Abstract
Plasmacytoid dendritic cells (pDC) produce type I interferon (IFN-I) in response to viruses and are routinely identified in mice by SiglecH expression. SiglecH is a sialic acid-binding immunoglobulin-like lectin that has an immunomodulatory role during viral infections. Here, we evaluated the impact of SiglecH deficiency on cytokine responses in the presence and absence of pDC. We found that lack of SiglecH enhanced IFN-I responses to viral infection regardless of whether pDC were depleted or not. We also examined the expression pattern of SiglecH in this study. We observed that SiglecH was expressed by specialized macrophages and progenitors of classical DC (cDC) and pDC. Accordingly, marginal zone macrophages and pDC precursors were eliminated in newly generated SiglecH-DTR Tg mice but not CLEC4C-DTR Tg mice after diphtheria toxin (DT) treatment. Using two different bacterial models, we found that SiglecH-DTR Tg mice injected with DT had altered bacterial uptake and were more susceptible to lethal Listeria monocytogenes infection than DT-treated CLEC4C-DTR Tg mice. Taken together, our findings suggest that lack of SiglecH may affect cytokine responses by cell types other than pDC during viral infections perhaps by altering viral distribution or burden and that cell depletion in SiglecH-DTR Tg mice encompasses more than pDC.
Keywords: SiglecH, plasmacytoid, dendritic cell, macrophage, interferon, virus
Introduction
Plasmacytoid dendritic cells (pDC) are bone marrow-derived cells that are able to secrete large amounts of type I interferons (IFN-I) and thus, are considered important mediators of antiviral responses (1, 2). There is a great deal of controversy in the field regarding pDC as a major source of IFN-I and as antigen-presenting cells in vivo. Our previous work has shown that pDC are an early, transient source of IFN-I and have a modest impact on virus-specific CD8 T cell responses (3–5). In contrast, another study has demonstrated that pDC have a more dramatic role in IFN-I production and promoting T cell-mediated immunity to viral infection (6).
In our model, pDC express the diphtheria toxin receptor (DTR) under the control of the CLEC4C promoter (CLEC4C-DTR Tg) and can be eliminated with diphtheria toxin (DT) administration (3). CLEC4C, also known as blood dendritic cell antigen 2, is a type II C type lectin that is specifically expressed by human pDC (7, 8). In the second model, DTR has been inserted into the SiglecH locus (SiglecHDTR/DTR) (6). SiglecHDTR/DTR mice lack SiglecH expression and can be depleted of pDC with DT. SiglecH is a member of the sialic acid-binding immunoglobulin-like lectin family that is commonly used to discriminate pDC from other cell types in mice and appears to have an immunomodulatory role during viral infections (6, 9–11). Because the results and conclusions obtained from these two inducible pDC depletion models differ in magnitude, it is important to investigate reasons for discrepancies.
In this study, we evaluated the impact of SiglecH deficiency on cytokine responses and the expression pattern of SiglecH in vivo using wild-type (WT) mice, previously generated SiglecH-eGFP knockin mice (3), CLEC4C-DTR Tg mice and newly generated SiglecH-DTR Tg mice. We found that purified pDC from SiglecHeGFP/eGFP (SiglecH-deficient) and WT mice produced similar amounts of IFN-α after stimulation with CpGA or murine cytomegalovirus (MCMV) ex vivo. In vivo, infection with MCMV, but not CpGA administration, resulted in increased serum IFN-α levels in SiglecHeGFP/eGFP mice. These data suggested that lack of SiglecH might influence cytokine responses by pDC or perhaps other cell types in vivo during an active viral infection. To investigate this we bred heterozygous SiglecHeGFP/+ and homozygous SiglecHeGFP/eGFP mice to CLEC4C-DTR Tg mice to distinguish the effect of SiglecH deletion from pDC depletion. Ablation of pDC in CLEC4C-DTR Tg × SiglecHeGFP/eGFP mice revealed that lack of SiglecH enhanced IFN-I responses to viral infection in the presence and absence of pDC.
Given that SiglecH deficiency may affect cytokine responses by cells other than pDC, we decided to evaluate the expression pattern of SiglecH in vivo using anti-SiglecH monoclonal antibodies and heterozygous SiglecHeGFP/+ mice. We found that SiglecH was expressed by pDC and specialized macrophage subsets such as marginal zone macrophages (MZM), lymph node (LN) medullary macrophages and microglia. SiglecH was also expressed by immediate precursors of pDC (pre-pDC) in the bone marrow (BM), which have the plasticity to differentiate into pDC and classical DC (cDC) (12, 13). Analysis of SiglecH-DTR Tg and CLEC4-DTR Tg mice indicated that MZM and pre-pDC were eliminated in DT-treated SiglecH-DTR Tg mice but not CLEC4C-DTR Tg mice. Therefore, administration of DT to mice that express DTR under the control of the SiglecH promoter mice might affect a large number of antigen-presenting cells. Indeed, using two different bacterial infection models, we found that SiglecH-DTR Tg mice injected with DT had altered bacterial uptake and were more susceptible to lethal Listeria monocytogenes infection than DT-treated CLEC4C-DTR Tg mice. Thus, we envision that the broad expression pattern of SiglecH potentially explains why data derived from inducible pDC ablation models may be different.
Materials and Methods
Mice, treatments and infections
Animal studies were approved by the Washington University Animal Studies Committee. SiglecH-eGFP knockin mice and CLEC4C-DTR Tg mice, both on a C57BL/6 background, were bred in house (3). SiglecH-DTR Tg mice were generated and bred at NIH (C57BL/6) or at Nanyang Technological University (BALB/c). CLEC4C-DTR Tg mice and SiglecH-DTR Tg mice were injected i.p. with 100–200 ng or 200–500 ng of DT (Sigma-Aldrich), respectively. Non-Tg control mice were also injected with DT in some experiments. CpGA 2216 (Operon, 6 µg/mouse) was complexed with DOTAP and injected i.v. Herpes simplex virus 1 (HSV-1) KOS strain was injected i.v. at 1×107 pfu. MCMV Smith strain was injected i.p. at 5×104 pfu. L. monocytogenes expressing OVA (LM-OVA) (14) was injected i.p. at 2.5×107 cfu. Alexa Fluor 647 labeled, heat-killed S. pneumoniae R36A was a generous gift from J. F. Kearney (University of Alabama at Birmingham) and injected i.v. at ~1×108 cfu per mouse.
Generation of SiglecH-DTR Tg mice
C57BL/6-Tg(SiglecH-hDTR-EGFP)NCr transgenic mice were generated by Bacterial Artificial Chromosome (BAC) recombineering. The BAC clone encoding the complete SiglecH gene locus (RPA24-163A12) was obtained from the BACPAC Resources Center at Children's Hospital Oakland Research Institute (Oakland, CA). The BAC clone was modified by recombination using a shuttle vector containing a bicistronic cassette consisting of the cDNA sequences encoding for the human DTR and eGFP. The cassette was flanked by two homologous regions targeting the transgenes to the desired site of insertion (SiglecH exon I, after the second triplet of the open reading frame). The modified BAC clone was linearized and injected into the pronuclei of fertilized C57BL/6NCr oocytes at the Laboratory Animal Science Program facility (National Cancer Institute, Frederick, MD). Single cell-embryos were implanted in pseudogravid females and litters were screened to select transgenic mouse founders. Two transgenic mouse lines with high transgene expression were established. The plasmid containing the hDTR sequence used in the shuttle vector preparation was a generous gift of Dr. T. Walzer (Université de Lyon, France). SiglecH-DTR Tg mice on a BALB/c background were generated via BALB/c ES cells transfected with recombineered BAC clones (Siglec-H: RP24-265E12) carrying insertions of human DTR sequence with its pA site in the initiation codons replacing the first coding exon of the SiglecH gene (15).
Generation of SiglecH-DTR Tg BM chimeras
BM from C57BL/6 SiglecH-DTR Tg mice was prepared from tibias and femurs. Red blood cells were lysed with RBC lysis buffer (Sigma-Aldrich). BM cells were injected i.v. into irradiated age/gender matched C57BL/6 mice purchased from The Jackson Laboratory (5–10 million cells per mouse) 8–10 h after irradiation. Chimeric mice were used in experiments 4–5 months later.
Cell preparations
Spleens were processed as previously described (3). BM was harvested from tibias and femurs. Microglia was isolated as described (16). pDC were enriched from BM by negative selection using the Plasmacytoid Dendritic Cell Isolation Kit II from Miltenyi Biotec and stimulated with CpGA (3 or 6 µg/ml). Purity was ~50% after enrichment. FACS-sorted pDC (purity >98%) were stimulated with CpGA (6 µg/ml) or MCMV tissue culture stock (MOI 10:1). Cells were cultured in 96-well flat bottom plates at 0.25–1×105 cells/well with CpGA or MCMV. Sorted pre-pDC (1×105 cells/well) were cultured in 96-well flat bottom plates in complete medium with GM-CSF (1 ng/ml) or Flt3L (10 ng/ml) (PeproTech) for 3 days.
Antibodies, flow cytometry and cell sorting
Antibodies were purchased from BioLegend, eBioscience or BD Biosciences. The following clones were used: SiglecH (551 or 440c), CD11c (HL3), Ly6C (AL-21), CCR9 (eBioCW-1.2), B220 (RA3-6B2), CD11b (M1/70), CD45 30-F11), CD4 (GK1.5), CD8α (53-6.7), Gr-1 (RB6-8C5), F4/80 (BM8), IA/IE (M5/114.15.2). Flow cytometry was conducted on a FACSCalibur or FACSCanto (BD Biosciences) and analyzed with FlowJo (Tree Star, Inc.). Cell sorting was performed on a FACSAria II (BD Biosciences). Mature pDC and pre-pDC were defined/sorted as SiglecH+B220+CCR9+ and SiglecH+B220loCCR9lo/− cells, respectively. cDC were sorted from spleens into CD11chiMHCII+CD8α+ and CD11chiMHCII+CD8α− subsets.
Cytokine analysis and qPCR
IFN-α levels were determined by ELISA (PBL Interferon Source). Cytokines were measured by Cytometric Bead Array (BD Biosciences). Expression levels of SiglecH and E2-2 were measured by qPCR and normalized to GAPDH. SiglecH forward primers: 5’-ATT TCT GTG AGG AAA GGA TC-3’ and 5’-AAT TCA CAG AAC TCC ACA GC-3’. SiglecH reverse primers: 5’-TAG GAC GAC CAA GCT CCA GT-3’ and 5’-GAT CCC AAG AAG CAG GAA TT-3’. E2-2 forward primers: 5’-TGA GAT CAA ATC CGA CGA-3’ and 5’-ACA ACG GAG CGA TGG GTA G-3’. E2-2 reverse primers: 5’-CGT TAT TGC TAG ATC TTG ACC T-3’ and 5’-GCA GGA GAG AAT GGC TGC CT-3’.
Bloodwork and kidney histology
Whole blood was collected by cardiac puncture into EDTA tubes for complete blood counts or in serum collection tubes for measurements of blood urea nitrogen, creatinine and total protein. Tests were performed by the Division of Comparative Medicine at Washington University School of Medicine. For H&E staining, kidneys were fixed with 10% buffered formalin solution and embedded in paraffin. 5 µm sections were collected then stained with hematoxylin and eosin.
Immunohistochemistry (IHC) and Immunofluorescence (IF)
For IHC: five micron frozen tissue sections were used for immunohistochemical staining to visualize SiglecH+ cells in spleens and lymph nodes. Digital images were taken using the Olympus BX60 microscope and captured using a DP-70 Olympus digital camera and processed using Analysis Image Processing software (Olympus). For IF: eight micron frozen spleen sections were fixed in acetone for 5 minutes at room temperature (RT) and stored at −80°C. Frozen sections were blocked with 10% horse serum for 20 min at RT. Primary antibodies to MARCO, Gr-1, SIGN-R1 and Sialoadhesin were applied to tissue sections for 30 min at RT. After washing with PBS, fluorescent-conjugated anti-rat secondary antibodies were added to slides for 30 min at RT. Slides were mounted with Fluoromount-G and imaged on a Zeiss LSM 510 META Confocal Laser Scanning Microscope. IF images were adjusted globally for brightness and contrast using Adobe Photoshop CS6.
Statistical analysis
Statistical significance was analyzed with unpaired, two-tailed Student’s t-test or Mann-Whitney test. P values less than 0.05 were considered statistically significant. For susceptibility studies, p values were determined by Log-rank test.
Results
SiglecH effectively identifies pDC in steady state and during infection
SiglecH is a DAP12-associated receptor used to discriminate pDC from other cell types in mice (9, 10). Our previous work has shown that pDC numbers are reduced in spleens of infected mice, which appeared to be a consequence of cell death (17). A recent study has indicated that following stimulation with CpGA or infection with MCMV that SiglecH is downregulated in a TLR9/MyD88-dependent manner (11). To determine if reduced pDC numbers during infection are due to reduced detection of SiglecH expression, we compared SiglecH and eGFP expression in pDC from spleens of SiglecHeGFP/+ mice infected or not with HSV-1. Using markers to identify pDC such as Ly6C and CD11c, we found that frequencies of Ly6C+eGFP+ and Ly6C+SiglecH+ cells were comparable and reduced in spleen to a similar extent 8 and 24 h after infection (Figure 1A and data not shown), indicating that anti-SiglecH antibodies are effective at identifying pDC in steady state and during viral infection.
Figure 1. Analysis of WT and SiglecH-eGFP reporter mice.
(A) SiglecHeGFP/+ mice were infected or not with HSV-1 and spleens were analyzed 8 and 24 h later for pDC (Ly6C+SiglecH+eGFP+). (B) Body weights and levels of blood urea nitrogen, creatinine and total protein in blood from WT and SiglecHeGFP/eGFP mice. Data are from two independent experiments. (C) Representative light microscopy of H&E stained kidney sections revealed no pathologic abnormalities of SiglecH-deficient mice. Magnification 40×.
SiglecHeGFP/eGFP mice are healthy in steady state
A recent report suggested that an independently derived SiglecH-deficient mouse strain had abnormal kidney pathology and function (18). Therefore, we evaluated body weight and performed bloodwork analyses on WT and SiglecHeGFP/eGFP mice (Figure 1). Both WT and age/gender matched SiglecHeGFP/eGFP mice had comparable body weights, percentages of segmented neutrophils and lymphocytes in circulation as well as similar levels of blood urea nitrogen, creatinine and total protein (Figure 1B and data not shown). Consistent with the absence of biochemical abnormalities in renal function, no pathologic changes were observed by light microscopy in the glomeruli or tubules of SiglecHeGFP/eGFP mice (Figure 1C). Thus, SiglecHeGFP/eGFP mice appear to be healthy and have normal kidney function in steady state.
SiglecH deficiency does not impact IFN-I production by pDC ex vivo
It has been shown that SiglecH has an immunomodulatory role during inflammation and viral infections (6, 9, 11). To determine whether SiglecH deficiency altered cytokine production by pDC, we measured IFN-α levels in supernatants from enriched or sort-purified pDC from WT and SiglecHeGFP/eGFP mice stimulated ex vivo with CpGA. CpGA is a synthetic Toll-like receptor (TLR) ligand that induces IFN-I production by pDC through TLR9 (19). pDC from WT and SiglecHeGFP/eGFP mice produced comparable amounts of IFN-α in response to CpGA (Figure2A and 2B), suggesting that lack of SiglecH does not strongly alter IFN-I production by pDC ex vivo. We next evaluated IFN-I responses to CpGA in vivo. WT and SiglecHeGFP/eGFP mice were injected i.v. with CpGA complexed with DOTAP and serum IFN-α was measured 6 h later (Figure 2C). Analyses of several mice revealed no significant differences in serum IFN-α levels between WT and SiglecHeGFP/eGFP mice. IFN-α was not detectable in serum from naïve WT or SiglecHeGFP/eGFP mice (data not shown).
Figure 2. Effect of SiglecH deficiency on pDC responses to CpGA and MCMV.
(A) pDC from WT and SiglecHeGFP/eGFP mice were enriched from BM and stimulated with CpGA for 48 h. (B) pDC from WT and SiglecHeGFP/eGFP mice were sort-purified from BM and stimulated with CpGA for 24 h. (A, B) IFN-α was measured in supernatants by ELISA. (C) WT and SiglecHeGFP/eGFP mice were injected i.v. with CpGA and serum IFN-α levels were measured 6 h later. (D) pDC were sort-purified from WT and SiglecHeGFP/eGFP mice and stimulated for 24 h with MCMV. IFN-α was measured in supernatants by ELISA. (E) WT and SiglecHeGFP/eGFP mice were infected i.p. with MCMV and serum IFN-α levels were measured 48 h p.i. Statistical significance is indicated by the p value. Data are from two (A, B, D, E) or three (C) experiments.
We next evaluated whether SiglecH deficiency influenced IFN-I production by pDC after exposure to a live virus. MCMV is sensed by pDC through TLR9 (20). Moreover, it has been reported that pDC are an important and early source of IFN-I during MCMV infection (3, 20–23). Thus, we sort-purified pDC from WT and SiglecHeGFP/eGFP mice and cultured them with MCMV. pDC from both groups of mice produced similar levels of IFN-α in response MCMV (Figure 2D), indicating that SiglecH deficiency did not affect IFN-I production by virus-stimulated pDC ex vivo. We next evaluated whether mice lacking SiglecH had altered IFN-I responses in vivo during MCMV infection. We found that SiglecHeGFP/eGFP mice had increased levels of systemic IFN-α compared to WT mice at 48 h p.i. (Figure 2E), similar to a recent study (11). Taken together, these data suggest that SiglecH deficiency does not affect IFN-I secretion by pDC in response to a synthetic TLR9 ligand or virus ex vivo but may influence IFN-I production by pDC during viral infection in vivo.
Enhanced cytokine responses to HSV-1 in SiglecHeGFP/eGFP mice occurs in the presence and absence of pDC
SiglecHDTR/DTR mice were reported to have increased cytokine responses to systemic HSV-1 infection (6). Corroborating these findings, SiglecHeGFP/eGFP mice also had slightly elevated levels of systemic IFN-α and proinflammatory cytokines compared to WT mice after HSV-1 infection (Figure 3A–C). These results are consistent with an immunomodulatory role for SiglecH in antiviral responses (6, 9, 11). To determine whether this effect was due to pDC, we bred CLEC4C-DTR Tg mice to SiglecHeGFP/+ and SiglecHeGFP/eGFP mice and infected them with HSV-1 in the presence or absence of pDC. We found that SiglecHeGFP/eGFP mice depleted of pDC produced more IFN-α than pDC-depleted SiglecHeGFP/+ mice or pDC-depleted CLEC4C-DTR Tg mice (Figure 3D). In all three lines of depleted mice, there was a ~2 ng reduction in serum IFN-α levels relative to their undepleted counterparts. These findings suggest that lack of SiglecH may affect cytokine secretion by cells other than pDC during viral infection or that viral burden and/or distribution is altered in the absence of SiglecH.
Figure 3. Lack of SiglecH impacts innate immune responses independently of pDC in vivo.
WT and SiglecHeGFP/eGFP mice were infected i.v. with HSV-1. Serum IFN-α (A), TNF-α (B) and IL-6 (C) were measured 6 h p.i. (D) CLEC4C-DTR Tg mice were bred to SiglecHeGFP/+ and SiglecHeGFP/eGFP mice. Mice were injected with phosphate buffered saline (PBS) or DT 24 h before infection with HSV-1. Serum IFN-α levels were measured 6 h p.i. Data are from two or three independent experiments with at least 3 mice per group in each experiment. Statistical significance is indicated by p values.
SiglecH is expressed by marginal zone macrophages, medullary macrophages, microglia and progenitors of cDC and pDC
Although SiglecH expression is mainly confined to pDC in cell suspensions from primary and secondary lymphoid organs, it was also observed by microscopy that specialized macrophage subsets in the spleen and LN express SiglecH (10). Corroborating this, we found that MZM in spleen (Figure 4A) and medullary macrophages in LN (data not shown) were SiglecH+ by immunohistochemistry. In addition, we observed that SiglecH was expressed by CD45intCD11b+ microglia in brain (Figure 4B), in agreement with a recent study (24).
Figure 4. SiglecH is expressed by specialized macrophages.
(A) SiglecH is expressed by pDC and MZM in spleen. Black arrowheads denote pDC in the T cell area and red arrowheads indicate MZM. Magnification: top panels, 100× scale bar 200 micron; bottom panels, 400× scale bar 50 micron. (B) Microglia were isolated from WT mice and stained with CD45, CD11b and SiglecH. The dotplot shows CD45intCD11b+ microglia and histograms show SiglecH expression. Data are representative of two independent experiments with 3–5 mice per experiment.
We previously reported that cDC are eGFP+ in SiglecHeGFP/+ mice (3). Closer examination revealed that both CD4+ and CD4− cDC in spleen were eGFP+ (Figure 5A), however, they did not express SiglecH on the surface or at the transcript level (data not shown and Figure 5B). These findings indicated that the SiglecH promoter was active in DC progenitors during development, consistent with a recent study (25) and that eGFP persists in differentiated or mature cDC. In the BM, there is a subset of SiglecH+ cells that has been defined as pre-pDC (Figure 5C), which can differentiate into pDC and cDC in vitro and in vivo (Figure 5D) (12, 13). Both mature pDC and pre-pDC expressed the pDC-specific transcription factor Tcf4/E2-2 (Figure 5E) (12, 26) and were eGFP+ in SiglecHeGFP/+ mice (data not shown). Taken together, these data indicate that SiglecH is expressed by mature pDC, specialized macrophages and progenitors of cDC and pDC.
Figure 5. SiglecH expression in progenitors of cDC and pDC.
(A) CD11chi cells in SiglecHeGFP/+ mice express eGFP. The dotplot shows live spleen cells stained with CD4 and CD11c. Histograms show eGFP expression in CD4+CD11chi DC and CD4−CD11chi DC in WT and SiglecHeGFP/+ mice. (B) pDC, CD8α+ and CD8α− DC were sorted from spleens and SiglecH expression was measured by qPCR. (C) Precursors of pDC (pre-pDC) in BM express SiglecH. The dotplot shows B220hiSiglecH+ pDC and B220loSiglecH+ pre-pDC and histograms denote expression of CCR9, Ly6C, CD11c and MHCII on each subset. (D) Sorted pre-pDC from BM differentiate into cDC or pDC after culture in GM-CSF or Flt3L, respectively. (E) E2-2 expression in pDC, CD8α+ DC and CD8α− DC sorted from spleen and mature pDC and pre-pDC sorted from BM. (F) SiglecH-DTR Tg mice, CLEC4C-DTR Tg mice and their littermates (DTR−) were injected with DT and BM was analyzed for mature pDC and pre-pDC 48 h later. (A, C, F) Dotplots are representative of 5–10 mice. (B, D, E) Cells were sorted from 5–6 mice for qPCR or in vitro differentiation.
Pre-pDC and MZM are depleted in SiglecH-DTR Tg mice
Given that SiglecH expression is not restricted to mature pDC, we hypothesized that prepDC and MZM may also be depleted in mice that express DTR under the control of the SiglecH promoter. To test this hypothesis, we utilized newly generated C57BL/6 SiglecH-DTR Tg mice that express DTR under the control of the SiglecH promoter. After DT injection, both mature pDC and pre-pDC were ablated in SiglecH-DTR Tg mice (Figure 5F). In contrast, only mature pDC were eliminated in CLEC4C-DTR Tg mice (Figure 5F). Furthermore, when CLEC4C-DTR Tg mice were bred to SiglecHeGFP/+ mice, eGFP+ cDC were not depleted after DT administration (3).
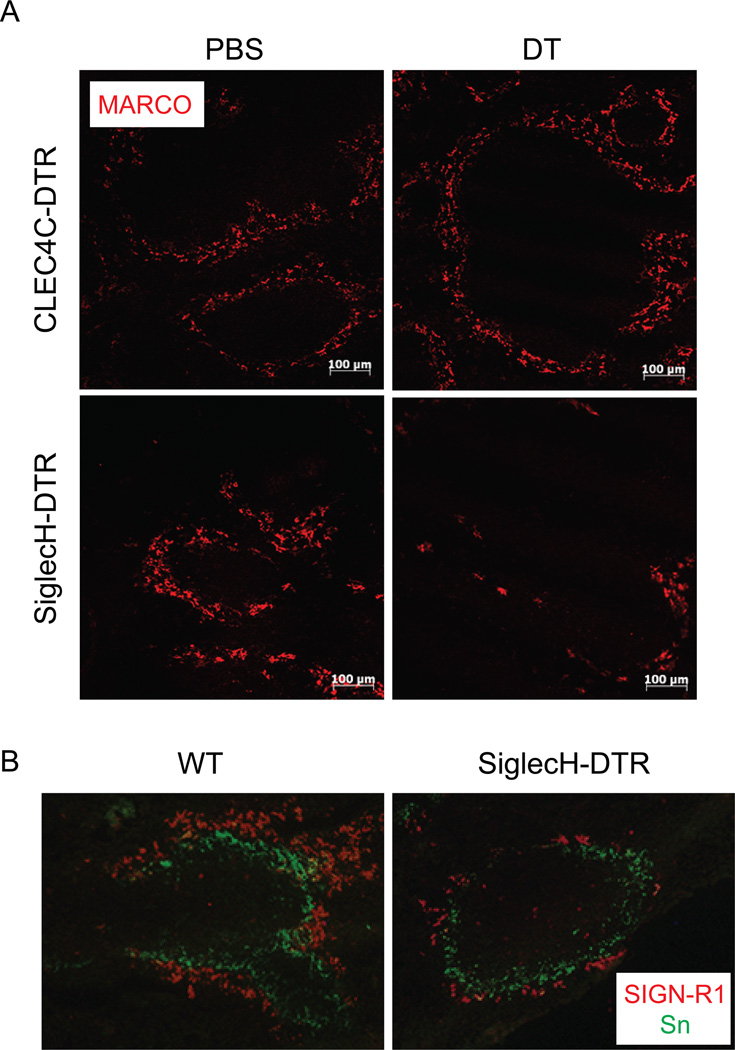
We next asked whether MZM were depleted in SiglecH-DTR Tg mice after DT treatment. To address this, we generated C57BL/6 SiglecH-DTR Tg BM chimeras because normal C57BL/6 SiglecH-DTR Tg mice do not tolerate DT very well. After 4–5 months of rest, we injected CLEC4C-DTR Tg mice and SiglecH-DTR Tg chimeras with PBS or DT then harvested spleens 24–36 h later. Spleen sections were stained with antibody against the scavenger receptor MARCO, which is highly expressed by MZM (Figure 6A) (27, 28). Results indicated that MZM were intact in CLEC4C-DTR Tg mice treated with either PBS or DT. In contrast, very few MZM could be identified in spleen sections from SiglecH-DTR Tg chimeras injected with DT. The depletion of MZM in SiglecH-DTR Tg mice was validated in a second SiglecH-DTR Tg line that was generated on a BALB/c background (15). Spleen sections stained for both MZM and metallophilic macrophages (MM) revealed that MZM but not MM were reduced in BALB/c SiglecH-DTR Tg mice injected with DT (Figure 6B).
Figure 6. MZM are depleted in SiglecH-DTR Tg mice.
(A) Spleen sections from PBS or DT-treated CLEC4C-DTR Tg mice and C57BL/6 SiglecH-DTR Tg chimeras were stained for MARCO to identify MZM (10× magnification). (B) Spleen sections from BALB/c SiglecH-DTR Tg mice and their non-Tg littermates (WT) injected with DT were stained with antibodies to SIGN-R1 and Sialoadhesin (Sn) to detect MZM and MM, respectively (20× magnification). Data are representative of two-three independent experiments.
Altered bacterial uptake and increased susceptibility to LM-OVA infection in depleted SiglecH-DTR Tg mice
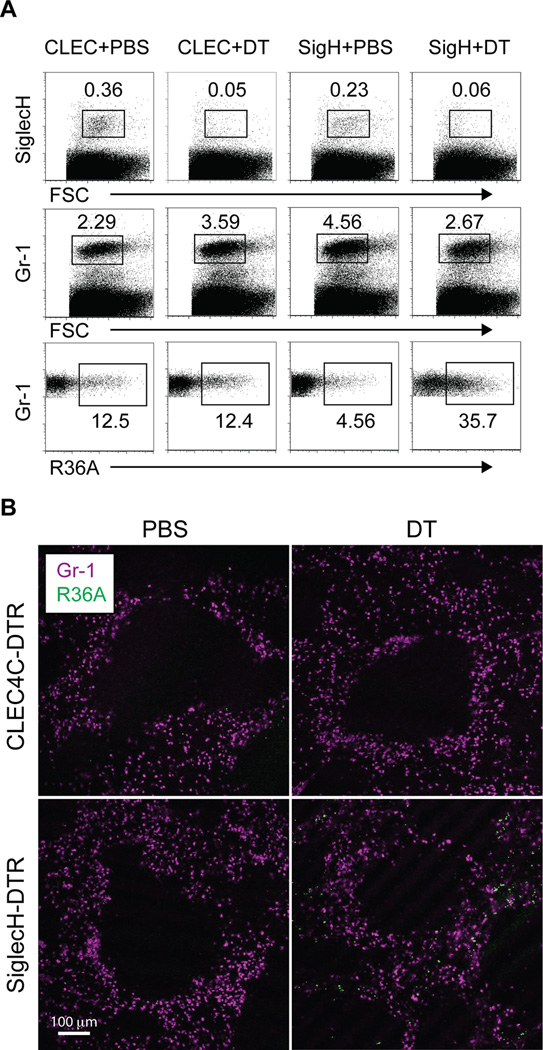
MZM are important for the clearance of apoptotic cells (29–31) and for the uptake of bacteria such as S. pneumoniae (32–34). Mice lacking SIGN-R1, a receptor expressed by MZM, are more susceptible to systemic S. pneumoniae infection and show signs of altered bacterial distribution in the spleen upon infection (33, 34). Therefore, we hypothesized that SiglecH-DTR Tg mice, which appear to lack MZM after DT treatment, would also exhibit altered bacterial distribution and perhaps be more susceptible to infection. To test this, we injected control or depleted CLEC4C-DTR Tg mice and SiglecH-DTR Tg chimeras with fluorescent-labeled S. pneumoniae and examined spleens 1 h after infection by flow cytometry. We first confirmed depletion in mice treated with DT by staining for pDC (Figure 7A, top panels). Next, using a variety of antibodies we evaluated which cells from each group of mice were associated with fluorescent bacteria. In all groups of mice, bacteria were mainly found among CD11b+Gr-1+F4/80− cells but not pDC (Figure 7A, bottom panels and data not shown). However, we noted that in SiglecH-DTR Tg chimeras treated with DT, there was a 3-fold increase in the frequency of Gr-1+ cells, presumably neutrophils, which were associated with bacteria. These findings suggested that the absence of MZM in SiglecH-DTR Tg mice resulted in increased bacterial burden in the red pulp. To confirm this, we analyzed spleens by microscopy 1 h after injection of fluorescent-labeled S. pneumoniae (Figure 7B). Very few fluorescent bacteria could be detected in CLEC4C-DTR Tg mice injected with PBS or DT or in SiglecH-DTR Tg chimeras injected with PBS. In contrast, SiglecH-DTR Tg chimeras treated with DT had an abundance of bacteria located in the red pulp as visualized by Gr-1 staining, confirming altered bacterial distribution and impaired clearance in the absence of MZM.
Figure 7. Distribution and clearance of bacteria is altered in depleted SiglecH-DTR Tg mice.
PBS or DT-treated CLEC4C-DTR Tg mice and C57BL/6 SiglecH-DTR Tg chimeras were injected with Alexa Fluor 647 labeled S. pneumoniae R36A. One hour after injection, spleens were harvested for flow cytometry and tissue sections. (A) Top panels show pDC frequencies in PBS and DT-treated mice. Middle panels show frequencies of live, Gr-1+ cells and bottom panels show frequencies of Alexa Fluor 647+ cells among live, Gr-1+ cells. (B) Spleen sections were stained with Gr-1 and show increased numbers of bacteria in the red pulp of DT-treated SiglecH-DTR Tg chimeras (10× magnification). Data are representative of three independent experiments.
Given the differences in bacterial uptake and clearance between CLEC4C-DTR Tg and SiglecH-DTR Tg chimeras we sought to determine whether SiglecH-DTR Tg mice were more or less susceptible to bacterial infection after DT administration. A study by Takagi et al., found that SiglecHDTR/DTR mice treated with DT were more resistant to lethal infection with LM-OVA than non-depleted mice (6). Thus, we performed a similar experiment in our CLEC4C-DTR Tg mice and SiglecH-DTR Tg chimeras. SiglecH-DTR Tg chimeras treated with DT succumbed to LM-OVA infection faster than mice in the other three groups (Figure 8A). Moreover, it did not appear that lack of pDC was responsible for this phenotype since CLEC4C-DTR Tg mice depleted or not of pDC exhibited identical rates of survival and died later than DT-treated SiglecH-DTR Tg chimeras (Figure 8A). Analysis of serum cytokine levels revealed that DT-treated SiglecH-DTR Tg chimeras produced very little IL-12p70 and had exaggerated levels of TNF-α and IL-6 in their serum compared to the other groups of mice (Figure 8B). Thus, the increased susceptibility and cytokine storm in DT-treated SiglecH-DTR Tg mice was most likely a consequence of altered bacterial distribution in the absence of SiglecH+ cells that were not pDC.
Figure 8. Susceptibility of CLEC4C-DTR Tg mice and SiglecH-DTR Tg mice to LM-OVA.
Groups of five mice: PBS or DT-treated CLEC4C-DTR Tg mice and SiglecH-DTR Tg chimeras were infected i.p. with LM-OVA. (A) Survival of mice was monitored every 12 h and (B) serum was collected from three mice in each group 24 h p.i. for cytokine analysis. Statistical significance is indicated by p values. NS, not significant.
Discussion
In this study, we found that SiglecH deficiency resulted in increased cytokine responses during HSV-1 infection and other viral infections, which may be pDC-independent. Indeed, SiglecH was expressed by MZM, which have been reported as major producers of IFN-I during systemic HSV-1 infection (35). SiglecH was also expressed by LN medullary macrophages, microglia and pre-pDC in the BM, which can differentiate into pDC and cDC (10, 12, 13, 24). Although cDC did not express SiglecH on their surface or at the transcript level, they were eGFP+ in SiglecH-eGFP knockin mice (3), suggesting that the SiglecH promoter was active in a common DC progenitor during DC development (25). In addition, it was recently shown using newly generated SiglecH-RFP reporter mice that a fraction of B-, T-, NK- and NKT cells are RFP+, indicating SiglecH promoter activity in a common lymphoid progenitor (11). Thus, lack of SiglecH may affect cytokine production by a variety of cell types during a viral infection perhaps by altering viral burden and/or distribution.
The semi-promiscuous expression of SiglecH potentially explains data inconsistencies between inducible pDC ablation models. It is possible that certain macrophage subsets and DC progenitors are depleted in SiglecH-DTR knockin or Tg mice thus yielding stronger phenotypes than observed in CLEC4C-DTR Tg mice. Indeed, using two different SiglecH-DTR Tg mouse lines, we found that pre-pDC and MZM were reduced after DT treatment in contrast to CLEC4C-DTR Tg mice. Furthermore, depleted SiglecH-DTR Tg mice showed altered bacterial distribution and impaired clearance after injection of S. pneumoniae compared to control mice and DT-treated CLEC4C-DTR Tg mice. These results are consistent with a role for MZM in responses to S. pneumoniae (32–34). Moreover, we found that depleted SiglecH-DTR Tg mice were highly susceptible to lethal LM-OVA infection. Previous studies have shown that early after infection, Listeria can be found associated with SIGN-R1+ cells in the spleen (36, 37) and that mice depleted of MZM and MM are impaired in their ability to control and survive Listeria infection (38). We envision that lack of MZM in DT-treated SiglecH-DTR Tg mice may account for the cytokine storm and rapid death following LM-OVA infection.
The results we obtained in our LM-OVA experiments using SiglecH-DTR Tg mice differ from those published in an earlier report that depleted pDC in SiglecHDTR/DTR mice in two ways. First, our depleted SiglecH-DTR Tg mice were more susceptible to infection while depleted SiglecHDTR/DTR mice appeared to be more resistant (6). And second, our model of specific pDC depletion using CLEC4C-DTR Tg mice revealed no major role for pDC in lethal LM-OVA infection as PBS- and DT-treated mice had identical survival rates and similar levels of systemic cytokines. It should be noted that SiglecHDTR/DTR mice lack SiglecH expression (6). Therefore, the reported findings by Takagi et al. do not discriminate effects of SiglecH-deficiency from pDC depletion. Although it has been suggested that pDC may be detrimental during Listeria infection because of their ability to produce IFN-I, studies have shown that myeloid cells and CD8α+ DC are important in pathogenesis, as either IFN-I producing cells or initial cellular entry points that establish productive infection (39, 40). Taken together, we conclude that the broad expression pattern of SiglecH should be considered when using SiglecH-DTR mice to evaluate pDC functions in vivo.
Acknowledgments
We would like to thank C. A. Stewart (National Cancer Institute, Frederick, MD) for advice on BAC recombineering; D. Leib (Geisel School of Medicine at Dartmouth) for HSV-1; A. French (Washington University School of Medicine, St. Louis, MO) for MCMV; C. Rossini for technical support; J. F. Kearney and L. Jia (University of Alabama at Birmingham) for R36A; S. Srivatsan and E. Lantelme (Washington University School of Medicine, St. Louis, MO) for help with BM chimeras and cell sorting.
Footnotes
M. S. was supported by 1K01DK095972-01A1 from NIDDK; Y. W. was supported by the Pulmonary and Critical Care training grant 2T32HL007317-31 from NHLBI; W. V. was supported by PRIN and AIRC; C. R. was supported by National Medical Research Council grants NMMR/1253/2010.
References
- 1.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11:631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swiecki M, Wang Y, Gilfillan S, Colonna M. Plasmacytoid Dendritic Cells Contribute to Systemic but Not Local Antiviral Responses to HSV Infections. PLoS Pathog. 2013;9:e1003728. doi: 10.1371/journal.ppat.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, Otsuka H, Hijikata A, Watanabe T, Ohara O, Kaisho T, Malissen B, Sato K. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 8.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 11.Puttur F, Arnold-Schrauf C, Lahl K, Solmaz G, Lindenberg M, Mayer CT, Gohmert M, Swallow M, van Helt C, Schmitt H, Nitschke L, Lambrecht BN, Lang R, Messerle M, Sparwasser T. Absence of Siglec-H in MCMV Infection Elevates Interferon Alpha Production but Does Not Enhance Viral Clearance. PLoS Pathog. 2013;9:e1003648. doi: 10.1371/journal.ppat.1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlitzer A, Loschko J, Mair K, Vogelmann R, Henkel L, Einwachter H, Schiemann M, Niess JH, Reindl W, Krug A. Identification of CCR9- murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood. 2011;117:6562–6570. doi: 10.1182/blood-2010-12-326678. [DOI] [PubMed] [Google Scholar]
- 13.Schlitzer A, Heiseke AF, Einwachter H, Reindl W, Schiemann M, Manta CP, See P, Niess JH, Suter T, Ginhoux F, Krug AB. Tissue-specific differentiation of a circulating CCR9- pDC-like common dendritic cell precursor. Blood. 2012;119:6063–6071. doi: 10.1182/blood-2012-03-418400. [DOI] [PubMed] [Google Scholar]
- 14.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 15.Piva L, Tetlak P, Claser C, Karjalainen K, Renia L, Ruedl C. Cutting edge: Clec9A+ dendritic cells mediate the development of experimental cerebral malaria. J Immunol. 2012;189:1128–1132. doi: 10.4049/jimmunol.1201171. [DOI] [PubMed] [Google Scholar]
- 16.Cardona AE, Huang D, Sasse ME, Ransohoff RM. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat Protoc. 2006;1:1947–1951. doi: 10.1038/nprot.2006.327. [DOI] [PubMed] [Google Scholar]
- 17.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med. 2011;208:2367–2374. doi: 10.1084/jem.20110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orr SL, Le D, Long JM, Sobieszczuk P, Ma B, Tian H, Fang X, Paulson JC, Marth JD, Varki N. A phenotype survey of 36 mutant mouse strains with gene-targeted defects in glycosyltransferases or glycan-binding proteins. Glycobiology. 2013;23:363–380. doi: 10.1093/glycob/cws150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Briere F. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol. 2005;175:6723–6732. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- 23.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 24.Kopatz J, Beutner C, Welle K, Bodea LG, Reinhardt J, Claude J, Linnartz-Gerlach B, Neumann H. Siglec-h on activated microglia for recognition and engulfment of glioma cells. Glia. 2013;61:1122–1133. doi: 10.1002/glia.22501. [DOI] [PubMed] [Google Scholar]
- 25.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, Holter W, Rauch A, Zhuang Y, Reizis B. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Naito M, Kobayashi Y, Takatsuka H, Jiang S, Usuda H, Umezu H, Hasegawa G, Arakawa M, Shultz LD, Elomaa O, Tryggvason K. Roles of a macrophage receptor with collagenous structure (MARCO) in host defense and heterogeneity of splenic marginal zone macrophages. Arch Histol Cytol. 1999;62:83–95. doi: 10.1679/aohc.62.83. [DOI] [PubMed] [Google Scholar]
- 29.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 30.Prabagar MG, Do Y, Ryu S, Park JY, Choi HJ, Choi WS, Yun TJ, Moon J, Choi IS, Ko K, Ko K, Young Shin C, Cheong C, Kang YS. SIGN-R1, a C-type lectin, enhances apoptotic cell clearance through the complement deposition pathway by interacting with C1q in the spleen. Cell Death Differ. 2013;20:535–545. doi: 10.1038/cdd.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang YS, Kim JY, Bruening SA, Pack M, Charalambous A, Pritsker A, Moran TM, Loeffler JM, Steinman RM, Park CG. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci U S A. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanoue A, Clatworthy MR, Smith P, Green S, Townsend MJ, Jolin HE, Smith KG, Fallon PG, McKenzie AN. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2004;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 35.Eloranta ML, Alm GV. Splenic marginal metallophilic macrophages and marginal zone macrophages are the major interferon-alpha/beta producers in mice upon intravenous challenge with herpes simplex virus. Scand J Immunol. 1999;49:391–394. doi: 10.1046/j.1365-3083.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- 36.Jablonska J, Dittmar KE, Kleinke T, Buer J, Weiss S. Essential role of CCL2 in clustering of splenic ERTR-9+ macrophages during infection of BALB/c mice by Listeria monocytogenes. Infection and immunity. 2007;75:462–470. doi: 10.1128/IAI.00443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyszkiewicz M, Zietara N, Rohde M, Gekara NO, Jablonska J, Dittmar KE, Weiss S. SIGN-R1+MHC II+ cells of the splenic marginal zone--a novel type of resident dendritic cells. J Leukoc Biol. 2011;89:607–615. doi: 10.1189/jlb.0610368. [DOI] [PubMed] [Google Scholar]
- 38.Aichele P, Zinke J, Grode L, Schwendener RA, Kaufmann SH, Seiler P. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J Immunol. 2003;171:1148–1155. doi: 10.4049/jimmunol.171.3.1148. [DOI] [PubMed] [Google Scholar]
- 39.Stockinger S, Kastner R, Kernbauer E, Pilz A, Westermayer S, Reutterer B, Soulat D, Stengl G, Vogl C, Frenz T, Waibler Z, Taniguchi T, Rulicke T, Kalinke U, Muller M, Decker T. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 2009;5:e1000355. doi: 10.1371/journal.ppat.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, Belizaire R, Aoshi T, Schreiber RD, Miller MJ, Murphy TL, Unanue ER, Murphy KM. CD8alpha(+) dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]