Abstract
The sophistication of the editing mechanisms that prevent gene translation errors indicates that amino acid misincorporation is generally a problem to be avoided. Mistranslation is considered invariably deleterious and often caused by confusion between similar proteogenic amino acids. These views are being challenged. The evidence linking misincorporation of dietary non-proteogenic amino acids to human disease continues to grow, and a report in this issue of The EMBO Journal demonstrates the importance of preventing non-proteogenic amino acid misincorporation for cellular homeostasis (Cvetesic et al, 2014).
See also: N Cvetesic et al (August 2014)
The genetic code holds the key to translate 64 codons into 20-odd amino acids. The enzymes that aminoacylate tRNAs, aminoacyl-tRNA synthetases (ARS), are the keepers of the code as they create the molecular link between amino acids and triplet information in the tRNA. ARS form two families of enzymes with a peculiar symmetric organization that clusters them in groups that recognize chemically similar amino acids. These two families possibly emerged from an ancestral complex of two proteins around a single tRNA molecule that evolved to increase the number of cognate substrates as the genetic code grew to its extant size. This expansion in cognate substrates logically involved the gradual incorporation of relatively similar side chains to those that were previously used (Ribas de Pouplana & Schimmel, 2001).
The extent to which some proteogenic amino acids are similar to each other—as well as the structural organization of the ARS themselves—explain the difficulty in discriminating between certain residues during tRNA aminoacylation. To make matters worse, several nonprotein amino acids, which are ubiquitous in many cellular metabolic pathways, can also be mistakenly incorporated into proteins through ARS recognition errors that also require editing reactions to be corrected (Jakubowski, 2012).
Linus Pauling was the first to note that the chemical proximity between some side chains makes it impossible for ARS to discriminate between them with a tolerable error rate (Pauling, 1958). Hence the necessity of editing activities to remove incorrectly charged amino acids was postulated. A “second sieve” model for aminoacylation editing was proposed by Alan Fersht, and later proven to exist in several ARS (reviewed in Yadavalli & Ibba, 2012).
Valine, isoleucine, and leucine are good examples of amino acids requiring proofreading due to their chemical similarity. The discovery of a common editing domain shared by the ARS cognate to these three residues reinforced the notion that misincorporations would mostly involve related proteogenic amino acids, and that such errors always need to be corrected. However, mistranslation need not be limited to proteogenic amino acids and, in some cases, it may offer adaptive advantages to cells.
In this issue of The EMBO Journal Gruic-Sovulj and colleagues elegantly demonstrate that the editing domain of leucyl-tRNA synthetase (LeuRS) is not designed to fend off the misincorporation of isoleucine, as was previously thought. Earlier reports that suggested otherwise were marred by an unsuspected contamination of leucine in commercial preparations of isoleucine. Once the contaminating cognate amino acid is removed from the reaction the authors clearly show, by kinetic, structural, thermodynamic and in vivo approaches, that isoleucine is in fact a very poor substrate for LeuRS, which gets discriminated early in the reaction cycle and is not incorporated (substantially) to tRNA (Cvetesic et al, 2014). This clearly obviates a need for isoleucine editing (Fig1).
Figure 1. Three different recognition pathways are used by leucyl-tRNA synthetase to discriminate among the amino acids leucine, isoleucine, and norvaline#.
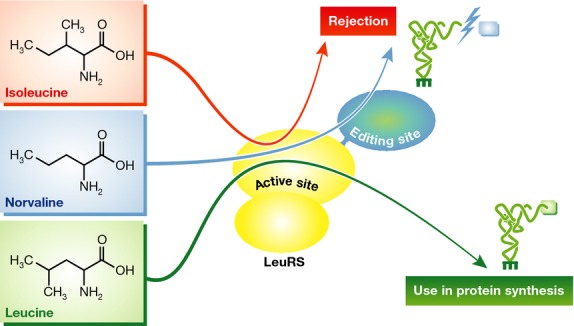
Contrary to previous belief isoleucine is very effectively discriminated by the synthetic active site of LeuRS, and is not activated nor transferred to tRNALeu. Norvaline is easily charged to tRNA, and requires a posterior docking into the editing domain of the enzyme to prevent its incorporation into proteins.
Norvaline, a non proteogenic amino acid that in microaerobic conditions accumulates in the cytosol of E. coli (Soini et al, 2008) is, on the other hand, an excellent analog of leucine and is readily mischarged to tRNALeu by LeuRS. However, accumulation of norvaline-tRNALeu is prevented by the editing domain of LeuRS (Cvetesic et al, 2012).
A beautiful physiologic explanation to this biochemistry is offered in the paper when the authors show that E. coli grown under aerobic conditions do not require editing by LeuRS, whereas this activity becomes essential when intracellular concentrations of norvaline increase as a result of growth in microaerobic conditions.
Norvaline thus joins the ranks of non-proteogenic amino acids that can be misincorporated into proteins and cause toxicity. Indeed, recent reports have established links between several types of human neurodegeneration and the ingestion of non-proteogenic amino acids. For example, beta-methylamino-L-alanine is an amino acid analog taken up in the diet, and mischarged by seryl-tRNA synthetases respectively due to its similarity to serine (Dunlop et al, 2013). It is still unclear why the nervous system is more affected by this insult than other tissues.
Opposite to the previous examples, a body of literature is also starting to accumulate that reports on cellular strategies that utilize mistranslation to improve biologic fitness. For example, the adaptive nature of random variations in the proteome caused by amino acid misincorporation has been demonstrated in Candida albicans. The proteome of this pathogenic fungus undergoes generalized serine to leucine substitutions as an adaptive strategy that increases the virulence of this species (Moura et al, 2010).
The existence of an adaptive mistranslation has been confirmed in bacteria and human cells, and we now know that the mis-methiolation of proteins is a strategy used across the phylogenetic tree to minimize the damage caused by oxidative stress (Pan, 2013). Thus, amino acid misincorporation needs not be a deleterious mistake, but can sometimes be seen as a beneficial relaxation in translation fidelity that increases the fitness of the organism.
Correction added on 30 June 2014, after first online publication. In the figure, “Valine” was corrected to “Leucine”, and “editing side” was corrected to “editing site”.
References
- Cvetesic N, Palencia A, Halasz I, Cusack S, Gruic-Sovulj I. The physiological target for LeuRS translational quality control is norvaline, not isoleucine. EMBO J. 2014;33:1639–1653. doi: 10.15252/embj.201488199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetesic N, Perona JJ, Gruic-Sovulj I. J Biol Chem. 2012;287:25381–25394. doi: 10.1074/jbc.M112.372151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop RA, Cox PA, Banack SA, Rodgers KJ. The non-protein amino acid BMAA is misincorporated into human proteins in place of L-serine causing protein misfolding and aggregation. PLoS ONE. 2013;8:e75376. doi: 10.1371/journal.pone.0075376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski H. Quality control in tRNA charging. Wiley Interdiscip Rev RNA. 2012;3:295–310. doi: 10.1002/wrna.122. [DOI] [PubMed] [Google Scholar]
- Moura GR, Paredes JA, Santos MA. Development of the genetic code: insights from a fungal codon reassignment. FEBS Lett. 2010;584:334–341. doi: 10.1016/j.febslet.2009.11.066. [DOI] [PubMed] [Google Scholar]
- Pan T. Adaptive translation as a mechanism of stress response and adaptation. Annu Rev Genet. 2013;47:121–137. doi: 10.1146/annurev-genet-111212-133522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L. The probability of errors in protein synthesis. In: Pauling L, editor. Festschrift Arthur Stöll Siebzigsten Geburtstag. Basel: Birkhauser Verlag; 1958. p. 597. (ed) [Google Scholar]
- Ribas de Pouplana L, Schimmel P. Aminoacyl-tRNA synthetases: potential markers of genetic code development. Trends Biochem Sci. 2001;26:591–596. doi: 10.1016/s0968-0004(01)01932-6. [DOI] [PubMed] [Google Scholar]
- Soini J, Falschlehner C, Liedert C, Bernhardt J, Vuoristo J, Neubauer P. Norvaline is accumulated after a down-shift of oxygen in Escherichia coli W3110. Microb Cell Fact. 2008;7:30. doi: 10.1186/1475-2859-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadavalli SS, Ibba M. Quality control in aminoacyl-tRNA synthesis its role in translational fidelity. Adv Protein Chem Struct Biol. 2012;86:1–43. doi: 10.1016/B978-0-12-386497-0.00001-3. [DOI] [PubMed] [Google Scholar]


