Abstract
One of the most common abnormalities of cancer cells is their predilection to engage in a high rate of glycolysis despite the continued availability of oxygen. First described by Otto Warburg, this phenomenon of aerobic glycolysis (or the Warburg effect) has recently been proposed to result from cancer-associated alterations in signal transduction pathways. In this issue of The EMBO Journal, Pate et al provide further support for this hypothesis by demonstrating that Wnt signaling plays an important role in establishing aerobic glycolysis as a mechanism to support in vivo cancer cell proliferation.
See also: KT Pate et al (July 2014)
There has been a renewed interest in how signal transduction regulates cellular metabolism to promote cell survival, growth, and proliferation (Vander Heiden et al, 2009). In mammals, cell survival is dependent on the maintenance of sufficient glucose uptake to sustain ATP production through coupled glycolysis/oxidative phosphorylation. In contrast, proliferating cells engage in the seemingly wasteful metabolism of glucose through a process termed aerobic glycolysis or the Warburg effect. In cells exhibiting aerobic glycolysis, the uptake of glucose exceeds the bioenergetic needs of the cell. Despite the continued availability of oxygen, the majority of pyruvate produced by glycolysis is secreted from the cell in the form of lactate rather than oxidized in the mitochondrial TCA cycle. A partial explanation for the contrasting metabolism of quiescent versus proliferating cells came with the discovery that the level of glucose uptake is directly controlled by the magnitude of growth factor signal transduction (Rathmell et al, 2000).
Cancer cells with activating mutations in the PI3K/AKT pathway take up glucose in excess of their ATP production needs. PI3K/AKT signaling rewires glucose metabolism to support lipid and protein synthesis (Vander Heiden et al, 2009). As a result, cells grow bigger and the cell size increase is proportional to the PI3K/AKT-induced increase in glucose metabolism. However, a negative consequence of this enhanced glucose metabolism is that oxidative metabolism is also increased, resulting in the production of potentially damaging reactive oxygen species (ROS). AKT-transformed cells protect against ROS stress by inducing HIF-1α which suppresses glucose entry into the TCA cycle (Lum et al, 2007). HIF-1α-induced pyruvate dehydrogenase kinase 1 (PDK1) phosphorylates pyruvate dehydrogenase (PDH), inhibiting its activity and resulting in cytosolic pyruvate being shunted into lactate through induction of lactate dehydrogenase A (LDH-A) (Suda et al, 2011). While the activation of PI3K/AKT results in aerobic glycolysis, the induction of HIF-1α suppresses cell proliferation. Furthermore, HIF-1α reduces but does not completely suppress mitochondrial ROS. The residual ROS over time can result in either cellular senescence or apoptosis. Together, these findings raise the question of whether PI3K activation was a sufficient explanation for the aerobic glycolysis seen in proliferating cells, particularly stem cells.
In this issue of The EMBO Journal, Pate et al (2014) identify Wnt signaling as a mechanism that suppresses pyruvate oxidation in the TCA cycle and promotes rather than inhibits cell proliferation. As such, Wnt signaling is a candidate for the signal transduction pathway that could synergize with PI3K/AKT signaling in proliferating cells. Wnt signaling is already well characterized as a regulator of cell proliferation. First, Wnt-induced LEF/TCF/β-catenin transcription complexes have been implicated in controlling cell proliferation through the induction of Myc and Cyclin D. Cyclin D levels are critical to cell cycle progression through G1, and Myc has been implicated in the stimulation of glutamine metabolism and nucleotide synthesis necessary to support S-phase (Dang, 2010; Niehrs & Acebron, 2012).
Pate et al (2014) identify additional transcriptional targets of LEF/TCF/β-catenin complexes. They report that the genes connected to metabolism are the most highly overrepresented category of Wnt-target genes. Two relevant Wnt targets were identified: pyruvate dehydrogenase kinase 1 (PDK1) and the lactate transporter (MCT-1). These two proteins along with the Myc-induced gene LDH-A allow Wnt-activated cells to divert glycolytic pyruvate away from the TCA cycle by converting it into lactate and promoting lactate secretion from the cell. Induction of PDK1 was found to be required for Wnt-induced aerobic glycolysis, in vivo tumor cell accumulation, and VEGF-independent angiogenesis.
Pate et al did not test for whether Wnt induction of Myc and/or glutamine metabolism contributes to Wnt-induced tumor cell accumulation. However, it is reasonable to suspect that Wnt induction of PDK1 cooperates with Myc-induced glutaminolysis to facilitate a cellular transition from growth to proliferation since Myc is a well-characterized effector of Wnt signaling (Nusse, 2008; Niehrs & Acebron, 2012). The combined effects of Wnt-facilitated aerobic glycolysis (Pate et al, 2014) and Myc-induced glutaminolysis (Wise et al, 2008) provide the cell with a potent ability to engage in de novo nucleotide biosynthesis (see Fig1). Thus, Wnt activation is likely to not only convert glycolytic pyruvate away from the TCA cycle but also promote Myc-induced glutaminolysis to support the increase in nucleotide biosynthesis required for the execution of S-phase and cell division. These data may also explain why Wnt signaling plays such an important role in the maintenance of stem cells. The ability of Wnt to directly induce PDK1 provides a mechanism by which stem cells can engage in aerobic glycolysis in support of cell division, without exposing themselves to the damaging effects of mitochondrial ROS generation secondary to enhanced TCA cycle activity.
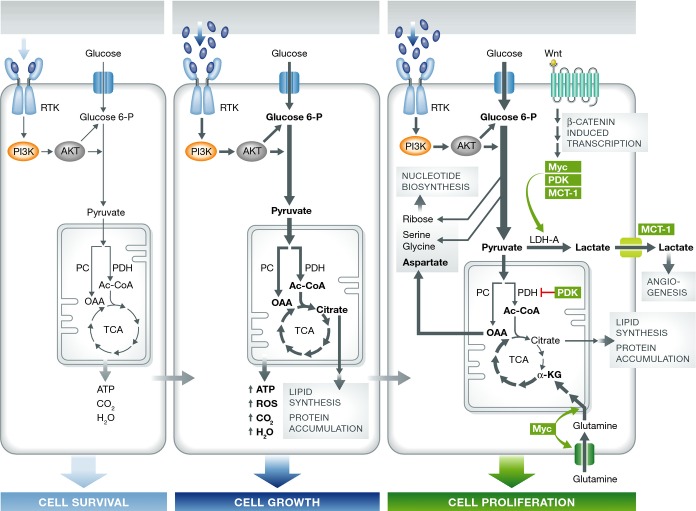
Figure 1. A model for growth factor regulation of mammalian cell survival, growth, and proliferation.
In the left-hand panel, ligand-induced activation of receptor tyrosine kinases (RTK) is required for quiescent cells to take up sufficient glucose to survive. PI3K/AKT-directed glucose metabolism results in sufficient ATP to maintain cell survival. In the middle panel, as a result of increased ligand stimulation, receptor tyrosine kinase signaling stimulates increased glucose uptake which if completely oxidized would exceed the cellular needs for ATP. When cellular ATP needs are satisfied, excess pyruvate which enters the TCA cycle is converted to citrate which is exported to the cytosol. The cytosolic metabolism of citrate promotes lipid synthesis and protein accumulation. In the right-hand panel, Wnt signal transduction is proposed to convert the cell from growth to proliferation as a result of induction of Myc, PDK1, and MCT1. PDK1, Myc-induced LDH-A, and MCT1 cooperate to divert glycolytically derived pyruvate into secretion from the cell as lactate which can stimulate angiogenesis (Hunt et al, 2007). In turn, Myc-induced glutamine uptake and glutaminolysis support mitochondrial integrity and the production of aspartate (Wise et al, 2008). The cytosolic accumulation of pyruvate/lactate results in turn to accumulation of glycolytic intermediates that are diverted into ribose, serine, and glycine biosynthesis providing the cells with the building blocks necessary to engage in de novo nucleotide biosynthesis. The resulting induction of de novo nucleotide biosynthesis promotes the ability of cells to enter S-phase and complete cell division.
Although there is much more to be done to flesh out the complete effects of Wnt signaling on metabolism, it appears that Wnt signaling can be added to the growing list of signal transduction pathways that directly contribute to the regulation of cellular metabolism.
Conflict of interest
The author declares that he has no conflict of interest.
References
- Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010;70:859–862. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Aslam RS, Beckert S, Wagner S, Ghani QP, Hussain MZ, Roy S, Sen CK. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9:1115–1124. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signalling. EMBO J. 2012;31:2705–2713. doi: 10.1038/emboj.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- Pate K, Stringari C, Sprowl Tanio S, Wang K, TeSlaa T, Hoverter N, McQuade M, Garner C, Digman M, Teitell M, Edwards R, Gratton E, Waterman M. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]