Abstract
Why and how we age remains a topic that has kept molecular biologists busy for the past few decades. In recent years, several studies pointed to sirtuins as important players in lifespan regulation, yet, in vivo proof for most of the mammalian homologs was missing. In this issue of The EMBO Journal, Sinclair and colleagues provide novel evidence that SIRT2 is indeed a critical modulator of aging in vivo.
See also: BJ North et al (July 2014)
Organismal aging is associated with a decline of cellular function in tissues, leading to the so-called age-related diseases, including cardiovascular and metabolic diseases, neurodegeneration and cancer (Cosentino & Mostoslavsky, 2013). The molecular mechanisms leading to aging are not yet fully understood and neither is clear which are the players modulating life span in mammals. In this issue, North et al present compelling evidence that the NAD+-dependent deacetylase SIRT2 has a profound impact on life span and onset of age-related diseases. Multiple studies have demonstrated that sirtuins may play a protective role against age-related diseases (Haigis & Sinclair, 2010; Sebastian et al, 2012). Although sirtuins could extend life span in lower organisms, including yeast, worms, and flies, such a role has been disputed (Cosentino & Mostoslavsky, 2013), and therefore, extensive efforts were applied to demonstrate that mammalian sirtuins can also modulate life span. Recent studies have shown that both overexpression of SIRT6 in male mice and brain-specific overexpression of SIRT1 extend life span (Kanfi et al, 2012; Satoh et al, 2013). Yet, whether other mammalian sirtuins may also have a role in aging was not known.
BubR1 is a mitotic checkpoint kinase. Previous studies have shown that BubR1 levels decrease with age (Baker et al, 2004; Hartman et al, 2007; Matsumoto et al, 2007), and overexpression of BubR1 extends life span (Baker et al, 2013). On the other hand, mice hypomorphic for BubR1 exhibit progeroid characteristics, and clearance of senescent cells delayed the onset of some age-related phenotypes (e.g., lordokyphosis and cataract); however, it did not have any effect on overall life span (Baker et al, 2011). This study was the first one showing a causal link between senescence and aging, but it left important questions unanswered, including ‘why did clearance of senescent cells improve the age related phenotype, but not extend the lifespan of the mice?’ North et al provide critical new answers to this question.
The authors start their study presenting molecular evidence convincingly connecting SIRT2 and BubR1. The authors show that reducing SIRT2 activity or levels diminishes BubR1 protein levels. In a biochemical tour de force, they describe BubR1 as a novel deacetylation target of SIRT2. Using a combination of biochemistry and mass spectrometry analysis, they identified that BubR1 is acetylated specifically by CBP on lysine 668, and this acetylation primes the protein for ubiquitination and subsequent degradation. By deacetylating K668, the authors showed that SIRT2 stabilizes BubR1.
In the second part of the study, North and colleagues presented in vivo studies to provide physiological significance for their findings. Using a transgenic SIRT2 mouse strain (SIRT2tg) crossed with the hypomorphic BubR1 mice (BubR1H/H), they found that SIRT2tg/BubR1H/H mice remained as small as the BubR1H/H; however, they observed a striking 58%increase (122%for male mice) in the median life span and a 21%increase in maximal life span compared to BubR1H/H mice.
North et al then investigated how SIRT2 rescues BubR1H/H mice. BubR1 mice die of cardiac dysfunction, and indeed SIRT2 does improve cardiac function in male mice. The observation that male mice are more prone than female ones to cardiac dysfunction (Du, 2004) might at least in part explain why SIRT2 is more effective in increasing life span of male mice. It is important to notice that BubR1H/H mice do not show any gender bias (Baker et al, 2004). Moreover, as discussed below, BubR1 mutations are part of an autosomal disease (Wijshake et al, 2012); therefore, the reason behind this sexual dimorphism remains to be fully understood. As mentioned above, SIRT6 also exhibited sexual dimorphism with regard to lifespan benefits; therefore, it will be interesting to determine whether there is a common mechanism behind such effects.
It is worth noting that SIRT2 fails to fully restore wild-type levels of BubR1, and in fact, the mice hypomorphic for BubR1 and overexpressing SIRT2 still present some of the progeroid phenotypes (e.g., lordokyphosis, cataract, and small size). The authors propose that there is a critical threshold for the levels of BubR1 that is tissue specific, so the amount of protein reached in the heart is sufficient to improve the cardiac function (and life span), but not so in other tissues. In this context, it remains to be tested whether heart, compared to other tissues, exhibits more senescent cells during aging, a possibility that could explain this phenotype. Based on these results, the authors also concluded that there must be other factors regulating BubR1 levels. It will be essential to identify these other factors, in order to obtain a full picture on how this key lifespan factor is regulated.
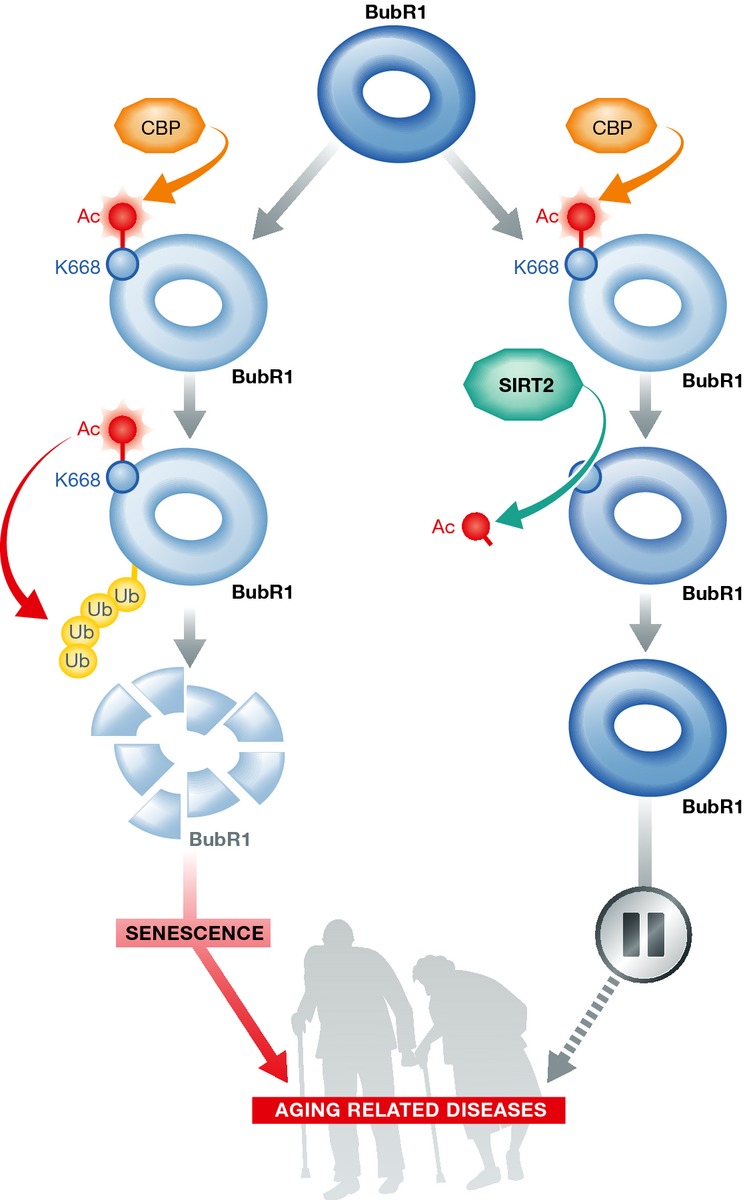
Considering that SIRT2 overexpression increases BubR1 levels also in wild-type MEFs and that aging is accompanied by a decrease of BubR1, we may predict that SIRT2 overexpression would increase life span also in wild-type mice, as SIRT6 and SIRT1 do, a possibility that remains to be tested. Although the potential therapeutic benefits of activating SIRT2 in the context of aging will require further studies, the finding by North et al could already find a clinical readout in the treatment of patients affected by mosaic variegated aneuploidy (MVA). MVA is a rare recessive disorder due to BubR1 mutations (Wijshake et al, 2012). Children affected by this disease are cancer prone, exhibit a progeroid phenotype, and die at an early age. In that context, increasing levels of BubR1 in those patients by means of increasing SIRT2 activity could provide an alternative therapeutic possibility.
This study opens other intriguing questions. BubR1 levels decreased with age, yet no data suggest that SIRT2 levels change with age as well. In that regard, rather than sirtuin levels to being affected, it is possible that availability of the cofactor NAD may change with age, in turn affecting SIRT2 activity instead of levels. Indeed, two recent studies provided strong evidence for such a case (Gomes et al, 2013; Stein & Imai, 2014). Although treatments with NAD precursors (NMN, NR) have been proposed to improve healthspan through SIRT1, this new study indicates SIRT2 as another strong candidate behind the beneficial effects of these small molecules. Undoubtedly, North et al shed new light into our understanding of aging and age-related diseases, while setting ground for interesting future studies.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Baker DJ, Dawlaty MM, Wijshake T, Jeganathan KB, Malureanu L, van Ree JH, Crespo-Diaz R, Reyes S, Seaburg L, Shapiro V, Behfar A, Terzic A, van de Sluis B, van Deursen JM. Increased expression of BuR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat Cell Biol. 2013;15:96–102. doi: 10.1038/ncb2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino C, Mostoslavsky R. Metabolism, longevity and epigenetics. Cell Mol Life Sci. 2013;70:1525–1541. doi: 10.1007/s00018-013-1295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XJ. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc Res. 2004;63:510–519. doi: 10.1016/j.cardiores.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, deCabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;19:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Ann Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TK, Wengenack TM, Poduslo JF, van Deursen JM. Mutant mice with small amounts of BubR1 display accelerated age-related gliosis. Neurobiol Aging. 2007;28:921–927. doi: 10.1016/j.neurobiolaging.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Baker DJ, d’Uscio LV, Mozammel G, Katusic ZS, van Deursen JM. Aging-associated vascular phenotype in mutant mice with low levels of BubR1. Stroke. 2007;38:1050–1056. doi: 10.1161/01.STR.0000257967.86132.01. [DOI] [PubMed] [Google Scholar]
- North BJ, Rosenberg MA, Jeganathan KB, Hafner AV, Michan S, Dai J, Baker DJ, Cen Y, Wu LE, Sauve AA, van Deursen JM, Rosenzweig A, Sinclair DA. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014;33:1438–1453. doi: 10.15252/embj.201386907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, WOzniak DF, Herzog ED, Yamada KA, Imai S. SIRT1 extends lifespan and delays aging in mice through regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287:42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijshake T, Malureanu LA, Baker DJ, Jeganathan KB, van de Sluis B, van Deursen JM. Reduced Life- and healthspan in mice carrying a mono-allelic BubR1 MVA mutation. PLoS Genet. 2012;8:e1003138. doi: 10.1371/journal.pgen.1003138. [DOI] [PMC free article] [PubMed] [Google Scholar]


