Abstract
Src family kinases (SFKs) have long been implicated in tumorigenesis, but the exact requirement for individual kinase members, and their specific spatially and temporally defined roles in the maintenance of epithelial homeostasis remained unclear. A study by Cordero et al (2014) now combines the strengths of Drosophila and mouse models for intestinal epithelial regeneration to characterize the role of individual SFKs in epithelial stem cells, tissue regeneration, and tumorigenesis.
See also: JB Cordero et al (July 2014)
Expression and activation of the tyrosine kinase Src have long been linked with proliferation, invasion, and metastasis of multiple types of human tumors, including colorectal, breast, ovarian, lung, and pancreatic cancers (Summy & Gallick, 2003). Colorectal cancer (CRC) is the third most common malignancy diagnosed worldwide and a leading cause of cancer deaths in the developed world (Jemal et al, 2011). Twelve percent of advanced human colon cancer cases contain mutations activating Src, and Src expression is increased in about 80%of CRC specimens compared with normal colonic epithelium (Sirvent et al, 2012). Furthermore, high Src activity has also been shown to be an independent indicator of poor clinical prognosis in all stages of colon cancer (Sirvent et al, 2012). Given the strong link between Src activity and cancer, a number of inhibitors targeting Src family kinases (SFK) have been developed in recent years, yet their use as a monotherapy in CRC has proven ineffective (Gargalionis et al, 2014). The reason for the low efficacy of CRC monotherapy with Src inhibitors is still not clear, and mechanistic insights are hampered by the fact that these inhibitors act on a broad number of targets.
Clearly, further dissection of the physiological role of SFKs in epithelial regeneration and homeostasis, and of their integration into signaling networks controlling cell proliferation and differentiation in vivo are required if rational therapies for CRC targeting these kinases are to be developed. In this issue, Cordero et al (2014) significantly advance our understanding of this physiological role of SFKs by combining Drosophila and mouse models of intestinal regeneration to examine the effects of SFK mutations on tissue homeostasis, damage-induced regeneration, and hyperplasia of the intestinal epithelium.
Similar to the human intestine, the Drosophila adult midgut epithelium is self-renewed by intestinal stem cells (ISC) (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006), making it a useful and productive model to explore cellular and molecular signaling interactions governing intestinal homeostasis, regeneration, and disease (Biteau et al, 2011). Using this paradigm, Cordero et al (2014) dissect the contribution of individual SFKs to the control of ISC proliferation, identify EGFR/Ras/MAPK and Stat signaling as mediators of this function, and place Src downstream of Wnt signaling during tumorigenesis.
The authors show that SFK activity from either Drosophila SFK ortholog (Src42A and Src64B) is sufficient to drive ISC hyperproliferation in the Drosophila intestine, but that only one ortholog (Src42A) is required for homeostatic ISC proliferation and for the proliferative response to damage induced by pathogenic bacteria. This specificity is also observed in pro-tumorigenic conditions associated with elevated Wingless (Wg) signaling. Integrating Src42A into established pro-proliferative signaling networks in ISCs, the authors show that Src42A acts upstream of EGFR/MAPK and Jak/Stat signaling to promote ISC proliferation (Fig1).
Figure 1. Model for the integration of SFKs in the control of ISC proliferation in flies and mice.
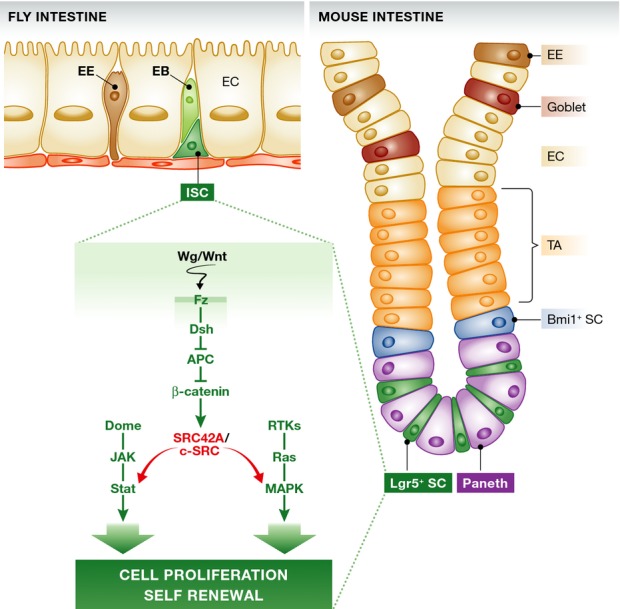
See text for details. APC, adenomatous polyposis coli; EC, enterocyte; EE, enteroendocrine cells; EB, enteroblast; ISC, intestinal stem cell; TA, transit amplifying cell population; SFKs, Src family kinases.
Importantly, the authors show that most of these functional links are conserved in mammals: Mammalian SFKs (c-Src, Fyn, Yes) act redundantly to control homeostasis of the intestinal epithelium (loss of all three kinases results in loss of Paneth cells and increased apoptosis in villae, as well as an inability to form organoids in culture), but c-Src is uniquely required for regeneration following gamma irradiation and in tumors resulting from adenomatous polyposis coli (APC) loss. As in flies, c-Src thus acts downstream of Wnt to promote tumorigenesis, and the authors provide evidence that it also acts upstream of EGFR/MAPK/Erk and Stat3 signaling during regeneration following damage (Fig1).
This report uncovers a new mechanism for Wnt-mediated pro-tumorigenic function involving Src activity that is very similar to the mechanism proposed by the same group for myc-mediated control of ISC proliferation downstream of Wnt signaling (Cordero et al, 2012). Both pathways converge on EGFR/MAPK and Stat signaling activation, suggesting alternative ways to activate common pro-tumorigenic signaling cascades. Understanding this signaling cross talk in the control of SC proliferation and tumorigenesis in more detail is expected to identify key downstream regulators that can be effective therapeutic targets.
The findings further highlight the intimate relationship between processes that regulate ISC proliferation in response to regeneration cues and conditions that promote tumorigenesis. ISC-like cells have recently been established as critical drivers of tumor growth in models for adenoma formation (Schepers et al, 2012), and the data reported here further support the idea that endogenous signaling networks governing epithelial regeneration are being hijacked during the establishment and growth of intestinal tumors.
Finally, the evident conservation of the Src-mediated regulation of ISC function and epithelial regeneration corroborates the relevance of the Drosophila intestine as a model for discovery of pathways and drug targets relevant for human disease.
Acknowledgments
Work in Dr. Jasper’s laboratory is supported by the National Institute on Aging (RO1 AG028127), the National Institute on General Medical Sciences (RO1 GM100196), and the National Eye Institute (R01 EY018177). The authors would like to thank Dr. Jason Karpac for helpful comments on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9:402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero JB, Stefanatos RK, Myant K, Vidal M, Sansom OJ. Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development. 2012;139:4524–4535. doi: 10.1242/dev.078261. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Ridgway RA, Valeri N, Nixon C, Frame MC, Muller WJ, Vidal M, Sansom OJ. c-Src drives intestinal regeneration and transformation. EMBO J. 2014;33:1474–1491. doi: 10.1002/embj.201387454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargalionis AN, Karamouzis MV, Papavassiliou AG. The molecular rationale of Src inhibition in colorectal carcinomas. Int J Cancer. 2014;134:2019–2029. doi: 10.1002/ijc.28299. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Sirvent A, Benistant C, Roche S. Oncogenic signaling by tyrosine kinases of the SRC family in advanced colorectal cancer. Am J Cancer Res. 2012;2:357–371. [PMC free article] [PubMed] [Google Scholar]
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]


