Abstract
microRNAs (miRNAs) are a family of small, non-coding RNAs, which provides broad silencing activity of mRNA targets in a sequence-dependent fashion. This review explores the hypothesis that the miRNA machinery is intimately linked with the cellular stress pathway and apparatus. Stress signaling potentially alters the function of the miRNA-bioprocessing core components and decompensates regulation. In addition, dysregulation of miRNA activity renders the cell more prone to stress and emerges as a new pathway for age-related insults and diseases, such as neurodegeneration.
Keywords: cellular stress, dicer, disease, micrornas, stress granules
Introduction
microRNAs
miRNAs are genome-encoded small RNAs that mediate post-transcriptional silencing. Considering hundreds of miRNA genes, each with many dozens of targets, regulation by miRNAs is very broad and met in any cellular activity in health or in disease (Bushati & Cohen, 2008; Bartel, 2009; Carthew & Sontheimer, 2009; Fabian et al, 2010). miRNA precursors are processed in two steps (Fig1). The initial miRNA transcript (pri-miRNA) is subjected to a nuclear processing by the Drosha–DGCR8 ‘microprocessor’ complex. The resulting intermediate precursor (pre-miRNA) is exported to the cytoplasm and then further identified and cut by Dicer, yielding a 22-nt mature miRNA (Gregory et al, 2005). Partners of Dicer are required for efficient pre-miRNA processing and include Argonaute (AGO), protein kinase interferon-inducible double-stranded RNA-dependent activator (Pact) and TAR RNA-binding protein (Trbp; Chendrimada et al, 2005; Haase et al, 2005; Diederichs & Haber, 2007; Koscianska et al, 2011). Dicer and its co-factors load the mature miRNA onto AGO in the RNA-induced silencing complex (RISC), providing sequence-specific silencing activity.
Figure 1. Canonical pathways for miRNA maturation and loading of the RISC.
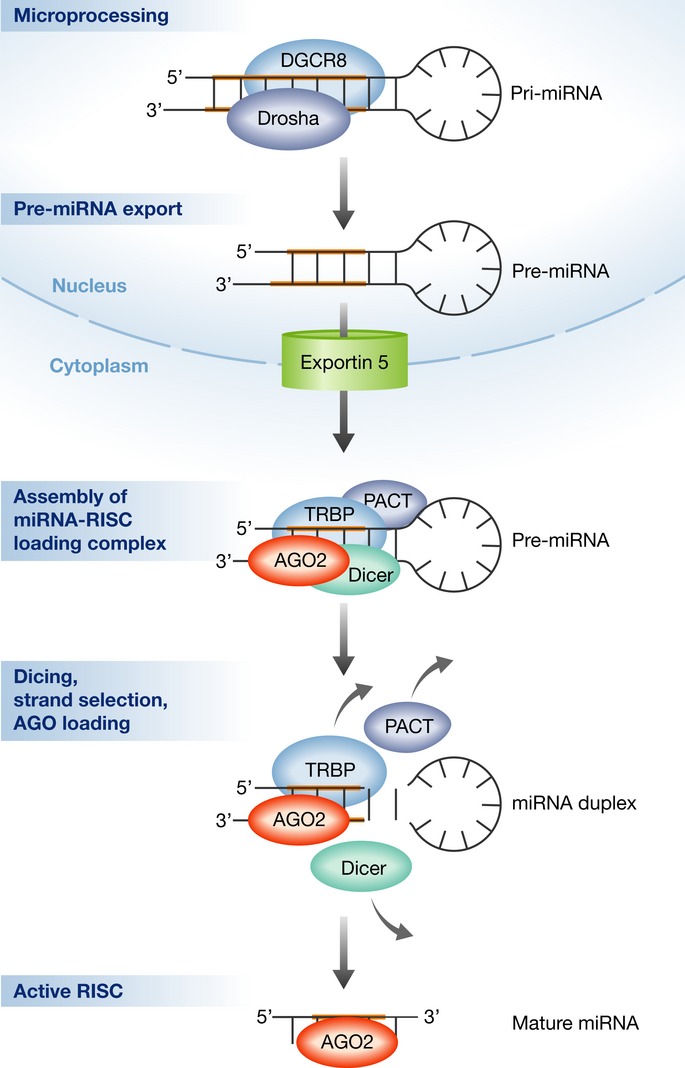
After transcription, an initial pri-miRNA transcript is subject to microprocessing by the Drosha/DGCR8 complex. The resultant intermediate precursor, pre-miRNA, is a ˜70-nt hairpin that is exported from the nucleus into the cytoplasm in a regulated manner by Exportin 5. Assembly of the Dicer complex/miRNA-RISC-loading complex is required for further processing and for AGO loading. Upon disassembly of the RISC-loading complex, AGO is the chief protein factor of an active RISC, which is programmed with a specific miRNA guide strand.
Argonaute RISC catalytic component 2 (AGO2) has dual functions in the processing of miRNA precursors and in target silencing. First, AGO2 functions as a Dicer co-factor in pre-miRNA processing, as part of the RISC-loading complex. Next, AGO2 is loaded with a guide miRNA strand, making an active RISC (Chendrimada et al, 2005; Gregory et al, 2005). Once programmed with a particular miRNA sequence, RISC acts as an effector that facilitates miRNA-dependent mRNA silencing (Fig1). Therefore, miRNA processing and target-RNA repression are physically and functionally interlinked by sharing many common protein components.
miRNA circuitry buffers unwanted gene expression, and loss of miRNA activity enables drift from normal cellular function (Hornstein & Shomron, 2006; Li et al, 2009; Herranz & Cohen, 2010; Mukherji et al, 2011; Pelaez & Carthew, 2012; Cassidy et al, 2013; Siciliano et al, 2013). In recent years, an intimate link between miRNA activity and the cellular stress response has been uncovered and is the focus of this review. We explore the evidence that stress signaling and the miRNA biosynthesis machinery are interlinked at various levels of activity. miRNAs emerge as critical regulators of the stress response, and dysregulation of miRNA expression or activity renders the cell more prone to stress and to its insults.
Stress and stress granule formation
Extreme environmental conditions and chemical stressors invoke a cellular cascade, which reduces the damage and conserves integrity. This adaptive response for coping with stress is executed primarily by transient blockade in translation of most cellular mRNAs and directing cellular RNA metabolism toward damage repair (Ron & Walter, 2007). The phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2a) is the canonical signal for blocking translation initiation and promoting polysome disassembly. Accordingly, the phosphorylation of eIF2a is controlled by stress-activated kinases, PKR, HRI, PERK, or GCN2 (Chen et al, 1991; Dever et al, 1992; Carroll et al, 1993; Harding et al, 1999).
mRNA from disassembling polysomes is often found in cytoplasmic stress granules (SGs), the structural correlate of the stress response (Kedersha et al, 1999; Anderson & Kedersha, 2006; Anderson & Kedersha, 2008; Buchan & Parker, 2009). SGs are composed of stalled translation preinitiation complexes: 40S ribosomal subunits, translation initiation factors (eIFs) and RNA-binding proteins that are involved in other facets of RNA metabolism (Fig 2). Recruitment of proteins into SGs is regulated and in specific conditions is facilitated by so-called ‘piggyback’ protein-protein interactions (Anderson & Kedersha, 2008). Several of the reported SG inhabitant RNA-binding proteins are: poly(A)-binding protein (PABP1), GTPase activating protein (SH3 domain) binding protein (G3BP), Tristetraprolin (TTP), Pumilio, cytoplasmic polyadenylation element binding protein (CPEB), Ataxin-2 (ATXN2), ELAV like RNA-binding protein (HuR), 5′-to-3′ exonuclease (Xrn1), fragile X mental retardation protein (FMRP) and its autosomal homolog FXR, DEAD box polypeptide 6 (DDX6/RCK), polysomal ribonuclease 1 (PMR1/PXDNL), Zipcode-binding protein 1 (ZBP1), TIA-1 and its homolog TIAR, STAUFEN and Fas-activated serine/threonine kinase (FAST). PACT and AGO2, two RISC-loading complex proteins are also found in SGs (Leung et al, 2006; Pare et al, 2009; Johnston et al, 2010).
Figure 2. A diagram of representative components present in stress granules, grouped according to the known protein function.
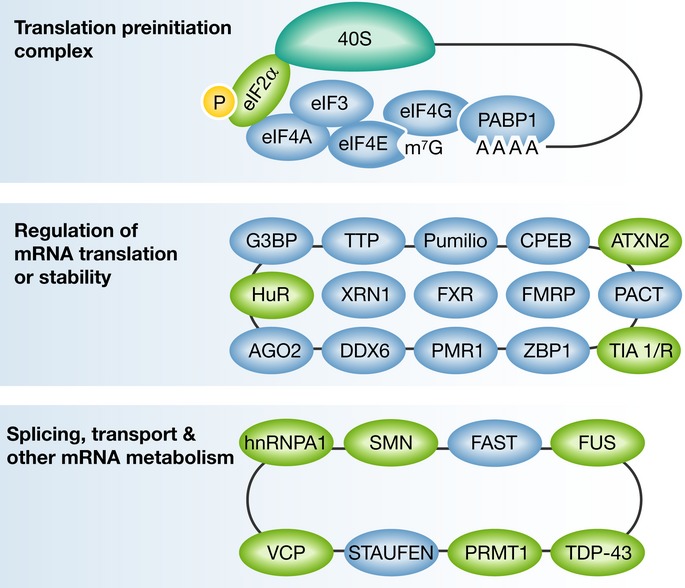
The translation preinitiation complex, including the small ribosomal subunit (40S; upper panel); RNA-binding proteins with role in regulation of mRNA translation or stability (middle panel); Splicing and other mRNA metabolism activities (lower panel). For comprehensive reviews see Anderson & Kedersha (2006, 2008), Buchan & Parker (2009) and references therein. Several of the presented RNA-binding proteins, depicted in green, were suggested to be involved in neurodegeneration. Incomplete list of references, linking particular stress granule components to motor neuron diseases include: eIF2α (Kim et al, 2014); HuR (Lu et al, 2009); TIA1 (Lu et al, 2009); SMN (Hua & Zhou, 2004); TDP-43 (Liu-Yesucevitz et al, 2010; McDonald et al, 2011); FUS (Bosco et al, 2010; Vance et al, 2013); hnRNPA1 (Kim et al, 2013); ATXN2 (Farg et al, 2013); VCP (Johnson et al, 2010; Buchan et al, 2013); PRMT1 (Tradewell et al, 2012; Yamaguchi & Kitajo, 2012).
Several RNA-binding proteins that are identified in SGs are encoded by genes that are mutated in different neurodegenerative states, including TAR DNA-binding protein 43 (TDP-43; Liu-Yesucevitz et al, 2010; McDonald et al, 2011), fused in sarcoma (FUS; Bosco et al, 2010; Vance et al, 2013), heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1; Kim et al, 2013), valosin containing protein (VCP; Johnson et al, 2010; Buchan et al, 2013) and SMN (survival motor neuron 1; Hua & Zhou, 2004). Other SG resident proteins were also linked to the molecular pathogenesis of neurodegeneration, including eukaryotic translation initiation factor 2A (eIF2a; Kim et al, 2014), HuR, TIA1 (Lu et al, 2009), ATXN2 (Farg et al, 2013) and protein arginine methyltransferase 1 (PRMT1; Tradewell et al, 2012; Yamaguchi & Kitajo, 2012). Therefore, SG proteins are intriguingly linked to human neurodegeneration.
The interconnection between miRNAs and the cellular stress response
Association between the Dicer complex and the cellular stress response
Several lines of evidence reveal links between the Dicer complex and the cellular stress response. Stress may be experimentally induced by many reagents. Stressors, including reactive oxygen species (ROS), phorbol ester, UV radiation or type I interferon, cause a reduction in the expression of Dicer (Wiesen & Tomasi, 2009; Mori et al, 2012). These changes in Dicer levels appear to be particularly relevant for further coping with stress, since loss of Dicer function reduces stress tolerance, whereas Dicer over-expression confers stress resistance, at least in invertebrates (Lim et al, 2011; Mori et al, 2012). Therefore, Dicer activity contributes to stress resistance. In addition, Dicer co-factors AGO, PACT and TRBP participate at multiple levels of stress signaling, as summarized in Fig3, substantiating the connection between the Dicer/miRNA-RISC loading complex and the stress response.
Figure 3. Interrelations between the miRNA-bioprocessing machinery, stress and stress granules.
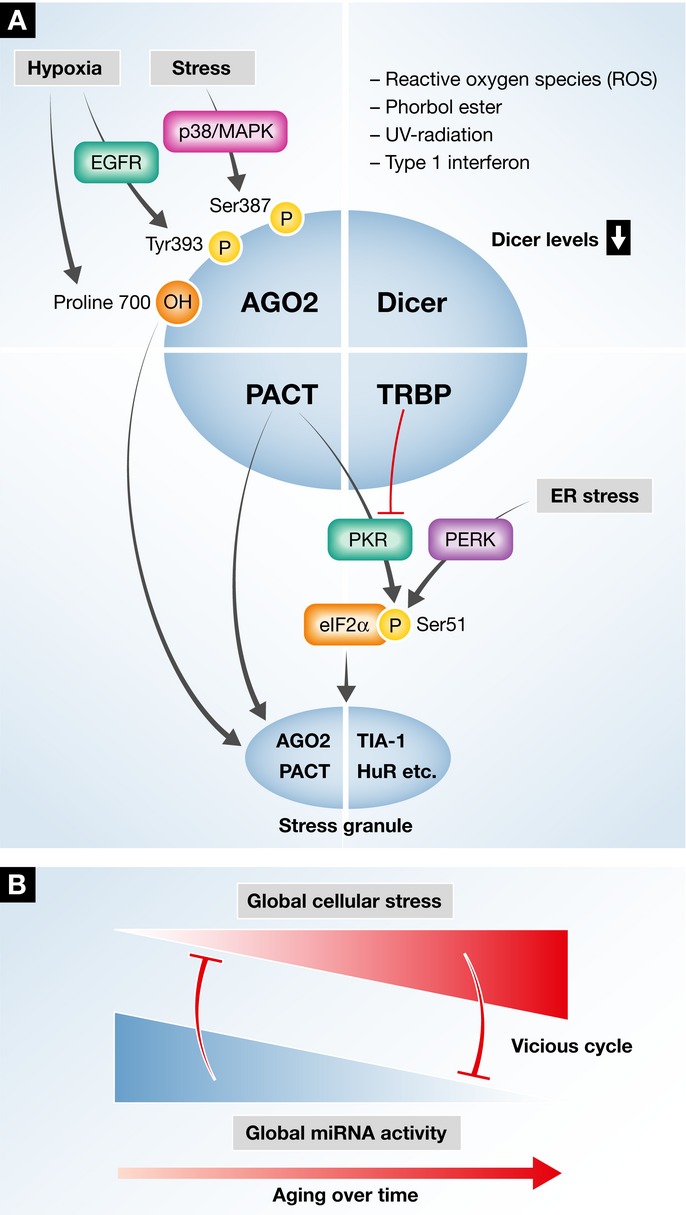
(A) A simplified illustration of the Dicing complex with its documented components: Dicer, AGO2, TRBP and PACT. Stress was suggested to modify AGO2 at a post-translational level: Hypoxia induces Proline 700 hydroxylation, which recruits AGO2 into stress granules. Likewise, EGFR-mediated phosphorylation of Tyrosine 393 leads to impaired miRNA processing of specific pre-miRNAs. Stress-mediated p38/MAPK signaling phosphorylates AGO2 on Serine 387, but the impact of this phosphorylation event on miRNA maturation is not characterized. Several types of cellular stress down-regulate Dicer levels and inhibit miRNA processing. Kinases of eukaryotic translation initiation factor 2 alpha (eIF2a) phosphorylate it on Serine 51, which is a critical step in halting translation and inducing stress granules. eIF2a is phosphorylated by eIF2aK3/PERK as part of the unfolded protein response, in response to endoplasmic reticulum stress (ER stress), or by eIF2aK2/PKR in response to viral doubled-stranded RNA. For simplicity, eIF2aK1 and eIF2aK4 are not mentioned. eIF2aK2/PKR activity is modulated by PACT and TRBP in a reciprocal manner. When stress granules are formed, both PACT and AGO2 are recruited into them. The interactions between cellular stress and the Dicing complex/miRNA-RISC-loading complex core components point toward a dynamic regulation of Dicing activity. (B) Stress signaling potentially alters the function of the miRNA-bioprocessing core components and leads to decompensated regulation. The consequences may include a vicious cycle, whereby stress impairs miRNA processing which in turn renders cells even more prone to stress.
Stress signaling alters AGO2 function by post-translational modifications, and these are suggested to impact RISC and Dicer activity. Epidermal growth factor receptor (EGFR) signaling phosphorylates tyrosine 393 under hypoxic conditions, which reduces the binding of AGO2 to Dicer and inhibits pre-miRNA processing (Shen et al, 2013). In addition, stress-induced P38/MAPK signaling phosphorylates AGO2 at Serine 387 (Zeng et al, 2008). Hypoxic stress regulates AGO2 post-translationally as well, via hydroxylation of proline 700 (Qi et al, 2008), which leads to AGO2 SG localization (Wu et al, 2011). Taken together, it appears that various stress signaling cascades control AGO2 activity and contribute to AGO2 translocation into SGs and to attenuation of miRNA processing. However, further mechanistic studies will be required for understanding of the full picture.
PACT and TRBP are two co-factors of Dicer, which are engaged in the cellular stress response via direct interaction with PKR. PKR is a dsRNA-dependent serine/threonine protein kinase sensor of viral dsRNA that phosphorylates eIF2a on Ser51, thereby causing global reduction of protein synthesis (Taylor et al, 2005; Garcia et al, 2006). PACT and TRBP both contribute to Dicer complex functions but possess distinct, non-redundant, activities (Lee et al, 2013). Intriguingly, they reciprocally modulate PKR, as PACT activates PKR, whereas TRBP inhibits PKR via dsRNA sequestration and direct protein-protein interactions (Park et al, 1994; Cosentino et al, 1995; Patel & Sen, 1998; Lee et al, 2006; Daniels & Gatignol, 2012). The interaction of both TRBP and PACT with PKR links miRNA processing to stress signaling, but it is not known whether or how TRBP and PACT provide crosstalk between these systems.
Stress granules and the miRNA machinery are interlinked
SGs recruit proteins, which are involved in miRNA regulation pathways. This further suggests that SGs are integrated with miRNA-induced translational silencing pathways. Indeed, fragile X mental retardation protein (Caudy et al, 2002; Jin et al, 2004; Li et al, 2008) and Papb1 (Moretti et al, 2012) interact with the miRNA machinery. Furthermore, AGO2 and PACT are recruited into stress granules, a process that depends at least partially on HSP90 activity (Leung et al, 2006; Pare et al, 2009; Johnston et al, 2010). In this context, Leung and Sharp suggested that AGO translocation into SGs may reflect a dynamic change in its activity (Leung et al, 2006). Stress response is extended also to the regulation of RISC activity, by poly-ADP ribosylation and probably other mechanisms. Additional investigations will substantiate initial reports in this field that focus on specific subsets of mRNAs (Leung et al, 2011; Karginov & Hannon, 2013). In addition, we believe that it will be indeed important to test whether the translocation of AGO2 and/or PACT into SGs impairs the various activities of Dicer and the RISC complex, for example, pre-miRNA processing.
The activity of specific miRNA species is involved in the stress response
miRNAs contribute in different contexts to modulation of the stress response, by adjusting the levels of key unfolded protein response (UPR) components. For example, miR-214 regulates ATF4 levels (Wang et al, 2013), and miR-30c-2* is upstream of Xbp1 (Byrd et al, 2012). In addition, maturation of a few miRNA species is downstream of IRE1, one of the three arms of the cellular stress response. IRE1, commonly known for its activity in splicing Xbp1 in the cytoplasm, terminates specific pre-miRNAs by preventing them from being further processed by Dicer into their mature forms (Upton et al, 2012). Therefore, miRNAs fine-tune the expression of components of the UPR signaling cascade and modulate cellular adaptation to stress, which is further reviewed in (Byrd & Brewer, 2013; Chitnis et al, 2013; Maurel & Chevet, 2013).
Additional influential reviews (Mendell & Olson, 2012 and Leung & Sharp, 2007, 2010) describe potential network architectures for the influence of miRNAs on stress signals: (i) miRNAs may dampen a stress signal if wired in a negative feedback loop or (ii) miRNAs may promote pathway activation via the inhibition of negative regulators within a positive-feedback loop. (iii) Finally, miRNAs may generate a threshold in responsiveness, because the relative levels of miRNAs and mRNA targets determine how much target protein is effectively produced (See Fig4).
Figure 4. Potential network architecture for miRNA influence on stress signals.
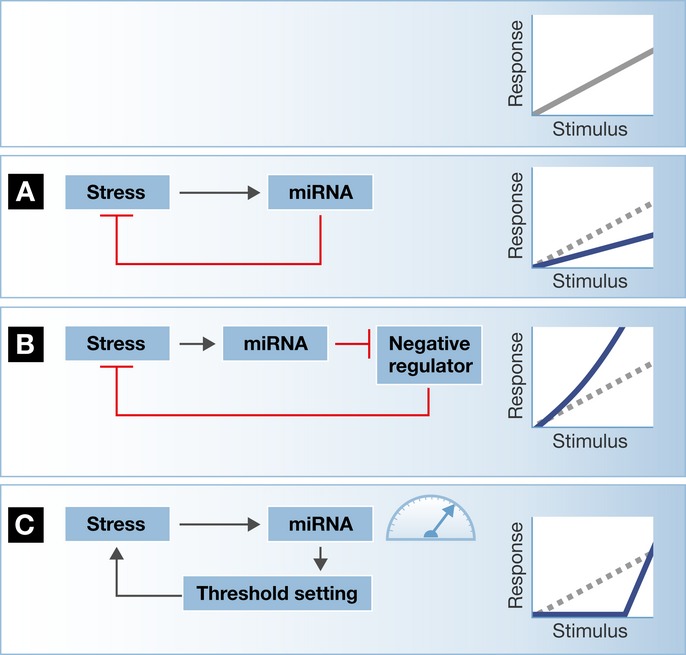
The cellular response to stressful stimuli is modulated by miRNAs. (A) A miRNA may be wired in a negative-feedback loop, inhibiting the expression of a target that is involved in stress signaling. In this case, miRNA activity dampens the cellular stress cascade activity. (B) However, a miRNA may contribute to pathway activation via the inhibition of negative regulators, as part of a positive-feedback loop. (C) The relative levels of miRNAs and their cognate target mRNAs determine how much target protein is effectively produced. Therefore, if the miRNA is in excess, there is effective silencing of the target, which activates the stress cascade with defined threshold. However, either a continuous stress stimulus or a dynamic change in the levels of the miRNA may enable adaptation or sensitization of the cellular stress response. See also reviews by Mendell & Olson (2012) and Leung & Sharp (2007, 2010).
This last aspect of miRNA activity warrants further discussion. Long-lived miRNAs generate threshold for activation, which contributes to filtering transient or low-amplitude signals. Dynamic increase in the expression of a miRNA would contribute to a higher threshold and hence to tolerance toward specific stressor agents or to stress pre-conditioning. Examples for buffering may be observed in regulation of MICA and MICB, two stress-induced ligands that mark cells for destruction. The miR-17˜92 family chronically represses MICA/MICB mRNAs levels, thereby filtering fluctuations in expression under normal conditions. However, when MICA/MICB transcription is induced at higher levels, to an extent that overcomes the threshold of miRNAs, cell destruction ensues (Stern-Ginossar et al, 2008). In another intriguing example, miRNAs encoded by herpes simplex virus 1 continuously repress viral proteins and provide the molecular basis of long-term latency in neurons. In this case, stress enables breakdown of the miRNA-based inhibitory mechanism and allows for reactivation of dormant viruses (Umbach et al, 2008).
miRNA activity and stress signaling in human disease
miRNA activity and stress signaling in animal models
Under favorable laboratory conditions, the loss of miRNA function infrequently exhibits dramatic phenotypes, unless aggravated by stress. Horvitz and co-workers have shown that many C. elegans miRNAs are dispensable under normal conditions and even the elimination of whole miRNA gene families was of surprisingly limited impact. However, genetic knockout of specific miRNAs renders the mutant animals incapable of coping with stress (Miska et al, 2007; Alvarez-Saavedra & Horvitz, 2010). This conclusion holds true also in mammals. For example, loss of miR-208a activity does not affect cardiovascular function, yet it abrogates stress-responsive cardiac remodeling (van Rooij et al, 2007). Likewise, nullification of miR-375, a miRNA highly expressed in the endocrine pancreas, exhibits mild impairments in glucose homeostasis, but these are aggravated by leptin-resistance obesity (Poy et al, 2009). These examples suggest that miRNAs will be particularly relevant to investigations of the response of fully developed tissues to stress (Leung & Sharp, 2010). In the same train of thought, it is plausible that miRNA insufficiency will be particularly evident in disease states.
Stress is a component of many adult-onset diseases
Cellular stress is often considered a vector of pathology. Aberrant protein folding and aggregation ignite UPR, which is evident in many chronic diseases including cardiovascular diseases (Minamino et al, 2010) and diabetes. Diabetes is a common metabolic disease, whereby insulin supply does not meet the body’s demands. The endocrine pancreas is under high demand for insulin synthesis in diabetes. The typical progressive reduction in beta cell mass observed in diabetes requires that the remaining beta cells increase insulin synthesis, which subjects them to increased UPR stress. Accordingly, modulation of the stress response is considered a potential therapeutic target in diabetes. For example, a chemical chaperone of unfolded proteins, sodium phenylbutyrate, was able to reduce UPR/ER stress and to restore glucose homeostasis in a mouse model of diabetes (Ozcan et al, 2006; Ozcan & Tabas, 2012).
Stress is evident also in brain diseases and particularly in neurodegeneration. Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease of the motor system (Al-Chalabi & Hardiman, 2013). Motor neurons in ALS are thought to agonize under stress (Atkin et al, 2008), and ER stress components including Perk (Wang et al, 2011), Gadd34 (Wang et al, 2014), and phosphorylation of eIF2a (Kim et al, 2013) were shown to modulate disease progression in a mouse model of ALS. Moreover, Salubrinal, an ER stress-protective agent, attenuates ALS manifestations and delayed progression in similar disease models (Saxena et al, 2009). Stress is plausibly playing comparable roles in other neurodegenerative states, including Alzheimer’s disease, where deposition of aggregated beta-amyloid triggers ER stress (Lee do et al, 2010).
RNA-binding proteins and stress granules in neurodegeneration
Accumulation of cytoplasmic granules is a patho-histological hallmark of several neurodegenerative diseases, including ALS. Mutations in the genes encoding for RNA-binding proteins TDP-43 and FUS were recently identified as causative in human ALS (Sreedharan et al, 2008; Kwiatkowski et al, 2009; Vance et al, 2009). These RNA-binding proteins are commonly found within SGs (Bosco et al, 2010; Parker et al, 2012; Daigle et al, 2013). TDP-43 is essential for proper SG assembly, and both TDP-43 and FUS interact with SG core components (McDonald et al, 2011; Aulas et al, 2012; Vance et al, 2013). Additionally, the mutated forms of TDP-43 and FUS both have the propensity to enhance aggregation (Bosco et al, 2010; Liu-Yesucevitz et al, 2010), and SGs containing TDP-43 or FUS tend to be more persistent, larger, and more abundant (Vance et al, 2009; Baron et al, 2013). Altogether, ALS-causing mutations in RNA-binding proteins contribute to the emergence of stress granules, which may aggravate other cues for SG formation in neurodegeneration. Importantly, TDP-43 and FUS are involved in miRNA processing. TDP-43 is a co-factor of both Drosha and Dicer, promoting the biogenesis of a subset of miRNAs (Buratti et al, 2010; Kawahara & Mieda-Sato, 2012; Li et al, 2013), and FUS stimulates biogenesis of a specific subset of miRNAs as well (Morlando et al, 2012). Therefore, these specific RNA-binding proteins provide a new intriguing link between miRNA bioprocessing, SGs and neurodegeneration.
Altered SG dynamics occur also in other neurodegenerative diseases. ATXN2 concentration impacts SG assembly by interaction with SG components PABP1 and Dead box helicase 6 (DDX6/RCK) and by regulating the translocation of FUS to the cytoplasm (Nonhoff et al, 2007; Farg et al, 2013). This may be relevant to the increased risk for ALS observed in ATXN2 intermediate-length polyglutamine expansions (Elden et al, 2010). SGs also play a role in Alzheimer’s disease (Vanderweyde et al, 2012), where SG components TIA-1 and tristetraprolin (TTP) bind phospho-tau. DDX6/RCK and ribosomal protein S6 were observed in degenerating granulovacuolar pyramidal neurons (Castellani et al, 2011). Altogether, the modulation of stress and SG formation plays a role in several neurodegenerative diseases, suggesting potential molecular commonalities for these conditions, regardless of the neuronal cell type involved or the particular clinical manifestations.
miRNAs in control of aging and age-related diseases
Aging is often characterized by the declined ability to cope with cellular stress. Several sources of internal cellular stress were suggested to impact longevity. Such insults include impaired mitochondrial activity, excessive production of ROS, DNA damage, and excessive accumulation of misfolded proteins (Finkel & Holbrook, 2000; Ishii et al, 2002; Gerstbrein et al, 2005; Heidler et al, 2009; Brown & Naidoo, 2012; Van Raamsdonk & Hekimi, 2012; Taylor & Dillin, 2013).
miRNAs ensure developmental robustness and homeostasis (Hornstein & Shomron, 2006) and reinforce cellular programs also in the adult life (Li et al, 2009; Pelaez & Carthew, 2012; Cassidy et al, 2013). Therefore, dysregulation of miRNA function may contribute to aging by allowing the activation of aberrant pathways that are repressed by normal miRNA activity in younger individuals (Ibanez-Ventoso & Driscoll, 2009).
One of the most established examples in this emerging field focuses on miR-34, a specific miRNA family with conserved functions in control of aging and age-related diseases. miR-34 is up-regulated in aging nematodes (Kato et al, 2011), and mir-34 over-expression extends Drosophila life span and reduces the propensity to acquire age-related neurodegeneration (Liu et al, 2012). In human neurodegenerative diseases, both up- and down-regulation of miR-34 have been observed. miR-34 is down-regulated in human Parkinson’s disease brains (Minones-Moyano et al, 2011). In contrast, miR-34c is up-regulated in aging mice, in mouse models for Alzheimer’s disease, and in patients. Furthermore, miR-34c over-expression impairs memory, whereas targeting miR-34c rescues memory function in a mouse model for AD-linked amyloid pathology (Zovoilis et al, 2011). miR-34 is also up-regulated in plasma of patients of Huntington’s disease (Gaughwin et al, 2011) and in the organ of Corti in a mouse model of age-related hearing loss (Zhang et al, 2013). Altogether, it appears that miR-34 is involved in regulation of proper aging and of age-related brain diseases, suggesting potentially complex roles, which may include compensatory mechanisms.
miRNA activity per se is also critical in the C. elegans life cycle. Dysregulation of many miRNAs was observed in aging C. elegans with impact on longevity (Ibanez-Ventoso et al, 2006; Kato et al, 2011). A series of exciting studies on this topic from the laboratory of Frank Slack are reviewed in Smith-Vikos & Slack (2012) and Inukai & Slack (2013). One of the earliest works in this field showed that lin-4 and its target, lin-14, regulate the insulin growth factor (IGF)/DAF-16 pathway, which is a primary cascade for control of aging in nematodes (Boehm & Slack, 2005). Intriguingly, the expression of other miRNAs is up-regulated in aging animals, which may be a protective, compensatory response. For example, miR-71, miR-238, and miR-246 prolong life span and at least miR-71 functions via the IGF/DAF-16 (de Lencastre et al, 2010; Pincus et al, 2011; Boulias & Horvitz, 2012).
In addition to the function of individual miRNA genes, a global decrease in miRNA abundance or activity has been observed in aging organisms and suggests that the whole miRNA regulatory network may play a role in aging and late-onset diseases (Ibanez-Ventoso et al, 2006; Drummond et al, 2011; ElSharawy et al, 2012; Inukai et al, 2012; Liu et al, 2012; Mori et al, 2012). In nematodes, miRNA-binding proteins Argonaute-like 1 (alg-1), Dicer, Drosha, and DGCR8/Pasha regulate aging and control life span (Kato et al, 2011; Lehrbach et al, 2012; Mori et al, 2012). The activity of at least one of these miRNA-binding proteins, Dicer, decreases also in aging mammals; Dicer was found to be globally down-regulated in old mouse tissues in comparison with their younger adult counterparts (Nidadavolu et al, 2013).
In another study, Dicer activity was reduced in aging adipocytes of rodents and humans, noting concomitant reduction of miRNA expression (Mori et al, 2012). Likewise, Dicer activity is reduced in cerebro-microvascular endothelial cells of aged rats (Ungvari et al, 2013) and in age-related macular degeneration in humans (Kaneko et al, 2011; Tarallo et al, 2012), although the critical role of Dicer in this model was suggested to regulate ALU mobile elements.
Together, these observations suggest a link between age-related Dicer dysfunction and loss of robust cellular function. Reduction in Dicer levels lessens stress tolerance, whereas increased stress further inhibits Dicer activity (Lim et al, 2011; Mori et al, 2012). Because normal Dicer activity contributes to stress resistance, our proposed model suggests a vicious cycle, in which Dicer activity is progressively reduced as part of the aging process, whereas stress is increasingly built. Over time, cells and organisms are rendered more prone to disease (Fig3).
Conclusion
miRNA regulation and various aspects of the cellular stress response are tied together. The data reviewed above suggest the presence of compensatory mechanisms, whereby miRNA functionality takes part in the physiological stress response, which are required for appropriate execution of the stress response and for proper cellular adaptation. However, stress signaling potentially alters the function of the miRNA-bioprocessing core components and leads to decompensated regulation. The consequences may include a potentially vicious cycle, whereby stress in the aging organism impairs miRNA processing, which in turn renders cells even more prone to stress.
Aging is characterized by progressive decline of robustness and stress resistance. miRNAs are used to ensure cellular robustness and homeostasis (Hornstein & Shomron, 2006; Ibanez-Ventoso & Driscoll, 2009; Pelaez & Carthew, 2012; Cassidy et al, 2013). Alterations of miRNA expression can occur as a response to stress-related phenomena in a variety of diseases. However, currently, it is not clear whether the dysregulation of a single miRNA or a subset of miRNAs presents the cause or the consequence of an altered cellular phenotype. What pathways may modulate dysregulation of miRNAs in aging and disease? Many research efforts will be directed in coming years towards deciphering the role of miRNAs of various aspects of human disease and to uncovering the potential therapeutic activity of manipulating miRNA expression.
Acknowledgments
We apologize to those authors whose articles we were unable to cite because of space limitations. We thank Richard Carthew and Igor Ulitsky for comments on the manuscript. This work at the Hornstein laboratory was supported by grants to E.H. from the ERC consolidator program, Israel Science Foundation, Bruno Frick foundation, Kekst Family Institute for Medical Genetics, David and Fela Shapell Family Center for Genetic Disorders Research, Crown Human Genome Center, Yeda Sela Center, Nella and Leon Benoziyo Center for Neurological Diseases, Y. Leon Benoziyo Institute for Molecular Medicine, Helen and Martin Kimmel Institute for Stem Cell Research, Nathan, Shirley, Philip and Charlene Vener New Scientist Fund, Julius and Ray Charlestein Foundation, Celia Benattar Memorial Fund for Juvenile Diabetes, Fraida Foundation, Wolfson Family Charitable Trust, Legacy Heritage Fund, Adelis Foundation, Minna-James-Heineman Stiftung, MERCK UK, Maria Halphen, Estates of Fannie Sherr, Lola Asseof, Lilly Fulop, Yeda–Sela fund Yeda–CEO fund, Teva NNE program, miCure Therapeutics. A.E. was supported by Deutsche Forschungsgemeinschaft (DFG). E.H. is the incumbent of the Helen and Milton A. Kimmelman Career Development Chair and the laboratory is further supported by Dr. Sydney Brenner and Friends.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- Alvarez-Saavedra E, Horvitz HR. Many families of C. elegans microRNAs are not essential for development or viability. Curr Biol. 2010;20:367–373. doi: 10.1016/j.cub.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis. 2008;30:400–407. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Aulas A, Stabile S, Vande Velde C. Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP. Mol Neurodegener. 2012;7:54. doi: 10.1186/1750-1326-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron DM, Kaushansky LJ, Ward CL, Sama RR, Chian RJ, Boggio KJ, Quaresma AJ, Nickerson JA, Bosco DA. Amyotrophic lateral sclerosis-linked FUS/TLS alters stress granule assembly and dynamics. Mol Neurodegener. 2013;8:30. doi: 10.1186/1750-1326-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Jr, Sapp P, McKenna-Yasek D, Brown RH, Jr, Hayward LJ. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010;19:4160–4175. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulias K, Horvitz HR. The C. elegans microRNA mir-71 acts in neurons to promote germline-mediated longevity through regulation of DAF-16/FOXO. Cell Metab. 2012;15:439–450. doi: 10.1016/j.cmet.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MK, Naidoo N. The endoplasmic reticulum stress response in aging and age-related diseases. Front Physiol. 2012;3:263. doi: 10.3389/fphys.2012.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Kolaitis RM, Taylor JP, Parker R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013;153:1461–1474. doi: 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E, De Conti L, Stuani C, Romano M, Baralle M, Baralle F. Nuclear factor TDP-43 can affect selected microRNA levels. FEBS J. 2010;277:2268–2281. doi: 10.1111/j.1742-4658.2010.07643.x. [DOI] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. MicroRNAs in neurodegeneration. Curr Opin Neurobiol. 2008;18:292–296. doi: 10.1016/j.conb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Byrd AE, Aragon IV, Brewer JW. MicroRNA-30c-2* limits expression of proadaptive factor XBP1 in the unfolded protein response. J Cell Biol. 2012;196:689–698. doi: 10.1083/jcb.201201077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AE, Brewer JW. Micro(RNA)managing endoplasmic reticulum stress. IUBMB Life. 2013;65:373–381. doi: 10.1002/iub.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Elroy-Stein O, Moss B, Jagus R. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2 alpha-specific protein kinase. J Biol Chem. 1993;268:12837–12842. [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JJ, Jha AR, Posadas DM, Giri R, Venken KJ, Ji J, Jiang H, Bellen HJ, White KP, Carthew RW. miR-9a minimizes the phenotypic impact of genomic diversity by buffering a transcription factor. Cell. 2013;155:1556–1567. doi: 10.1016/j.cell.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Gupta Y, Sheng B, Siedlak SL, Harris PL, Coller JM, Perry G, Lee HG, Tabaton M, Smith MA, Wang X, Zhu X. A novel origin for granulovacuolar degeneration in aging and Alzheimer’s disease: parallels to stress granules. Lab Invest. 2011;91:1777–1786. doi: 10.1038/labinvest.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy AA, Myers M, Hannon GJ, Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Throop MS, Gehrke L, Kuo I, Pal JK, Brodsky M, London IM. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc Natl Acad Sci U S A. 1991;88:7729–7733. doi: 10.1073/pnas.88.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to AGO2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis N, Pytel D, Diehl JA. UPR-inducible miRNAs contribute to stressful situations. Trends Biochem Sci. 2013;38:447–452. doi: 10.1016/j.tibs.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino GP, Venkatesan S, Serluca FC, Green SR, Mathews MB, Sonenberg N. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc Natl Acad Sci U S A. 1995;92:9445–9449. doi: 10.1073/pnas.92.21.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle JG, Lanson NA, Jr, Smith RB, Casci I, Maltare A, Monaghan J, Nichols CD, Kryndushkin D, Shewmaker F, Pandey UB. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum Mol Genet. 2013;22:1193–1205. doi: 10.1093/hmg/dds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SM, Gatignol A. The multiple functions of TRBP, at the hub of cell responses to viruses, stress, and cancer. Microbiol Mol Biol Rev. 2012;76:652–666. doi: 10.1128/MMBR.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA, Rasmussen BB. Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genomics. 2011;43:595–603. doi: 10.1152/physiolgenomics.00148.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSharawy A, Keller A, Flachsbart F, Wendschlag A, Jacobs G, Kefer N, Brefort T, Leidinger P, Backes C, Meese E, Schreiber S, Rosenstiel P, Franke A, Nebel A. Genome-wide miRNA signatures of human longevity. Aging Cell. 2012;11:607–616. doi: 10.1111/j.1474-9726.2012.00824.x. [DOI] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Farg MA, Soo KY, Warraich ST, Sundaramoorthy V, Blair IP, Atkin JD. Ataxin-2 interacts with FUS and intermediate-length polyglutamine expansions enhance FUS-related pathology in amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22:717–728. doi: 10.1093/hmg/dds479. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughwin PM, Ciesla M, Lahiri N, Tabrizi SJ, Brundin P, Bjorkqvist M. Hsa-miR-34b is a plasma-stable microRNA that is elevated in pre-manifest Huntington’s disease. Hum Mol Genet. 2011;20:2225–2237. doi: 10.1093/hmg/ddr111. [DOI] [PubMed] [Google Scholar]
- Gerstbrein B, Stamatas G, Kollias N, Driscoll M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 2005;4:127–137. doi: 10.1111/j.1474-9726.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Haase AD, Jaskiewicz L, Zhang H, Lainé S, Sack R, Gatignol A, Filipowicz W. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Heidler T, Hartwig K, Daniel H, Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2009;11:183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- Herranz H, Cohen SM. MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev. 2010;24:1339–1344. doi: 10.1101/gad.1937010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet. 2006;38(Suppl):S20–S24. doi: 10.1038/ng1803. [DOI] [PubMed] [Google Scholar]
- Hua Y, Zhou J. Survival motor neuron protein facilitates assembly of stress granules. FEBS Lett. 2004;572:69–74. doi: 10.1016/j.febslet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Ibanez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ibanez-Ventoso C, Driscoll M. MicroRNAs in C. elegans aging: molecular insurance for robustness? Curr Genomics. 2009;10:144–153. doi: 10.2174/138920209788185243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai S, de Lencastre A, Turner M, Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS One. 2012;7:e40028. doi: 10.1371/journal.pone.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai S, Slack F. MicroRNAs and the genetic network in aging. J Mol Biol. 2013;425:3601–3608. doi: 10.1016/j.jmb.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Goto S, Hartman PS. Protein oxidation during aging of the nematode Caenorhabditis elegans. Free Radic Biol Med. 2002;33:1021–1025. doi: 10.1016/s0891-5849(02)00857-2. [DOI] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, Ding J, McCluskey L, Martinez-Lage M, Falcone D, Hernandez DG, Arepalli S, Chong S, Schymick JC, Rothstein J, Landi F, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M, Geoffroy MC, Sobala A, Hay R, Hutvagner G. HSP90 protein stabilizes unloaded argonaute complexes and microscopic P-bodies in human cells. Mol Biol Cell. 2010;21:1462–1469. doi: 10.1091/mbc.E09-10-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, Kariko K, Yoo JW, Lee DK, Hadziahmetovic M, Song Y, Misra S, Chaudhuri G, Buaas FW, Braun RE, Hinton DR, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karginov FV, Hannon GJ. Remodeling of AGO2-mRNA interactions upon cellular stress reflects miRNA complementarity and correlates with altered translation rates. Genes Dev. 2013;27:1624–1632. doi: 10.1101/gad.215939.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Chen X, Inukai S, Zhao H, Slack FJ. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA. 2011;17:1804–1820. doi: 10.1261/rna.2714411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Mieda-Sato A. TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc Natl Acad Sci U S A. 2012;109:3347–3352. doi: 10.1073/pnas.1112427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002;13:195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, Kanagaraj AP, Carter R, Boylan KB, Wojtas AM, Rademakers R, Pinkus JL, Greenberg SA, Trojanowski JQ, Traynor BJ, Smith BN, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Raphael AR, Ladow ES, McGurk L, Weber RA, Trojanowski JQ, Lee VM, Finkbeiner S, Gitler AD, Bonini NM. Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet. 2014;46:152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscianska E, Starega-Roslan J, Krzyzosiak WJ. The role of Dicer protein partners in the processing of microRNA precursors. PLoS One. 2011;6:e28548. doi: 10.1371/journal.pone.0028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee do Y, Lee KS, Lee HJ, Kim do H, Noh YH, Yu K, Jung HY, Lee SH, Lee JY, Youn YC, Jeong Y, Kim DK, Lee WB, Kim SS. Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS One. 2010;5:e10489. doi: 10.1371/journal.pone.0010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Zhou K, Smith AM, Noland CL, Doudna JA. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013;41:6568–6576. doi: 10.1093/nar/gkt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach NJ, Castro C, Murfitt KJ, Abreu-Goodger C, Griffin JL, Miska EA. Post-developmental microRNA expression is required for normal physiology, and regulates aging in parallel to insulin/IGF-1 signaling in C. elegans. RNA. 2012;18:2220–2235. doi: 10.1261/rna.035402.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lencastre A, Pincus Z, Zhou K, Kato M, Lee SS, Slack FJ. MicroRNAs both promote and antagonize longevity in C. elegans. Curr Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Calabrese JM, Sharp PA. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci U S A. 2006;103:18125–18130. doi: 10.1073/pnas.0608845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. microRNAs: a safeguard against turmoil? Cell. 2007;130:581–585. doi: 10.1016/j.cell.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lin L, Jin P. The microRNA pathway and fragile X mental retardation protein. Biochim Biophys Acta. 2008;1779:702–705. doi: 10.1016/j.bbagrm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cassidy JJ, Reinke CA, Fischboeck S, Carthew RW. A microRNA imparts robustness against environmental fluctuation during development. Cell. 2009;137:273–282. doi: 10.1016/j.cell.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lu Y, Xu XL, Gao FB. The FTD/ALS-associated RNA-binding protein TDP-43 regulates the robustness of neuronal specification through microRNA-9a in Drosophila. Hum Mol Genet. 2013;22:218–225. doi: 10.1093/hmg/dds420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DH, Oh CT, Lee L, Hong JS, Noh SH, Hwang S, Kim S, Han SJ, Lee YS. The endogenous siRNA pathway in Drosophila impacts stress resistance and lifespan by regulating metabolic homeostasis. FEBS Lett. 2011;585:3079–3085. doi: 10.1016/j.febslet.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, Petrucelli L, Wolozin B. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Landreh M, Cao K, Abe M, Hendriks GJ, Kennerdell JR, Zhu Y, Wang LS, Bonini NM. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature. 2012;482:519–523. doi: 10.1038/nature10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang S, Zheng L, Li X, Suswam EA, Zhang X, Wheeler CG, Nabors LB, Filippova N, King PH. Amyotrophic lateral sclerosis-linked mutant SOD1 sequesters Hu antigen R (HuR) and TIA-1-related protein (TIAR): implications for impaired post-transcriptional regulation of vascular endothelial growth factor. J Biol Chem. 2009;284:33989–33998. doi: 10.1074/jbc.M109.067918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M, Chevet E. Endoplasmic reticulum stress signaling: the microRNA connection. Am J Physiol Cell Physiol. 2013;304:C1117–C1126. doi: 10.1152/ajpcell.00061.2013. [DOI] [PubMed] [Google Scholar]
- Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet. 2002;11:3007–3017. doi: 10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, Rouleau GA, Vande Velde C. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 2011;20:1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- Minones-Moyano E, Porta S, Escaramis G, Rabionet R, Iraola S, Kagerbauer B, Espinosa-Parrilla Y, Ferrer I, Estivill X, Marti E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum Mol Genet. 2011;20:3067–3078. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Abbott AL, Lau NC, Hellman AB, McGonagle SM, Bartel DP, Ambros VR, Horvitz HR. Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet. 2007;3:e215. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti F, Kaiser C, Zdanowicz-Specht A, Hentze MW. PABP and the poly(A) tail augment microRNA repression by facilitated miRISC binding. Nat Struct Mol Biol. 2012;19:603–608. doi: 10.1038/nsmb.2309. [DOI] [PubMed] [Google Scholar]
- Mori MA, Raghavan P, Thomou T, Boucher J, Robida-Stubbs S, Macotela Y, Russell SJ, Kirkland JL, Blackwell TK, Kahn CR. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16:336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlando M, Dini Modigliani S, Torrelli G, Rosa A, Di Carlo V, Caffarelli E, Bozzoni I. FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. EMBO J. 2012;2012:4502–4510. doi: 10.1038/emboj.2012.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nat Genet. 2011;43:854–859. doi: 10.1038/ng.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidadavolu LS, Niedernhofer LJ, Khan SA. Identification of microRNAs dysregulated in cellular senescence driven by endogenous genotoxic stress. Aging (Albany NY) 2013;5:460–473. doi: 10.18632/aging.100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonhoff U, Ralser M, Welzel F, Piccini I, Balzereit D, Yaspo ML, Lehrach H, Krobitsch S. Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol Biol Cell. 2007;18:1385–1396. doi: 10.1091/mbc.E06-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–328. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare JM, Tahbaz N, Lopez-Orozco J, LaPointe P, Lasko P, Hobman TC. Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies. Mol Biol Cell. 2009;20:3273–3284. doi: 10.1091/mbc.E09-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Davies MV, Langland JO, Chang HW, Nam YS, Tartaglia J, Paoletti E, Jacobs BL, Kaufman RJ, Venkatesan S. TAR RNA-binding protein is an inhibitor of the interferon-induced protein kinase PKR. Proc Natl Acad Sci U S A. 1994;91:4713–4717. doi: 10.1073/pnas.91.11.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SJ, Meyerowitz J, James JL, Liddell JR, Crouch PJ, Kanninen KM, White AR. Endogenous TDP-43 localized to stress granules can subsequently form protein aggregates. Neurochem Int. 2012;60:415–424. doi: 10.1016/j.neuint.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaez N, Carthew RW. Biological robustness and the role of microRNAs: a network perspective. Curr Top Dev Biol. 2012;99:237–255. doi: 10.1016/B978-0-12-387038-4.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus Z, Smith-Vikos T, Slack FJ. MicroRNA predictors of longevity in Caenorhabditis elegans. PLoS Genet. 2011;7:e1002306. doi: 10.1371/journal.pgen.1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi HH, Ongusaha PP, Myllyharju J, Cheng D, Pakkanen O, Shi Y, Lee SW, Peng J. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature. 2008;455:421–424. doi: 10.1038/nature07186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, Du Y, Wang Y, Chang WC, Chen CH, Hsu JL, Wu Y, Lam YC, James BP, Liu X, Liu CG, Patel DJ, Hung MC. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–387. doi: 10.1038/nature12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano V, Garzilli I, Fracassi C, Criscuolo S, Ventre S, di Bernardo D. MiRNAs confer phenotypic robustness to gene networks by suppressing biological noise. Nat Commun. 2013;4:2364. doi: 10.1038/ncomms3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 2012;125(Pt 1):7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9:1065–1073. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Haste NM, Ghosh G. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell. 2005;122:823–825. doi: 10.1016/j.cell.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153:1435–1447. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tradewell ML, Yu Z, Tibshirani M, Boulanger MC, Durham HD, Richard S. Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations. Hum Mol Genet. 2012;21:136–149. doi: 10.1093/hmg/ddr448. [DOI] [PubMed] [Google Scholar]
- Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:877–891. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, Oakes SA. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci U S A. 2012;109:5785–5790. doi: 10.1073/pnas.1116158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Scotter EL, Nishimura AL, Troakes C, Mitchell JC, Kathe C, Urwin H, Manser C, Miller CC, Hortobagyi T, Dragunow M, Rogelj B, Shaw CE. ALS mutant FUS disrupts nuclear localization and sequesters wild-type FUS within cytoplasmic stress granules. Hum Mol Genet. 2013;22:2676–2688. doi: 10.1093/hmg/ddt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderweyde T, Yu H, Varnum M, Liu-Yesucevitz L, Citro A, Ikezu T, Duff K, Wolozin B. Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies. J Neurosci. 2012;32:8270–8283. doi: 10.1523/JNEUROSCI.1592-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Popko B, Roos RP. The unfolded protein response in familial amyotrophic lateral sclerosis. Hum Mol Genet. 2011;20:1008–1015. doi: 10.1093/hmg/ddq546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Popko B, Roos RP. An enhanced integrated stress response ameliorates mutant SOD1-induced ALS. Hum Mol Genet. 2014;23:2629–2638. doi: 10.1093/hmg/ddt658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A, Li D, Hou Z, Lv K, Kan G, Cao H, Wu H, Song J, Pan X, Sun Q, Ling S, Li Y, Zhu M, Zhang P, Peng S, et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19:93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- Wiesen JL, Tomasi TB. Dicer is regulated by cellular stresses and interferons. Mol Immunol. 2009;46:1222–1228. doi: 10.1016/j.molimm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, So J, Davis-Dusenbery BN, Qi HH, Bloch DB, Shi Y, Lagna G, Hata A. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol. 2011;31:4760–4774. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kitajo K. The effect of PRMT1-mediated arginine methylation on the subcellular localization, stress granules, and detergent-insoluble aggregates of FUS/TLS. PLoS One. 2012;7:e49267. doi: 10.1371/journal.pone.0049267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Sankala H, Zhang X, Graves PR. Phosphorylation of Argonaute 2 at serine-387 facilitates its localization to processing bodies. Biochem J. 2008;413:429–436. doi: 10.1042/BJ20080599. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu H, McGee J, Walsh EJ, Soukup GA, He DZ. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS One. 2013;8:e62786. doi: 10.1371/journal.pone.0062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I, Schmitt A, Falkai P, Bahari-Javan S, Burkhardt S, Sananbenesi F, Fischer A. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30:4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]


