Abstract
Interleukin-6 (IL-6) is a pleiotropic cytokine with complex roles in inflammation and metabolic disease. While typically regarded as a pro-inflammatory cytokine, multiple studies in the last 20 years have generated conflicting data on the role of IL-6 in inflammation and metabolism. In a recent study in Nature Immunology, Brüning and collaborators demonstrate that IL-6 signaling in myeloid cells attenuates obesity-induced inflammation and insulin resistance by promoting macrophage alternative activation (Mauer et al, 2014). This study unveils a new and surprising anti-inflammatory action of IL-6 and further highlights the complex actions of this cytokine.
See also: J Mauer et al (May 2014)
The Good: IL-6 promotes alternative activation of macrophages
Evidence from animal and human studies indicates that the pathogenesis of obesity-related metabolic dysfunction involves the development of a systemic, low-grade inflammatory reaction, which is largely mediated by macrophages. Macrophages are frequently classified as pro-inflammatory, M1 or ‘classically activated’ or anti-inflammatory, M2 or ‘alternatively activated’. Although macrophages exist along the M1/M2 spectrum and frequently have mixed phenotypes in vivo, this binary classification has proven to be useful in many pathophysiological settings, such as white adipose tissue (WAT) inflammation. Resident adipose tissue macrophages (ATMs) in lean organisms tend to express genes associated with a M2-like phenotype, whereas ATMs in obese organisms typically express genes associated with a M1-like phenotype and contribute to obesity-induced adipose tissue inflammation and associated systemic insulin resistance dysfunction. It is generally accepted that M1-like macrophages promote obesity-induced insulin resistance, whereas M2-like macrophages protect against it. In their study, Mauer and collaborators show that myeloid-restricted deficiency of the IL-6 receptor IL-6Rα exacerbates glucose metabolism dysfunction in obese mice, in parallel with a pattern of gene expression in WAT that is suggestive of enhanced M1 polarization of ATMs. Although to a lesser extent, this pattern was also present in other metabolic tissues such as brown adipose tissue and liver. Through a series of in vitro and in vivo experiments with recombinant IL-6 and IL-4 proteins, it is shown that IL-6 primes macrophages to IL-4-mediated alternative activation by inducing the expression of its receptor IL-4Rα via STAT3-mediated activation of the IL4ra promoter. The authors also provide evidence suggesting that IL-6 contributes to alternative activation of macrophages in other settings by demonstrating that myeloid-restricted IL-6Rα ablation limits LPS-induced inflammation in several tissues. Overall, the authors provide strong evidence supporting an anti-inflammatory role of IL-6 signaling in myeloid cells via modulation of macrophage phenotype.
The findings by Mauer et al strongly support the anti-inflammatory role of IL-6, which had been suggested long ago (Xing et al, 1998). In addition, the finding of exacerbated insulin resistance in myeloid IL-6Rα-deficient mice is consistent with previous studies that identified IL-6 as a myokine that mediates the anti-inflammatory and insulin-sensitizing effects of physical exercise (Pedersen & Fischer, 2007). Furthermore, some studies with mice deficient in IL-6 have reported that it protects against the progression of inflammatory vascular disorders such as atherosclerosis (Elhage et al, 2001; Schieffer et al, 2004) (Fig1).
Figure 1. The Janus-like actions of IL-6.
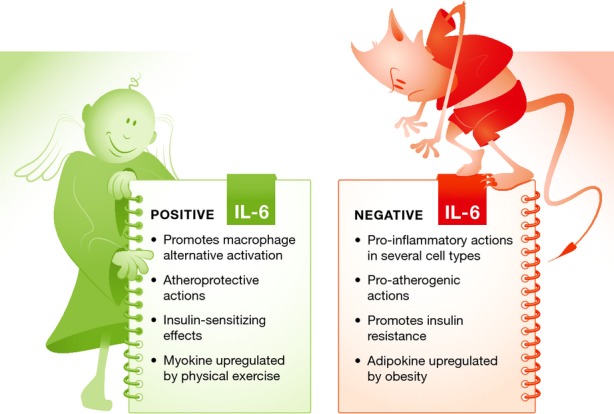
IL-6 has complex and often conflicting activities. As shown by Mauer et al (2014), it promotes an anti-inflammatory (M2-like) state in macrophages. Consistent with these observations, others have reported that IL-6 functions to limit atheroma formation and that it is secreted in response to physical exercise, mediating its insulin-sensitizing actions. On the other hand, IL-6 also acts as a pro-inflammatory cytokine involved in the acute phase reaction to tissue injury. It has a contributory role in a number of inflammatory and autoimmune diseases, and its secretion by the adipose tissues of obese organisms contributes to metabolic dysfunction.
The Bad: IL-6 is a pro-inflammatory cytokine
In marked contrast with the notion that IL-6 controls macrophage alternative activation, numerous studies have shown that it is also a powerful pro-inflammatory cytokine that is essential for the inflammatory acute phase response induced by tissue damage (Kopf et al, 1994). It has been reported to exert direct pro-inflammatory actions in lymphocytes (McLoughlin et al, 2005) and other cell types. Furthermore, IL-6 is an adipokine, which is greatly upregulated in obese adipose tissue, and has been proposed to be an essential part of a signaling axis that links WAT dysfunction with hepatic insulin resistance (Sabio et al, 2008). Human studies also suggest that IL-6R signaling has a role in the development of coronary heart disease (Sarwar et al, 2012), a notion supported by some mouse studies (Huber et al, 1999; Schuett et al, 2012), but not all (Elhage et al, 2001; Schieffer et al, 2004), as mentioned above (Fig1).
The Ugly: fitting IL-6 signaling into a simple model
The study by Mauer and collaborators strongly suggests a macrophage-mediated anti-inflammatory effect of IL-6. In contrast, extensive evidence in the literature suggests that IL-6 induces inflammation in various settings. How can we interpret this? There are several potential explanations for this apparent discrepancy. One possibility is that IL-6 plays a pro-inflammatory action in acute inflammation, but also exerts immunosuppressive/anti-inflammatory actions when expressed at lower levels. Another option is that IL-6 exerts opposite roles in different cell types. Indeed, this has already been suggested to account for the conflicting effects of IL-6 in insulin signaling, since IL-6 seems to promote insulin resistance in hepatocytes (Senn et al, 2002) and endothelial cells (Yuen et al, 2009), but increases insulin sensitivity in skeletal muscle (Carey et al, 2006; Yuen et al, 2009). This could be related to differences in the expression of intracellular mediators of IL-6 signaling, as it has been shown that ablation of one of these mediators (SOCS3) shifts the cellular effects of IL-6 from pro-inflammatory to anti-inflammatory in macrophages (Yasukawa et al, 2003). Finally, the complex actions of IL-6 may be linked to the different manners by which this cytokine signals at the plasma membrane. IL-6-induced cell signaling can be classified as either classic or trans-signaling, and these two variants can lead to markedly different cellular responses. In classic signaling, IL-6 stimulates target cells via the membrane-bound isoform of IL-6Rα, which upon ligand binding forms a complex with the signaling receptor protein gp130. Only a few cell types express membrane-bound IL-6Rα, whereas essentially every cell type expresses gp130. While the cells that only express gp130 are not responsive to IL-6 alone, they can be stimulated by a complex of IL-6 bound to a naturally occurring soluble form of IL-6Rα. This type of signaling, which is known as IL-6 trans-signaling, greatly expands the spectrum of IL-6 actions and target cells. Importantly, selective blockade of IL-6 trans-signaling has been shown to blunt inflammatory responses in mice (Rabe et al, 2008). Whether classic, trans-signaling or both contribute to the effect of IL-6 on macrophage polarization was not assessed in the study by Mauer et al (2014).
Finally, what are the potential therapeutic implications of the pro- and anti-inflammatory actions of IL-6? Antibodies targeting IL-6 signaling are currently used as a treatment against autoimmune disorders such as rheumatoid arthritis. Whether the role of IL-6 in macrophage polarization can be exploited therapeutically remains to be investigated. Given that mouse and human IL-6 proteins exhibit only 41%sequence identity, future studies are warranted to address whether the role of IL-6 in macrophage polarization is conserved in humans.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, Maret A, Bayard F. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–320. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Bruning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci USA. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Fischer CP. Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28:152–156. doi: 10.1016/j.tips.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Rabe B, Chalaris A, May U, Waetzig GH, Seegert D, Williams AS, Jones SA, Rose-John S, Scheller J. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111:1021–1028. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJ, Drexler H. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D, Kempf T, Wollert KC, Seegert D, Rose-John S, Tietge UJ, Schieffer B, Grote K. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:281–290. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Yuen DY, Dwyer RM, Matthews VB, Zhang L, Drew BG, Neill B, Kingwell BA, Clark MG, Rattigan S, Febbraio MA. Interleukin-6 attenuates insulin-mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor-alpha expression. Diabetes. 2009;58:1086–1095. doi: 10.2337/db08-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]


