 | 24.2 |
| Moderate | 1128 | 37.3 |
| Somewhat high | 361 | 11.5 |
| Very high | 123 | 4.5 |
| Cancer-related worry‡ | 3366 | |
| Low | 3110 | 92.4 |
| High | 256 | 7.6 |
Total sample N = 3,375; decreased and unequal n for individual variables due to excluded and missing data.
Percentages weighted to the 2003 U.S. census.
Four-category Likert responses collapsed to binary categories “low” and “high”.
Relationships Between Perceived Ambiguity and Perceived Preventability of Cancer
Main Effects
A strong negative relationship (OR .29, 95% CI: .22–.40, p < .01) was found between perceived ambiguity and perceived preventability of cancer, controlling for sociodemographic variables (Table 2). As predicted and consistent with the phenomenon of ambiguity aversion, higher levels of perceived ambiguity were associated with lower preventability beliefs, indicating a pessimistic bias in the interpretation of ambiguity. Significant associations also were found between preventability beliefs and age (p < .01), race (p = .02), and education level (p < .01), with older age and “other” race predicting lower perceived preventability, and higher education associated with higher perceived preventability.
Table 2.
Multivariate logistic regression models of the relationship between perceived ambiguity and perceived cancer preventability, perceived cancer risk, and cancer worry (2002–2003 HINTS)*
| Model 1 (n = 2,688) |
Model 2 (n = 2,710) |
Model 3 (n = 2,877) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Perceived cancer preventability |
Perceived cancer risk† |
Cancer-related worry |
|||||||
| Variables | OR | 95% C.I. | PV‡ | OR | 95% C.I. | PV‡ | OR | 95% C.I. | PV‡ |
| Age | .00 | .00 | .01 | ||||||
| 40–49 | 1.00 | 1.00 | 1.00 | ||||||
| 50–59 | 0.71 | 0.54–0.94 | 1.23 | 0.95–1.60 | 1.32 | 0.88–1.97 | |||
| 60–69 | 0.55 | 0.40–0.77 | 0.85 | 0.67–1.06 | 1.04 | 0.63–1.72 | |||
| 70+ | 0.54 | 0.38–0.78 | 0.53 | 0.40–0.70 | 0.38 | 0.19–0.75 | |||
| Education level | .00 | .03 | .00 | ||||||
| Less than high school | 1.00 | 1.00 | 1.00 | ||||||
| High school graduate | 1.65 | 1.12–2.44 | 0.98 | 0.66–1.44 | 1.24 | 0.71–2.15 | |||
| Some college | 2.75 | 1.70–4.45 | 0.97 | 0.70–1.35 | 0.91 | 0.48–1.72 | |||
| College graduate | 4.01 | 2.79–5.75 | 0.75 | 0.55–1.03 | 0.48 | 0.26–0.88 | |||
| Race | .02 | .56 | .23 | ||||||
| White | 1.00 | 1.00 | 1.00 | ||||||
| Black | 0.76 | 0.51–1.11 | 0.81 | 0.54–1.21 | 0.97 | 0.57–1.62 | |||
| Other | 0.46 | 0.25–0.84 | 0.93 | 0.60–1.47 | 1.67 | 0.91–3.05 | |||
| Gender | .99 | .36 | .04 | ||||||
| Female | 1.00 | 1.00 | 1.00 | ||||||
| Male | 1.00 | 0.81–1.24 | 0.91 | 0.74–1.12 | 0.64 | 0.41–0.99 | |||
| Perceived ambiguity | .00 | .00 | .04 | ||||||
| Low | 1.00 | 1.00 | 1.00 | ||||||
| High | 0.29 | 0.22–0.40 | 1.46 | 1.18–1.80 | 1.53 | 1.01–2.32 | |||
Total sample N = 3,375; decreased and unequal n for individual models due to excluded and missing data.
Analysis using ordinal regression.
P value for Wald chi-square test of association.
Interactions
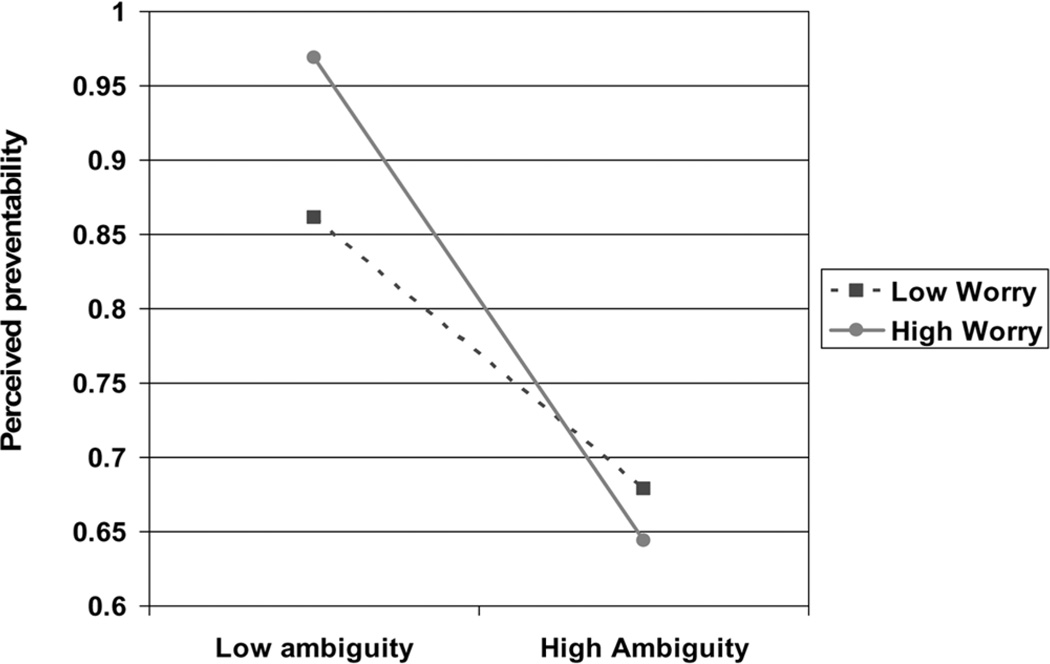
Consistent with predictions, a significant interaction (p < .01) was found between perceived ambiguity and worry in their relationship to perceived cancer preventability (Figure 2). Higher levels of worry were associated with stronger negative relationships between perceived ambiguity and perceived preventability, consistent with the hypothesis that worry predisposes individuals to greater ambiguity aversion—that is, more pessimistic interpretations of ambiguity.
Figure 2.
Interaction between perceived ambiguity and cancer worry in relation to perceived cancer preventability, adjusted for sociodemographic covariates (p < .00)
Relationships Between Perceived Ambiguity and Perceived Cancer Risk
Main Effects
A positive relationship (OR 1.46, 95% CI: 1.18–1.80, p < .01) was found between perceived ambiguity and perceived cancer risk, controlling for sociodemographic variables (Table 2). As predicted, higher levels of perceived ambiguity were associated with higher levels of perceived cancer risk. A significant negative association was found between perceived risk and both age (p < .01) and education (p = .03), with greater age and higher education being associated with lower perceived risk.
Mediational Effects
We predicted that perceived preventability of cancer would mediate the relationship between perceived ambiguity and perceived risk. Mediational analysis, however, failed to support this prediction. Significant associations were found between both perceived ambiguity and perceived cancer preventability (p < .01) and perceived cancer preventability and perceived risk (p = .02). The association between perceived ambiguity and perceived risk (p < .01), however, remained significant when controlling for perceived preventability, suggesting that any potential mediating effect of this variable was not significant (Baron & Kenny, 1986).
Relationships Between Perceived Ambiguity and Cancer-related Worry
Main Effects
A positive relationship (OR 1.53, 95% CI: 1.01–2.32, p = .04) was found between perceived ambiguity and cancer-related worry, controlling for sociodemographic variables (Table 2). Consistent with predictions, higher levels of perceived ambiguity were associated with greater worry. Significant negative associations were found between cancer-related worry and age (p = .01), gender (p = .04), and education level (p < .01), with greater age, male gender, and higher education level all being associated with lower worry.
Mediational Effects
Using Baron and Kenny’s procedure for testing mediation (Baron & Kenny, 1986), we obtained evidence consistent with the prediction that perceived ambiguity would influence worry not only directly but indirectly, through its effects upon risk perceptions. In separate logistic regression models, we first confirmed significant associations between perceived ambiguity and worry (p = .04), perceived ambiguity and perceived risk (p < .01), and perceived risk and worry (p < .01). Next, we regressed worry on perceived ambiguity while controlling for perceived risk; this reduced the previously significant association between perceived ambiguity and worry to nonsignificance (p = .20), consistent with a mediational effect of perceived risk. The mediational effect was modest; the odds ratio decreased from 1.53 (95% C.I., 1.01–2.32) to 1.36 (95% C.I., 0.84–2.21), suggesting only partial mediation. Supporting the hypothesized causal direction of this effect, however, we also found no evidence that worry mediated the relationship between perceived ambiguity and perceived risk. The association between perceived ambiguity and perceived risk remained significant (p < .01) even when controlling for worry.
Discussion
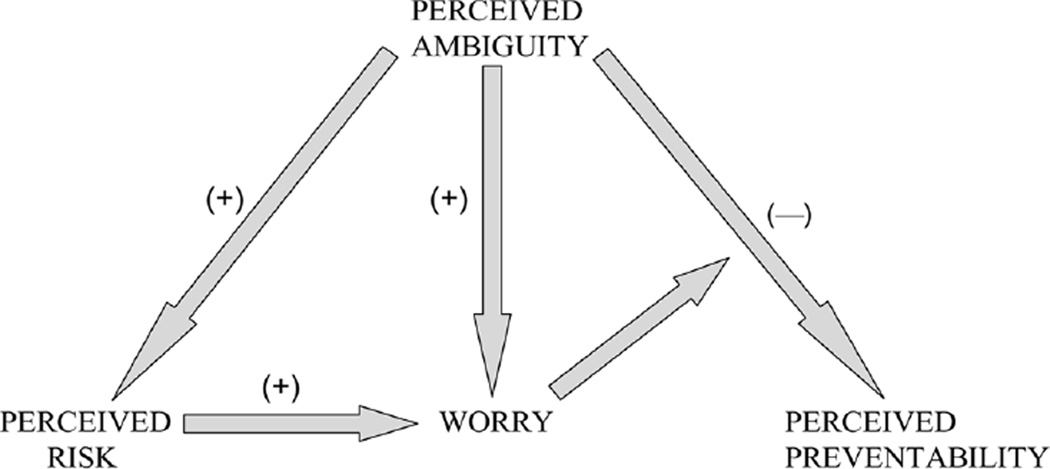
In this nationally representative cross-sectional survey, we found important relationships between perceived ambiguity about cancer prevention recommendations and other cancer-related cognitions and emotions (Figure 3). Perceived ambiguity was negatively related to perceived preventability and positively related to both perceived risk and worry. Important effect-modifying relationships were present among these variables. Worry moderated the extent of ambiguity aversion—that is, the negative relationship between perceived ambiguity and preventability. At the same time, perceived risk partially mediated the potential effect of perceived ambiguity on worry.
Figure 3.
Tentative model of relationships among perceived ambiguity, perceived cancer preventability, perceived cancer risk, and cancer worry.
To our knowledge, this study is the first to explicitly operationalize perceived ambiguity about cancer prevention recommendations, while exploring its relationships with other health cognitions. Taplin and colleagues (1997) examined perceptions of scientific conflict over mammography recommendations, and found these perceptions to be unrelated to past mammography use in women 50 years and older. They also found that of women who perceived conflicting recommendations, only a minority reported feeling confused or believing that mammography is not important. Relationships with other health cognitions were not examined, however, and the study’s retrospective design limited its conclusions.
Clearly, methodological issues impose limitations upon our study as well. The relatively low response rate for the HINTS reflects an unfortunate trend seen in many random digit dialing surveys (de Leeuw & de Heer, 2002; Goyder, Warriner, & Miller, 2002), and may have biased our findings. We believe, however, that any such bias would most likely have led to an underestimation of the associations we found, because the study sample probably over-represents individuals who are aware of health issues and therefore are more capable of processing ambiguous health messages. Furthermore, the effect of the low response rate may have been partially mitigated by the population weighting procedures used for the HINTS sample.
Additional methodological limitations are introduced by the small sample sizes for some response categories of the cognitive variables—for example, higher levels of perceived risk and worry. Furthermore, subjects with undecided responses on the perceived ambiguity item (N = 271) were excluded; however, because undecided responses may reflect high ambiguity perceptions, excluding such responses might have biased our findings. In fact, this subgroup of respondents did demonstrate a degree of ambiguity aversion similar to that of respondents with the highest levels of perceived ambiguity (OR .35, 95% CI: .18–.67) for association between perceived ambiguity and preventability. Additional analyses categorizing undecided respondents with the high perceived ambiguity group, however, demonstrated no significant changes in any of the relationships examined.
We also cannot rule out other measurement artifacts—for example, the influence of survey question order upon participants’ responses. Because the perceived ambiguity item was asked after the items used to measure the other cognitive constructs (perceived cancer preventability, risk, and worry), however, we believe that order effects are a less likely explanation for the relationships found between these constructs.
Another methodological concern is that the perceived ambiguity construct and the constructs of perceived cancer preventability, risk, and worry were each measured by single items with unknown reliability and validity. This may limit the strength of our conclusions; however, cognitions such as risk perceptions have been shown to be highly reliable over time (Shepperd, Helweg-Larsen, & Ortega, 2003), and single-item measures of constructs such as cancer risk and cancer worry have been shown to be strong predictors of behavioral outcomes such as cancer screening (Diefenbach, Miller, & Daly, 1999; Lipkus, Iden, Terrenoire, & Feaganes, 1999; Stefanek & Wilcox, 1991).
Most importantly, the cross-sectional design of HINTS prevents us from establishing causal directions for the significant relationships identified. While we cannot confirm our causal hypotheses, however, they are consistent with a large body of past research on ambiguity. Thus we believe our study provides a plausible and promising framework for future research.
A number of questions remain to be addressed. Above all, causal directions need to be confirmed through longitudinal and experimental studies. Although the proposed relationships are consistent with past research, alternative hypotheses are plausible. For example, both cancer risk perceptions and worry might influence perceptions of ambiguity about cancer prevention recommendations—rather than vice versa—through processes of “motivated reasoning” (Kunda, 1990; Liberman & Chaiken, 1992). People who feel more vulnerable to cancer might process cancer prevention messages defensively, searching for flaws—that is, magnifying their ambiguity—in order to refute its undesirable implications (Croyle, Sun, & Louie, 1993). Such alternative explanations cannot be excluded, and may point to coexisting bidirectional causal processes. Indeed, the modest effect sizes (odds ratios < 2) observed among perceived ambiguity, risk, and worry suggest the existence of intervening pathways that remain to be elucidated.
The significance of the associations between sociodemographic characteristics and health cognitions (Table 1) is also unclear. Various sociocultural variables have been associated with cognitive factors such as cancer risk perceptions and beliefs about the benefits of cancer prevention and screening measures (Demark-Wahnefried et al., 1995; Myers et al., 1996; Wardle, McCaffery, Nadel, & Atkin, 2004; Wolf et al., 1997). These relationships were beyond the scope of the present study, but they warrant further examination.
It also remains unclear how significant ambiguity aversion really is in judgment and decision making about cancer prevention and control. Limited studies of mammography (Nekhlyudov et al., 2003; Taplin et al., 1997; Woloshin et al., 2000) and PSA screening (Farrell et al., 2002) have suggested that perceived professional conflict about screening recommendations has little effect on people’s beliefs about the good of screening. Ambiguity aversion may vary in magnitude or importance depending on both individual psychological differences and situational factors (Kahn & Sarin, 1988; Kruglanski & Webster, 1996), which may overpower the effect of ambiguity perceptions on specific health cognitions and behaviors. For example, the influence of mass media and lay advocacy groups (Katz et al., 2004; Schwartz, Woloshin, Fowler, & Welch, 2004; Yamey & Wilkes, 2002) and of cultural values regarding the good of cancer prevention and screening (Farrell et al., 2002; Schwartz & Woloshin, 2002) may legitimate screening sufficiently to make decisionmakers resistant to the ambiguity surrounding these interventions. Other factors specific to recommended interventions may also moderate the importance of ambiguity aversion. Whether the intervention is for cancer screening versus cancer prevention, for example, may influence people’s responses to ambiguity surrounding these interventions (Schwartz & Woloshin, 2002). These factors require further exploration.
Finally, ambiguity perceptions need to be understood in terms of their effects upon not only health cognitions, but also health-related behaviors. Further research is needed to examine how perceived ambiguity about cancer prevention and screening recommendations influences cancer-protective behaviors. Perceived ambiguity may ultimately need to be incorporated as a discrete construct in health behavior theories.
While our study raises intriguing questions for future research, we believe it also has important implications for health communication efforts. Although increasing people’s awareness of ambiguity may be a morally justifiable endeavor, the provision of ambiguous information is not necessarily a benign undertaking. Fischhoff (1987) has cautioned that “treating people with information about hazardous aspects of their lives can mean taking significant gambles with their welfare” (p. 14). Our study shows how perceptions of ambiguity about cancer prevention recommendations may have broad complex effects on other cognitions tied to people’s sense of well-being, decreasing people’s beliefs in the preventability of cancer, while increasing their sense of vulnerability to cancer. This effect may be strongest for people who worry the most about cancer—and who also show greater reliance upon heuristical reasoning and increased susceptibility to persuasion (Bohner et al., 1994; Jepson & Chaiken, 1990).
We need to know how best to communicate ambiguity in cancer prevention and screening. A 55-year-old man contemplating PSA screening, for example, could be presented with a broad array of ambiguous information: varying recommendations of professional organizations, widespread physician support for screening despite the lack of direct evidence that PSA screening saves lives (Cooper, Merritt, Ross, John, & Jorgensen, 2004), and various estimates—accompanied by wide confidence intervals—of the potential harms of screening (Harris & Lohr, 2002). For cases like this, our study suggests the need for caution in designing interventions aimed at promoting informed decision making and educating the public. Communicating ambiguity may influence people in unintended and undesirable ways. In some people, it may foster a sense of pessimism, resignation, or helplessness. The end result may be to dissuade people from undertaking potentially beneficial interventions, or to make them paradoxically more dependent upon professional recommendations—contrary to the intent of the informed decision-making ideal. Communicating ambiguity may also increase feelings of vulnerability in some people, thereby decreasing their capacity for thoughtful deliberation while increasing their susceptibility to persuasion and manipulation. In these ways and perhaps more, communicating ambiguity may ultimately undermine—rather than promote—informed decision making and patient autonomy and well-being.
The communication of ambiguity is an increasingly important and problematic task in cancer prevention and control, and in health care generally. Our study provides preliminary evidence that ambiguity may have potentially important effects, and suggests the need for further research to elucidate these effects.
Acknowledgments
We thank Kevin McCaul, Wendy Nelson, Ellen Peters, Whitney Randolph Steele, and Michael Stefanek for helpful input at various stages of this project.
Contributor Information
Paul K. J. Han, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland, USA
Richard P. Moser, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland, USA
William M. P. Klein, Department of Psychology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
References
- Angela E, Raffle F. Information about screening—is it to achieve high uptake or to ensure informed choice? Health Expect. 2001;4(2):92–98. doi: 10.1046/j.1369-6513.2001.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell M, Kassirer JP. Clinical research—what should the public believe? N Engl J Med. 1994;331:189–190. doi: 10.1056/NEJM199407213310309. [DOI] [PubMed] [Google Scholar]
- Austoker J. Gaining informed consent for screening. Is difficult—but many misconceptions need to be undone. BMJ. 1999;319(7212):722–723. doi: 10.1136/bmj.319.7212.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G, Greenberg RC. Anxiety disorders and phobias: A cognitive perspective. New York: Guilford Press; 1986. [Google Scholar]
- Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al. Informed decision making: An annotated bibliography and systematic review. Health Technol Assess. 1999;3(1):1–156. [PubMed] [Google Scholar]
- Bohner G, Chaiken S, Hunyadi P. The role of mood and message ambiguity in the interplay of heuristic and systematic processing. European Journal of Social Psychology. 1994;24:207–221. [Google Scholar]
- Briss P, Rimer B, Reilley B, Coates RC, Lee NC, Mullen P, et al. Promoting informed decisions about cancer screening in communities and healthcare systems. Am J Prev Med. 2004;26(1):67–80. doi: 10.1016/j.amepre.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Brouwers MC, Sorrentino RM. Uncertainty orientation and protection motivation theory: The role of individual differences in health compliance. Journal of Personality and Social Psychology. 1993;65(1):102–112. [Google Scholar]
- Calvo MG, Castillo MD. Mood-congruent bias in interpretation of ambiguity: strategic processes and temporary activation. Quarterly Journal of Experimental Psychology. 1997;50A(1):163–182. [Google Scholar]
- Camerer C, Weber M. Recent developments in modeling Preferences uncertainty and ambiguity. Journal of Risk and Uncertainty. 1992;5:325–370. [Google Scholar]
- Cameron LD. Screening for cancer: Illness perceptions and illness worry. In: Petrie KJ, Weinman JA, editors. Perceptions of health and illness: Current research and applications. Amsterdam: Harwood; 1997. pp. 291–322. [Google Scholar]
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (Or it takes at least two to tango) Soc Sci Med. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- Cooper CP, Merritt TL, Ross LE, John LV, Jorgensen CM. To screen or not to screen, when clinical guidelines disagree: Primary care physicians’ use of the PSA test. Prev Med. 2004;38(2004):182–191. doi: 10.1016/j.ypmed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Croyle RT, Sun YC, Louie DH. Psychological minimization of cholesterol test results: moderators of appraisal in college students and community residents. Health Psychol. 1993;12(6):503–507. doi: 10.1037//0278-6133.12.6.503. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Andrykowski MA, Wilson JF, McGrath PC, Sloan DA, Kenady DE. Physical symptoms, distress, and breast cancer risk perceptions in women with benign breast problems. Health Psychol. 1998;17(4):371–375. doi: 10.1037//0278-6133.17.4.371. [DOI] [PubMed] [Google Scholar]
- Curley SP, Yates JF. The center and range of the probability interval as factors affecting ambiguity preferences. Organizational Behavior and Human Decision Processes. 1985;36:273–287. [Google Scholar]
- Curley SP, Yates JF. An empirical evaluation of descriptive models of ambiguity reactions in choice situations. Journal of Mathematical Psychology. 1989;33:397–427. [Google Scholar]
- Curley SP, Yates JF, Abrams RA. Psychological sources of ambiguity avoidance. Organizational Behavior and Human Decision Processes. 1986;38:230–256. [Google Scholar]
- de Leeuw E, de Heer W. Trends in household survey nonresponse: A longitudinal and international comparison. In: Groves DADRM, Eltinge JL, Little RJA, editors. Survey nonresponse. New York: John Wiley; 2002. pp. 121–134. [Google Scholar]
- Demark-Wahnefried W, Strigo T, Catoe K, Conaway M, Brunetti M, Rimer BK, et al. Knowledge, beliefs, and prior screening behavior among blacks and whites reporting for prostate cancer screening. Urology. 1995;46(3):346–351. doi: 10.1016/S0090-4295(99)80218-0. [DOI] [PubMed] [Google Scholar]
- Diefenbach MA, Miller SM, Daly MB. Specific worry about breast cancer predicts mammography use in women at risk for breast and ovarian cancer. Health Psychol. 1999;18(5):532–536. doi: 10.1037//0278-6133.18.5.532. [DOI] [PubMed] [Google Scholar]
- Dobias KS, Moyer CA, McAchran SE, Katz SJ, Sonnad SS. Mammography messages in popular media: Implications for patient expectations and shared clinical decision-making. Health Expect. 2001;4:131–139. doi: 10.1046/j.1369-6513.2001.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn HJ, Hogarth RM. Ambiguity and uncertainty in probabilistic inference. Psychological Review. 1985;92(4):433–461. [Google Scholar]
- Einhorn HJ, Hogarth RM. Decision making under ambiguity. Journal of Business. 1986;59(4):S225–S250. [Google Scholar]
- Ellsberg D. Risk, ambiguity, and the savage axioms. Quart J Econ. 1961;75:643–669. [Google Scholar]
- Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. JAMA. 1992;267(16):2221–2226. [PubMed] [Google Scholar]
- Farrell MH, Murphy MA, Schneider CE. How underlying patient beliefs can affect physician-patient communication about prostate-specific antigen testing. Effective Clinical Practice. 2002;5(3):120–129. [PubMed] [Google Scholar]
- Fischhoff B. Treating the public with risk communications: A public health perspective. Science, Technology, and Human Values. 1987;12(3&4):13–19. [PubMed] [Google Scholar]
- Fox RC. The evolution of medical uncertainty. Milbank Mem Fund Q Health Soc. 1980;58(1):1–49. [PubMed] [Google Scholar]
- Frisch D, Baron J. Ambiguity and rationality. Journal of Behavioral Decision Making. 1988;1:149–157. [Google Scholar]
- Frosch DL, Kaplan RM, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med. 2001;16(6):391–398. doi: 10.1046/j.1525-1497.2001.016006391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnham A, Ribchester T. Tolerance of ambiguity: A review of the concept, its measurement and applications. Current Psychology. 1995;14(3):179–199. [Google Scholar]
- Gardenfors P, Sahlin N. Decision making with unreliable probabilities. British Journal of Mathematical and Statistical Psychology. 1983;36:240–251. [Google Scholar]
- Goyder J, Warriner K, Miller S. Evaluating socio-economic status (SES) bias in survey nonresponse. Journal of Official Statistics. 2002;18:1–12. [Google Scholar]
- Harris R, Lohr KN. Screening for prostate cancer: An update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(11):917–929. doi: 10.7326/0003-4819-137-11-200212030-00014. [DOI] [PubMed] [Google Scholar]
- Hazlett-Stevens H, Borkovec TD. Interpretive cues and ambiguity in generalized anxiety disorder. Behav Res Ther. 2004;42(8):881–892. doi: 10.1016/S0005-7967(03)00204-3. [DOI] [PubMed] [Google Scholar]
- Heath C, Tversky A. Preference and belief: Ambiguity and competence in choice under uncertainty. Journal of Risk and Uncertainty. 1991;4:5–28. [Google Scholar]
- Holmes-Rovner M, Charles S. The mammography screening controversy: Who and what is heard in the press? Patient Educ Couns. 2003;51(1):75–81. doi: 10.1016/s0738-3991(02)00167-2. [DOI] [PubMed] [Google Scholar]
- Jepson C, Chaiken S. Chronic issue-specific fear inhibits systematic processing of persuasive communications. Journal of Social Behavior and Personality. 1990;5(2):61–84. [Google Scholar]
- Jepson RG, Forbes CA, Sowden AJ, Lewis RA. Increasing informed uptake and non-uptake of screening: Evidence from a systematic review. Health Expect. 2001;4(2):116–126. doi: 10.1046/j.1369-6513.2001.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BE, Sarin RK. Modeling ambiguity in decisions under uncertainty. Journal of Consumer Research. 1988;15(2):265–272. [Google Scholar]
- Kaplan RM. Shared medical decisionmaking. A new tool for preventive medicine. Am J Prev Med. 2004;26(1):81–83. doi: 10.1016/j.amepre.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Katz ML, Sheridan S, Pignone M, Lewis C, Battle J, Gollop C, et al. Prostate and colon cancer screening messages in popular magazines. J Gen Intern Med. 2004;19(8):843–848. doi: 10.1111/j.1525-1497.2004.30504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren G, Gerritsen LEM. On the robustness and possible accounts of ambiguity aversion. Acta Psychologica. 1999;103:149–172. [Google Scholar]
- Kruglanski AW, Webster DM. Motivated closing of the mind: “Seizing” and “freezing”. Psychological Review. 1996;103(2):263–283. doi: 10.1037/0033-295x.103.2.263. [DOI] [PubMed] [Google Scholar]
- Kunda Z. The case for motivated reasoning. Psychological Bulletin. 1990;108:480–498. doi: 10.1037/0033-2909.108.3.480. [DOI] [PubMed] [Google Scholar]
- Kunreuther H, Meszaros J, Hogarth RM, Spranca M. Ambiguity and underwriter decision processes. Journal of Economic Behavior and Organization. 1995;26:337–352. [Google Scholar]
- Lauriola M, Levin IP. Relating individual differences in attitude toward ambiguity to risky choices. Journal of Behavioral Decision Making. 2001;14(2):107–122. [Google Scholar]
- Lawson C, MacLeod C. Depression and the interpretation of ambiguity. Behav Res Ther. 1999;37(5):463–474. doi: 10.1016/s0005-7967(98)00131-4. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Keltner D. Beyond valence: Toward a model of emotion-specific influences on judgement and choice. Cognition and Emotion. 2000;14(4):473–493. [Google Scholar]
- Lerner JS, Keltner D. Fear, anger, and risk. J Pers Soc Psychol. 2001;81(1):146–159. doi: 10.1037//0022-3514.81.1.146. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. London: Routledge; 2003. pp. 42–65. [Google Scholar]
- Liberman A, Chaiken S. Defensive processing of personally relevant health messages. Personality and Social Psychology Bulletin. 1992;18(6):669–679. [Google Scholar]
- Lipkus IM, Iden D, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(6):533–539. [PubMed] [Google Scholar]
- Lipkus IM, Klein WMP, Skinner CS, Rimer BK. Breast cancer risk perceptions and breast cancer worry: What predicts what? Journal of Risk Research. 2005;8(5):439–452. [Google Scholar]
- MacLeod C, Cohen IL. Anxiety and the interpretation of ambiguity: A text comprehension study. J Abnorm Psychol. 1993;102(2):238–247. doi: 10.1037//0021-843x.102.2.238. [DOI] [PubMed] [Google Scholar]
- Mathews AM, MacLeod C. Cognitive approaches to emotions and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Meszaros JR, Asch DA, Baron J, Hershey JC, Kunreuther H, Schwartz-Buzaglo J. Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. J Clin Epidemiol. 1996;49(6):697–703. doi: 10.1016/0895-4356(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Myers RE, Wolf TA, McKee L, McGrory G, Burgh DY, Nelson G, et al. Factors associated with intention to undergo annual prostate cancer screening among African American men in Philadelphia. Cancer. 1996;78(3):471–479. doi: 10.1002/(SICI)1097-0142(19960801)78:3<471::AID-CNCR14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Nekhlyudov L, Ross-Degnan D, Fletcher SW. Beliefs and expectations of women under 50 years old regarding screening mammography: A qualitative study. J Gen Intern Med. 2003;18(3):182–189. doi: 10.1046/j.1525-1497.2003.20112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Kreps GL, Hesse BW, Croyle RT, Willis G, Arora NK, et al. The Health Information National Trends Survey (HINTS): Development, design, and dissemination. J Health Commun. 2004;9(5):443–460. doi: 10.1080/10810730490504233. discussion 481-444. [DOI] [PubMed] [Google Scholar]
- Nijs HG, Essink-Bot ML, DeKoning HJ, Kirkels WJ, Schroder FH. Why do men refuse or attend population-based screening for prostate cancer? J Public Health Med. 2000;22(3):312–316. doi: 10.1093/pubmed/22.3.312. [DOI] [PubMed] [Google Scholar]
- Rimer BK. Interventions to increase breast screening. Lifespan and ethnicity issues. Cancer. 1994;74(1 Suppl):323–328. doi: 10.1002/cncr.2820741317. [DOI] [PubMed] [Google Scholar]
- Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: What is its role in cancer screening? Cancer. 2004;101(Suppl. 5):1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- Ritov I, Baron J. Reluctance to vaccinate: Omission bias and ambiguity. Journal of Behavioral Decision Making. 1990;3:263–277. [Google Scholar]
- Schwartz LM, Woloshin S. News media coverage of screening mammography for women in their 40s and tamoxifen for primary prevention of breast cancer. JAMA. 2002;287(23):3136–3142. doi: 10.1001/jama.287.23.3136. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Woloshin S, Fowler FJ, Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- Shah B, Barnwell B, Bieler G. SUDAAN (Version 8.0.2) [Computer Software] Research Triangle Park, NC: Research Triangle Institute; 1997. [Google Scholar]
- Shepperd JA, Helweg-Larsen M, Ortega L. Are comparative risk judgments stable across time and events? Personality and Social Psychology Bulletin. 2003;29(9):1169–1180. doi: 10.1177/0146167203254598. [DOI] [PubMed] [Google Scholar]
- Sorrentino RM, Roney CJR. The uncertain mind: Individual differences in facing the unknown. Philadelphia: Taylor & Francis; 2000. [Google Scholar]
- Stefanek ME, Wilcox P. First degree relatives of breast cancer patients: screening practices and provision of risk information. Cancer Detect Prev. 1991;15(5):379–384. [PubMed] [Google Scholar]
- Taplin SH, Urban N, Taylor VM, Savarino J. Conflicting national recommendations and the use of screening mammography: does the physician’s recommendation matter? J Am Board Fam Pract. 1997;10(2):88–95. [PubMed] [Google Scholar]
- Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169. doi: 10.1126/science.7618077. [DOI] [PubMed] [Google Scholar]
- Viscusi WK. Alarmist decisions with divergent risk information. The Economic Journal. 1997;107:1657–1670. [Google Scholar]
- Viscusi WK, Magat WA, Huber J. Communication of ambiguous risk information. Theory and Decision. 1991;31:159–173. [Google Scholar]
- Viscusi WK, Magat WA, Huber J. Smoking status and public responses to ambiguous scientific risk evidence. Southern Economic Journal. 1999;66(2):250–270. [Google Scholar]
- Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: A randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1(1):22–28. doi: 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald N. Populist instead of professional. J Med Screen. 2000;7(1):1. doi: 10.1136/jms.7.1.1. [DOI] [PubMed] [Google Scholar]
- Wardle J, McCaffery K, Nadel M, Atkin W. Socioeconomic differences in cancer screening participation: Comparing cognitive and psychosocial explanations. Soc Sci Med. 2004;59(2):249–261. doi: 10.1016/j.socscimed.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Whitney SN. A new model of medical decisions: Exploring the limits of shared decision making. Med Decis Making. 2003;23(4):275–280. doi: 10.1177/0272989X03256006. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Nasser JF, Schorling JB. The impact of informed consent on patient interest in prostate-specific antigen screening. Arch Intern Med. 1996;156(12):1333–1336. [PubMed] [Google Scholar]
- Wolf AM, Philbrick JT, Schorling JB. Predictors of interest in prostate-specific antigen screening and the impact of informed consent: What should we tell our patients? Am J Med. 1997;103(4):308–314. doi: 10.1016/s0002-9343(97)00155-1. [DOI] [PubMed] [Google Scholar]
- Woloshin S, Schwartz LM, Byram SJ, Sox HC, Fischhoff B, Welch HG. Women’s understanding of the mammography screening debate. Arch Intern Med. 2000;160(10):1434–1440. doi: 10.1001/archinte.160.10.1434. [DOI] [PubMed] [Google Scholar]
- Yamey G, Wilkes M. The PSA storm. BMJ. 2002;324(7334):431. [Google Scholar]
- Yates JF, Zukowski LG. Characterization of ambiguity in decision making. Behavioral Science. 1976;21:19–25. [Google Scholar]