Abstract
Evidence suggests an association between exposure to cadmium and dysglycemia. To investigate this matter, we examined the relationship between urinary cadmium and prediabetes in the cross sectional National Health and Nutrition Examination Survey (NHANES). NHANES participants for the years 2005 through 2010 aged ≥40 years were included in the analysis. Participants with nephropathy, overt diabetes, or missing required data were excluded. To assess the non-linear relationship between cadmium and Prediabetes, non-parametric logistic regression with B spline expansion of urinary cadmium/creatinine ratio was performed. This analysis revealed a complex non-linear association between higher cadmium levels and prediabetes. This relationship persisted, though with varying magnitudes across smoking groups (never smokers, moderate smokers, heavy smokers). In a conventional logistic regression analysis, this relationship was less evident with significantly increased OR for prediabetes was found in the highest quintile of urine cadmium compared to the lowest quintile in the overall population and in moderate smokers. In an age stratified analysis, a significant linear association was found only in the age groups 60–69 and ≥70. We conclude that there is a significant non-linear, complex relationship between urinary Cd levels, age, smoking habits and odds of prediabetes.
Keywords: Cadmium, Diabetes mellitus, Prediabetes, NHANES
Introduction
Type-2 diabetes mellitus is a multifactorial disease, with the interaction of genetic susceptibility, age, lifestyle, and environmental factors contributing to its development. Some epidemiologic and experimental evidence suggests that chronic exposure to cadmium (Cd) may be associated with an increased risk for developing dysglycemia and diabetes mellitus (DM) (Afridi et al., 2008; Chang et al., 2013; El Muayed et al., 2012; Schwartz et al., 2003; Swaddiwudhipong et al., 2012). However, other epidemiologic studies have shown mixed or no such association (Barregard et al., 2013; Moon, 2013). There are several important differences in the study populations and methods that likely contributed to these mixed results as we detail in the discussion of this manuscript. However, we hypothesized that beyond the more obvious differences in these studies, two factors are particularly important when examining the relationship between Cd exposure and dysglycemia and were not sufficiently addressed in prior studies. Firstly, it is possible that the relationship is of a more complex, non-linear nature, thereby being underestimated when using conventional analysis methods. Secondly, age is likely to play an important role, especially given the long half-life of Cd of 7–30 years in various tissues (Amzal et al., 2009; Elinder et al., 1976; Jarup et al., 1983). These factors may have contributed to the mixed results in prior studies examining this relationship. The goal of this study was to examine the relationship between age, Cd exposure, and the prevalence of prediabetes in the NHANES population from 2005 through 2010 with special emphasis on examining the data for the presence of a complex non-linear relationship between these parameters using spline analysis, while also examining the role of age.
Materials and methods
Study population
Participants 40 years old or older in the 2005–2006, 2007–2008, and 2009–2010 National Health and Nutrition Examination Survey (NHANES) cycles were examined. Participants with laboratory evidence of macroalbuminuria (urine albumin to creatinine ratio ≥ 300 mg/g), evidence of chronic kidney disease (GFR < 30 ml/min/1.73 m2), or DM (self-report of diabetes medication use and/or one of the following: hemoglobin A1c ≥ 6.5% (48 mmol/mol), fasting plasma glucose ≥7.0 mmol/L, 2 h OGTT ≥ 11.1 mmol/L) were excluded from the analyses given the uncertain effect of these conditions on urinary Cd excretion (supplemental Table 1). A description of the laboratory analysis can be found on the NHANES resources (CDC, 2012).
Definitions
The urinary creatinine corrected Cd concentration – a well recognized surrogate marker for Cd exposure (Amzal et al., 2009; Choudhury et al., 2001; Jarup et al., 1998; Orlowski et al., 1998) – was defined as the exposure variable. Urinary creatinine corrected Cd was expressed as μg Cd/g creatinine. Glycated Hemoglobin (HbA1c) – a indirect marker of average serum glucose levels – is expressed as % of total hemoglobin.
The outcome variable prediabetes was defined as any one of the following: 5.7% (39 mmol/mol) ≥ hemoglobin A1c < 6.5% (48 mmol/mol), fasting plasma glucose between 5.6 mmol/L and 7.0 mmol/L, 2 h glucose value between 7.8 mmol/L and 11.1 mmol/L on a 2 h 75 g oral glucose tolerance test in accordance with the current guidelines (American-Diabetes-Association, 2013). Never, moderate, and heavy smokers were defined as individuals with 0, 0.1–20, or >20 pack years of smoking history respectively without consideration of current smoking status.
Calculation of HOMA-IR and HOMA-β
HOMA-IR and HOMA-β were calculated using software for calculating the HOMA IR and HOMA-β using the updated HOMA2 formula (Levy et al., 1998) from http://www.dtu.ox.ac.uk/homacalculator/index.php
Statistical analysis
Weighted means and standard errors were calculated for continuous variables, weighted percentages for categorical variables. Logistic regression was used to determine the association between quintile of urine Cd and prediabetes. Quintiles were set at 0.014–0.183 (Q1), 0.183–0.285 (Q2), 0.285–0.420 (Q3), 0.420–0.656 (Q4), and 0.656–3.74 μg Cd/g creatinine (Q5). Regression models were fit overall, stratified by 10-year age category, and stratified by smoking intensity (never, moderate, and heavy smokers). Model-I represents the unadjusted data. In model-II the data were adjusted for age, race/ethnicity, gender, education, BMI, hypertension, smoking status, pack-years, and survey year. Age, BMI (kg/m2) and pack-years were included in the model as continuous covariates. According the NHANES protocols race/ethnicity was entered as a categorical variable including Non-Hispanic white, Non-Hispanic African American, Mexican American and Others. A self-reported diagnosis of hypertension was modeled dichotomously. Survey year (2005–2006, 2007–2008, and 2009–2010), education (less than 9th grade, some high school, high school graduate, some college, or college graduate), and smoking status (current, former and never) were modeled as categorical variables. Pack-years and current smoking status (as a marker of the time span of smoking) were included as covariates in the final model. Interaction of urine Cd quintiles with age category was assessed by including a multiplicative interaction term in the regression model. To assess a non-linear relationship between urine Cd levels and prediabetes, non-parametric logistic regression with B-spline expansion of urine cadmium was used. Knots for the spline analysis were set at the quintile transition points from the overall population (0.183, 0.285, 0.420, 0.656) and the reference was set at the 20th percentile (0.183). Spline regression macros used in this analysis were written by Gregory et al. (2008). All analyses were performed using SAS 9.3 (SAS Institute, NC). Survey procedures were used to take the complex, multistage sampling design of NHANES into account. GFR was estimated using the MDRD equation (GFR = 175 × serumCr−1.154 × age−0.203 × 1.212 (if patient is black) × 0.742 (if female)).
Results
Baseline characteristics
2398 participants met the inclusion criteria (supplemental Table 1). The characteristics of the population are summarized in Table 1.
Table 1.
Characteristics of NHANES 2005–2010 participants included in the analysis.
| Overall |
Males |
Females |
||||
|---|---|---|---|---|---|---|
| N | Weighted mean or frequency |
N | Weighted mean or frequency |
N | Weighted mean or frequency |
|
| Age (years) | 2398 | 55.9 | 1194 | 55.3 | 1204 | 56.4 |
| Race/ethnicity (%) | ||||||
| Non-Hispanic White | 1339 | 78.4 | 673 | 37.0 | 666 | 41.5 |
| Non-Hispanic Black | 434 | 8.9 | 225 | 4.0 | 209 | 4.9 |
| Mexican American | 347 | 4.9 | 167 | 2.5 | 180 | 2.4 |
| Other | 278 | 7.7 | 129 | 3.5 | 149 | 4.2 |
| Education (%) | ||||||
| Less than 9th grade | 302 | 6.4 | 143 | 3.0 | 159 | 3.4 |
| 9–11th grade | 353 | 10.6 | 184 | 5.2 | 169 | 5.4 |
| High School Grad/GED | 558 | 24.8 | 294 | 12.5 | 264 | 12.3 |
| Some College or AA degree | 647 | 28.9 | 307 | 12.9 | 340 | 16.0 |
| College Grad or Above | 538 | 29.3 | 266 | 13.3 | 272 | 16.0 |
| BMI (kg/m2) | 2398 | 28.3 | 1194 | 28.2 | 1204 | 28.4 |
| Smoking Status (%) | ||||||
| Never | 1185 | 49.5 | 457 | 19.1 | 728 | 30.4 |
| Former | 735 | 20.0 | 467 | 23.6 | 268 | 15.5 |
| Pack-years | ||||||
| 0.1–20 | 449 | 63.0 | 269 | 32.4 | 180 | 30.7 |
| 20+ | 271 | 34.7 | 192 | 22.5 | 790 | 12.2 |
| Current | 478 | 29.1 | 270 | 32.6 | 208 | 24.9 |
| Pack-years | ||||||
| 0.1–20 | 224 | 40.5 | 113 | 19.0 | 111 | 21.5 |
| 20+ | 254 | 59.5 | 157 | 35.4 | 97 | 24.0 |
| Hypertension (%) | 1028 | 38.5 | 507 | 17.2 | 521 | 21.3 |
| Diabetes status (%) | ||||||
| No diabetes | 1191 | 55.8 | 552 | 25.3 | 639 | 30.5 |
| Prediabetes | 1207 | 44.2 | 642 | 21.6 | 565 | 22.6 |
| Fasting glucose (mmol/L) | 1101 | 99.7 | 546 | 102.0 | 555 | 97.7 |
| 2 h OGTT (mmol/L) | 967 | 112.9 | 485 | 111.5 | 482 | 114.1 |
| % Hemoglobin A1c | 2396 | 5.5 | 1194 | 5.4 | 1202 | 5.5 |
| Urine Cd (μg/g creatinine) | 2398 | 0.4 | 1194 | 0.4 | 1204 | 0.5 |
Spline analysis of the relationship between urinary Cd and prediabetes
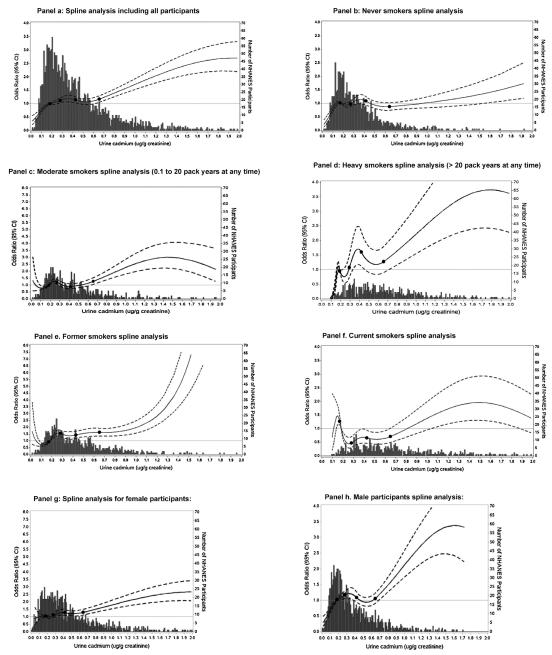
Quadratic restricted spline regression with knots at the 20th, 40th, 60th, and 80th percentiles of the overall Cd distribution with the OR adjusted according to Model-II is seen in Fig. 1, and suggests a complex, non-linear relationship between Cd exposure and the odds ratio for prediabetes. Urinary Cd concentrations below the reference level of 0.183 μg/g creatinine were associated with a decrease in prediabetes OR compared to the reference level in the whole population (panel a) and in never smokers (panel b), but not in moderate (panel c) or heavy smokers (panel d). In the overall population (panel a), a non-linear, progressive rise in prediabetes OR is observed between urinary Cd levels 0.262–0.452 μg/g creatinine. The association then disappears until Cd level of 0.622 μg/g creatinine. A similar pattern was observed in never smokers (panel b), where an increased Odds Ratio (OR) for prediabetes was observed at urinary Cd/Cr ratios above 1.375 μg/g creatinine. In moderate smokers (panel c), the OR for prediabetes was increased above 0.646 μg/g creatinine and continued to rise above this threshold. In heavy smokers (panel d) an increased OR for prediabetes was observed between urinary Cd values of 0.329 and 0.454 μg/g creatinine and above 0.711 μg/g creatinine. Similar patterns were seen when the former smokers (panel e) and current smokers (panel f) were analyzed as separate groups. The association was evident in both genders, though less prominent in women (panel g) than in men (panel h). Across all groups, the data suggest a positive association between creatinine corrected Cd levels higher than 0.7–0.9 μg/g creatinine and an increased OR for prediabetes.
Fig. 1.
Odds ratios for prediabetes by creatinine-corrected urine cadmium level adjusted for age, race/ethnicity, gender, education, BMI, hypertension, smoking status, packyears, and survey year. Knots are at quintile of urine cadmium distribution (0.183, 0.284, 0.420, 0.654 μg/g). Reference value is set at the 20th percentile of urine cadmium distribution (0.183 μg/g). Odds ratio is displayed as a solid line. The upper and lower limit of the 95% confidence interval is displayed as a dotted line. The histogram illustrates the number of participants at each Cd concentration point. Panel a represents the data from all participants. Panel b represents the data from never smokers. Panels c and d represent data from moderate (0.1–20 pack years and heavy smokers (>20 pack years) irrespective of current smoking status. Panels e, f, g, and h represent the data from former and current smokers, females, and males respectively.
Stratified analysis relationship between urinary Cd and the rate of prediabetes
The OR for prediabetes were increased in the 3rd, 4th, and 5th quintile of urinary Cd in the unadjusted model as compared to the lowest quintile (Model-I, Table 2). After adjustment for age, race/ethnicity, gender, education, BMI, hypertension, smoking status, pack-years, and survey year in Model-II, the odds for prediabetes remained elevated in the 5th quintile of urinary Cd. In an analysis stratified by smoking intensity, a relationship between urinary Cd and prediabetes was only found in the 5th quintile of the unadjusted Model-I and adjusted Model-II in moderate smokers only. There was an interaction between age and urinary cadmium levels (supplemental Table 3). Urine cadmium levels were associated with the presence of prediabetes only in the elderly (age 60–70+). Specifically, in participants aged 60–69, an association with prediabetes was found in the 5th quintile of urinary Cd in the unadjusted Model-I and the adjusted Model-II. In participants aged ≥70, an association with prediabetes was found in the 2nd, 3rd, and 5th quintiles in the unadjusted Model-I. In the adjusted Model-II, the association was evident in the 2nd through 5th quintiles.
Table 2.
Odds ratio (OR) for prediabetes in quintiles of urine cadmium (μg/g creatinine) in the entire study population and stratified by age, pack year, or gender category.
| N | Model-I OR (95% CI) | Model-II OR (95% CI) | |
|---|---|---|---|
| All participants | 1207/2398 | ||
| Quintile 1 (0.014–0.183) | 206/479 | 1 (−) | 1 (−) |
| Quintile 2 (0.183–0.285) | 244/180 | 1.13 (0.82,1.54) | 0.99 (0.71,1.36) |
| Quintile 3 (0.285–0.420) | 246/179 | 1.35 (1.003,1.81) | 1.25 (0.91,1.73) |
| Quintile 4 (0.420–0.656) | 240/180 | 1.37 (1.01,1.87) | 1.25 (0.83,1.86) |
| Quintile 5 (0.656–3.74) | 271/480 | 1.62 (1.17,2.25) | 1.67 (1.12, 2.47) |
| Never smoker | 585/1200 | ||
| Quintile 1 (0.014–0.183) | 152/348 | 1 (−) | 1 (−) |
| Quintile 2 (0.183–0.285) | 136/281 | 1.06 (0.73, 1.56) | 0.91 (0.60, 1.39) |
| Quintile 3 (0.285–0.420) | 138/265 | 1.42 (0.96, 2.10) | 1.07 (0.67, 1.71) |
| Quintile 4 (0.420–0.656) | 95/185 | 1.43 (0.93, 2.21) | 1.04 (0.58, 1.86) |
| Quintile 5 (0.656–3.74) | 64/121 | 1.51 (0.91, 2.51) | 1.03 (0.55, 1.93) |
| Moderate smoker (0.1 –20 PY) | 321/673 | ||
| Quintile 1 (0.014–0.183) | 46/113 | 1 (−) | 1 (−) |
| Quintile 2 (0.183–0.285) | 82/154 | 1.21 (0.71, 2.08) | 1.12 (0.68, 1.84) |
| Quintile 3 (0.285–0.420) | 56/125 | 1.29 (0.79, 2.11) | 1.40 (0.76, 2.58) |
| Quintile 4 (0.420–0.656) | 66/152 | 1.03 (0.61, 1.75) | 1.12 (0.59, 2.13) |
| Quintile 5 (0.656–3.74) | 71/129 | 1.80 (1.04, 3.12) | 1.95 (1.02, 3.72) |
| Heavy smoker > 20 PY | 301/525 | ||
| Quintile 1 (0.014–0.183) | 8/18 | 1 (−) | 1 (−) |
| Quintile 2 (0.183–0.285) | 26/45 | 1.04 (0.29, 3.72) | 0.85 (0.20, 3.64) |
| Quintile 3 (0.285–0.420) | 52/89 | 0.94 (0.27, 3.22) | 1.80 (0.48, 6.73) |
| Quintile 4 (0.420–0.656) | 79/143 | 1.29 (0.37, 4.54) | 2.28 (0.60, 8.68) |
| Quintile 5 (0.656–3.74) | 136/230 | 1.16 (0.36, 3.73) | 3.14 (0.91, 10.79) |
| Age 40–49 | 307/757 | ||
| Quintile 1 (0.014–0.183) | 92/237 | 1.0 (−) | 1.0 (−) |
| Quintile 2 (0.183–0.285) | 67/174 | 0.83 (0.52, 1.31) | 0.78 (0.44, 1.37) |
| Quintile 3 (0.285–0.420) | 63/150 | 0.97 (0.59, 1.61) | 1.05 (0.55, 1.98) |
| Quintile 4 (0.420–0.656) | 41/103 | 0.89 (0.54, 1.47) | 0.82 (0.40, 1.67) |
| Quintile 5 (0.656–3.74) | 44/93 | 1.10 (0.61, 2.00) | 1.09 (0.48, 2.46) |
| Age 50–59 | 290/556 | ||
| Quintile 1 (0.014–0.183) | 57/113 | 1.0 (−) | 1.0 (−) |
| Quintile 2 (0.183–0.285) | 62/114 | 0.92 (0.45, 1.87) | 0.73 (0.36, 1.49) |
| Quintile 3 (0.285–0.420) | 51/99 | 1.39 (0.74, 2.60) | 1.15 (0.60, 2.22) |
| Quintile 4 (0.420–0.656) | 55/105 | 1.43 (0.81, 2.52) | 1.22 (0.60, 2.48) |
| Quintile 5 (0.656–3.74) | 65/125 | 1.11 (0.64, 1.94) | 0.97 (0.49, 1.91) |
| Age 60–69 | 282/517 | ||
| Quintile 1 (0.014–0.183) | 33/70 | 1.0 (−) | 1.0 (−) |
| Quintile 2 (0.183–0.285) | 56/105 | 1.48 (0.68, 3.20) | 1.75 (0.80, 3.83) |
| Quintile 3 (0.285–0.420) | 59/105 | 1.61 (0.75, 3.45) | 1.96 (0.87, 4.43) |
| Quintile 4 (0.420–0.656) | 65/123 | 1.45 (0.68, 3.11) | 1.85 (0.76, 4.49) |
| Quintile 5 (0.656–3.74) | 69/114 | 2.50 (1.25, 4.99) | 3.95 (1.82, 8.58) |
| Age ≥ 70 | 328/568 | ||
| Quintile 1 (0.014–0.183) | 24/59 | 1.0 (−) | 1.0 (−) |
| Quintile 2 (0.183–0.285) | 59/87 | 3.54 (1.80, 6.97) | 3.52 (1.89, 6.56) |
| Quintile 3 (0.285–0.420) | 73/125 | 1.90 (1.03, 3.50) | 2.33 (1.27, 4.30) |
| Quintile 4 (0.420–0.656) | 79/149 | 1.65 (0.84,3.26) | 2.29 (1.21, 4.33) |
| Quintile 5 (0.656–3.74) | 93/148 | 2.32 (1.02, 5.26) | 4.92 (1.96, 12.38) |
| Female | 565/1204 | ||
| Quintile 1 (0.014–0.183 | 59/161 | 1.0 (−) | 1.0 (−) |
| Quintile 2 (0.183–0.285 | 93/207 | 1.42 (0.91, 2.21) | 1.33 (0.83, 2.11) |
| Quintile 3 (0.285–0.420) | 117/263 | 1.82 (1.18, 2.82) | 1.55 (0.95, 2.52) |
| Quintile 4 (0.420–0.656) | 130/266 | 2.06 (1.35, 3.14) | 1.75 (1.09, 2.81) |
| Quintile 5 (0.656–3.74) | 166/307 | 2.62 (1.60, 4.29) | 2.29 (1.27, 4.11) |
| Male | 642/1194 | ||
| Quintile 1 (0.014–0.183 | 147/318 | 1.0 (−) | 1.0 (−) |
| Quintile 2 (0.183–0.285 | 151/273 | 1.07 (0.71, 1.60) | 0.80 (0.51, 1.26) |
| Quintile 3 (0.285–0.420) | 129/216 | 1.31 (0.90, 1.91) | 1.16 (0.71, 1.91) |
| Quintile 4 (0.420–0.656) | 110/214 | 1.13 (0.73, 1.77) | 0.95 (0.53, 1.71) |
| Quintile 5 (0.656–3.74) | 105/173 | 1.17 (0.75, 1.81) | 1.45 (0.88, 2.39) |
Model-I is unadjusted. Model-II is adjusted for age, race/ethnicity, gender, education, BMI, hypertension, smoking status, pack-years, and survey year. Values with a p value < 0.05 are highlighted in bold.
HOMA-IR and HOMA-β
The Homeostatic Model Assessment (HOMA) is a method used to estimate insulin resistance (HOMA-IR) and beta-cell function (HOMA-β)(Matthews et al., 1985). Decrease in HOMA-IR and HOMA-β values of −0.31 (SE 0.11) and −12.09 (SE 4.97) were found in the 5th urinary Cd quintile in the non-adjusted Model-I only. No changes in either HOMA-IR and HOMA-β were found across the urinary Cd quintiles in the adjusted Model-II (Table 3).
Table 3.
Association of urine cadmium (μg/g) with HOMA-IR and HOMA-β by urinary Cd quintile.
| Outcome: HOMA-IR | N | Weighted mean (SE) | Model-I-unadjusted β (SE) | p | Model-IIa β (SE) | p |
|---|---|---|---|---|---|---|
| Total | 1096 | 1.2 (0.04) | ||||
| Quintile 1 | 144 | 1.4 (0.10) | 0 | 0 | ||
| Quintile 2 | 204 | 1.3 (0.09) | -0.10 (0.11) | 0.38 | 0.01 (0.10) | 0.89 |
| Quintile 3 | 244 | 1.3 (0.07) | −0.11 (0.10) | 0.30 | −0.03 (0.08) | 0.74 |
| Quintile 4 | 225 | 1.3 (0.10) | −0.04 (0.13) | 0.78 | 0.11 (0.11) | 0.33 |
| Quintile 5 | 279 | 1.1 (0.06) | −0.31 (0.11) | 0.005 | −0.04 (0.12) | 0.72 |
| Outcome: HOMA-β | N | Weighted mean (SE) | Model-I-unadjusted β (SE) | p | Model-IIa β (SE) | p |
|
| ||||||
| Total | 1096 | 85.0 (2.18) | ||||
| Quintile 1 | 144 | 88.8 (4.55) | 0 | 0 | ||
| Quintile 2 | 204 | 89.0 (5.15) | 0.16 (5.80) | 0.98 | 3.37 (5.07) | 0.51 |
| Quintile 3 | 244 | 85.7 (3.83) | −3.11 (4.97) | 0.53 | −1.44 (3.86) | 0.71 |
| Quintile 4 | 225 | 87.8 (4.12) | −1.09 (6.08) | 0.86 | 3.49 (5.39) | 0.52 |
| Quintile 5 | 279 | 76.8 (2.85) | −12.09 (4.97) | 0.02 | −3.22 (6.21) | 0.61 |
Model-II is adjusted for age, race/ethnicity, gender, education, BMI, hypertension, smoking status, pack-years, and survey year. Values with a p value < 0.05 are highlighted in bold.
Discussion
The results reported herein suggest a complex non-linear association between urinary Cd excretion – a widely accepted surrogate marker for Cd exposure (Amzal et al., 2009; Choudhury et al., 2001) – and dysglycemia in the US based adult NHANES population. The association was particularly strong among elderly individuals. These results may point toward a threshold effect, whereby a certain level of Cd accumulation has to be reached over time before a detrimental effect on glycemia is observed. Indeed, across all smoking and gender strata, the spline analysis results suggest an association between creatinine corrected Cd levels higher than 1.7–0.9 μg/g creatinine and an increased OR for prediabetes. We previously reported that human insulin producing islets contain measurable quantities of Cd under normal environmental exposure (El Muayed et al., 2012). Others and we previously demonstrated that Cd accumulation in beta cells results in a deterioration of beta cell function once a certain toxic level is exceeded (Edwards and Prozialeck, 2009; El Muayed et al., 2012). The hypothesis of a gradual accumulation of Cd with a resulting decline in beta cell function upon reaching a toxic threshold may therefore provide a possible explanation for our observation of a more pronounced relationship between urinary Cd and prediabetes in the elderly given their longer exposure duration. This is especially plausible given the long biological half-life of Cd of up to 30 years (Amzal et al., 2009; Benemann et al., 2003; Elinder et al., 1976; Hoffmann et al., 1999; Jarup et al., 1983).
Of particular importance is our observation that there seems to be a complex relationship between urinary Cd and cigarette smoking. Smoking is known to be a common source of cadmium exposure while at the same time being associated with an increased incidence of prediabetes and diabetes through mechanisms other than cadmium exposure (Benemann et al., 2003; Elinder et al., 1983; Hoffmann et al., 1999; Lewis et al., 1972; Willi et al., 2007). In our study, we observed a stronger association between urinary Cd levels and prediabetes risk in moderate and heavy smokers than in never smokers despite adjustment for the known diabetogenic effect of smoking. Given that no differences in HOMA-IR or HOMA-β were observed across the exposure quintiles, it is unlikely that changes in insulin resistance or major changes in beta cell function are the main cause for the observed association. This possibility should however be further explored in future studies using more accurate methods given the known limitations of using the HOMA-IR or HOMA-β as surrogate markers of insulin resistance and beta-cell function respectively.
The results of prior studies examining the relationship between various markers of Cd exposure and dysglycemia are mixed. Schwartz et al. showed an association between elevated urinary Cd levels and impaired fasting glucose levels as well as DM in 8722 participants in the Third National Health and Nutrition Examination Survey (NHANES-III) observational cohort study (Schwartz et al., 2003). Swaddiwudhipong et al. reported an increase incidence of diabetes in 217 persons with continued high Cd exposure compared to 219 persons who lowered their Cd intake through dietary interventions in a 5-year observational study in a cohort of 436 persons exposed to high environmental Cd concentrations (Swaddiwudhipong et al., 2012). Afridi et al. reported increased Cd levels in scalp hair of 238 subjects with diabetes mellitus compared to 196 control subjects without diabetes mellitus (Afridi et al., 2008). However other epidemiologic studies showed no such association (Barregard et al., 2013; Moon, 2013). It is likely differences in the study design, sample size, exposure levels and durations in these studies contributed to the varying results. Specifically, the larger study by Moon et al. in 3184 participants of the Korea National Health and Nutritional Examination Surveys (KNHANES 2009–2010) used blood Cd levels to estimate the level of Cd exposure while most other studies used creatinine corrected urinary Cd, or scalp hair Cd (Moon, 2013). It is likely that this important difference may have influenced this outcome of this study given the differences in toxicokinetics between blood Cd and other modalities of Cd measurement, especially creatinine corrected urinary Cd (Choudhury et al., 2001; Elinder et al., 1976; Jarup et al., 1983). Additionally, it is unclear what effect the hyperglycemia associated with overt diabetes has on Cd concentrations in blood and urine, which is the rationale of limiting out study to the outcome variable of prediabetes without the inclusion of overt diabetes as an outcome variable. The study by Barregard et al. performed a cross sectional analysis in a cohort of 590 64 year old women from Gothenburg, Sweden. 244 of the participants not diagnosed with diabetes at baseline were reexamined on average 5.4 years later. This study showed no association between urinary or blood Cd and dysglycemia (diabetes or prediabetes) in either of the cross sectional or the prospective portion of the study (Barregard et al., 2013). This study was limited in power given the small sample size. Beyond these obvious methodological and sampling differences, we think that an important, previously overlooked aspect is the complex, non-linear interplay between age, smoking, Cd accumulation, and the risk for developing dysglycemia that was uncovered in our present study.
Low-level human exposure to environmental Cd is highly prevalent (Afridi et al., 2008; Benemann et al., 2003; Benoff et al., 2009; Hoffmann et al., 1999; Olsson et al., 2002). Human Cd exposure in the general population below the threshold generally considered toxic is highly prevalent. Aside from cigarette smoke, the main sources of human exposure in non-smokers is dietary Cd contamination, occupational exposure, and Cd containing house dust inhalation (Afridi et al., 2008; Benemann et al., 2003; Benoff et al., 2009; Bulat et al., 2009; Dakeshita et al., 2009; Ebert-McNeill et al., 2012; Hoffmann et al., 1999; Hogervorst et al., 2007; Jarup et al., 1983; Link et al., 2007; Olsson et al., 2002; Ruiz et al., 2010). Reports of serum or blood Cd concentrations in the population range from 0.0009 to 0.087 μmol/L (Afridi et al., 2008; Benoff et al., 2009; Bulat et al., 2009; Dakeshita et al., 2009; Ebert-McNeill et al., 2012; Jarup et al., 1983; Link et al., 2007; Olsson et al., 2002; Ruiz et al., 2010). These exposure levels are traditionally considered to be mostly below the toxic exposure levels as defined based on the threshold for nephrotoxic effects of Cd (Maret and Moulis, 2013; Schulz et al., 2011). However, recent findings – including our current study – warrant the investigation of a causal relationship between low-level chronic Cd exposure below the traditional safe thresholds and the risk of prediabetes and diabetes.
Steps to reduce Cd exposure of the general public should be undertaken if a causal link between Cd exposure and dysglycemia is established in future, larger cohort prospective studies. Given the intracellular location and long half-life of Cd, primary prevention of Cd accumulation by modifying the allowable exposure standards is the only viable method for avoiding the negative health consequences resulting from Cd exposure.
A major limitation of our study is the cross sectional design of the underlying NHANES study. Additionally, it is possible that a change in Cd exposure levels over the years resulted in an altered age distribution within the population. No causal link between Cd exposure and the risk for dysglycemia can be drawn from our findings alone. Also, the association with overt DM was not included in the analysis given the uncertain effect of overt, sustained hyperglycemia on renal Cd excretion. Furthermore, at the extremes of the distribution of cadmium our sample size is limited. The resulting lower power is reflected in the wide confidence bands around the curve at the highest and lowest Cd concentrations. Nevertheless, the fact that the non-linear correlation between urinary Cd levels and OR for prediabetes mostly persisted in the spline analysis at the extremes of urinary Cd ranges across all strata of gender and smoking history provides additional evidence of a relevant and persistent correlation.
Prospective studies with sufficient power to perform analyses that take into account the complex, non-linear interactions uncovered in our current study will be required to validate the findings reported herein.
We conclude from our study that urinary Cd excretion is associated with a non-linear increase in odds ratio for prediabetes. This effect is most pronounced in the elderly. Further studies investigating the underlying cause for the observed association between Cd and dysglycemia, especially in the elderly are warranted.
Supplementary Material
Acknowledgments
Funding
This study was funded by grant 1K08ES020880-01 from the National Institute of Environmental Health Sciences (NIEHS)/National Institute of Health for ME.
The NHANES study from which the current study was derived was approved by the Centers for Disease Control (CDC) affiliated National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB).
Abbreviations
- Cd
cadmium
- DM
diabetes mellitus
- NHANES
National Health and Nutrition Examination Survey
- HOMA-IR
homeostatic model assessment for insulin resistance
- HOMA-β
homeostatic model assessment for beta cell function
- GFR
glomerular filtration rate
- OGTT
oral glucose tolerance test
- HbA1c
percentage of glycated hemoglobin.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijheh.2014.06.005.
References
- Afridi HI, Kazi TG, Kazi N, Jamali MK, Arain MB, Jalbani N, Baig JA, Sarfraz RA. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res. Clin. Pract. 2008;80:280–288. doi: 10.1016/j.diabres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- American-Diabetes-Association Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(Suppl. 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzal B, Julin B, Vahter M, Wolk A, Johanson G, Akesson A. Population toxicokinetic modeling of cadmium for health risk assessment. Environ. Health Perspect. 2009;117:1293–1301. doi: 10.1289/ehp.0800317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barregard L, Bergström G, Fagerberg B. Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: A cross-sectional and prospective study in women. Environ. Res. 2013;121:104–109. doi: 10.1016/j.envres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Benemann J, Bromen K, Lehmann N, Marr A, Jockel K-H. Bewertung wesentlicher Pfade der Schadstoffbelastung der Allgemeinbevolkerung mit Hilfe multivariater Analysen Teilprojekt E: Arsen, Blei, Cadmium, Quecksilber und Edelmetalle im Blut und/oder im Urin. Institut für Wasser-Bodenund Lufthygiene (WaBoLu) Berlin Hefte Forschungsbericht. 2003;201(62):214/04. [Google Scholar]
- Benoff S, Hauser R, Marmar JL, Hurley IR, Napolitano B, Centola GM. Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers) Mol. Med. 2009;15:248–262. doi: 10.2119/molmed.2008.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulat ZP, Dukic-Cosic D, Dokic M, Bulat P, Matovic V. Blood and urine cadmium and bioelements profile in nickel–cadmium battery workers in Serbia. Toxicol. Ind. Health. 2009;25:129–135. doi: 10.1177/0748233709104488. [DOI] [PubMed] [Google Scholar]
- CDC National Health and Nutrition Examination Survey 1999–2012 Survey Content Brochure. 2012.
- Chang KC, Hsu CC, Liu SH, Su CC, Yen CC, Lee MJ, Chen KL, Ho TJ, Hung DZ, Wu CC, Lu TH, Su YC, Chen YW, Huang CF. Cadmium induces apoptosis in pancreatic beta-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-jun N-terminal kinase activation. PLoS ONE. 2013;8:e54374. doi: 10.1371/journal.pone.0054374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury H, Harvey T, Thayer WC, Lockwood TF, Stiteler WM, Goodrum PE, Hassett JM, Diamond GL. Urinary cadmium elimination as a biomarker of exposure for evaluating a cadmium dietary exposure – biokinetics model. J. Toxicol. Environ. Health A. 2001;63:321–350. doi: 10.1080/15287390152103643. [DOI] [PubMed] [Google Scholar]
- Dakeshita S, Kawai T, Uemura H, Hiyoshi M, Oguma E, Horiguchi H, Kayama F, Aoshima K, Shirahama S, Rokutan K, Arisawa K. Gene expression signatures in peripheral blood cells from Japanese women exposed to environmental cadmium. Toxicology. 2009;257:25–32. doi: 10.1016/j.tox.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Ebert-McNeill A, Clark S, Miller J, Birdsall P, Chandar M, Wu L, Cerny E, Hall P, Johnson M, Isales C, Chutkan N, Bhattacharyya MH. Cadmium intake and systemic exposure in postmenopausal women and age-matched men who smoke cigarettes. Toxicol. Sci. 2012;130:191–204. doi: 10.1093/toxsci/kfs226. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009;238:289–293. doi: 10.1016/j.taap.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Muayed M, Raja MR, Zhang X, Macrenaris KW, Bhatt S, Chen X, Urbanek M, O’Halloran TV, Lowe WL., Jr. Accumulation of cadmium in insulin-producing beta cells. Islets. 2012;4:405–416. doi: 10.4161/isl.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder CG, Lind B, Kjellstrom T, Linnman L, Friberg L. Cadmium in kidney cortex, liver, and pancreas from Swedish autopsies. Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch. Environ. Health. 1976;31:292–302. doi: 10.1080/00039896.1976.10667239. [DOI] [PubMed] [Google Scholar]
- Elinder CG, Kjellstrom T, Lind B, Linnman L, Piscator M, Sundstedt K. Cadmium exposure from smoking cigarettes: variations with time and country where purchased. Environ. Res. 1983;32:220–227. doi: 10.1016/0013-9351(83)90209-8. [DOI] [PubMed] [Google Scholar]
- Gregory M, Ulmer H, Pfeiffer KP, Lang S, Strasak AM. A set of SAS macros for calculating and displaying adjusted odds ratios (with confidence intervals) for continuous covariates in logistic B-spline regression models. Comput. Methods Prog. Biomed. 2008;92:109–114. doi: 10.1016/j.cmpb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Helm D, Becker K, Friedrich C, Krause C, Nollke P, Seiwert M, Seifert B. 1999. Umwelt-Survey 1990/1992. Band IX: Cadmium – Zusammenhangsanalyse. Institut für Wasser-, Bodenund Lufthygiene (WaBoLu) Berlin Hefte.
- Hogervorst J, Plusquin M, Vangronsveld J, Nawrot T, Cuypers A, Van Hecke E, Roels HA, Carleer R, Staessen JA. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ. Res. 2007;103:30–37. doi: 10.1016/j.envres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Jarup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellstrom T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand. J. Work Environ. Health. 1983;9:327–331. doi: 10.5271/sjweh.2404. [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure – a review of the literature and a risk estimate. Scand. J. Work Environ. Health. 1998;24(Suppl. 1):1–51. [PubMed] [Google Scholar]
- Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Coughlin LL, Jusko WJ, Hartz S. Contribution of cigarette smoking to cadmium accumulation in man. Lancet. 1972;1:291–292. doi: 10.1016/s0140-6736(72)90294-2. [DOI] [PubMed] [Google Scholar]
- Link B, Gabrio T, Piechotowski I, Zollner I, Schwenk M. Baden-Wuerttemberg Environmental Health Survey (BW-EHS) from 1996 to 2003: toxic metals in blood and urine of children. Int. J. Hyg. Environ. Health. 2007;210:357–371. doi: 10.1016/j.ijheh.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Maret W, Moulis JM. The bioinorganic chemistry of cadmium in the context of its toxicity. Metal Ions Life Sci. 2013;11:1–29. doi: 10.1007/978-94-007-5179-8_1. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Moon SS. Association of lead, mercury and cadmium with diabetes in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Diabet. Med. 2013;30:e143–e148. doi: 10.1111/dme.12103. [DOI] [PubMed] [Google Scholar]
- Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine – impact of sex, age, dietary intake, iron status, and former smoking – association of renal effects. Environ. Health Perspect. 2002;110:1185–1190. doi: 10.1289/ehp.021101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski C, Piotrowski JK, Subdys JK, Gross A. Urinary cadmium as indicator of renal cadmium in humans: an autopsy study. Hum. Exp. Toxicol. 1998;17:302–306. doi: 10.1177/096032719801700603. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Mumtaz M, Osterloh J, Fisher J, Fowler BA. Interpreting NHANES biomonitoring data, cadmium. Toxicol. Lett. 2010;198:44–48. doi: 10.1016/j.toxlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Schulz C, Wilhelm M, Heudorf U, Kolossa-Gehring M. Human biomonitoring commission of the German federal environment: a update of the reference and HBM values derived by the German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health. 2011;215:26–35. doi: 10.1016/j.ijheh.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26:468–470. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Punta B, Funkhiew T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: a five-year follow-up. Environ. Res. 2012;112:194–198. doi: 10.1016/j.envres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. J. Am. Med. Assoc. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.