Abstract
Frontotemporal dementia (FTD) was documented over a century ago. The last decade, however, has seen substantial changes in our conceptions of this increasingly recognized disorder. Different clinical variants have been delineated, the most common of which is the behavioral variant (bvFTD). Updated diagnostic criteria have been established. New histopathological findings and genetic etiologies have been discovered. Research continues to uncover molecular mechanisms by which abnormal proteins accumulate in degenerating brain tissue. Novel neuroimaging techniques suggest that functional networks are diminished in bvFTD that may be relevant to empathy and social behavior. Despite rapid advances in our understanding of bvFTD, the disease is still under-recognized and commonly misdiagnosed. The result is inappropriate patient care. Recognizing the various presentations of bvFTD and its histological and genetic subtypes may further diagnosis, treatment and research.
Keywords: frontotemporal dementia, frontotemporal lobar degeneration, Pick's disease, behavioral variant frontotemporal dementia, bvFTD, FTD
Introduction
The brain's frontal lobes influence human personality, motivation, attention, executive function, emotions, social capability and language. Although the Czech neuropsychiatrist Arnold Pick first described a progressive aphasia in 1892, selective degeneration of the frontal and temporal lobes remained relatively obscure for approximately a century. (1, 2) In the last few decades, Pick's disease, now called frontotemporal dementia (FTD), has become recognized as the second most common neurodegenerative dementia in patients under the age of 65 and the third most common neurodegenerative dementia in all age groups.(3, 4)
The term FTD refers to a heterogeneous group of syndromes caused by progressive and selective degeneration of the frontal lobes, temporal lobes, or both with relative preservation of the posterior cortical regions. This focal anterior-predominant neurodegeneration leads to disorders of behavior, language and executive function.
FTD has acquired several different names over the years, including Pick's disease, frontal lobe dementia of the non-Alzheimer type, frontotemporal lobar degeneration, and dementia of the frontal type. Frontotemporal dementia remains the most common term in use. We use the term FTD to describe the clinical syndrome and frontotemporal lobar degeneration (FTLD) for the pathological diagnosis.(5) Pick's disease is now reserved for pathological findings of argyrophilic, tau-positive neuronal inclusions (Pick bodies).(6) This clear separation between syndromic and pathological diagnoses is fundamental to a clear understanding of FTD, as identical symptoms may be caused by many different molecular and cellular mechanisms.
FTD is currently divided into three major subtypes: behavioral variant frontotemporal dementia (bvFTD), semantic variant of primary progressive aphasia (svPPA), and nonfluent variant primary progressive aphasia (nfvPPA).(7,8) (Supplement: Table S1) A third variant of PPA, logopenic variant primary progressive aphasia (lvPPA) is associated with atrophy of the temporoparietal junction and histopathological findings of Alzheimer's disease (AD).
The focus of this review is on the most common FTD subtype, bvFTD. This dementia shares many symptoms with primary psychiatric disorders including schizophrenia, obsessive-compulsive disorder, borderline personality disorder and bipolar disorder. In addition, neurodegenerative diseases with prominent movement abnormalities including progressive supranuclear palsy (PSP),(9, 10) corticobasal degeneration (CBD),(11, 12) and amyotrophic lateral sclerosis (ALS) often present as bvFTD or nfvPPA.(13)
The neuropathological classification of FTLD subtypes is based upon the types of misfolded protein aggregates found within the brain such as tau, trans-activator regulatory deoxyribonucleic acid (TAR-DNA) binding protein 43 (TDP-43),(14) or fused in sarcoma (FUS).(15, 16) The neuropathological changes in patients with ALS, CBD and PSP are strongly linked with the presence of tau (PSP, CBD) or TDP-43 (ALS) aggregates.
Symptomatically, patients with FTD may present at a relatively young age, and many are mistaken for non-progressive psychiatric diseases more commonly seen in young adulthood such as schizophrenia and bipolar disorder. Approximately 50% of patients in our bvFTD cohort were initially diagnosed with a psychiatric illness prior to being recognized as having bvFTD.(17) Improper diagnosis prevents patients and families from properly planning for the future and often leads to inappropriate and potentially harmful treatments.
Epidemiology
The incidence of FTD seems to peak during the sixth and seventh decades of life, at 2.2, 3.3, and 8.9 per 100,000 person-years at 40-49, 50-59, and 60-69 respectively. Mean age at onset varies somewhat across FTD subtypes, with bvFTD having the earliest onset, followed by svPPA and then nvfPPA.(18) The average survival time from diagnosis to death ranges from three years for bvFTD patients with motor neuron disease to approximately twelve years in patients with semantic variant PPA.(19) The mean age at presentation for bvFTD is approximately 58 years, although cases presenting as early as the second decade and as late as the ninth decade have been reported.(18, 20)
The overall prevalence of FTD has likely been underestimated due to misdiagnosis, but may range from 15–22/100,000.(21) The prevalence in Cambridgeshire in the UK was estimated at 15 per 100,000 in the age group of 45-64 years, similar to early-onset Alzheimer's disease.(4) Among patients with progressive dementia, the prevalence of a non-Alzheimer's related frontotemporal atrophy with neuronal loss and gliosis on autopsy has been estimated at between 12% to 16%, but prevalence varies enormously from center to center based upon the age of the cohort and the interest of the clinicians and neuropathologists in FTLD.(22)
In one study from the State of California AD centers, the clinical frequency of FTD in patients with dementia varied across different ethnicities, present in 4.7% of Caucasians, 4.2% of Asians and Pacific Islanders, 2.4% in Latinos and 2.4 % in African –Americans. Many of these cases were diagnosed during the 1990s when FTD was rarely recognized, and the frequencies for all ethnicities are low.(23) There may be a slight male preponderance, although this finding has varied between reports.
Clinical Presentations
In 2011 an international consortium developed revised criteria for the diagnosis of behavioral variant frontotemporal dementia.(24) (Supplement: Table S2) The new criteria were designed to increase diagnostic sensitivity compared to previous criteria by Neary et al.(25) When comparing the new criteria to histological evidence of FTLD the sensitivity was 85% and specificity was 95% for probable bvFTD.(26)
People with bvFTD frequently have limited insight into their illness, making an informant critically important when collecting the patient history.(27) Patients’ family members often do not initially recognize personality changes as being due to a degenerative illness. Patients may present first to psychiatrists or primary care physicians with behavioral symptoms. They may also present to marriage counselors, to human resources offices in the work environment, to addiction programs, and to the criminal system. Symptoms of a global dementia become more apparent as the disease progresses.
Initial personality changes often include social disinhibition and impulsivity. Social norms may be infringed upon with offensive remarks or inappropriate behaviors. Patients may lose the ability to empathize with others, be insensitive to someone else's distress, or become distant and detached in their relationships. Loss of empathy correlated with atrophy in the right anterior temporal and medial frontal regions in a large-scale voxel-based morphometry analysis.(28) An impaired recognition of emotions has been found to correlate with volume loss in the right lateral inferior temporal and right middle temporal gyri.(29)
Patients may become more irritable, and may commit antisocial or even criminal acts. Such acts, however, are usually not malevolent but rather poorly thought out or impulsive in nature, such as driving through a stop sign while being followed by a policeman. In some instances, patients are overly friendly and start conversations that are inappropriately explicit or personal. Often, these patients are overly trusting and become susceptible to financial scams. Such behavior may reflect an inability to judge potential for reward or punishment. Often, this correlates with atrophy in the orbitofrontal cortex, a region that has been associated with weighting potential outcomes of future actions. The subgenual cingulate, medial prefrontal and anterior temporal cortices may also be involved.(30)
Patients with bvFTD often display obsessive-compulsive behavior that varies from simple repetitive motions, like foot tapping or pacing, to frequent playing of computer games or bizarre rituals. Hoarding is common. Some patients become more mentally rigid and resistant to changes in scheduled routines or plans.(31)
Eating habits frequently change in patients with bvFTD. Some patients develop a strong preference for sweets or eat beyond the point of satiety.(32) Family members may have to lock kitchen cabinets and be wary in grocery stores to stop the patient from stealing food. Hyperorality and attempts to eat nonedible objects may also occur.
While patients may exhibit either increased or decreased activity in the beginning of their disease course, all eventually develop symptoms of apathy and inertia. This may progress to mutism and immobility in the end stages of the disease. Anatomically, apathy is thought to correlate with dysfunction of brain circuits that involve the right anterior cingulate and caudate. (30, 33)
Neuropsychological testing in patients with bvFTD frequently reveals executive dysfunction, which often correlates with tissue loss in the dorsolateral prefrontal cortex. Although memory can be better than in Alzheimer's disease, episodic memory can be impaired even in early stages of the disease, which may contribute to misdiagnoses of AD.(34) By contrast, drawing and other visuospatial functions are often remarkably spared in all of the FTD clinical subtypes.
In addition, many patients with bvFTD demonstrate changes with language, including but not restricted to stereotypy of speech and echolalia.(25) As the disease progresses, semantic deficits and progressive reduction of speech occur. These changes represent a disruption of dominant lobar language networks.
There are few physical examination findings specific for bvFTD. FTD may be associated with classical findings of typical Parkinson's disease, and it is also associated with two atypical parkinsonian disorders, PSP and CBD. The classical PSP findings of vertical gaze palsy and axial-predominant parkinsonism may occur early or emerge later in some patients with tau-related FTLD. Similarly, asymmetric parkinsonism, alien limb phenomena, hemineglect and apraxia, the corticobasal syndrome, is often associated with FTLD neuropathology. Amyotrophic lateral sclerosis accompanies bvFTD in about 15% of cases,(35, 36) and can be suggested by the presence of upper and lower motor neuron findings. Both parkinsonism and motor neuron disease, however, can be caused by a variety of other disorders which must be taken into consideration.
Clinicopathological Correlation: Histopathology and Genetics
Behavioral variant FTD involves atrophy of the orbitofrontal, anterior cingulate, anterior insular and anterior temporal cortices, particularly within the right hemisphere.(37) In addition to modular and structural atrophy, functional studies have increasingly demonstrated network connectivity disruption. Different degenerative diseases have been associated with unique networks that may explain particular symptoms. For example, Alzheimer's disease has been tied to changes in the default mode network, progressive supranuclear palsy with changes beginning in the midbrain, and primary progressive aphasia with dominant hemisphere language networks. (38-40) Behavioral variant FTD is associated with changes in the salience network, which is comprised of the orbital-frontal cortex, anterior cingulate cortex, anterior insula and presupplementary motor area. This network is posited to facilitate selection and prioritization of the myriad stimuli presented to us, many laden with potential rewards or punishments. An executive control network linking dorsolateral frontal and parietal neocortices may then mediate planning and actions based on this salient information.(41)
Histologically, components of the salience network such as the anterior insula and anterior cingulate contain Von Economo neurons. These spindle shaped neurons are greatly expanded in number in socially complex animals including cetaceans, elephants and the great apes, which has led to the hypothesis that von Economo neurons have evolved in relation to social behaviors. bvFTD is associated with selective loss of these neurons, the reason for which is a very active area of research.(42) Furthermore, FTLD is associated with frontal and anterior temporal atrophy with microvacuolation and astrogliosis most prominent in layers II and III. (36, 43)
Neurodegenerative diseases are characterized by the accumulation of abnormal protein deposits on immunohistochemistry. Nearly all bvFTD cases fall within one of three histopathological groupings: tau, TDP-43, or fused-in sarcoma (FUS).(5) (Supplement: Table S3) In addition, Alzheimer's disease may occasionally mimic FTD with pathology found in the frontal lobes.(44) For each proteinopathy, there are possible genetic contributions. Genetics may play a role in up to 40% of cases, including direct inheritance in at least 10%.(45) While there is significant variation between individuals with a mutation, on a population level each genetic variant is associated with slightly different symptoms and patterns of neurodegeneration.
Tau
Tau is important in the pathogenesis of a variety of neurodegenerative conditions, including AD and FTD. Tau protein normally helps to maintain microtubular structure. There are six isoforms of tau, three of which have three microtubule binding repeats (3R), and three of which have four repeats (4R). Approximately one-half of all patients with bvFTD have tau aggregates: 3R tau in Pick's disease, and 4R tau in PSP and CBD. (Supplement: Figures S1-S3) In addition to bvFTD, tauopathies are a cause of about 70% of nfvPPA cases in our center.
Mutations in the microtubule-associated protein tau gene (MAPT) have been found in in one series to be present in about 32% of patients with both FTD and a positive family history.(20) We have found MAPT mutations to be less common and they account for approximately 17% of familial forms of FTD in our center. Over 40 different mutations of the MAPT gene have been identified. These mutations tend to cause bifrontal and anterior temporal atrophy.(46)
TDP-43
TDP-43 protein is found in approximately one-half of bvFTD cases on histological examination, and is seen in all cases of FTD-ALS. There are three major patterns of TDP-43 pathology: Type A, Type B, and Type C, which correlate with different forms of FTD. FTLD TDP-43 type A is characterized by inclusions that occur with progranulin mutations but can be seen in other patients with bvFTD or nfvPPA in whom progranulin mutations are absent. Type B is typical for FTD with motor neuron disease, and type C is present in the vast majority of patients with svPPA. (Supplement: Figures S4-S6)
Mutations in the TDP-43 gene itself rarely cause FTD, usually with ALS. The two more common genetic mutations associated with TDP-43 pathology are progranulin and C9orf72 (chromosome 9 open reading frame 72).(47, 48) Mutations in the progranulin gene (GRN) accounted for approximately 8% of all familial forms of FTD. At 62 years, the mean age of onset is slightly higher than other forms of FTD. In contrast to MAPT mutations, GRN mutations usually lead to asymmetric cerebral atrophy, and in addition to bvFTD may be associated with nfvPPA. Like MAPT mutations, progranulin mutations may lead to parkinsonism.(47)
GRN mutations cause haploinsufficiency, resulting in levels of serum progranulin that are approximately one-third of normal.(49) How low progranulin levels mediate neurodegeneration is unknown but is under active study. Mouse models suggest that low levels of progranulin are associated with decreased neural connectivity,(50) and progranulin seems to play a role as a neuronal growth factor. Additionally, low progranulin levels lead to accelerated inflammation.(51, 52) In a recent study Zachary Miller and colleagues demonstrated that patients with GRN mutations exhibited a higher frequency of autoimmune disorders including sarcoid, Sjogren syndrome, rheumatoid arthritis, lupus and chronic lymphocytic colitis and a high peripheral tumor necrosis factor level.(53)
A non-coding GGGGCC hexanucleotide expansion in the C9 open reading frame is strongly associated with both FTD and ALS.(54, 55) Aggregates of a dipeptide- repeat protein generated from the GGGGCC hexanucleotide are found with C9orf72 mutations in various brain regions including the cerebellum. (56, 57) C9orf72 mutations account for roughly 50% of familial FTD cases in our center. Other reports give a range of 13% to 26% among familial FTD cases, compared to 11% to 22% for MAPT and 6% to 22% for GRN. (58)
Many C9orf72 bvFTD patients exhibit bizarre behavior including obsessive-compulsive behaviors, odd rituals, and psychotic features, making this mutation particularly relevant to psychiatric practitioners.(59, 60) Some relatively young patients with slowly progressive psychiatric symptoms may actually suffer from early FTD. Clinical testing is now commercially available for C9orf72 mutations.(60, 61) On MRI, patients with the C9orf72 mutation are more likely to have atrophy in dorsolateral, medial orbitofrontal, anterior temporal, parietal, occipital and cerebellar regions, compared to anteromedial temporal atrophy in MAPT gene mutations.(46)
A smaller proportion of patients with FTD have pathology without TDP-43 or tau aggregates (5%). The majority of these cases have FUS protein deposits.(62, 63) Age of onset in this population tends to be younger (mean 48 years), and they may present with psychiatric symptoms.
These distinctive genetic and neuropathological subtypes may demand different treatment options in the future. For example, because GRN mutations cause a protein deficiency, studies are underway to elevate levels of that protein in patients with the mutation.(64) As with progranulin mutations, TDP-43 type C has been associated with an increased risk of autoimmune disorders, suggesting a unique biochemical pathway which might respond to immunomodulation.(53) Even while these treatments are in development, recognition of the genetic, histologic, and syndromic variability of these diseases may help prevent misdiagnosis.
Differential Diagnosis
A careful history, combined with laboratory studies and neuroimaging, can usually exclude reversible mimics of FTD such as neurological infections, metabolic disorders, vascular disease and paraneoplastic conditions.
Patients with bvFTD may exhibit symptoms suggestive of obsessive-compulsive disorder, bipolar disorder, depression or schizophrenia. An onset of symptoms in patients in middle age should lead to consideration of bvFTD's inclusion in the differential diagnosis.(17) Other neurodegenerative disorders can be mistaken for FTD-spectrum disorders, particularly AD, vascular dementia and dementia with Lewy bodies. Both normal pressure hydrocephalus and low intracranial pressure syndromes have sometimes been mistaken for FTD, although their imaging features should immediately draw attention to a non-FTD diagnosis. (65)
An entity initially described by John Hodges and Chris Kipps, “slowly progressive FTD” can be a difficult diagnostic dilemma.(66) These are patients who seem to meet the bvFTD phenotype but progress slowly or not at all, and do not show atrophy on MRI. Some of these individuals have FTD, and gene carriers can progress very slowly. In other patients the initial history is misleading or incomplete, and further history taking clarifies the bvFTD diagnosis. Psychiatric disorders can also account for some of these bvFTD phenotypical patients. While sometimes a psychiatric syndrome may be misdiagnosed as FTD, in our experience it is more common to initially diagnose a dementia as being non-degenerative. (67, 68)
Statistically, someone under the age of 65 is approximately as likely to have AD as FTD.(4) Early-onset AD disease frequently does not have the classical amnestic presentation of later-onset AD, but may instead have prominent executive dysfunction or language dysfunction.(69) Conversely, memory loss may be a feature of FTD. Interpersonal dysfunction is unusual for AD, and may be a useful clinical distinguishing feature of bvFTD.
Diagnosis of Frontotemporal Dementia
A diagnosis of FTD is made primarily by clinical assessment. Despite potential diagnostic pitfalls, the presence of abnormal social conduct, change of eating habits, stereotyped behaviors, and apathy in the relative absence of memory or visuospatial deficit will usually lead to the correct diagnosis. (24)
A short neuropsychological battery to compare different cognitive domains can be an efficient way of aiding the differential diagnosis. Neuropsychological evaluation should show primarily executive dysfunction, with relative sparing of visuospatial ability. Memory should be relatively spared, but may be involved especially later in the disease. The NIH EXAMINER battery is designed to test executive functions, and can classify bvFTD and AD with 73% accuracy.(70)
The 2011 bvFTD criteria require neuroimaging data in the form of selective atrophy on MRI or hypometabolism on PET scan to transform a diagnosis from “possible” into “probable” bvFTD.(24) Early in the disease, however, imaging may be normal. bvFTD patients typically show atrophy in the frontoinsular structures and other regions of the salience network. (Figure 1). svPPA patients show atrophy at the temporal poles, and nfvPPA will show volume loss in the left perisylvian region. PSP classically shows midbrain atrophy with relative sparing of the pons. (46, 71) CBS often demonstrates dorsal atrophy around the central sulcus.(11) Patterns of atrophy may also suggest an underlying histological or genetic etiology. Furthermore, neuroimaging studies may help exclude alternative disorders such as vascular dementia or normal pressure hydrocephalus.
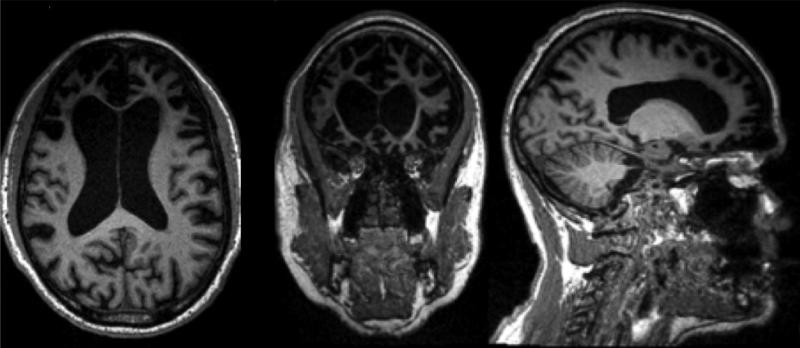
Figure 1.
A T1 magnetic resonance image of the brain in axial, coronal, and sagittal cuts, shown in neurological convention, showing severe anterior predominant atrophy with hydrocephalus ex vacuo in a patient with advanced behavioral variant frontotemporal dementia (bvFTD).
Functional neuroimaging studies such as fluorodeoxyglucose positron emission tomography (FDG-PET) may be more sensitive than MRI in early stages, and can be clinically useful in distinguishing FTD from AD.(72) FDG-PET reveals hypometabolism of frontal, anterior cingulate and anterior temporal regions in FTD, in contrast to temporoparietal and posterior cingulate hypometabolism in AD. (Figure 2) The increasing prominence of ligand imaging such as florbetapir in the diagnosis of Alzheimer's disease can help suggest the presence of AD pathology, but should not necessarily be thought to exclude co-morbid frontotemporal dementia. Tau ligands may also become more widely available for research in the near future.(73)
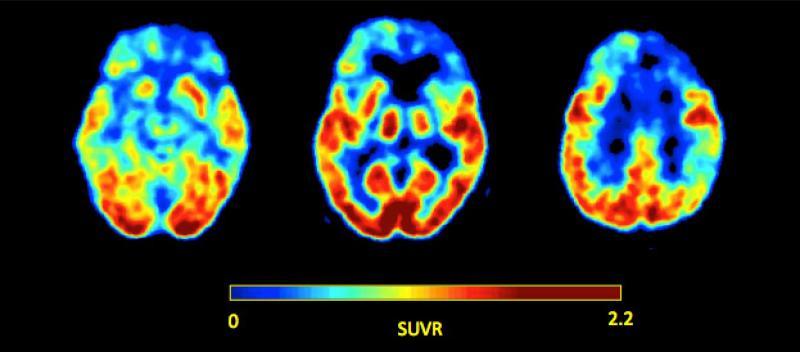
Figure 2.
Axial fluorodeoxyglucose positron emission tomography (FDG-PET) images of a patient with advanced bvFTD, shown in neurological convention, demonstrating hypometabolism in the anterior lobes bilaterally, but right more so than left. Image courtesy of Pia Ghosh of the UCSF Memory and Aging Center.
Other functional imaging techniques such as functional MRI have shown promise in distinguishing affected systems in research, but have not yet been used in the diagnosis of individuals. Similarly, electroencephalograms have distinguished FTD from AD, but are not used in routine diagnosis.
Cerebrospinal fluid (CSF) tau levels can be high or low in FTD and cannot help to rule in FTD. A cutoff of 1.06 in the tau/amyloid-beta ratio had a 79% sensitivity and 97% specificity in distinguishing syndromic FTD from AD.(74)
Genetic testing is available for several mutations that cause familial FTD. Such testing should be done selectively, and guided by the family history, clinical syndrome and results of any pathological studies. Genetic counseling is advisable for those who wish to know the results of any testing done.(75)
Treatment
The proper treatment of FTD requires an individually tailored approach. Because no treatment is yet available that changes the course of the neurodegenerative disease, the focus of medical therapy is on symptomatic relief.(76) The difficult behaviors typical for bvFTD can place a great deal of stress on caregivers. Education and counseling about the neurological basis of the disease can offer some relief to frustrated friends and family. Resources such as The Association for Frontotemporal Degeneration (http://www.theaftd.org/) may be useful.
Many non-pharmacologic interventions can assist the management of a patient with frontotemporal dementia.(77) Safety is a serious concern due to possible impulsivity and impaired judgment. Financial guidance and driving safety should be discussed early in the disease course.(78) Physical safety around the home should also be considered, as well as early retirement if the patient is still working.(79)
Exercise has been shown to benefit mood, cognition and overall health in patients with dementia.(80) Physical therapy may be helpful in patients with mobility problems such as parkinsonism, including reducing the risk of falls. Speech therapy may have some benefits for patients with language deficits. (81) Problematic behavior can be managed by assessing for causes of pain or delirium, and may also be handled with a combination of redirection, distraction, and offering simple choices. Most patients benefit from a stable and structured environment. Finally, caregiver stress is significant in FTD and should be addressed as early as possible.
Pharmacological treatment of neurobehavioral symptoms in frontotemporal dementia should ideally suit typical changes in neurotransmitter systems occurring with the disease.(82, 83) In nondemented populations, decreased brain levels of serotonin are associated with many behavioral problems seen in FTD.(84) FTD has been shown to have serotonergic network disruption based on autopsy, neuroimaging and CSF studies. (84-87) Some studies have suggested that serotonin selective reuptake inhibitors (SSRIs) are effective in helping with various symptoms of FTD, including disinhibition, impulsivity, repetitive behaviors and eating disorders.(88, 89) Trazodone has been effective in treating agitation and aggression in FTD, and may also function as a sleep aid if necessary.(90)
Dopaminergic function is altered not only in association with extrapyramidal symptoms, but may also be related to agitated behavior.(88, 91) Antipsychotic medications can help with neurobehavioral symptoms in FTD.(92, 93) Since many patients with FTD are predisposed towards parkinsonian symptoms, however, they are likely vulnerable to extrapyramidal side effects.(94) For this reason, antipsychotics are usually used after behavioral modification and SSRIs. Agents with relatively less D2 receptor antagonism such as quetiapine are preferred if antipsychotics are used.(95) Furthermore, patients and family members should be cautioned about the increased mortality associated with antipsychotic use in the elderly with dementia.
Unlike AD, the cholinergic system is relatively preserved in FTD.(96) Trials have not shown a convincing benefit for cholinesterase inhibitors in cognition or behavior.(97, 98) Several other drugs, including memantine, lithium and some anticonvulsants, have been tested in FTD with negative or inconclusive results.(99, 100) Risks of toxicity are generally deemed to outweigh theoretical benefits.
Conclusions
FTD is increasingly recognized as not only a common cause of early-onset dementia, but also as a cause of manic, depressive, obsessive-compulsive, or psychotic symptoms. The syndrome of bvFTD can result from a number of different proteinopathies and some cases have a genetic etiology. Recognizing the core features of bvFTD as well as the variety of possible presentations can avoid misdiagnosis. This will permit the appropriate selection of symptomatic therapies and may identify subtypes that may benefit from future therapies. Promising areas of research include the role of von Economo neurons, PET imaging with tau ligands, autoimmune treatments for TDP-C disorders, and protein replacement for progranulin mutations.
Supplementary Material
Acknowledgements
We would like to thank the Tau Consortium, the Consortium for Frontotemporal Dementia Research, and the teams of the Primary Program Grant and the Alzheimer's Disease Research Center of the Memory and Aging Center of the University of California, San Francisco. This publication was made possible by grant number P50AG023501 and grant number P01AG019724 from NIH National Institute on Aging. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Aging or NIH.
Dr. Miller is a board member of the Larry L. Hillblom Foundation, the John Douglas French Foundation, and the Tau Consortium. Dr. Miller has consulted for Tau Rx, LTD, Allon Therapeutics, Bristol-Myers Squibb, Siemens Molecular Imaging, and Eli Lilly US. He receives funding from the National Institute of Health/National Institute of Aging. Dr. Miller receives royalties from Cambridge University Press, Neurocase, and Guilford Publications, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Pressman reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Pick A. Uber die beziehungen der senilen hirnatropie zur aphasie. Pragen Medizinischen Wochenschrift. 1892;17:165–167. [Google Scholar]
- 2.Pasquier F, Petit H. Frontotemporal dementia: its rediscovery. European Neurology. 1997;38:1–6. doi: 10.1159/000112894. [DOI] [PubMed] [Google Scholar]
- 3.Brunnstrom H, Gustafson L, Passant U, Englund E. Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Archives of Gerontology and Geriatriatrics. 2009;49:146–149. doi: 10.1016/j.archger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 5.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathologica. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kertesz A, Hillis A, Munoz D. Frontotemporal degeneration, Pick's disease, Pick complex, and Ravel. Annals of Neurology. 2003;54(Suppl 5):S1. doi: 10.1002/ana.10595. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS drugs. 2010;24:375–398. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu WZ, Papma JM, de Koning I, Donker Kaat L, Seelaar H, Reijs AEM, et al. Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2012;83:910–915. doi: 10.1136/jnnp-2011-302035. [DOI] [PubMed] [Google Scholar]
- 10.Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, et al. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick's disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathologica. 2001;101:167–173. doi: 10.1007/s004010000283. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, et al. Clinicopathological correlations in corticobasal degeneration. Annals of Neurology. 2011;70:327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rankin KP, Mayo MC, Seeley WW, Lee S, Rabinovici G, Gorno-Tempini ML, et al. Behavioral variant frontotemporal dementia with corticobasal degeneration pathology: phenotypic comparison to bvFTD with Pick's disease. Journal of Molecular Neuroscience. 2011;45:594–608. doi: 10.1007/s12031-011-9615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lomen-Hoerth C. Clinical phenomenology and neuroimaging correlates in ALS-FTD. Journal of Molecular Neuroscience. 2011;45:656–662. doi: 10.1007/s12031-011-9636-x. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong RA, Carter D, Cairns NJ. A quantitative study of the neuropathology of 32 sporadic and familial cases of frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP). Neuropathology & Applied Neurobiology. 2012;38:25–38. doi: 10.1111/j.1365-2990.2011.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SE, Seeley WW, Poorzand P, Rademakers R, Karydas A, Stanley CM, et al. Clinical characterization of bvFTD due to FUS neuropathology. Neurocase. 2012;18:305–317. doi: 10.1080/13554794.2011.604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain : a journal of neurology. 2011;134:2565–2581. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. Journal of Clinical Psychiatry. 2011;72:126–133. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Archives of Neurology. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 19.Roberson ED, Hesse JH, Rose KD, Slama H, Johnson JK, Yaffe K, et al. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65:719–725. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- 20.Rosso SM, Donker Kaat L, Baks T, Joosse M, de Koning I, Pijnenburg Y, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain : a journal of neurology. 2003;126:2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 21.Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. International Review of Psychiatry. 2013;25:130–137. doi: 10.3109/09540261.2013.776523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greicius MD, Geschwind MD, Miller BL. Presenile dementia syndromes: an update on taxonomy and diagnosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:691–700. doi: 10.1136/jnnp.72.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou C, Yaffe K, Perrez-Stable E, Miller B. Frequency of dementia etiologies in four ethnic groups. Dementia and Geriatric Cognitive Disorders. 2006;22:42–47. doi: 10.1159/000093217. [DOI] [PubMed] [Google Scholar]
- 24.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : a journal of neurology. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neary D, Snowden J, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 26.Harris JM, Gall C, Thompson JC, Richardson AMT, Neary D, Plessis Dd, et al. Sensitivity and specificity of FTDC criteria for behavioral variant frontotemporal dementia. Neurology. 2013;80:1–7. doi: 10.1212/WNL.0b013e318292a342. [DOI] [PubMed] [Google Scholar]
- 27.Mendez M, Shapira JS. Loss of Insight and Functional Neuroimaging in Frontotemporal Dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:413–416. doi: 10.1176/jnp.17.3.413. [DOI] [PubMed] [Google Scholar]
- 28.Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain : a journal of neurology. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen HJ, Wilson MR, Schauer GF, Allison S, Gorno-Tempini ML, Pace-Savitsky C, et al. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44:365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Rosen H, Allison S, Schauer G, Gorno-Tempini M, Weiner M, Miller B. Neuroanatomical correlates of behavioural disorders in dementia. Brain : a journal of neurology. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry DC, Whitwell JL, Boeve BF, Pankratz VS, Knopman DS, Petersen RC, et al. Voxel-based morphometry in patients with obsessive-compulsive behaviors in behavioral variant frontotemporal dementia. European Journal of Neurology. 2012;19:911–917. doi: 10.1111/j.1468-1331.2011.03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wooley J, Gorno-Tempini M, Seeley W, Rankin K, Lee S, Mathews B, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 33.Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behavioural Neurology. 2012;25:127–136. doi: 10.3233/BEN-2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Annals of Neurology. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 35.Gustafson L. Frontal lobe degeneration of non-Alzheimer type. II. Clinical picture and differential diagnosis. Archives of Gerontology & Geriatrics. 1987;6:209–223. doi: 10.1016/0167-4943(87)90022-7. [DOI] [PubMed] [Google Scholar]
- 36.Brun A. Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology. Archives of Gerontology & Geriatrics. 1987;6:193–208. doi: 10.1016/0167-4943(87)90021-5. [DOI] [PubMed] [Google Scholar]
- 37.Seeley W, Crawford R, Rascovsky K, Kramer J, Weiner M, Miller B, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Annals of Neurology. 2013 doi: 10.1002/ana.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Procedures of the National Academy of Science U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Annals of nNeurology. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- 43.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 44.Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, et al. Focal cortical presentations of Alzheimer's disease. Brain : a journal of neurology. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 45.Goldman JS, Rademakers R, Huey ED, Boxer AL, Mayeux R, Miller BL, et al. An algorithm for genetic testing of frontotemporal lobar degeneration. Neurology. 2011;76:475–483. doi: 10.1212/WNL.0b013e31820a0d13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain : a journal of neurology. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 48.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 49.Rademakers R, Baker M, Gass J, Adamson J, Huey E, Mommeni P, et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C-->T (Arg493X) mutation: An international initiative. Lancet Neurology. 2007;6:857–868. doi: 10.1016/S1474-4422(07)70221-1. [DOI] [PubMed] [Google Scholar]
- 50.Tapia L, Milnerwood A, Guo A, Mills F, Yoshida E, Vasuta C, et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. Journal of Neuroscience. 2011;31:11126–11132. doi: 10.1523/JNEUROSCI.6244-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, et al. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Procedures of the National Academy of Science U S A. 2011;108:4441–4446. doi: 10.1073/pnas.1100650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward ME, Miller BL. Potential mechanisms of progranulin-deficient FTLD. Journal of Moledular Neuroscience. 2011;45:574–582. doi: 10.1007/s12031-011-9622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller Z, Rankin K, Graff-Radford N, Takada L, Sturm V, Cleveland C, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. Journal of Neurology, Neurosurgery and Psychiatry. 2013 doi: 10.1136/jnnp-2012-304644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 58.Majounie E, Abramzon Y, Renton AE, Perry R, Bassett SS, Pletnikova O, et al. Repeat expansion in C9ORF72 in Alzheimer's disease. New England Journal of Medicine. 2012;366:283–284. doi: 10.1056/NEJMc1113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, et al. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology. 2012;79:1002–1011. doi: 10.1212/WNL.0b013e318268452e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain : a journal of neurology. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. Journal of Neurology, Neurosurgery and Psychiatry. 2012;83:358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain : a journal of neurology. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urwin H, Josephs KA, Rohrer JD, Mackenzie IR, Neumann M, Authier A, et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathologica. 2010;120:33–41. doi: 10.1007/s00401-010-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cenik B, Sephton CF, Dewey CM, Xian X, Wei S, Yu K, et al. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. Journal of Biological Chemistry. 2011;286:16101–16108. doi: 10.1074/jbc.M110.193433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wicklund MR, Mokri B, Drubach DA, Boeve BF, Parisi JE, Josephs KA. Frontotemporal brain sagging syndrome: an SIH-like presentation mimicking FTD. Neurology. 2011;76:1377–1382. doi: 10.1212/WNL.0b013e3182166e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kipps CM, Hodges JR, Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the ‘bvFTD phenocopy syndrome’. Current Opinion in Neurology. 2010;23:628–632. doi: 10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- 67.Davies R, Kipps C, Mitchell J, Kril J, Halliday G, Hodges J. Progression in frontotemporal dementia: Identifying a benign behavioral variant by magnetic resonance imaging. Archives of Neurology. 2006;63:1627–1631. doi: 10.1001/archneur.63.11.1627. [DOI] [PubMed] [Google Scholar]
- 68.Woolley J, Wilson M, Hung E, Gorno-Tempini M, Miller B, Shim J. Frontotemporal Dementia and Mania. American Journal of Psychiatry. 2007;164:1811–1816. doi: 10.1176/appi.ajp.2007.07061001. [DOI] [PubMed] [Google Scholar]
- 69.Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic presentations of early-onset Alzheimer's disease. American Journal of Alzheimer's Disease and other Dementias. 2012;27:413–420. doi: 10.1177/1533317512454711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Possin K, Feigenbaum D, Rankin K, Smith G, Boxer A, Wood K, et al. Neurology. American Academy of Neurology; San Diego: 2013. Select Executive Function Measures Distinguish Behavioral Variant Frontotemporal from Alzheimer Dementia. p. P03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Massey L, Miscallef C, Paviour D, O'Sullivan S, Ling H, Williams D. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Movement Disorders : Official Journal of the Movement Disorder Society. 2012 doi: 10.1002/mds.24968. [DOI] [PubMed] [Google Scholar]
- 72.Womack KB, Diaz-Arrastia R, Aizenstein HJ, Arnold SE, Barbas NR, Boeve BF, et al. Temporoparietal hypometabolism in frontotemporal lobar degeneration and associated imaging diagnostic errors. Archives of Neurology. 2011;68:329–337. doi: 10.1001/archneurol.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bian H, Swieten JV, Leight S, Massimo L, Wood E, Forman M, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldman JS, Adamson J, Karydas A, Miller BL, Hutton M. New genes, new dilemmas: FTLD genetics and its implications for families. American Journal of Alzheimer's Disease & Other Dementias. 2007;22:507–515. doi: 10.1177/1533317507306662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perry R, Miller B. Behavior and treatment in frontotemporal dementia. Neurology. 2001;56:S46–51. doi: 10.1212/wnl.56.suppl_4.s46. [DOI] [PubMed] [Google Scholar]
- 77.Merrilees J. A model for management of behavioral symptoms in frontotemporal lobar degeneration. Alzheimer's Disease and Associated Disorders. 2007;21:S64–69. doi: 10.1097/WAD.0b013e31815bf774. [DOI] [PubMed] [Google Scholar]
- 78.Piguet O, Hornberger M, Mioshi E, Hodges J. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurology. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- 79.Talerico K, Evans L. Responding to safety issues in frontotemporal dementias. Neurology. 2001;56:S52–55. doi: 10.1212/wnl.56.suppl_4.s52. [DOI] [PubMed] [Google Scholar]
- 80.Cheng ST, Chow PK, Song YQ, Yu EC, Chan AC, Lee TM, et al. Mental and Physical Activities Delay Cognitive Decline in Older Persons With Dementia. The American Journal of Geriatric Psychiatry : official Journal of the American Association for Geriatric Psychiatry. 2012 doi: 10.1016/j.jagp.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 81.Robinson K. Rehabilitation applications in caring for patients with Pick's disease and frontotemporal dementias. Neurology. 2001;56:S56. doi: 10.1212/wnl.56.suppl_4.s56. [DOI] [PubMed] [Google Scholar]
- 82.Huey E, Putnam K, Grafman J. A systematic reviw of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bowen D, Procter A, Mann D. Imbalance of a serotonergic system in frontotemporal dementia: implication for pharmacotherapy. Psychopharmacology. 2008;196:603–610. doi: 10.1007/s00213-007-0992-8. [DOI] [PubMed] [Google Scholar]
- 84.Sparks D, Markesbery W. Altered serotonergic and cholinergic synaptic markers in Pick's disease. Archives of Neurology. 1991;48:796–799. doi: 10.1001/archneur.1991.00530200032014. [DOI] [PubMed] [Google Scholar]
- 85.Engelborghs S, Vloeberghs E, Maertens K. Evidence for an association between the CSF HVA:5-HIAA ratio and aggressiveness in frontotemporal dementia but not in Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:1080. [PMC free article] [PubMed] [Google Scholar]
- 86.Procter A, Qurne M, Francis P. Neurochemical features of frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 1999;10(Suppl 1):80–84. doi: 10.1159/000051219. [DOI] [PubMed] [Google Scholar]
- 87.Franceschi M, Anchisi D, Pelati O. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. A. Annals of Neurology. 2005;57:216–225. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- 88.Manoochehri M, Huey ED. Diagnosis and management of behavioral issues in frontotemporal dementia. Current Neurology and Neuroscience Reports. 2012;12:528–536. doi: 10.1007/s11910-012-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swartz J, Miller B, Lesser I. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. Journal of Clinical Psychiatry. 1997;58:212–216. [published erratum appears in J Clin Psychiatry 1997 Jun; 58 (6): 275].
- 90.Lebert F, Stekke W, Hasenbroekz C. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dementia and Geriatric Cognitive Disorders. 2004;17:355–359. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 91.Engelborghs S, Vloeberghs E, Le Bastard N, Van Buggenhout M, Marien P, Somers N, et al. The dopaminergic neurotransmitter system is associated with aggression and agitation in frontotemporal dementia. Neurochemistry International. 2008;52:1052–1060. doi: 10.1016/j.neuint.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 92.Moretti R, Torre P, Antonello R, Cazzato G, Griggio S, Bava A. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer's disease and other dementias: a 24-month follow-up of 68 patients. American Journal of Alzheimer's Disease and other Dementias. 2003:18. doi: 10.1177/153331750301800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fellgiebel A, Muller M, Hiemke C, Bartenstein P, Schreckenberger M. Clinical improvement in a case of frontotemporal dementia under aripiprazole treatment corresponds to partial recovery of disturbed frontal glucose metabolism. World Journal of Biological Psychiatry. 2007;8:123–126. doi: 10.1080/15622970601016538. [DOI] [PubMed] [Google Scholar]
- 94.Pijnenburg Y, Sampson E, Harvey R, Fox N, Rossor M. Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. International Journal of Geriatric Psychiatry. 2003;18:67–72. doi: 10.1002/gps.774. [DOI] [PubMed] [Google Scholar]
- 95.Komossa K, Rummel-Kluge C, Schmid F. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Systematic Revue. 2010 doi: 10.1002/14651858.CD006625.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hansen L, Deteresa R, Tobias H, Alford M, Terry R. Neocortical morphometry and cholinergic neurochemistry in Pick's disease. Americal Journal of Pathology. 1988;131:507–518. [PMC free article] [PubMed] [Google Scholar]
- 97.Hu B, Ross L, Neuhaus J, Knopman D, Kramer J, Boeve B, et al. Off-label medication use in frontotemporal dementia. American Journal of Alzheimer's Disease and Other Dementias. 2010;25:128. doi: 10.1177/1533317509356692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mendez M, Shapira J, MucMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. American Journal of Geriatric Psychiatry. 2007:15. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 99.Boxer AL, Knopman DS, Kaufer DI, Grossman M, Onyike C, Graf-Radford N, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. The Lancet Neurology. 2013;12:149–156. doi: 10.1016/S1474-4422(12)70320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Freedman M. Frontotemporal dementia: recommendations for therapeutic studies, designs, and approaches. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2007;34(Suppl 1):S118–124. doi: 10.1017/s0317167100005680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.