Abstract
Emotional experiences can strengthen memories so that they can be used to guide future behavior. Emotional arousal, mediated by the amygdala, is thought to modulate storage by the hippocampus, which may encode unique episodic memories via pattern separation – the process by which similar memories are stored using non-overlapping representations. While prior work has examined mnemonic interference due to similarity and emotional modulation of memory independently, examining the mechanisms by which emotion influences mnemonic interference has not been previously accomplished in humans. To this end, we developed an emotional memory task where emotional content and stimulus similarity were varied to examine the effect of emotion on fine mnemonic discrimination (a putative behavioral correlate of hippocampal pattern separation). When tested immediately after encoding, discrimination was reduced for similar emotional items compared to similar neutral items, consistent with a reduced bias towards pattern separation. After 24 h, recognition of emotional target items was preserved compared to neutral items, whereas similar emotional item discrimination was further diminished. This suggests a potential mechanism for the emotional modulation of memory with a selective remembering of gist, as well as a selective forgetting of detail, indicating an emotion-induced reduction in pattern separation. This can potentially increase the effective signal-to-noise ratio in any given situation to promote survival. Furthermore, we found that individuals with depressive symptoms hyper-discriminate negative items, which correlated with their symptom severity. This suggests that utilizing mnemonic discrimination paradigms allows us to tease apart the nuances of disorders with aberrant emotional mnemonic processing.
Keywords: Emotion, Amygdala, Memory, Discrimination, Hippocampus, Interference, Pattern separation
1. Introduction
Emotions color our memories and can afford them a special status that preserves them from loss and forgetting. This relationship is thought to be adaptive; the association of memories with positive or negative affect allows them to more successfully guide future action. While studies in animals have reliably demonstrated a facilitatory effect of arousal on memory consolidation (McGaugh, 2004), studies in humans suggest a more complicated picture. Flashbulb memories (memories for the circumstances in which one heard about a newsworthy event) initially suggested that memory was better for emotional events and their context compared to neutral events (Brown & Kulik, 1977). These memories were thought to be vivid due to inclusion of many peripheral details. However, Heuer and Reisberg (1990) showed that while emotion leads to vivid recollections, these recollections are not completely accurate and may result from post hoc reconstructions of the emotional event. Thus, the hallmark of an emotional memory may be the subjective vividness with which it is remembered rather than the accuracy with which it is retained (Kensinger, 2009).
More recently, several studies have suggested that emotion's effects on memory are asymmetrical, such that emotional modulation of memory for the gist is enhanced, while memory for details is impaired (Kensinger, 2009; Loftus, Loftus, & Messo, 1987; Mather & Sutherland, 2011). Such selectivity suggests an emotion-induced memory trade-off, where individuals remember the central emotional content of an experience but often forget the details (Buchanan & Adolphs, 2002). An example of this phenomenon is the “weapon focus” effect, where eye-witnesses often recall the weapon used in a crime with great detail but fail to encode (or perhaps more quickly forget) peripheral details such as the perpetrator's clothing (Loftus et al., 1987). Typically these effects are most robust after a delay, where emotional arousal can influence the consolidation of information (McGaugh, 2004). However, several studies have shown that explicit memory for emotional stimuli can be enhanced even when tested immediately, which suggests that emotion affects both encoding and consolidation mechanisms (Hamann, 2001).
The effect of emotional arousal on memory is thought to be mediated by the influence of the amygdala on hippocampal processing (McGaugh, 2004). The amygdala is promiscuous in influencing the consolidation of memory for many kinds of motivationally arousing experiences, either appetitive (i.e. positive) or aversive (i.e. negative) (McGaugh, 2002, 2004). This is thought to occur through the amygdala's ability to modulate hippocampal representations. The hippocampus is known to play a critical role in the encoding and storage of episodic memories (Squire, 1992). While there are several levels at which hippocampal functions can be described, many computational models have ascribed particular computational functions to subregions of the hippocampus in service of episodic memory. David Marr (1971) first suggested that the recurrent collaterals in the hippocampal CA3 enabled this region to act as an auto-associative network capable of pattern completion (the process by which previously stored representations are retrieved when presented with partial or degraded cues). In contrast, upstream of the CA3, the dentate gyrus (DG) uses a sparse firing pattern among its granule cells, which allows the region to perform pattern separation – the process of reducing interference among similar inputs by using non-overlapping representations (McClelland, McNaughton, & O'Reilly, 1995; O'Reilly and Norman, 2002; Shapiro & Olton, 1994; Treves & Rolls, 1994; Yassa & Stark, 2011).
While the effect of amygdala-mediated emotional arousal on hippocampal episodic memory has long been observed both in animal and human studies (Dolcos, LaBar, & Cabeza, 2004; McGaugh, 2004), the exact mechanism for this effect has remained quite elusive. The computational descriptions of hippocampal function offer a potential mechanistic account by which information storage may be modulated (i.e. either by enhancing pattern separation or pattern completion). Using this framework, we can reframe the question “How does emotion enhance gist memory and weaken detail memory?” into a more directed mechanistic hypothesis: “Does emotional arousal facilitate or impede pattern separation?”
While we cannot directly make inferences about the computational process, we make the assumption that pattern separation will have a behavioral consequence, namely mnemonic discrimination among similar stimuli. This logic has been previously applied to many studies examining hippocampal function (Hunsaker & Kesner, 2013; Yassa & Stark, 2011). In a recent study, we examined the effect of emotional arousal on discrimination (Segal, Stark, Kattan, Stark, & Yassa, 2012) and demonstrated that increased emotional arousal (measured using salivary alpha amylase, a biomarker for endogenous peripheral noradrenergic activation) was correlated with enhanced mnemonic discrimination for similar neutral objects. These findings suggest that emotion may modulate mnemonic discrimination abilities when interference is high (i.e. when test items are similar to study items), which is thought to rely on hippocampal pattern separation (Yassa & Stark, 2011). However, this prior study only evaluated emotion as a pre-study state effect (i.e. a state of increased arousal could have enhanced attention or increased vigilance) and not on a trial-by-trial basis (thereby losing stimulus specificity). In addition, the study only investigated the immediate effects of emotional arousal on memory, whereas many studies of emotional memory have tested participants after a delay to allow for consolidation to occur.
In the current study, we systematically examined the effect of emotional modulation on individual stimuli (transient effects) using a paradigm where both emotional content (negative, neutral, positive) and stimulus similarity (high and low) were varied in a parametric fashion, and testing was conducted both immediately and after a 24-h delay. Given the wealth of recent empirical data in favor of the role of the hippocampus in reducing mnemonic interference (Yassa & Stark, 2011), the current investigation offers an alternative conceptual framework by which to examine the impact of emotion on hippocampal computations. We hypothesized that emotional targets (i.e. repeated items) would be better remembered while emotional lures (i.e. similar but not identical items) would be more difficult to discriminate when tested immediately and that this effect would be exaggerated after a 24-h delay. Stimulus similarity was manipulated to create highly interfering test stimuli, with the high and low similarity conditions expected to alter performance on the task, where highly similar items should be more difficult to discriminate than low similarity items. We did not have any strong behavioral hypotheses for whether these similarity conditions would vary with emotion.
We also tested the utility of this approach in examining individuals with depressive symptoms. Depression is a neuropsychiatric phenotype involving a recognized disturbance in emotional memory processing. Many studies of major depressive disorder (MDD) have documented general deficits in episodic memory (Airaksinen, Wahlin, Forsell, & Larsson, 2007; Airaksinen, Wahlin, Larsson, & Forsell, 2006; Dere, Pause, & Pietrowsky, 2010). In addition, depressed individuals tend to better remember negative items compared to neutral or positive items (Gordon, Barnett, Cooper, Tran, & Williams, 2008; Haas & Canli, 2008; Hasler, Drevets, Manji, & Charney, 2004; Watkins, Martin, & Stern, 2000; Watkins, Vache, Verney, Muller, & Mathews, 1996). We examined these behavioral aberrations using our emotional discrimination task in a sample of individuals exhibiting depressive symptoms. Our results shed new light on how emotion affects mnemonic computations and how these processes may be affected in depression.
2. Materials and Methods
2.1. Participants
Participants were recruited from Johns Hopkins University and received either course credit or monetary remuneration for their participation for the primary experiments. In the immediate condition, 24 participants were tested (all mean age ± SD, 21 ± 3, 16 female). In the 24-h delayed condition, 14 participants were tested (20 ± 2, 6 female). In the depressive symptom condition, 15 participants were tested (22 ± 4, 11 female). Participants with depressive symptoms (DS) were recruited through local campus announcements and posted flyers. Supplementary experiments required additional participants (demographics listed in respective sections). Informed consent was obtained from all participants, with all procedures approved by the Johns Hopkins University Institutional Review Board.
2.2. Inclusion/exclusion criteria
All participants were screened against self-reported major medical, psychiatric, and substance use comorbidity. Participants did not receive a diagnostic psychiatric evaluation as part of this study. The Beck Depression Inventory-II was given to all participants. Assignment to healthy versus depressive symptom group was based on BDI-II cutoff (BDI-II < 7 = healthy group, BDI-II > 15 = depressive symptom group). These cutoff criteria were based on the BDI-II symptom severity scale in which 16 is the cutoff for a mild mood disturbance (scores above 16 are suggestive of clinical depression; the higher the score indicates greater severity of depressive symptoms) (Watkins et al., 2000). Participants with depressive symptoms were medication-free. All participants had normal or corrected to normal vision.
2.3. Valence, arousal, and similarity measures
An independent sample (N = 50, all means ± SD, 22 ± 5, 32 female) rated the images for emotional valence on a scale 1-9 (1 being the most negative, 9 being the most positive, and 5 being neutral). Images rated 1–3.5 were called Negative, images rated 3.6–6 were called Neutral, and images rated 6.1–9 were called Positive [all Tukey HSD P <.001; Fig. S1a]. Another sample (N= 16, 23 ± 5, 4 female) rated the images for emotional arousal on a scale 1–9 (1 being the least arousing, 9 being the most arousing). Negative and positive images received higher arousal ratings than neutral, although negative images were more arousing than positive images [all Tukey HSD P < .001; Fig. S1b]. A third sample (N= 17, 20 ± 1, 11 female) was used to examine relative similarity of negative, neutral, and positive images as measured by number of false alarms/total responses in a separate sample. Here, we found a significant difference between the two similarity bins (t(98) =−4.68, P <.001) when stimuli were collapsed across all emotional categories as well as within each emotional category [Negative t(31) = −2.74, P = .01; Neutral t(30) = −2.77, P =.01; Positive, t(33) = −2.51, P = .02; Fig. S1c].
While we had assigned a priori similarity classes to these items (high similarity vs. low similarity) we determined that our assignment was valid by collecting subjective similarity ratings on pairs of stimuli presented side-by-side in a fourth sample (N = 31, 19 ± 1, 21 female). We compared subjective ratings on the two classes of stimuli using a two-sample t-test and found a significant difference between the two similarity bins (t(97) = −4.95, P <.001) when stimuli were collapsed across all emotional categories as well as within each emotional category (Negative t(31) = −3.88, P = .001; Neutral t(30)= −2.10, P = .04; Positive, t(32) = −3.01, P = .01). Together, these experiments show that high and low similarity stimuli were both perceived subjectively by participants as such and influenced behavioral responses in the predicted manner.
2.4. Emotional discrimination task
The stimulus set was comprised of novel scenes freely available online, sized to a width of 600 pixels. As described above, images were rated for emotional valence, arousal, and similarity in orthogonal experiments with separate samples. The experimental paradigm consisted of 149 images during the encoding phase (divided roughly equally between negative, neutral, and positive) and 291 images during the retrieval phase (divided roughly equally between negative, neutral, and positive stimuli). Targets, lures, and foils were roughly evenly distributed across emotion and similarity level during retrieval. Lures were divided into 50% high similarity and 50% low similarity.
An Apple iMac equipped with MATLAB (Version R2010a, Natick, MA) software and PsychToolbox version 3.0 was used to present the stimuli and record keyboard responses. Each trial consisted of 2 displays: an image display and a fixation display. During both encoding and retrieval phases, images were presented on the center of the screen with a black background for 2500 ms. The fixation display consisted of a white fixation cross on the center of the screen with a black background for 500 ms.
Participants underwent an incidental encoding phase where they were shown emotional and non-emotional images, presented in randomized order, and were asked to rate the images for emotional valence using a 1–9 scale (1 being most negative, 9 being most positive, and 5 being neutral). Participants were told to spread their responses across the scale. Participants were given a subsequent surprise memory test either immediately after encoding or 24 h later, in which they saw another series of stimuli, some of which were seen once before in the incidental task (targets), some were similar to ones seen in the incidental task but not identical (lures), and some were new (foils) (Fig. 1). Some of the lures were very similar (high similarity) to the original images and some were less similar (low similarity) classified as such based on supplementary studies in a separate sample (Fig. S1c). Participants were asked to indicate whether items were “old” or “new” by button responses on the keyboard. Participants were explicitly told that in order for an image to be called “old,” it had to be the same image they saw before.
Fig. 1.
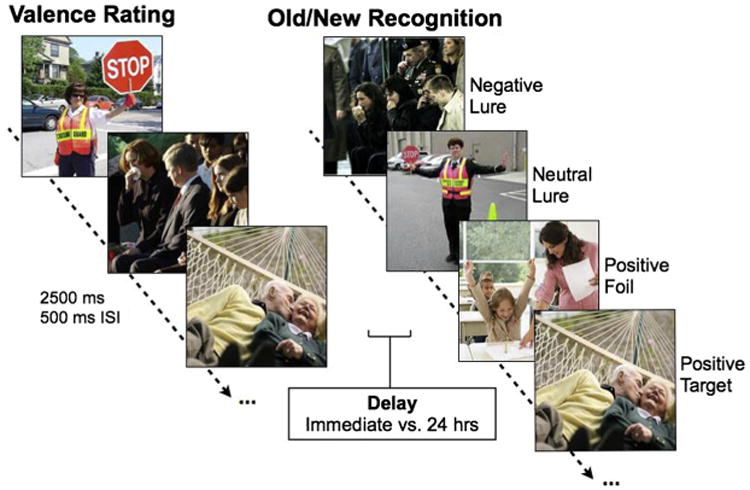
Emotional discrimination task design. During encoding, participants rated images according to their emotional valence from 1 (most negative) to 9 (most positive). Each image was presented for 2500 ms with a 500 ms inter-stimulus-interval (ISI). Either immediately after study or after a 24-h delay, participants underwent a surprise recognition test where they viewed negative, neutral, and positive targets, foils, and lures varying in similarity and were asked to indicate whether items were “old” or “new”.
Our two key outcome measures of interest were target recognition and lure discrimination index (LDI). Target recognition was measured by a discriminability index (d′), which was calculated as z(Hits) – z(False Alarms). Hits and false alarms refer to correct recognition of old items and false recognition of new items, respectively. D′ is calculated as the difference of z-transformed values. In order to measure how well participants discriminated similar items (lures), we examined performance using a bias-corrected LDI operationalized as p(‘New’|Lure) – p(‘New’|Target). This corrected for the general tendency to reject (i.e. call an item ‘New’) and is similar to other metrics we used in prior work (Yassa, Mattfeld, Stark, & Stark, 2011; Yassa et al., 2010a, 2010b).
2.5. Match-to-sample control task
A separate sample of 37 participants was used for this control task. Two conditions were tested: 2500 ms presentation time and 1000 ms presentation time (2500 ms: N = 18, 22 ± 3, 12 female, 1000 ms: N = 19, 21 ± 3, 13 female). The match-to-sample task consisted of trials that were composed of 4 sequential displays: first image, a pixelated noise mask display, a second image, and an inter-trial fixation display. Images were identical to those used in the discrimination experiments. Participants were told to determine whether the two images were exactly the same or different. Yoked images were either identical (repetitions) or similar (lures).
Each participant was tested in a single testing room in which the experimenter familiarized all participants with the task by providing oral and written instructions. Each trial began with the presentation of an image for 2500 ms or 1000 ms followed by the pixelated screen for 1000 ms (same across both presentation times), and then the second image for the same time as presented initially. This was followed by the fixation display (500 ms). Participants were told to respond while the second image was presented. Responses were recorded by keyboard press.
3. Results
3.1. Reduced discrimination of similar emotional items immediately after encoding
We assessed two key measures of performance. The first was target recognition (responding “old” to a previously viewed item), which is thought to assess gist knowledge or general familiarity (Norman, 2010; Yonelinas, Aly, Wang, & Koen, 2010). From a computational perspective, this process requires pattern completion but not pattern separation (Kim & Yassa, 2013; Yassa & Stark, 2011b). The second measure was lure discrimination (responding “new” to a previously unseen similar lure), which is thought to assess detail knowledge or specific recollection (Norman, 2010; Yonelinas et al., 2010). From a computational perspective, this process requires pattern completion as well as pattern separation (Kim & Yassa, 2013; Yassa & Stark, 2011b).
To assess the effect of emotion on target recognition, we used a repeated-measures one-way ANOVA (negative, neutral, and positive), which revealed that d′ differed significantly among positive, negative, and neutral stimuli [F(2, 46) = 6.35, P < .01]. Post-hoc contrasts showed that d′ was enhanced in negative and neutral compared to positive stimuli [F(1, 46) = 22.52, P < .01, critical Sche-ffé = 6.40; Fig. 2a]. To assess the effect of emotion on overall discrimination of similar items (i.e. lures collapsed across high and low similarity), we conducted another repeated measures ANOVA, which revealed that the lure discrimination index (LDI: see methods for how this response bias-corrected value is calculated) differed significantly among negative, neutral, and positive stimuli [F(2, 46) = 9.65, P <.001]. Post-hoc contrasts revealed that LDI was diminished for emotional stimuli (both positive and negative) compared to neutral stimuli [F(1, 46) = 18.20, P <.001, critical Scheffé = 6.40] (Fig. 2b). In order to assess the potential interaction between emotion and interference (i.e. lure similarity), we conducted a 2 × 2 ANOVA, which revealed a significant effect of emotion [F(2, 46) = 9.81, P < .001] as well as a significant effect of similarity level in which low similarity lures were easier to discriminate compared to high similarity lures [F(1, 23) = 70.8, P < .001] (Fig. S2a). Since there were no interactions with similarity, we decided to collapse across high and low similarity for all analyses (Fig. S2).
Fig. 2.
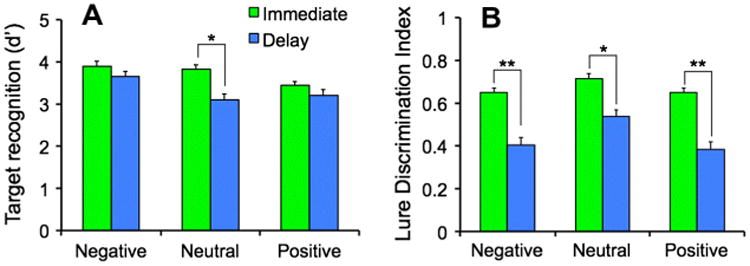
Performance in immediate and 24-h delayed testing (A) Target recognition (d′) was significantly better in negative and neutral compared to positive images in the immediate condition (N = 24).Overall, d′ was worse after 24 h, however, the difference was larger in the neutral images than in the emotional images (N = 14); (B) Lure Discrimination Index (LDI) was significantly worse in negative and positive compared to neutral images in the immediate condition. LDI overall was also worse after 24 h, however, the magnitude of the difference was larger in the emotional images than the neutral images. There was a significant interaction between emotion and time of testing, which is indicated by the different significance markers (* vs. **).
3.2. Emotional modulation not due to attention or perceptual effects
A potential interpretation of the data above is that the emotional effect is secondary to a shift in attentional focus and not necessarily due to a mnemonic process. For example, participants may not have perceptually encoded all of the details of the emotional images during the encoding phase and this lack of attention to detail may have affected subsequent memory performance. Consistent with this idea, Mather and Sutherland (2011) recently proposed that arousal during an event can either enhance or impair memory for events, depending on attentional factors that bias competition in favor of high priority stimuli.
To examine this possibility, we tested 37 new participants on a match-to-sample (MTS) task using the same stimuli. We measured target hit rate and lure rejection rate and found no significant differences across negative, neutral, and positive items for 2500 ms (Fig. S3a) and 1000 ms (Fig. S3b). This suggests that while attention may play a role in emotional processing, it did not significantly contribute to the effects observed here. A related possibility is that encoding and consolidation mechanisms interact so that emotionally arousing items are differentially processed during encoding, in such a manner that their long-term consolidation is also altered (Hamann, 2001).
3.3. Preserved emotional target recognition after 24 h
Previous studies have shown that the enhancement of emotional memories tends to be greater after a delay (Eysenck, 1976; Heuer & Reisberg, 1990; Kleinsmith & Kaplan, 1963; LaBar & Phelps, 1998; Sharot & Phelps, 2004). This delayed enhancement may be due to endogenous norepinephrine release during a narrow time window after encoding (i.e. during consolidation) that may enhance the strength of memories (Buchanan & Lovallo, 2001; McGaugh, 2002; McIntyre, Hatfield, & McGaugh, 2002; Segal et al., 2012). We tested this hypothesis in a separate sample (N = 14) that completed the same emotional discrimination task, but performed the memory test after a 24-h delay. We measured target recognition and LDI in these participants and compared their performance to the group tested immediately using an emotion *time of testing ANOVA. After a delay, overall target recognition was worse compared to immediate testing [F(1, 36) = 13.61, P = .001], as expected. There was a main effect of emotion [F(2, 72) = 8.30, P = .001] as well as an interaction between emotion and time of testing [F(2, 72) = 3.20, P = .047] such that memory for emotional stimuli was preserved over time (i.e. less forgetting), whereas target recognition for neutral items was impaired (i.e. more forgetting) [F(1, 72) = 6.39, P < .05, critical Scheffé = 6.24; Fig. 2a].
3.4. Impaired emotional lure discrimination after 24 h
Similar to d′, LDI was worse after a 24-h delay compared to immediate testing [F(1, 36) = 46.78, P < .001], consistent with forgetting. There was a main effect of emotion [F(2, 72) = 23.43, P < .001] as well as an interaction between emotion and time of testing [F(2, 72) = 3.45, P = .04]. However, the nature of the interaction was opposite of that observed with d′. Lure discrimination differences were greater for emotional stimuli compared to neutral stimuli over time [F(1, 72) = 6.28, P < .05, critical Scheffé = 6.24; Fig. 2b] such that there was more forgetting of emotional details after 24 h compared to neutral details.
We also analyzed the effect of similarity level across immediate and delay tested groups using a emotion * similarity * time of testing ANOVA and found a main effect of similarity (as found in the immediate study) [F(1, 36) = 125.61, P ≤ .001; Fig. S2a and b]. There was no significant interaction between similarity and time of testing and no significant three-way interaction between emotion, similarity, and time of testing.
Gender differences were examined in detail in all experiments and we did not find any significant gender differences or interactions with gender in any of our analyses (SI Text and Fig. S4). It is important to note that since our studies were not powered to detect gender differences, these results should not be taken as evidence for the absence of such differences. Prior studies have indeed noted gender differences on emotional memory tasks (Cahill, 2006). Complete analyses of reaction time data are also shown in supplementary materials (SI Text and Fig. S5).
3.5. Depression as a model of altered emotional discrimination
Our emotional discrimination paradigm may provide a sensitive measure for examining emotional memory in disorders with aberrant emotional mnemonic processing such as depression, anxiety, and Alzheimer's disease. We administered the emotional discrimination task (immediate testing) to 15 participants who showed moderate to severe depressive symptoms (according to the Beck Depression Inventory-II (BDI-II) and received a score > 15; average BDI-II = 26) (Watkins et al., 2000). We used participants from our previous experiment who were tested immediately as controls that received a score <7 (average BDI-II = 2.8). We conducted an emotion * group ANOVA of target recognition performance, which revealed a main effect of emotion [F(2, 74) = 6.92, P = .002] and a significant interaction between emotion and group [F(2, 74) = 4.19, P = .02]. Participants with depressive symptoms displayed worse target recognition for neutral items compared to healthy controls [F(1, 74) = 9.21, P < .05, critical Scheffé = 6.24; Fig. 3a]. Recognition of emotional items was not significantly different among groups.
Fig. 3.
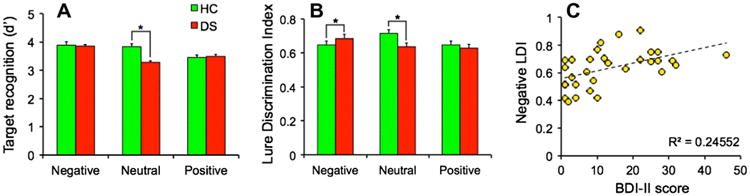
Performance in healthy controls (HC) and participants with depressive symptoms (DS) (A) Target recognition (d′) was significantly impaired in the DS group compared to HC only in the neutral condition but not in the emotional conditions (N = 15); (B) Lure Discrimination Index (LDI) was significantly impaired in the neutral condition and enhanced in the negative condition in the DS group relative to the HC group; (C) Positive correlation between depressive symptoms quantified by the Beck Depression Inventory (BDI-II) and Lure Discrimination Index (LDI) specifically on negative items (N = 31).
Next, we conducted an ANOVA of emotion * group with LDI as the dependent measure. While there were no main effects of either factor, we found a significant interaction [F(2, 74) = 5.99, P = .004]. Compared to healthy controls, participants with depressive symptoms showed an impairment in discrimination of neutral lures [F(1, 74) = 10.65, P < .05, critical Scheffé = 6.24], and an enhancement in discrimination of negative lures [F(1, 74) = 12.55, P < .05, critical Scheffé = 6.24; Fig. 3b]. There was no significant difference between groups in lure discrimination of positive items. We also repeated the same analyses but included similarity as a factor and found no interactions with between group and similarity, although the main effect of similarity was present, as in previous experiments [F(1, 37) = 106.48, P < .001] (Fig. S2c). Performance measures in participants with depressive symptoms stratified by gender are shown in Fig. S4c. Reaction time data for participants with depressive symptoms are shown in Fig. S5c.
Finally, we tested whether severity of depressive symptoms (measured by the BDI-II) was associated with negative lure discrimination performance. We expanded our sample to include any individuals showing depressive symptoms (BDI-II > 0), which increased our final sample size to 31 participants for this analysis. We found a robust positive correlation between BDI-II symptom severity and negative lure discrimination index (Pearson's r = .50, P = .005; Fig. 3c), consistent with the notion that enhanced memory for negative experiences is a core endophenotype of depression that becomes more exaggerated as depressive symptoms increase.
4. Discussion
Emotions have long been known to play a role in the persistence of memories (James, 1884; McGaugh, 2013). The goal of this study was to examine the relationship between emotion and the minimization of interference that is necessary for encoding unique conjunctive representations, a putative function of the hippocampal circuit. The emotional discrimination paradigm allowed us to investigate this interaction at a behavioral level and revealed a potential mechanistic basis for emotion's asymmetric effects on memory. We suggest that emotion results in a preservation of gist information (thought to rely solely on pattern completion) and a loss of detail information (thought to rely on both pattern completion and pattern separation).
Overall, our results suggest that emotion's effects on memory are magnified after a 24-h delay, consistent with a role in consolidation (McGaugh, 2004; Payne et al., 2008). For target recognition, emotional items were preserved from forgetting whereas neutral items were more likely to be forgotten, consistent with prior work (Kensinger, 2009; LaBar & Phelps, 1998). However, lure discrimination showed the opposite pattern after a 24-h delay, where emotional stimuli (in which performance was impaired in the immediate condition) were even more likely to be forgotten after 24 h. It appears that emotion plays at least two distinct roles in modulating memory strength: (1) an impairment of detail-based discrimination (taxing pattern separation) when tested immediately, and (2) a selective retention of gist information and forgetting of detail information over a 24-h period (presumably due to an effect on consolidation). Interestingly, we did not see selective retention of gist information for positive information when tested immediately. Our positive stimuli were not rated as arousing as the negative stimuli (Fig. S1b), which may be playing a role in the reduced memory for positive targets. Valence-based effects on memory specificity have been shown in the past (Kensinger & Schacter, 2006). In addition, while we saw better performance for low similarity lures compared to high similarity lures, we did not see any interactions between similarity and emotion. We might expect that the differences in overall accuracy between high and low stimuli may be reflected in hippocampal processing but may not vary as much behaviorally.
The emotional modulation effect reported here may be an adaptive mechanism, in which only the central and/or salient features of events (i.e. the gist) are strengthened while the peripheral and/or non-salient features (i.e. the details) are weakened. The latter weakening allows for the flexible generalization of gist information to novel situations, which may be required for survival behaviors (e.g. fight or flight). While emotion-induced gist versus detail trade-offs have been studied in the past (Kensinger, 2009; Kensinger & Schacter, 2007; Mather & Sutherland, 2011), the mechanisms underlying these behavioral effects have remained elusive. Viewing this trade-off as a shift in the balance of computational functions of the hippocampus (perhaps via modulation by the amygdala) provides a potential neurobiological context.
The basolateral amygdala (BLA) in particular is thought to be a major modulator of the hippocampus. The BLA projects to the hippocampus via multiple routes, including indirect connections through the entorhinal cortex (EC) as well as direct connections to CA3, CA1, and subiculum (Petrovich, Canteras, & Swanson, 2001; Pitkänen, Pikkarainen, Nurminen, & Ylinen, 2000). These connections are thought to modulate the strength of emotional memories (Ledoux, 2007; McGaugh, 2004). More specifically, stress hormones influence memory consolidation via neuromodulatory interactions with the BLA (i.e. norepinephrine or glucocorticoids) modulating the strength of memory for aversive or appetitive events (McGaugh, 2002). Also, it is worth noting that prior work in animals has suggested that the amygdala is sensitive to interference based on reward value (Gilbert & Kesner, 2002). We suggest that discriminating highly interfering emotional information may be supported by amygdala-hippocampal interactions such that the amygdala input to the hippocampus may bias the system away from pattern separation and towards pattern completion. Future studies using high-resolution functional MRI are necessary to understand the amygdala's potential influence on hippocampal computations.
It is possible that the effect of norepinephrine is twofold: a state-wide effect that enhances arousal and vigilance in stressful situations, as well as a transient effect that allocates resources to processing individual stimuli and their respective emotional value. We suspect that the enhanced vigilance state induced by norepinephrine would result in better overall encoding, thus explaining why in our prior work emotional arousal was associated with enhanced subsequent discrimination performance on neutral items (Segal et al., 2012). In the current experiment, however, brief stimuli were used to trigger arousal and thus the current manipulation may have been more sensitive to norepinephrine's transient effects. The latter may explain the apparent difference in the results between the two studies. Also, the prior study used object stimuli and not rich scene stimuli, which allow for detailed investigations of gist and detail information, thus subtle mnemonic effects could have been obscured.
Paradigms that vary mnemonic interference offer a robust empirical framework by which hippocampal function can be assessed (Hunsaker & Kesner, 2013). Indeed, much work has already been done using this framework including the assessment of changes in neurocognitive aging (Stark, Yassa, & Stark, 2010; Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa & Stark, 2011; Yassa et al., 2010a), mild cognitive impairment (Yassa et al., 2010b), perforant path degradation (Yassa, Muftuler, & Stark, 2010c; Yassa et al., 2011), and neurogenesis loss of function (Clelland et al., 2009) as well as gain of function (Sahay et al., 2011). In human high-resolution BOLD fMRI studies, behavior on discrimination tasks has been specifically associated with pattern separation signals in the hippocampal DG and CA3 (Yassa et al., 2011) as well as the integrity of the perforant path input to the hippocampus from the entorhinal cortex (Yassa et al., 2010c). Here, we extended the pattern separation framework to investigate the impact of emotional modulation on hippocampal memory. Manipulating of the similarity of lure stimuli allows us to examine a potential behavioral correlate of hippocampal pattern separation (Yassa & Stark, 2011).
Our emotional discrimination task also shows sensitivity to emotional memory dysfunction in individuals with depressive symptoms. While past studies have observed a negativity bias in depression, the interpretation has traditionally been that this is due to an overgeneralization of negative information (Fulford, Rosen, Johnson, & Carver, 2011). However, results from our interference paradigm offer an alternative account. In individuals with depressive symptoms, discrimination of similar neutral items was impaired, consistent with recently reported results using an object discrimination task (Shelton & Kirwan, 2013). At the same time, discrimination of similar negative items was enhanced, and the degree of such enhancement was correlated with symptom severity. It is possible that this emphasis on negative details is associated with the mood dysregulation that is characteristic of depression. Overemphasizing negative details can come at the cost of processing neutral or positive information, and thus may affect processing stimuli across a wide range of experiences. The aberration in negative item discrimination sheds new light on the negativity bias phenomenon and highlights the value of using this paradigm in the future to examine amygdala-hippocampal interactions in major depression to fully understand the nature of emotional memory abnormalities in the disorder.
Limitations of the study include sample sizes that were too small to thoroughly investigate gender differences, which have been demonstrated in prior studies of emotional memory (Cahill, Gorski, Belcher, & Huynh, 2004; Nielsen, Ahmed, & Cahill, 2013). This absence of evidence should not be taken as evidence of absence and we realize that there are likely gender differences here that need to be considered in future experiments. Also, we used naturalistic stimuli and not computer-generated, controlled morphs, thus specific features (e.g. orientation, color, etc.) were quite variable. It is possible that future studies with more controlled stimuli can be used to examine mnemonic asymmetry for emotional items in more detail by directly manipulating individual aspects of the images. Another limitation is that participants with depressive symptoms did not receive a formal psychiatric evaluation or a diagnosis of depression. Thus it is unknown whether the depressive symptoms reported are due to MDD or perhaps another etiology. Future work should attempt to extend the use of this task to a group of depressed participants with a confirmed diagnosis of major depression according to the Diagnostic and Statistical Manual (DSM).
In conclusion, our study suggests a novel mechanistic account by which emotional stimuli can have asymmetrical effects on long-term memory. Specifically, the results suggest that emotion influences hippocampal pattern separation. Although a large body of research has investigated the role of emotion on making memories stronger, none have attempted to use the pattern separation framework for hippocampal function to investigate the specific role of the amygdala on the computations of subregions of the hippocampus. The emotional discrimination paradigm used in the current study offers a window into how emotional arousal may alter pattern separation computations in service of episodic memory ultimately to promote survival. Equipped with a better understanding of hippocampal dynamics and a more detailed assessment of the behavioral effects of emotion on memory, future studies can investigate the specific relationship between amygdala-hippocampal connectivity and pattern separation in emotional contexts. This paradigm may also offer a novel tool to assess aberrations in emotional memory, perhaps offering a deeper understanding of abnormal emotional mnemonic processing associated with disorders with an abnormal mood component.
Supplementary Material
Acknowledgments
We thank Liz Murray, Eli Levitt, Gabrielle McNary, Ayobami Ward, and Allen Chang for help with participant recruitment and testing. We also acknowledge Clare King and the Johns Hopkins Student Counseling Center for help with participant recruitment. M.A.Y. is supported by US National Institute on Aging P50 AG05146and R01 AG034613. S.L.L. and S.K.T. are supported by National Institute on Aging Training Grant T32 AG027668 (PI: M. Albert).
Footnotes
Appendix A. Supplementary material: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nlm.2014.02.013.
References
- Airaksinen E, Wahlin A, Forsell Y, Larsson M. Low episodic memory performance as a premorbid marker of depression: Evidence from a 3-year follow-up. Acta Psychiatrica Scandinavica. 2007;115(6):458–465. doi: 10.1111/j.1600-0447.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- Airaksinen Eija, Wahlin A, Larsson M, Forsell Y. Cognitive and social functioning in recovery from depression: Results from a population-based three-year follow-up. Journal of Affective Disorders. 2006;96(1–2):107–110. doi: 10.1016/j.jad.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Brown R, Kulik J. Flashbulb memories. Cognition. 1977;5(1):73–99. [Google Scholar]
- Buchanan TonyW, Adolphs R. The role of the human amygdala in emotional modulation of long-term declarative memory. Emotional cognition from brain to behaviour. 2002:9–34. [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Belcher A, Huynh Q. The influence of sex versus sex-related traits on long-term memory for gist and detail from an emotional story. Consciousness and Cognition. 2004;13(2):391–400. doi: 10.1016/j.concog.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behavioural Brain Research. 2010;215(2):162–171. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Eysenck MW. Arousal, learning, and memory. Psychological Bulletin. 1976;83(3):389–404. [PubMed] [Google Scholar]
- Fulford D, Rosen R, Johnson S, Carver C. Negative generalization and symptoms of anxiety disorders. Journal of Experimental Psychopathology. 2011;3(1):62–68. doi: 10.5127/jep.019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The amygdala but not the hippocampus is involved in pattern separation based on reward value. Neurobiology of Learning and Memory. 2002;77(3):338–353. doi: 10.1006/nlme.2001.4033. [DOI] [PubMed] [Google Scholar]
- Gordon E, Barnett KJ, Cooper NJ, Tran N, Williams LM. An “integrative neuroscience” platform: Application to profiles of negativity and positivity bias. Journal of Integrative Neuroscience. 2008;7(3):345–366. [PubMed] [Google Scholar]
- Haas BW, Canli T. Emotional memory function, personality structure and psychopathology: A neural system approach to the identification of vulnerability markers. Brain Research Reviews. 2008;58:71–84. doi: 10.1016/j.brainresrev.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. TICS. 2001;5(9):394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10) doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heuer F, Reisberg D. Vivid memories of emotional events: The accuracy of remembered minutiae. Memory Cognition. 1990;18(5):496–506. doi: 10.3758/bf03198482. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and Biobehavioral Reviews. 2013;37(1):36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9(34):188–205. [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. 2009;1(2):99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: Effects of valence and arousal. Cognitive Affective and Behavioral Neuroscience. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Remembering the specific visual details of presented objects: Neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45(13):2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Kim J, Yassa MA. Assessing recollection and familiarity of similar lures in a behavioral pattern separation task. Hippocampus. 2013;23(4):287–294. doi: 10.1002/hipo.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology. 1963;65(2):190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9(6):490–493. [Google Scholar]
- Ledoux J. The amygdala. Current Biology. 2007;17(20):868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Loftus EF, Loftus GR, Messo J. Some facts about “weapon focus”? Law and Human Behavior. 1987;11:55–62. http://dx.doi.org/10.1007/BF01044839. [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Making lasting memories: Remembering the significant. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience. 2002;16(7):1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiology of Learning and Memory. 2013;106:56–65. doi: 10.1016/j.nlm.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: Revisiting the complementary learning systems model. Hippocampus. 2010;20(11):1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems framework. Trends in Cognitive Sciences. 2002;6(12):505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psych Science. 2008;8:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Brain Research Reviews. 2001;38(1–2):247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SK, Stark SM, Kattan D, Stark CE, Yassa MA. Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiology of Learning and Memory. 2012;97(4):465–469. doi: 10.1016/j.nlm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M, Olton D. Hippocampal function and interference. What are the memory systems of 1994? 1994:87–117. [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(3):294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Shelton DJ, Kirwan CB. A possible negative influence of depression on the ability to overcome memory interference. Behavioural Brain Research. 2013;256:20–26. doi: 10.1016/j.bbr.2013.08.016. http://dx.doi.org/10.1016/j.bbr.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning & Memory. 2010;17(6):284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & Memory. 2009;16(5):338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4(3):374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Watkins PC, Martin CK, Stern LD. Unconscious memory bias in depression: Perceptual and conceptual processes. Journal of Abnormal Psychology. 2000;109(2):282–289. [PubMed] [Google Scholar]
- Watkins PC, Vache K, Verney SP, Muller S, Mathews A. Unconscious mood-congruent memory bias in depression. Journal of Abnormal Psychology. 1996;105:34–41. doi: 10.1037//0021-843x.105.1.34. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010a;21(9):968–979. doi: 10.1002/hipo.20808. http://dx.doi.org/10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010c;107(28):12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High-resolution functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage. 2010b;51(3):1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


